Abstract
Three experiments examined whether a drug state serving as a positive feature for pairings between a discrete conditional stimulus (CS, 15-s light or 15-s noise) and sucrose could transfer facilitative control to a CS with which it had never been presented. To do so, a CS was paired with a sucrose reward in the nicotine (0.4 mg/kg), amphetamine (AMP, 1 mg/kg), or chlordiazepoxide (CDP, 5 mg/kg) drug state; in separate saline sessions the CS was presented but was not followed by any reward. All three drug states facilitated responding to a discrete CS; previous studies found that this facilitation did not depend on direct associations between the drug state and sucrose. When a second discrimination was trained (e.g., CDP: light-sucrose and nicotine: noise-sucrose) the drug states facilitated responding to the CS trained in that state (nicotine: noise) as well as the CS normally presented in the other drug state (e.g., nicotine: light). A novel drug state (e.g., amphetamine) did not affect responding to either CS, indicating that the originally trained drug states had acquired functional similarity based on learning history. Also, a novel or ambiguous CS did not evoke responding in the previously trained drug state, indicating that both the features (drug states) and target conditional stimuli had to be trained in discriminations before transfer could occur.
Introduction
Current models of drug addiction place a great deal of emphasis on the acquired reward of behaviors and environmental stimuli associated with drug effects [2, 19, 24, 35]. Cues and responses associated with drug effects acquire motivational value. The resulting change in valence is considered an important component of chronic drug use and the difficulty quitting experienced by drug-dependent individuals – the drug ‘cues’ and acts or rituals associated with drug taking become important contributors to the effect of the drug and may serve as triggers that evoke drug-seeking during periods of abstinence. Unfortunately, therapies that strive to alter the relation between the effects of drugs and their associated stimuli and behaviors (e.g., cue-exposure or extinction therapies) have not proven to be more successful than other treatments [17].
One reason for the equivocal success of exposure therapies may be the emerging complexity of associative learning; thus traditional exposure therapies targeting ‘drug cues’ may not fully divest drug-associated stimuli of their acquired meaning. A recent review by Conklin and Tiffany [17] focused on several findings that explain the resurgent or persistent effects of ‘cues’ despite treatments which attempt to reduce their value or change their meaning. They argued that exposure therapies, especially cue-exposure, are tantamount to extinction of Pavlovian conditioning – a decrease in conditional responding (CR; e.g., craving) occurs when reward-related conditional stimuli (CSs; e.g., cues) are presented repeatedly in the absence of the unconditional stimulus (US; e.g., drug effect). Even after extinction treatment, responding evoked by the CS can be re-invigorated by the simple passage of time (spontaneous recovery [12]), a change in physical ‘context’(renewal [10]), or after brief re-exposure to the US (reinstatement [13, 40]). This renewed CR would represent a threat to the success of exposure therapies (i.e., threats to treatments involving extinction) and a potential source of relapse [17].
Other threats to the success of exposure therapies may depend upon the circumstances in which the original learning occurred. For example, some stimuli set the occasion for associations between two other events, and are thus ignored or unaffected during extinction [6, 28]. The term ‘occasion setter’ typically refers to stimuli that inform the relation between a target CS and the occurrence or non-occurrence of a US. In feature-positive occasion setting, the CS and US are paired in the presence of an occasion setter. In contrast, presentations of the CS in the absence of the occasion setter are not followed by the US. Thus, the CS has been described as an ambiguous target; responding to this target depends on or is facilitated by presentation of the occasion setter.
Occasion setters have two important properties that are relevant to extinction based treatments such as exposure therapy. First, they can set the occasion for CS-US associations regardless of their own associative strength. For example, a feature-positive occasion setter precedes or occurs with the US on every learning trial, meaning that the occasion setter may have weak excitatory properties. However, even when this excitation is abolished with an extinction treatment, the occasion setter can still facilitate responding to the target CS. Second, within certain limits, occasion setters are able to transfer their control over responding to novel target CSs. For example, if an occasion setter signaled when a tone stimulus would be followed by a US (i.e., sucrose delivery) then this occasion setter would also facilitate conditional responses to other target CSs (e.g., light stimulus) that had been associated with sucrose delivery in the past. One limitation of this latter phenomenon is that the new target CS usually needs to participate in a comparable occasion setting discrimination before transfer can occur – meaning that some other feature must have previously set the occasion for responding to this CS.
These properties of occasion setters are relevant to addictions and drugs of abuse in any case where a drug-related CS is ambiguous and contextual stimuli may inform their relationship to a drug effect [6]. For example, cigarette packaging may be a CS for the effects of nicotine in some contexts (e.g., automobile, break area, etc.) but not in others (e.g., office or workplace, places with flammable or combustible materials, etc.). Under these circumstances, an occasion setting discrimination may inform when the CS is expected to evoke the strongest conditional response (i.e., craving).
We have previously extended the role of contextual occasion setters to include drug contexts [6, 28]. Drug states represent a class of stimuli that may be biased toward an occasion setting function; they have graded intensity, multimodal impact on sensory systems, and depending on their route of administration and pharmacological impact they turn ‘on’ and ‘off’ rather slowly. These characteristics make them particularly relevant as modulators of associative learning, perhaps sharing characteristics with external contextual stimuli [10]. Drug states serve as discriminative stimuli and can modulate the acquisition and expression of responses in Pavlovian and operant conditioning paradigms [4, 22, 23, 25, 31–34]. In recent studies we have extended the discriminant role of drug contexts to appetitive Pavlovian conditioning with rats using nicotine, amphetamine, and chlordiazepoxide (CDP) drug states [28–30]. In the drug context a discrete CS (15-s noise) was immediately followed by 4-s access to 26% sucrose. Outside of the drug context (vehicle injected before the session) the same CS was not followed by any scheduled outcome. The CS evoked anticipatory food-seeking (i.e., goal tracking [7, 20]) in a manner that was specific to the drug state. Initial studies found that maximal goal tracking was evoked at the training dose (e.g., 0.4 mg/kg nicotine, 1 mg/kg amphetamine, 5 mg/kg CDP) and decreasing each drug’s dose decreased the rate of goal tracking [29, 30]. Also, drugs with different pharmacological properties (i.e., act at different receptors) did not substitute for one another [30]. Finally, conditioned responding was evoked in the drug state by both visual (i.e., light-on) and auditory (i.e., noise-on) conditional stimuli [30].
The present studies sought to determine whether nicotine, amphetamine, and CDP functioned as occasion setters. These particular drugs were chosen because their dose-response and substitution profiles were already described [29, 30]. In a related series of experiments [28] we determined that facilitation by nicotine, amphetamine, and CDP did not depend on direct associations between the drug context and sucrose US. Repeated presentation of the drug context alone (i.e., drug extinction) attenuated drug-evoked goal tracking but did not change the facilitative function of the drug state (i.e., it still increased responding when the CS was reintroduced [28]). Therefore, in this series of experiments we investigated whether drug states would transfer their facilitative control over goal tracking to conditional stimuli with which they had never previously been presented. Experiments 1 and 2 trained two discriminations in the same subjects (e.g., nicotine: noise-sucrose and CDP: light-sucrose). After establishing that both drug states facilitated goal tracking to different stimuli, we determined whether novel drug/CS combinations evoked similar levels of goal tracking (i.e., transfer test). For example, the original drug/CS combinations were presented (nicotine: noise) or a CS that normally occurred in one drug state (CDP: light) was presented in the other drug state (nicotine: light).
One important aspect of these transfer tests is that there are a number of potential explanations for positive results. For example, drug states with similar pharmacological characteristics may ‘substitute’ for one another, even if they are not made functionally equivalent during the training phase. For this reason, additional tests determined whether a novel drug state would facilitate responding to the conditional stimulus. Another potential explanation for transfer is that, during the training phase rats learned something non-specific about the drug states. For example, they could learn that any stimulus presented in a drug state signals sucrose delivery. In discriminated taste aversion studies, a drug state that sets the occasion for saccharin-lithium pairings will also decrease intake of familiar (water) and novel (vinegar) taste stimuli [36]. Therefore, Experiment 3 determined whether novel or ambiguous conditional stimuli would be facilitated by a previously trained drug context.
