Abstract
The persistent effects of unilateral deep brain stimulation (DBS) of the globus pallidus interna (GPi) or subthalamic nucleus (STN) on specific movement parameters produced by Parkinson’s disease (PD) patient’s are poorly understood. The aim of this study was to determine the effects of unilateral GPi and STN DBS on the force producing capabilities of PD patients during maximal efforts and functional bimanual dexterity. Clinical and biomechanical data were collected from 14 unilaterally implanted patients (GPi=7; STN=7), at least 13 months post-DBS surgery, while On and Off stimulation in the absence of medication. Unilateral DBS of either location produced a 33% improvement in UPDRS Motor Scores. Significant gains in maximum force production were present in both limbs during unimanual efforts. The greatest increase in maximum force, for both limbs, was under bimanual conditions. Force in the contralateral limb increased more than 30% during bimanual efforts while ipsilateral force increased by 25%. Unilateral DBS improved grasping force control and consistency of digit placement during the performance of a bimanual dexterity task. The clinical and biomechanical data indicate that unilateral DBS of GPi or STN results in persistent improvements in the control and coordination of grasping forces during maximal efforts and functional dexterous actions. Unilateral DBS implantation of either site should be considered an option for those patients in which bilateral procedures are contraindicated.
Keywords: deep brain stimulation, globus pallidus pars interna, force control, Parkinson’s disease, subthalamic nucleus, bilateral deficit, hand function
Introduction
Deep brain stimulation (DBS) has been shown to improve motor functioning of advanced PD patients for whom traditional medical management is no longer effective[1,2]. Data are now becoming available that address the long-term effectiveness of subthalamic (STN) DBS; improvements in UPDRS ratings between 40–55% are typical[3–5].
Despite the lack of randomized clinical trials bilateral DBS become the standard surgical treatment for advanced PD in most centers worldwide. However, unilateral STN DBS has been shown to produce significant improvements in clinical rating scales[6,7] in advanced PD patients and has not been reported to be associated with the adverse cognitive events observed after bilateral STN DBS[7,8]. Recently, Germano and colleagues[6] reported that unilateral stimulation of the STN was effective in improving motor function of advanced PD patients as UPDRS Motor Scores decreased by 26% (50% and 17% decrease in the contra- and ipsilateral limbs respectively).
Relatively long-term beneficial effects of unilateral pallidotomy on parkinsonian symptoms have been reported[9] The short- and long-term effects of unilateral globus pallidus interna (GPi) DBS on the motor symptoms of PD patients are poorly understood. The largest study to assess the effects of unilateral GPi DBS has shown that in the short-term (approximately three months postoperatively) UPDRS Motor scores decreased by 50%, however during a long-term follow up (6–49 months) UPDRS Motor scores increased 8.3% compared to preoperative levels[10]. The limited data addressing the effects of unilateral GPi DBS on long-term motor function of PD patients necessitates additional study.
The effects of bilateral STN or GPi DBS on cognitive functions such as verbal fluency, working memory, planning and problem solving, depression and verbal memory are equivocal at best. Few well-controlled evidence-based studies have been completed in which the impact of unilateral or bilateral DBS on mood and cognition have been completed[11]. However, there is an emerging body of evidence to suggest that bilateral STN DBS does produce significant and permanent cognitive declines in PD patients who were followed for five years[5]. In general, GPi DBS is not associated with declines in cognitive functioning[12,13], however declines in word fluency and visuoconstruction scores have been reported[3]. Considering the potential cognitive declines associated with bilateral procedures and potential for motor symptom relief with DBS, unilateral procedures may be a viable option for advanced PD patients when bilateral procedures may be contraindicated as a result of age, symptom asymmetry or mild cognitive declines.
In the current study we sought to answer the following: what are the long-term effects of unilateral stimulation of GPi or STN on the force production capabilities of each limb under unimanual and bimanual maximal efforts; does unilateral DBS lead to improvements in bimanual dexterity; does unilateral stimulation lead to persistent clinical improvements in PD motor functioning (as assessed by the UPDRS Part III).
Subjects and methods
Subjects, medication and stimulation parameters
Fourteen advanced PD patients diagnosed with idiopathic PD (see Table 1 for demographics) with unilateral DBS devices implanted in the GPi (n = 7) or STN (n = 7) were enrolled and consented into the study which was approved by the Cleveland Clinic IRB. Twelve of these patients were part of an on-going randomized clinical trial in which surgical candidates were randomized to either a GPi or STN stimulation group; one patient from each group was implanted outside of study protocol. Following random group assignment, stimulation side was determined on a case-by-case basis. The more affected side of the patient was determined clinically and the stimulating electrode was placed contralateral to that side for maximal symptom relief. Details regarding the surgical and microelectrode mapping procedures have been provided previously[14]. The daily levodopa dose equivalents decreased 36 and 22 percent for the STN and GPi groups respectively. Prior to DBS mean levodopa dose was 1192±585 mg and 1011±698 mg for the STN and GPi groups respectively. At the time of testing the STN group’s mean levodopa dose equivalent was 764±407 mg while the STN group’s was 789±433 mg
Table 1.