Method
Subjects
Forty-four male Sprague Dawley rats were obtained from Harlan (Indianapolis, IN) and housed in a humidity and temperature controlled colony on a 12 h light:dark cycle. Rats were initially allowed free-access to food and water for 3 days in order to habituate them to the colony. They were subsequently food restricted to 85% of their free-feeding weight (Mean±SEM free-feeding weight = 356 ±19 g), water was always freely available. Feeding, housing and experimental conditions followed the followed the “Principles of laboratory animal care” (NIH #85–23, revised 1985).
Apparatus
Experiments were conducted in seven standard conditioning chambers (ENV-008CT; Med Associates, Georgia VT) each housed in a sound-attenuating cubicle (ENV-018). Each chamber had a Plexiglas ceiling and front and back walls with two aluminum sidewalls; all stimulus elements were attached to the aluminum walls. One side of each chamber was fitted with a liquid dipper in a receptacle (5.2 × 5.2 × 3.8 cm; l × w × d); the dipper arm had a 0.1-ml cup attached for sucrose delivery. The receptacle was fitted with an infrared emitter/detector unit to monitor head entries. On the same wall were two white stimulus lights (100 mA), these lights were mounted above and to the side of the receptacle and served as the light CS. A speaker and amplifier unit that provided white noise (70 dB) was mounted to the upper back corner of the opposite wall; 15 s of white noise from this speaker served as the noise CS. A personal computer with Med Associates interface and software controlled stimulus events and recorded dipper entries throughout each session.
Drugs
(−)-Nicotine hydrogen tartrate (Sigma, St Louis, MO) was dissolved in physiological saline, brought to a pH of 7.0±0.2 with a dilute NaOH solution, and injected subcutaneously (SC) at 1 ml/kg. D-amphetamine sulfate and chlordiazepoxide hydrochloride (Sigma, St Louis, MO) were dissolved in physiological saline and injected intraperitoneally (IP) at 1 ml/kg. Nicotine doses are expressed as the base form; other drug doses are expressed as the salt form. All drug or vehicle injections were given 15 min before the start of a session.
General Procedure
Dipper Training
In the first three sessions rats were trained to drink 26% sucrose (w/v) within 4 s. At the start of the first session sucrose had a 0.167 probability of sucrose occurring during each 4-s interval, which resulted in about 1 sucrose presentation every 6 intervals (24 s). Across this and two subsequent sessions the probability function was gradually decreased to 0.5 (1 sucrose presentation per 120 s). Robust dipper entry behavior was evident for all rats by the end of day 3.
Discrimination Training
Discrimination Training was separated into 8-day cycles during which each rat received 4 drug and 4 saline sessions in quasi-random order. The cycles were constructed such that the same session type (drug or saline) did not occur on more than 2 consecutive days. Rats received 8 presentations of a 15-s CS (noise or light) during each session. On drug sessions (0.4 mg/kg nicotine, 1 mg/kg amphetamine, or 5 mg/kg CDP injected before the session), each CS presentation was followed immediately by 4-s access to sucrose. On saline sessions 0.9% NaCl was injected before the session and CS presentations were not followed by any outcome. Sessions were controlled by one of 8 Med-State Notation (Med Associates, Georgia VT) programs that varied the first CS onset and the order of inter-trial intervals (ITIs). Varying these intervals within and across trials made CS onset less predictable. ITIs were defined as the time from CS offset to the next CS onset (mean=154 s, range=94–214 s), the first CS occurred on average 120 s after the session began (range=75–165 s).
Specific Experiments
Experiment 1
Sixteen rats were randomly assigned to one of four drug state/stimulus combinations (nicotine-light, CDP-light, nicotine-noise, or CDP-noise; n=4 per condition). Rats in each condition received 64 training sessions (8 cycles). During the first 24 sessions (first three cycles) one drug state was trained as a facilitator for one CS (e.g., nicotine: noise). Twelve sessions were preceded by drug injections, and the CS was always followed by sucrose. The remaining twelve sessions were preceded by saline injections; the CS was presented during these sessions but was not followed by any programmed event. During the second phase of training (next three cycles) a second drug state/CS combination was trained using the drug state and CS that had not previously been experienced. For example, rats assigned to the nicotine-light combination in the first phase were trained with the CDP-noise combination in the second phase. Training procedures were identical; however, the change in drug context always involved a change in the drug injection route (SC to IP or vice versa). To prevent any confounding relationships between the injection route and drug state, rats were given two injections (one via each route) before every session during this second training phase. On saline sessions this resulted in two vehicle injections, on drug sessions this included one vehicle injection and one drug injection.
During the final 16 training sessions (last two cycles) both drug feature-CS compounds were presented in intermixed order. No more than two drug or two saline sessions occurred in consecutive order. Also, no more than two sessions in which the same CS was presented could occur consecutively and only 1 of the conditional stimuli (noise or light) was presented in each session. Similar to previous training phases, sucrose delivery immediately followed CS presentations on drug sessions but not on saline sessions.
A 2-day test cycle followed each 8-day intermixed training cycle. On each day of the test cycle rats received a drug injection 15 min before a 4-min test session. During the test session, CS presentation occurred in extinction (i.e., no sucrose); test session parameters were identical to Discrimination Training except that only 1 CS was presented. Test drugs and CS presentations were delivered so that in each test cycle, rats received 1 session with a “normal” feature-CS configuration. This test session was included as a benchmark for conditioned responding under training procedures. The other session in each test cycle was a transfer or novel drug test. For transfer tests, rats were injected with one training drug and presented with the CS that had always occurred in the other drug (or saline) state during training. For the novel drug tests, rats were injected with 1 mg/kg amphetamine; this dose serves as a discriminative cue for CS-sucrose pairings under the present procedures [30]. Cycle order (transfer vs. novel), session order (normal vs. transfer/novel), and drug-CS configuration were counterbalanced in each group. For “normal” test sessions rats received each of the drug feature-CS configurations in counterbalanced order. For transfer/novel tests the CS was counterbalanced such that a CS presented on the transfer test differed from the CS presented on the novel drug test for each rat. For example, if a rat received CDP and the noise CS during the transfer test, the light CS was presented during the novel drug (amphetamine) test.
Experiment 2
Sixteen rats were randomly assigned to one of four drug state/stimulus combinations (amphetamine-light, CDP-light, amphetamine-noise, or CDP-noise; n=4 per condition). Training and testing were identical to Experiment 1 except that nicotine (0.1 mg/kg) was used as the novel drug state.
Experiment 3
Twelve rats were randomly assigned to one CS modality (light or noise) and drug feature (nicotine, amphetamine, or CDP) combination. The drug feature and CS modality were balanced so that in each drug feature condition (n=4 per drug) two rats were trained with the noise CS and two rats were trained with the light CS. After 32 discrimination training sessions (16 drug and 16 saline), each rat received a two-day test cycle similar to Experiments 1 and 2. During one test session, the CS from the training phase was presented (i.e., normal drug-CS configuration). On the other test session, a novel CS was presented to determine whether the drug states would facilitate a response to any discrete CS. During this latter test, we found a slight trend for rats to decrease goal tracking during the CS (see later), suggesting that orienting toward a novel CS may interfere with goal tracking. Therefore, we familiarized the rats with this CS during another 8-day training cycle (Ambiguous Phase). Each rat received repeated presentation of the “novel” CS; saline was injected IP or SC (according to previous phase) before each session. On 4 sessions, this previously novel CS was followed immediately by access to 26% sucrose (8 trials per session). On the remaining 4 sessions, each CS presentation was followed by a 4-s empty interval. This ‘ambiguous’ training was followed by a 2-day test cycle; the two discrete cues were tested in reversed order for each rat.
Dependent Measure & Data Analyses
Because goal-tracking topographies vary as a function of CS modality and drug feature [28], the analyses included both frequency and duration CR measures (i.e., elevation and duration scores; see [28]). Elevation and duration scores were calculated as frequency and duration of dipper entries that occurred during the 15-s CS minus frequency and duration of dipper entries that occurred during the 15 s before CS onset [12, 29]. Average elevation and duration scores for each session served as the main dependent measure. Scores of 0 indicate equal goal tracking during the CS and pre-CS periods across a session; positive scores indicate more goal tracking during the CS. Because the ‘maintained head poke’ CR topography was not expected at the start of the experiment (see [28]), duration scores were recorded beginning on session 13 during Experiment 1. For Discrimination Training, three-way mixed factors analysis of variance (ANOVA) compared mean goal-tracking scores across Drug (nicotine vs. saline), CS (light vs. noise), and Session.
Experiments 1 & 2
For Intermixed Training sessions a fourth factor, Order (Phase 1 vs. Phase 2), was added to examine whether drug feature-CS training order had any impact on goal tracking. For the normal configuration tests, paired-samples t-tests compared elevation and duration scores from the first and second tests (1st cycle vs. 2nd cycle). If there were no differences across cycles, scores from the two sessions were averaged and served as a pooled control. Subsequent one-way repeated measures ANOVA compared each of the test conditions (normal, transfer, and novel). Significant ANOVAs were followed by Dunnett’s Multiple Comparisons test to ascertain which test conditions (transfer or novel) differed from the pooled-normal control. Due to counterbalancing, each animal received different drug-stimulus combinations on the test sessions so the CS and Feature factors could not be included in the ANOVAs. Follow-up analyses were conducted to investigate the potential impact of CS modality and drug feature on test sessions.
Experiment 3
For Discrimination Training, analyses were similar to Experiments 1 and 2. For the Ambiguous Phase, ANOVAs compared CS (light or noise), Feature (drug from the original training phase), Sucrose (presented or withheld), and Session (1 to 4) for elevation and duration scores. For both test cycles, similar analyses were conducted however, Test (novel vs. normal) replaced Session as the repeated measure and the Drug/Sucrose factors were not included.
Results
Experiment 1
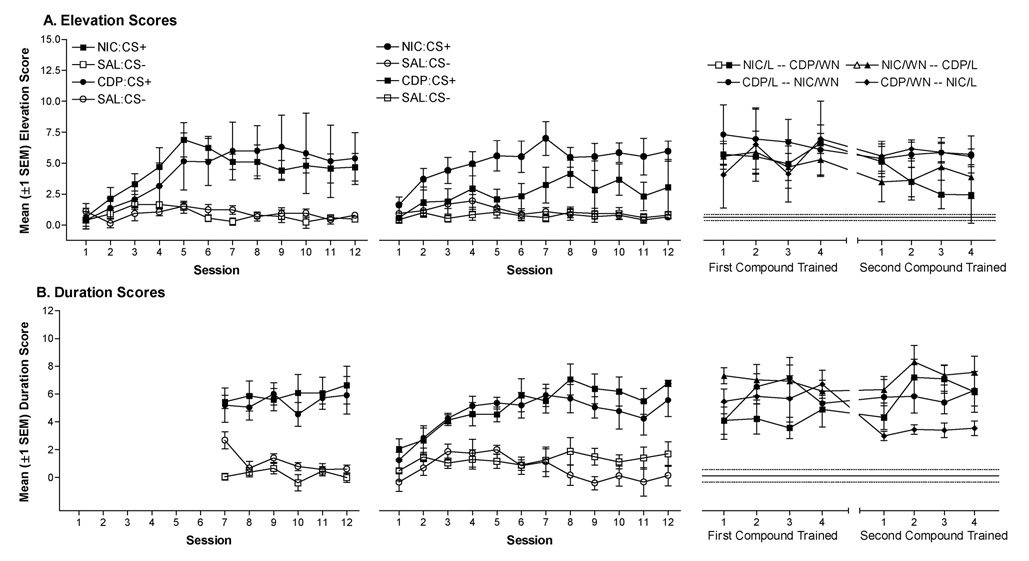
Rats acquired a drug state-specific goal tracking CR during the initial discrimination training phase. The ANOVA for elevation scores from the initial training phase (Figure 1A, left panel) revealed significant main effects of Drug, F(1,132)=39.62, p<0.001, of Session, F(11,132)=5.08, p<0.001, and a Drug × Session interaction, F(11,132)=6.16, p<0.001. The duration score ANOVA revealed a similar pattern in this phase (Figure 1B, left panel); there was a significant main effect of Drug, F(1,132)=118.63, p<0.001, and a Drug × Session interaction, F(11,132)=2.72, p=0.028.
Figure 1.
(A) Mean elevation scores (±1 SEM) for rats receiving NIC and CDP features with light or noise target CSs. (B) Mean duration scores (±1 SEM) for rats receiving NIC and CDP features with light or noise target CSs. Graphs are organized chronologically from left to right. Rats receiving discrimination training with one compound in the first training phase (left panels; e.g., NIC:L+/SAL:L−) received the opposite compound in the second training phase (center panels; e.g., CDP:WN+/SAL:WN−). Right panels represent elevation and duration scores from the intermixed training and test phase. These graphs are divided vertically to separate the first compound received (left half) from the second compound received (right half) by each animal. Filled symbols represent elevation or duration scores from drug sessions, open symbols represent elevation or duration scores from saline sessions. Each set (filled and open) represent elevation and duration scores from four rats (n=8 per compound in counterbalanced order).
Rats acquired a second drug state-specific goal tracking CR in which a new drug state (nicotine or CDP) facilitated responding to a new CS (light or noise; center panels of Figures 1A & 1B). For elevation scores, the four-way ANOVA revealed significant main effects of Drug, F(1,121)=47.29, p<0.001, of Session, F(11,121)=3.79, p<0.001, and a Drug × Session interaction, F(11,121)=4.79, p<0.001. The duration score ANOVA revealed a similar pattern with significant main effects of Drug, F(1,121)=151.81, p<0.001, and Session, F(11,121)=7.95, p<0.001, and a significant Drug × Session interaction, F(11,121)=7.48, p<0.001.
During the intermixed training phase, rats were able to respond appropriately under each discrimination condition. Elevation scores were higher on drug sessions relative to saline sessions [main effect of Drug, F(1,36)=65.89, p<0.001]. No other main effects or interactions were significant, Fs≤2.78, ps≥0.06. During this phase, duration scores were also higher on drug sessions relative to saline sessions [main effect of Drug, F(1,36)=342.95, p<0.001]. There was also a Drug × Session interaction, F(3,36)=4.82, p<0.01, this was probably due to a slight trend for increasing duration scores across drug sessions and decreasing across saline sessions, and may indicate continued acquisition of the discrimination during this phase. No other main effects or interactions were significant, Fs≤2.76, ps≥0.09.
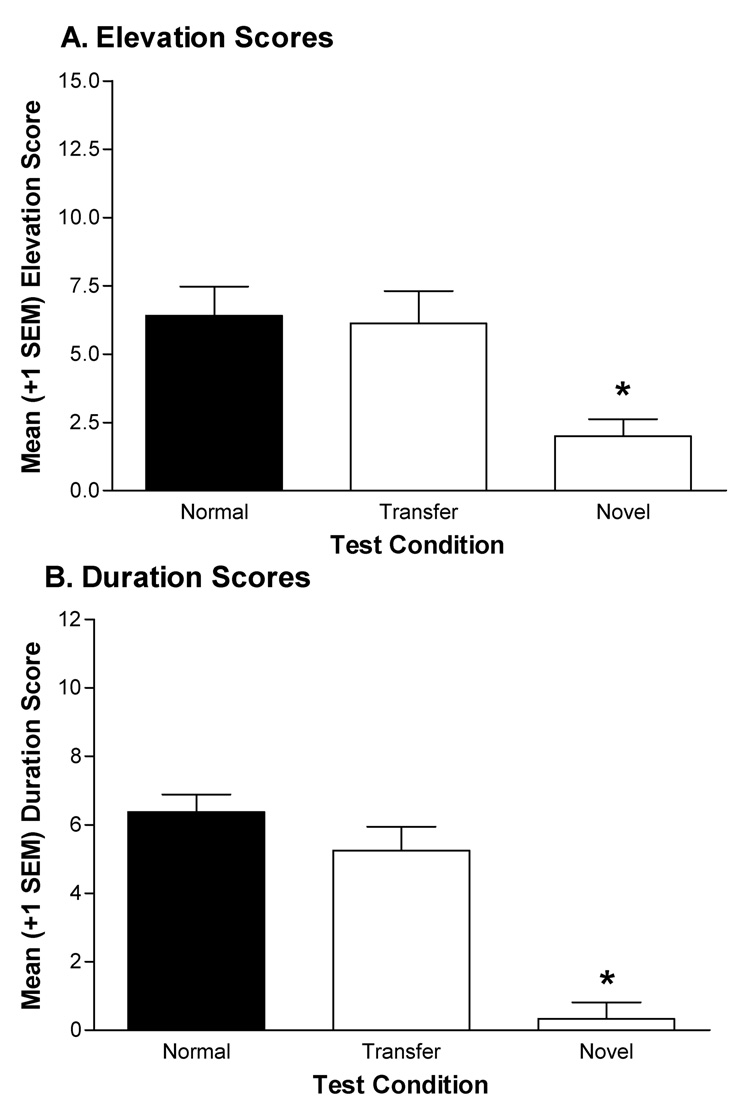
Nicotine and CDP transferred their facilitative control over responding to targets of a second discrimination. This transfer was specific to the nicotine and CDP drug states, as 1 mg/kg amphetamine did not facilitate conditional responding (Figures 2A & 2B). Normal configuration test data were pooled since they did not differ across test sessions, ts<1. For elevation scores (Figure 2A), the one-way repeated measures ANOVA revealed a significant main effect of Test, F(2,47)=5.95, p<0.01. Dunnett’s Multiple Comparisons test indicated that elevation scores from the novel drug test significantly differed from the pooled normal controls, p<0.01. However, elevation scores from the transfer test did not differ from this control condition, p>0.05. For duration scores (Figure 2B), there was also a significant main effect of Test, F(2,47)=44.45, p<0.01. Follow-ups revealed that duration scores from the novel drug test differed significantly from the normal control, p<0.01; however, duration scores from the transfer test did not differ from this control, p>0.05.