Group demographics and average UPDRS Part III Motor Scores
| Group | Gender | Ave. Age | Months post-surgery (range) | Ave. DBS Parameters | UPDRS Total Off/On | Rigidity Off/On | Tremor Off/On | Brady. Off/On | Gait Off/On | Postural Stability Off/On |
|---|---|---|---|---|---|---|---|---|---|---|
| GPi | 5 males 2 females | 52 (40–75) |
31.2 (18–55) |
148Hz 72µs 4.7V | 48/32 [33%] |
11/8 [34%] |
8/6 [33%] |
19/14 [28%] |
2/2 [0%] |
1/1 [0%] |
| STN | 4 males 3 females | 54 (38–68) |
23 (13–28) |
163Hz 84µs 3.1V | 51/34 [33%] |
11/6 [40%] |
10/5 [50%] |
22/16 [26%] |
1/1 [0%] |
1/1 [0%] |
Values within brackets represent percent change in UDPRS score from Off-DBS to On-DBS; Rigidity motor score taken from item 22, tremor taken from items 20 and 21, bradykinesia taken from items 23–26 and 31, gait taken from item 29, postural stability taken from item 30.
Patient age in the GPi group ranged from 40–75 years while the STN group ranged from 38–68 years. A two-tailed t-test indicated no significant difference in age between groups (P>0.1). No significant difference in postoperative time between groups was present (P>0.05) (see Table 1 for values). Patients’ medication and stimulation parameters were fully optimized, based on clinical response, for at least six months prior to data collection. Experimenters were blinded as to the site of stimulation during recruitment, data collection and analysis.
Experimental Setup
All experimental testing was performed while the patients were in the practically defined off stage in terms of antiparkinsonian medication[15] while the stimulator was On or Off. Patients reported to the laboratory while On stimulation. Upon completion of the informed consent process, stimulation parameters were documented using a Medtronic Console Programmer. The UPDRS Part III Motor Exam was then performed after which biomechanical testing was conducted. Upon completion of the biomechanical testing, the patient’s stimulator was turned Off. After three hours Off DBS, clinical and biomechanical tests were repeated.
Biomechanical Testing
Patients performed three unimanual maximum precision grip trials (e.g. using thumb and index finger only) with each hand separately while On and Off DBS. During bimanual trials, patients used a precision grip with both hands and exerted maximum force on each transducer. Unimanual and bimanual conditions were randomized between patients. During each maximum effort trial the patient was encouraged to squeeze the transducer with maximal force. Three minutes rest was given between each trial. The average of the three trials for each maximum testing condition was used for the analysis.
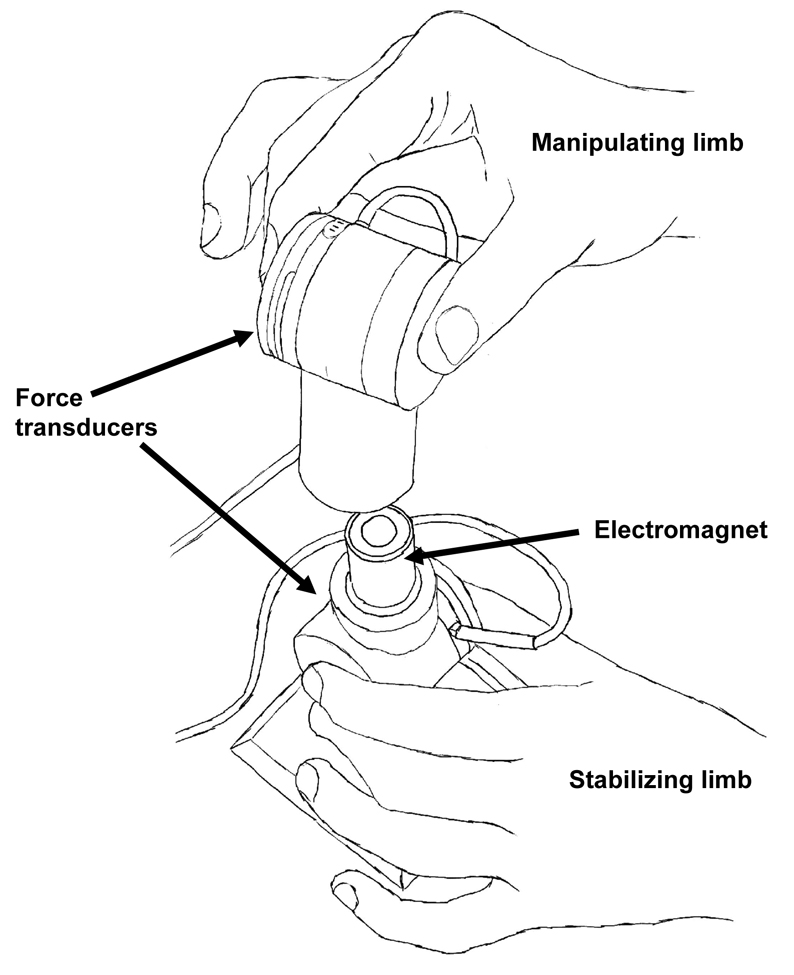
Bimanual dexterity was assessed using a paradigm developed in our laboratory (see Figure 1) to assess dexterity of PD patients, older adults and patients with stroke. Briefly, grasping forces, normal (grip) and tangential (load), for both limbs were recorded simultaneously using two identical six-dimensional force-torque transducers (ATI, Apex, NC) during the performance of the bimanual manipulation task. Patients were instructed to disconnect the two force transducers from one another using a precision grip; much like they would open a container. Ten trials were performed while On and Off DBS. An electromagnet exerted a resistive force of 8N between the upper and lower transducers. Grip force (Fz) was defined as the force along the axis normal to the flat surfaces of the transducer while load force (Fy) was defined as the force along the axis tangential to the flat surfaces of the transducer. Grip and load forces were recorded with an accuracy of 0.05N and 0.015N respectively. Force data were collected at 200 Hz using a customized LabView data acquisition program.