Figure 2.
Mean (±1 SEM) elevation scores (A) and duration scores (B) for the normal and transfer feature-target compound tests and the novel drug feature test from rats in the NIC/CDP group of Experiment 3A. For the “normal” test, rats received injections of nicotine or CDP 15 min before a single exposure to the CS normally followed by sucrose in that drug state. For the “transfer” test, rats received injections of nicotine or CDP 15 min before a single exposure to the CS followed by sucrose in the other drug state during training. For the novel feature test rats were injected with AMP (1 mg/kg IP) 15 min before a single exposure to one CS from the training phase. The CS presented on the novel drug test was counterbalanced according to modality (light vs. noise) and previous training drug state (nicotine vs. CDP). All tests were conducted in extinction. * indicates elevation or duration scores differ significantly from normal test condition, p<0.05.
Experiment 2
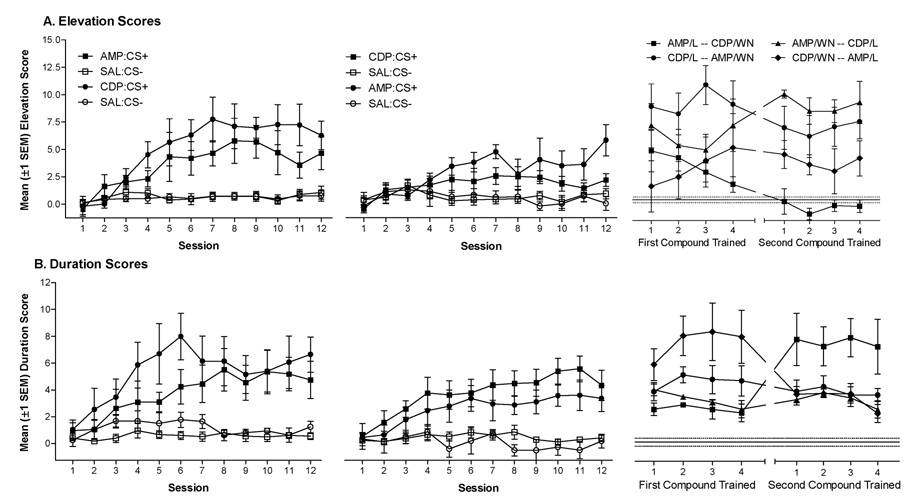
As in Experiment 1, rats acquired a drug state-specific conditioned response (Figures 3A & 3B, left panels). For elevation scores (Figure 3A) there were significant main effects of Drug, F(1,132)=65.70, p<0.001, of Session, F(11,132)=13.03, p<0.001, and a Drug × Session interaction, F(11,132)=11.18, p<0.001. For duration scores (Figure 3B) there were also significant main effects of Drug, F(1,132)=43.66, p<0.001, of Session, F(11,132)=8.50, p<0.001, and a Drug × Session interaction, F(11,132)=8.21, p<0.001.
Figure 3.
Mean (±1 SEM) elevation scores (A) and duration scores (B) for rats receiving AMP and CDP features with light or noise target CSs. Graphs are organized chronologically from left to right. Rats receiving discrimination training with one compound in the first training phase (left panels; e.g., AMP:L+/SAL:L−) received the opposite compound in the second training phase (center panels; e.g., CDP:WN+/SAL:WN−). Right panels represent elevation and duration scores from the intermixed training and test phase. These graphs are divided vertically to separate the first compound received (left half) from the second compound received (right half) by each animal. Filled symbols represent elevation or duration scores from drug sessions, open symbols represent elevation or duration scores from saline sessions. Each set (filled and open) represent elevation and duration scores from four rats (n=8 per compound in counterbalanced order).
Similar to Experiment 1, a second drug context-CS compound came to control goal tracking (center panels of Figures 3A & 3B). For elevation scores, the four-way ANOVA revealed significant main effects of Drug, F(1,132)=28.88, p<0.001, of Session, F(11,132)=4.09, p<0.001, and a Drug × Session interaction, F(11,132)=9.46, p<0.001. For duration scores, the four-way ANOVA also revealed significant main effects of Drug, F(1,132)=51.91, p<0.001, of Session, F(11,132)=8.74, p<0.001, and a Drug × Session interaction, F(11,132)=10.71, p<0.001.
Concurrent feature positive discriminations were maintained across an intermixed training phase (right panels of Figures 3A & 3B). Elevation scores were higher on drug sessions relative to saline sessions [main effect of Drug, F(1,36)=71.80, p<0.001] duration scores were also higher on drug sessions [main effect of Drug, F(1,36)=134.74, p<0.001], indicating that the drug features facilitated responding to the CS.
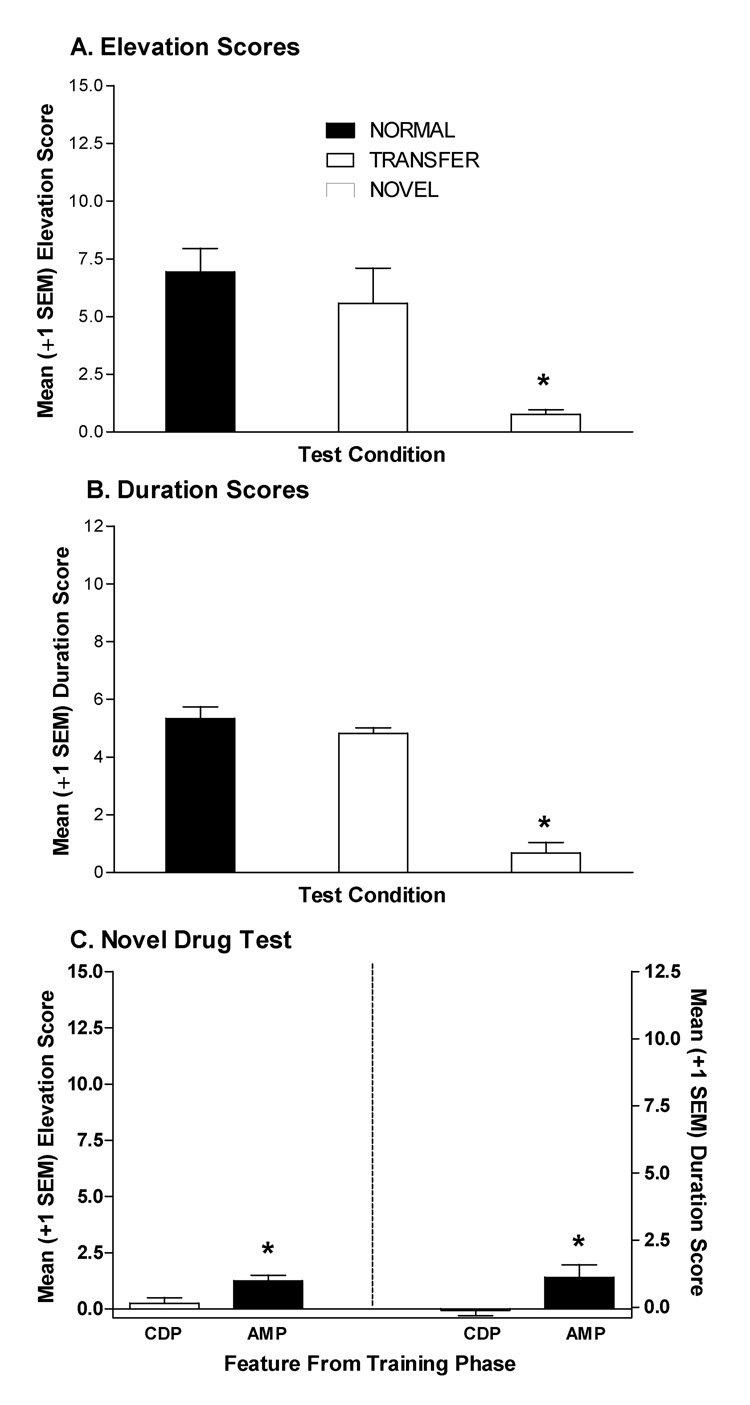
CDP and amphetamine transferred their facilitative control to conditional stimuli that had never been presented in those drug states (Figures 4A & 4B). This transfer was specific to the amphetamine and CDP drug states, as a novel drug (nicotine) did not facilitate goal tracking under similar test conditions. Normal configuration test data were pooled since they did not differ across test sessions, ts(15)≤1.16, ps≥0.26. One-way ANOVA for elevation scores revealed a significant main effect of Test, F(2,47)=11.07, p<0.001. Subsequent contrasts indicated that only elevation scores from the novel drug test differed significantly from normal controls, p<0.01. The one-way ANOVA on duration scores revealed a similar pattern; there was a significant main effect of Test, F(2,47)=56.48, p<0.0001, and only duration scores from the novel test differed significantly from the normal control, p<0.01.
Figure 4.
Mean (±1 SEM) elevation scores (A) and duration scores (B) for the normal and transfer feature-target compound tests and the novel drug feature test for rats in the AMP/CDP group of Experiment 3A. For the “normal” test, rats received injections of AMP or CDP 15 min before a single exposure to the CS normally followed by sucrose in that drug state. For the “transfer” test, rats received injections of AMP or CDP 15 min before a single exposure to the CS followed by sucrose in the other drug state during training. For the novel feature test rats were injected with nicotine (0.1 mg/kg SC) 15 min before a single exposure to one CS from the training phase. The CS presented on the novel drug test was counterbalanced according to modality (light vs. noise) and previous training drug state (AMP vs. CDP). (C) Illustrates mean (±1 SEM) elevation and duration scores from the novel drug test for rats receiving the CS previously presented in the AMP vs. CDP drug state on the novel drug test. All tests were conducted in extinction. * indicates elevation or duration scores differ significantly from normal test condition, p<0.05.
In Experiment 2, follow up analyses revealed that responding on the novel drug (i.e., nicotine) test varied as a function of the Feature/CS combination. When after injections with the novel drug, nicotine, the CS that was previously followed by sucrose in the amphetamine state evoked more goal tracking than the CS that was previously followed by sucrose in the CDP state (Figure 4C). This was confirmed by significant main effects of Feature (drug state that the test CS was originally presented in) for both dependent measures, Fs(1,16)≥ 4.78, p≤0.049. Follow-ups confirmed higher goal tracking scores for the CS previously reinforced in the amphetamine state (ps<0.05).
Experiment 3
Experiment 3 confirmed previous findings that drug states can set the occasion for a goal tracking CR evoked by a discrete CS (Figures 5A & 5B). The ANOVA on elevation scores (Figure 5A) revealed significant main effects of Drug, F(1,90)=50.86, p<0.001, of Session, F(15,90)=8.78, p<0.001, and a Drug × Session interaction, F(15,90)=8.80, p<0.001. The ANOVA on duration scores (Figure 5B) revealed significant main effects of Drug, F(1,90)=87.08, p<0.001, of Session, F(15,90)= 7.84, p<0.001, and a Drug × Session interaction, F(15,90)=7.61, p<0.001.
Figure 5.
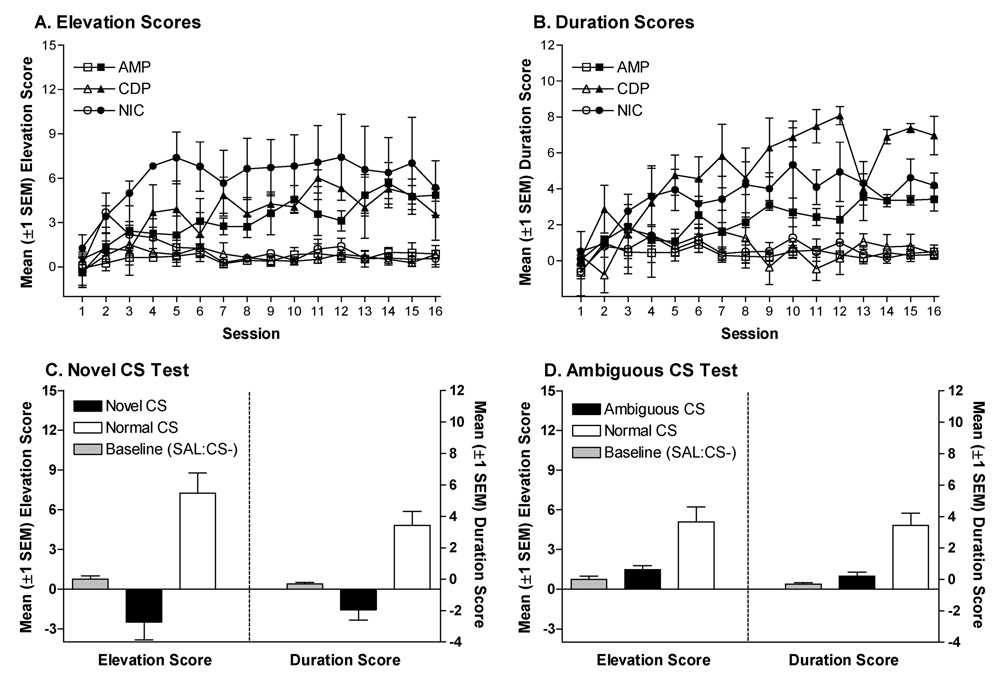
Mean (±1 SEM) elevation scores (A) and duration scores (B) from the discrimination training phase for rats in Experiment 3B. Mean (±1 SEM) elevation and duration scores from the novel CS (C) and ambiguous CS (D) test sessions. Initial tests with the novel CS indicated some orienting to the stimulus (negative elevation and duration scores, filled bars in C). After the test rats were injected with saline 15 min before 8 daily ‘ambiguous’ training sessions; the novel CS was presented 8 times during each session. On 4 of these sessions, this CS was immediately followed by 4-s access to sucrose; on the remaining sessions, the CS was followed by a 4-s empty interval. Subsequent tests (D) were conducted to examine whether the original training drug facilitated responding to this ‘ambiguous’ CS.
Presenting a novel CS in the feature positive drug state did not evoke a goal tracking CR (Figure 5C). For elevation scores, the ANOVA revealed a significant main effect of Test, F(1,6)=24.67, p<0.01. No other main effects or interactions were significant, Fs≤4.24, ps≥0.07. For duration scores, the ANOVA revealed significant main effects of Test, F(1,6)=24.19, p<0.01, and CS, F(1,6)=7.67, p=0.03. No other main effects or interactions were significant, Fs≤4.58, ps≥0.06. Rats had higher elevation and duration scores on the normal test session relative to the novel CS test session. Notably, the main effect of CS in the duration analyses reflects higher scores for rats tested with the noise CS (data not shown).
These results suggested that on the novel CS test, rats were spending more time away from the dipper during the CS relative to the pre-CS interval (i.e., negative scores). Although this pattern was not statistically reliable in one-sample t-tests (not reported), it suggests that orienting to the novel stimulus transiently interfered with goal tracking. In order to further examine the possibility that orienting responses might have interfered with transfer of occasion setting, the same rats were subsequently exposed to the novel CS under a 50% reinforcement contingency. For these additional sessions, the ANOVA on elevation scores revealed significant main effects of Sucrose, F(1,18)=45.02, p<0.001, and Session, F(3,18)=11.48, p<0.001, and a Sucrose × Session interaction, F(3,18)=6.73, p<0.01. There were also significant main effects of CS, F(1,6)=56.01, p<0.001, Feature, F(2,6)=5.18, p=0.05, a CS × Feature interaction, F(2,6)=9.03, p=0.02, and a Sucrose × Session × Feature interaction, F(6,18)=3.00, p=0.03. No other main effects or interactions were significant, Fs≤1.83, ps≥0.24. The 3-way interaction resulted from higher elevation scores on sucrose sessions for rats that had previously been trained with the CDP feature. The ANOVA on duration scores revealed significant main effects of Sucrose, F(1,18)=31.54, p<0.01, and Session, F(3,18)=11.52, p<0.001, and a Sucrose × Session interaction, F(3,18)=4.90, p=0.01. There was also a significant Sucrose × Session × CS interaction, F(6,18)=3.00, p=0.03. No other main effects or interactions were significant, Fs≤1.74, ps≥0.24. Duration scores for rats receiving the noise CS increased more across sucrose sessions, relative to rats receiving the light CS (data not shown). The Sucrose × Session interactions in both sets of analyses revealed that rats learned to anticipate when sucrose would be delivered based on the first trial of each session. However, this anticipatory food seeking emerged gradually and only after other discriminative stimuli (i.e., drug states) were removed.
Subsequent tests with the originally trained drug feature did not facilitate goal tracking to the “ambiguous” CS. Goal tracking to the original feature-CS compound was higher than goal tracking to the feature-ambiguous CS compound (Figure 5D). The ANOVA on elevation scores revealed a significant main effect of Test, F(1,6)=19.06, p<0.01, and a Test × Feature interaction, F(2,6)=5.64, p=0.04. No other main effects or interactions were significant, Fs≤3.99, ps≥0.08. The ANOVA on duration scores also revealed a significant main effect of Test, F(1,6)=14.05, p=0.01. No other main effects or interactions were significant, Fs≤3.12, ps≥0.13.
Response Topography Findings
Experiment 1
The present studies confirmed previous findings that conditional response topographies vary according to drug state and CS modality in this preparation (cf. [28]). For elevation scores, rats receiving the noise CS with the CDP facilitator in the first phase did not exhibit differential goal tracking across drug states. The pattern of main effects and interactions outlined in Table 3 illustrates that the lack of CR expressed in the elevation score (rate) measure for rats receiving the CDP feature and noise CS was caused by a maintained headpoke response topography across the first two phases of the study.
Interestingly, responding on the transfer test suggested that the Drug feature held more influence than the target CS over CR topography. Separate follow-up ANOVAs on elevation and duration scores revealed a significant CS × Feature interaction, F(1,16)=7.79, p=0.02, only on the duration score measure. No other main effects or interactions were significant, Fs≤2.74, ps≥0.12. Transfer tests in which the noise CS was presented in the nicotine state resulted in lower duration scores than the other drug-CS compounds (data not shown), ps<0.05. Under ‘normal’ training conditions, the light CS was followed by sucrose in the nicotine state; this compound yielded lower duration scores during the discrimination training phase. On the amphetamine (i.e., novel drug) test, the combination of a stimulant and visual CS evoked a higher frequency goal tracking response regardless of which drug (nicotine or CDP) had previously set the occasion for light-sucrose pairings. On the novel drug test rats tested with the light CS had higher elevation scores than rats tested with the noise CS (data not shown), p<0.05.
Experiment 2
Similar to Experiment 1, goal-tracking topography in Experiment 2 depended on the drug/CS compound. Elevation scores did not differ across drug states for rats receiving the CDP/noise compound in the first phase, however for each of the other compounds, goal tracking increased across drug sessions (e.g., 4-way interaction for elevation scores). The duration score analyses confirmed the ‘maintained’ headpoke topography for rats receiving the CDP/noise compound; these subjects expressed a conditioned response when the duration of goal tracking during the CS served as the dependent measure.
Experiment 3
The topography of this was consistent with Experiments 1 and 2 [see also 28]. Omnibus ANOVA’s included all factors and topography findings were comparable to Experiments 1 and 2. The F statistics and p values were not reported to conserve space.
Discussion
The present studies confirmed that drug states can transfer their facilitative control over responding to stimuli with which they have never been presented. Experiments 1 and 2 demonstrated that two drug states that do not share stimulus properties [30] can substitute for one another if they are functionally equated. Three additional findings of importance emerged from the present studies. First, transfer of drug facilitation did not depend on physical generalization between the two drug states. Second, the topography of conditional responses depended on drug/CS combination. And third, the drug states do not facilitate responding to any discrete stimulus presented during testing sessions (Experiment 3).
One important finding in these studies was that transfer of occasion setting was dissociated from stimulus generalization. Although there was some partial substitution by nicotine for amphetamine in Experiment 2, the non-specific generalization was marginal at best. Nicotine and amphetamine can partially substitute for each other when they are trained as discriminative stimuli in operant tasks [3, 15], suggesting that the two drugs have some common physiological properties. Although nicotine appeared to substitute for amphetamine, responding in the nicotine state did not differ from responding on saline sessions during the discrimination and intermixed training phases. This is perhaps best illustrated by comparisons between responding on ‘normal’ tests (Figures 4A & 4B) and those resulting from the novel drug test (Figure 4C). The finding that a novel drug did not facilitate responding in these studies is important for two reasons. First, in addition to the topographical differences in responding that depended on Feature/CS compounds, it indicates that rats could discriminate between the pharmacological effects of each drug facilitator. Second, it suggests that rats did not solve the discrimination by using drug vs. no-drug stimulus classes. For example, the way that each drug facilitator was trained could make the saline state an inhibitory stimulus. That is, ‘no-drug’ could signal that sucrose was not available, whereas any other internal context would facilitate responding to the CS. According to this hypothesis, novel pharmacological contexts should facilitate conditional responding to the CS, yet this did not occur.
One additional point of consideration is the use of only one drug/dose in the novel drug tests of Experiments 1 and 2. The reasons that rats were tested only one time and with one drug dose were threefold. First, initial exposure to 0.4 mg/kg nicotine base has a motor suppressant effect, even though it is the best-characterized discriminant dose using the subcutaneous route of administration [3, 6, 38]. These effects would not only decrease any facilitation engendered by nicotine (which depends on the animal being able to move to the goal area), but would also confound repeated testing. Second, when nicotine (0.4 mg/kg base), amphetamine (1 mg/kg), or CDP (5 mg/kg) are trained as facilitators, the doses that we tested as novel drug states produce the full conditional response [30] and were expected to produce a detectable stimulus with no motor suppressing effects. Third, the hypothesis under investigation was that any drug stimulus could facilitate responding based on the subjects’ learning history. Thus, detectable stimuli without confounding motoric effects should facilitate goal tracking.
A second finding of interest in these studies was the different topographical expression of the CR expressed under various drug state-CS combinations. Briefly, the combination of CDP and the noise produced a ‘maintained head poke’ CR which was not evident under any other stimulus conditions. In a previous report [28] we argued that experimental parameters such as being able to detect the auditory stimulus from within the dipper receptacle and the use of a temporally discrete US (4-s access to sucrose dipper) should bias rats toward this CR topography [14, 16, 20, 28]. Accordingly, psychomotor depressant drugs could decrease dipper entry frequency without interfering with anticipation of sucrose, thus the conditional response is expressed as a maintained head poke that is contemporaneous with CS presentation. For drugs that can increase response-rates (i.e., stimulants) frequency of dipper entries may be unaltered or increased. Although our findings are generally consistent with this hypothesis, additional psychomotor depressants should be investigated to determine whether they also promote the sustained response form.
The third finding of interest was that the drug states did not facilitate goal tracking to a novel or familiar discrete CS that was not explicitly trained in the discrimination. This finding is important because it confirms that the drug state did not simply change the probability of responding when any CS was presented. This differs somewhat from previous findings in which drug state facilitators can change the probability of conditional responses to familiar and novel taste stimuli that have not participated in the discrimination [36]. Skinner and colleagues [36] have suggested that this non-specific facilitation may be an artifact of the conditioned taste aversion paradigm. They have argued that the drug state sets the occasion for a drinking response-lithium association, in which case control over fluid intake is specific to the response (drinking) but not the taste CS [37]. In the present studies, the drug states control over responding was linked to the goal-tracking response, but depended more critically on the physical properties of the CS and their history of pairings with sucrose.
The current findings are in direct contrast with the hypothesis that associations between a discrete CS and sucrose are state-dependent or that the effects of the drug states are ‘dissociative’ in this paradigm. CDP can produce state-dependent learning or recall, however this usually occurs at higher doses (e.g., 40 mg/kg, [18]). Preliminary evidence from our laboratory has discouraged us from interpreting the drug-state specific CR as state-dependent learning (see [5, 29, 30, 34] for discussion). Perhaps the best evidence against state dependent learning are those of Experiments 1 and 2; if rats acquired state-dependent associations or CRs during the training phase, then one would not expect the drug states to transfer their conditional control over goal tracking.
Although we confirmed that these drug states were not functioning in a dissociative capacity, we cannot confirm what was learned about the drug states during training. For example, interference based theories of occasion setting [11] predict that the CSs acquire both excitatory and inhibitory associations with the US (sucrose). According to Bouton and Nelson [11] positive features interfere with the CS-no US (inhibitory) association or memory. In Experiments 1 and 2, the drug states may have increased responding by interfering with the memory that the US sometimes did not follow the CS. If a drug-state interferes with a CS-no US association, then that function may be generalized to other CSs with comparable histories. Perhaps a more parsimonious explanation is that the drugs acquired equivalence via pairing with the same US. Although we previously demonstrated that the drug-US association did not mediate facilitative control over responding [28] stimuli that share a common consequence (e.g., sucrose) can become functionally equated [9, 21] such that manipulations of the meaning of one stimulus can generalize to the other. Indeed, findings that occasion setting is often categorically specific to a US [8] support the acquired equivalence hypothesis. Regardless, the present studies demonstrate that an experience-mediated change in the meaning of a drug state can make it functionally equivalent to other drug states.
The implication of higher-order facilitation or equivalence of drug states could be substantial for the treatment of compulsive drug use and other addictive disorders. For example, humans can verbally and non-verbally distinguish between the effects of pharmacologically different compounds [22]. Similarly, in non-human animals, drug-occasioned operant behavior is generally specific to the pharmacological properties of the training drug [1]. Pharmacological specificity provides researchers and therapists with some general heuristics about drug states. For example, an abused drug will produce a characteristic and predictable set of stimulus conditions (i.e., Drug-US/UR). Accordingly, the cues and behaviors that are associated with the drug state should also produce a consistent set of physiological and behavioral circumstances (i.e., CSs-CRs). Both the pharmacological effects of the drug and the conditional stimuli/responses can be (and often are) targeted by behavioral and/or pharmacological therapies (see [17]). These therapies typically operate under the assumption that unrelated events will have little or no bearing on treatment outcome. For example, the pharmacological effects of caffeine should have no bearing on nicotine-cessation treatment unless smoking had previously occurred in the caffeine ‘context’.
The present studies, as well as others, suggest that this analysis may be incomplete. Drug-seeking behaviors may be evoked by circumstances without a previously established link to drug-taking history [17, 39]. Our finding that drug states can modulate reward-seeking in a “nonassociative” manner adds another layer of complexity. If interoceptive contexts are functionally equivalent, then situational specificity does not apply; the contexts produced by some drugs may facilitate reward-seeking that is assumed to be specific to another set of conditions. For example, if caffeine provided a context in which various stimuli (people, automobile, etc.) were associated with the reinforcing effects of nicotine, then a cue-exposure based tobacco cessation therapy may have to include exposure to such stimuli in the caffeine context. Otherwise the stimulus effects of caffeine could transfer their facilitative control to (i.e., increase reactivity to) other conditional stimuli associated with smoking (places, packaging/advertisements, etc.). This is an important consideration from a therapeutic perspective, given that the high relapse rates associated with cue-exposure may be associated with context shifts [17].
Table 1.
Design of Experiments 1 and 2.
| EXPT | GROUP | TRAINING PHASE | TESTING CONDITIONS | |||
|---|---|---|---|---|---|---|
| 1 | 2 | Intermixed | ||||
| 1 | 1 | NIC: A+ | Normal: | NIC: A –or– CDP: B | ||
| NIC: A+ | CDP:B+ | SAL: A− | ||||
| SAL: A− | SAL: B− | CDP: B+ | Transfer: | NIC: B –or– CDP: A | ||
| SAL: B− | Novel: | AMP: B –or– AMP: A | ||||
| 2 | CDP: A+ | Normal: | NIC: B –or– CDP: A | |||
| CDP: A+ | NIC: B+ | SAL: A− | ||||
| SAL: A− | SAL: B− | NIC: B+ | Transfer: | NIC: A –or– CDP: B | ||
| SAL: B− | Novel: | AMP: A –or– AMP: B | ||||
| 2 | 1 | AMP: A+ | Normal: | AMP: A –or– CDP: B | ||
| AMP: A+ | CDP: B+ | SAL: A− | ||||
| SAL: A− | SAL: B− | CDP: B+ | Transfer: | AMP: B –or– CDP: A | ||
| SAL: B− | Novel: | AMP: B –or– AMP: A | ||||
| 2 | CDP: A+ | Normal: | AMP: B –or– CDP: A | |||
| CDP: A+ | AMP: B+ | SAL: A− | ||||
| SAL: A− | SAL: B− | AMP: B+ | Transfer: | AMP: A –or– CDP: B | ||
| SAL: B− | Novel: | NIC: A –or– NIC: B | ||||
“EXPT”=experiment. GROUPs had identical procedures but differed in which drug state was trained first. “NIC:”=nicotine (0.4 mg/kg sc) injection before training/testing session. “CDP:”=chlordiazepoxide (10 mg/kg ip) injection before testing session. “SAL:”=0.9% saline injection (ip or sc) before training/testing session. “AMP:”=amphetamine (1 mg/kg ip) injection before training/testing session. “A” and “B” represent noise and stimulus light CS presentations; target CS/drug feature combinations were balanced in each group (see method). “+”=sessions in which sucrose was presented after each CS; “−”=empty interval (no sucrose) following CS presentations. “Normal:”=rats were tested with the original drug feature/target CS combination. “Transfer:”=rats were tested with a target CS that had previously only occurred in the other familiar drug state. “Novel:”=rats were tested with one of the target CSs in an unfamiliar drug state. Testing sessions were conducted in two 2-day cycles. For each subject, one cycle included a ‘Normal’ test and a ‘Transfer’ test, the other cycle included a ‘Normal’ test and a ‘Novel’ test. The first test cycle occurred after the 8th intermixed training session, the second test cycle occurred after the 16th intermixed training session.
Table 2.
Design of Experiment 3
| PHASE | ||||
|---|---|---|---|---|
| GROUP | Training | Novel CS Test | Ambig Training | Ambig CS Test |
| NIC | NIC: A | NIC: A | ||
| NIC: A+ | NIC: B | SAL: B+ | NIC: B | |
| SAL: A− | ------------- | SAL: B− | ---------- | |
| *SAL: A | *SAL: A | |||
| AMP | AMP: A | AMP: A | ||
| AMP: A+ | AMP: B | SAL: B+ | AMP: B | |
| SAL: A− | ------------- | SAL: B− | ------------- | |
| *SAL: A | *SAL: A | |||
| CDP | CDP: A | CDP: A | ||
| CDP: A+ | CDP: B | SAL: B+ | CDP: B | |
| SAL: A− | ------------- | SAL: B− | ----------- | |
| *SAL:A | *SAL: A | |||
Procedures and nomenclatures are similar to Experiments 1 & 2; “A” represents the originally trained CS (light or noise) and “B” represents the novel or ambiguous CS. The Novel CS Tests evaluated whether drug features set the occasion for responding to an unfamiliar CS (B). During the Ambig Training phase, the novel CSs were familiarized but explicit excitatory or inhibitory associations with the US were avoided by pairing the CS and sucrose on only half of the sessions (i.e., making CS B ambiguous). Subsequent Ambig CS Tests evaluated whether drug features set the occasion for responding to a familiar CS that had not participated in an occasion setting discrimination.
Baseline SAL: A data were taken from the first trial of the last SAL: A- session in the Training phase.
Acknowledgements
This research was partially supported by NIH (DA11893) and the UN-L Research Council. The experiments partially fulfilled the doctoral degree requirements of the first author, who was supported by NIH grant DA16179A. RA Bevins was supported by DA018114 while preparing this manuscript. We thank Cody Brooks for his thoughtful comments on a previous version of this manuscript. MedPC programs functionally equivalent to the ones used in this research are available upon request from RA Bevins at rbevins1@unl.edu.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Appel JB, et al. Increasing the selectivity of drug discrimination procedures. Pharmacol Biochem Behav. 1999;64:353–358. doi: 10.1016/s0091-3057(99)00089-1. [DOI] [PubMed] [Google Scholar]
- 2.Balfour DJ, et al. The putative role of extra-synaptic mesolimbic dopamine in the neurobiology of nicotine dependence. Behav Brain Res. 2000;113:73–83. doi: 10.1016/s0166-4328(00)00202-3. [DOI] [PubMed] [Google Scholar]
- 3.Bardo MT, et al. (−)-Nornicotine partially substitutes for (+)-amphetamine in a drug discrimination paradigm in rats. Pharmacol Biochem Behav. 1997;58(4):1083–1087. doi: 10.1016/s0091-3057(97)00303-1. [DOI] [PubMed] [Google Scholar]
- 4.Besheer J, et al. Nicotine as a signal for the presence or absence of sucrose reward: a Pavlovian drug appetitive conditioning preparation in rats. Psychopharmacology. 2004;172:108–117. doi: 10.1007/s00213-003-1621-9. [DOI] [PubMed] [Google Scholar]
- 5.Bevins R, Penrod R, Reichel C. Nicotine does not produce state-dependent effects on learning in a Pavlovian appetitive goal-tracking task with rats. Behavioural Brain Research. 2007;177:134–141. doi: 10.1016/j.bbr.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bevins RA, Palmatier MI. Extending the role of associative learning processes in nicotine addiction. Behav Cog Neurosci Rev. 2004;3:143–158. doi: 10.1177/1534582304272005. [DOI] [PubMed] [Google Scholar]
- 7.Boakes RA. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz H, editors. Operant-Pavlovian interactions. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1977. pp. 67–101. [Google Scholar]
- 8.Bonardi C. Occasion setting is specific to the CS-US association. Learning and Motivation. 2007;38(3):208–228. [Google Scholar]
- 9.et al. Bonardi C, et al. Acquired equivalence of cues in pigeon autoshaping: Effects of training with common consequences and with common antecedents. Animal Learning & Behavior. 1993;21(4):369–376. [Google Scholar]
- 10.Bouton ME, King DA. Effect of context on performance to conditioned stimuli with mixed histories of reinforcement and nonreinforcement. J Exp Psych: Anim Beh Proc. 1983;12:4–15. [Google Scholar]
- 11.Bouton ME, Nelson JB. Mechanisms of feature-positive and feature-negative discrimination learning in an appetitive conditioning paradigm. Schmajuk, Nestor A. 1998 [Google Scholar]
- 12.Brooks DC, Bouton ME. A retrieval cue for extinction attenuates response recovery (renewal) caused by a return to the conditioning context. J Exp Psych: Anim Behav Proc. 1994;20:366–379. [Google Scholar]
- 13.Brooks DC, et al. Reinstatement after counterconditioning. Animal Learning & Behavior. 1995;23(4):383–390. [Google Scholar]
- 14.Carrigan PF, Benedict JO, Ayres JJ. A comparison of lever press and head-poke discriminated Sidman avoidance. Behav Res Meth Inst. 1972;4:301–303. [Google Scholar]
- 15.Chance WT, et al. A description of the nicotine stimulus and tests of its generalization. Psychopharmacology (Berl) 1977;55(1):19–26. doi: 10.1007/BF00432812. [DOI] [PubMed] [Google Scholar]
- 16.Cleland GG, Davey GC. Autoshaping in the rat: The effects of localizable visual and auditory signals for food. J Exp Anal Beh. 1983;40:47–56. doi: 10.1901/jeab.1983.40-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- 18.Connelly JF, Connelly JM, Phifer R. Disruption of state-dependent learning (memory retrieval) by emotionally-important stimuli. Psychopharmacologia. 1975;41:139–143. doi: 10.1007/BF00421071. [DOI] [PubMed] [Google Scholar]
- 19.DiChiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- 20.Farwell BJ, Ayres JJ. Stimulus-reinforcer and response-reinforcer relations in the control of conditioned appetitive headpoking (goal tracking) in rats. Learn Motiv. 1979;10:295–312. [Google Scholar]
- 21.Hall G. Blackman, D. E.; 1990. Reasoning and associative learning. [Google Scholar]
- 22.Heishman SJ, Henningfield JE. Discriminative stimulus effects of d-amphetamine methylphenidate, and diazepam in humans. Psychopharmacology. 1991;103:436–442. doi: 10.1007/BF02244241. [DOI] [PubMed] [Google Scholar]
- 23.Jarbe TU, Lamb RJ. Effects of lithium dose (UCS) on the acquisition and extinction of a discriminated morphine aversion: tests with morphine and delta9-THC. Behav Pharmacol. 1999;10:349–358. doi: 10.1097/00008877-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Koob GF, LeMoal M. Plasticity of reward neurocircuitry and the 'dark side' of drug addiction. Nat Neurosci. 2001;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- 25.Martin GM, Gans M, van der Kooy D. Discriminative properties of morphine that modulate associations between tastes and lithium chloride. J Exp Psych: Anim Behav Proc. 1990;16:56–68. [PubMed] [Google Scholar]
- 26.Miller MA, et al. Searching for evidence of transfer between drug facilitators. Learn Motiv. 2002;33:197–229. [Google Scholar]
- 27.Murray JE, Bevins RA. Behavioral and neuropharmacological characterization of nicotine as a conditional stimulus. Eur J Pharmacol. 2007;561(1–3):91–104. doi: 10.1016/j.ejphar.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmatier MI, Bevins RA. Facilitation by drug states does not depend on acquired excitatory strength. Behav Brain Res. 2007;176(2):292–301. doi: 10.1016/j.bbr.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmatier MI, et al. Nicotine serves as a feature-positive modulator of Pavlovian appetitive conditioning in rats. Behav Pharmacol. 2004;15:183–194. [PubMed] [Google Scholar]
- 30.Palmatier MI, et al. Stimulus properties of nicotine, amphetamine, and chlordiazepoxide as positive features in a Pavlovian appetitive discrimination task in rats. Neuropsychopharmacol. 2005;30:731–741. doi: 10.1038/sj.npp.1300629. [DOI] [PubMed] [Google Scholar]
- 31.Parker BK, Schaal DW, Miller M. Drug discrimination using a Pavlovian conditional discrimination paradigm in pigeons. Pharmacol Biochem Behav. 1994;49(4):955–960. doi: 10.1016/0091-3057(94)90249-6. [DOI] [PubMed] [Google Scholar]
- 32.Quertemont E. Discriminative stimulus effects of ethanol with a conditioned taste aversion procedure: lack of acetaldehyde substitution. Behav Pharmacol. 2003;14:343–350. doi: 10.1097/01.fbp.0000082130.08343.47. [DOI] [PubMed] [Google Scholar]
- 33.Revusky S, Coombes S, Pohl RW. Drug states as discriminative stimuli in a flavor-aversion learning experiment. J Comp Physiol Psych. 1982;96:200–211. doi: 10.1037/h0077870. [DOI] [PubMed] [Google Scholar]
- 34.Rijnders HJ, Jarbe TU, Slangen JL. Extinction and reacquisition of differential responding in rats trained to discriminate between chlordiazepoxide and saline. Psychopharmacology. 1990;102:404–410. doi: 10.1007/BF02244111. [DOI] [PubMed] [Google Scholar]
- 35.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 36.Skinner DM. Modulation of taste aversions by a pentobarbital drug state: An assessment of its transfer properties. Learn Motiv. 2000;31:381–401. [Google Scholar]
- 37.Skinner DM, Goddard MJ, Holland PC. What can nontraditional features tell us about conditioning and occasion setting? In: Holland P, Schmajuk N, editors. Occasion setting: Associative learning and cognition in animals. Washington, DC: American Psychological Association; 1998. pp. 113–124. [Google Scholar]
- 38.Stolerman IP, et al. Selective antagonism of behavioural effects of nicotine by dihydro-beta-erythroidine in rats. Psychopharmacology. 1997;129:390–397. doi: 10.1007/s002130050205. [DOI] [PubMed] [Google Scholar]
- 39.et al. Wang J, et al. Region-specific effects of brain corticotropin-releasing factor receptor type 1 blockade on footshock-stress- or drug-priming-induced reinstatement of morphine conditioned place preference in rats. Psychopharmacology (Berl) 2006;185(1):19–28. doi: 10.1007/s00213-005-0262-6. [DOI] [PubMed] [Google Scholar]
- 40.Westbrook RF, et al. Reinstatement of fear to an extinguished conditioned stimulus: Two roles for context. Journal of Experimental Psychology: Animal Behavior Processes. 2002;28(1):97–110. [PubMed] [Google Scholar]