Figure 1.
Illustration of bimanual dexterity paradigm used to assess functional motor performance.
Data Processing and Analysis
A residual analysis was used to determine the optimal cut-off frequency for the force data; based on this analysis data were digitally filtered by a dual-pass through a second-order Butterworth filter with a 10.43-Hz cut-off. For the maximum force production task, maximum force produced by each hand was the primary outcome variable. Movement time (MT) for each limb and consistency of digit placement were the primary outcomes for the bimanual dexterity task. The center of pressure (COP) was determined through analysis of the torque data from each force-torque transducer during task performance. The first step in quantifying the COP was the creation of an ellipse that encompassed 95 percent of the COP data using principal component analysis. The area of the ellipse was calculated and used as an measure of the consistency of digit placement.
Statistical Analysis
The influence of stimulation site on force producing capabilities and manual dexterity was assessed through a group (GPi vs. STN) by stimulation condition (On vs. Off DBS) analysis using a repeated measures ANOVA. Paired t-tests were used to follow-up any significant interactions, main effects and differences between limbs. Statistical significance was set at (P<0.05).
Results
Results from the group by stimulation repeated measures ANOVA indicated that there were no significant interactions between these two sites (P>0.1) on any clinical or biomechanical variables. Furthermore, there were no main effects of group on any of these same variables (P>0.1). The remainder of the Results will focus on the overall effects of stimulation for each group and ipsilateral vs. contralateral effects during maximal efforts and manual dexterity.
Clinical Ratings
Table 1 contains UPDRS ratings for both patient groups. Stimulation led to a significant decrease in UPDRS Motor scores (P<0.01); on average stimulation led to a 33% improvement for both groups. The UPDRS was parsed into scores pertaining to the following symptoms: rigidity, tremor, bradykinesia, gait and postural stability. Changes in these subcategories while On and Off DBS are provided in Table 1. The GPi group exhibited improvement in rigidity (34%) tremor (33%) and bradykinesia (28%). Patients with STN stimulation showed the largest improvement in tremor (50%) while improvements of 40% and 26% were noted in the rigidity and bradykinesia. Gait and postural stability were largely unchanged.
The UPDRS data were further parsed to examine the effects of stimulation on rigidity, tremor and bradykinesia for each limb. For the GPi group rigidity, tremor and bradykinesa of the ipsilateral limb were reduced by 13%, 15% and 12% respectively due to stimulation. On average, the STN group showed ipsilateral benefits of 14%, 17% and 12% for rigidity, tremor and bradykinesia as a result of stimulation. Contralateral benefits of stimulation were significantly greater (P<0.01) for both groups compared to ipsilateral benefits. For the contralateral limb, rigidity, tremor and bradykinesa improved 45%, 46% and 40% respectively for the GPi group while the STN group improved 46%, 72% and 42% on these same variables.
Maximum force production
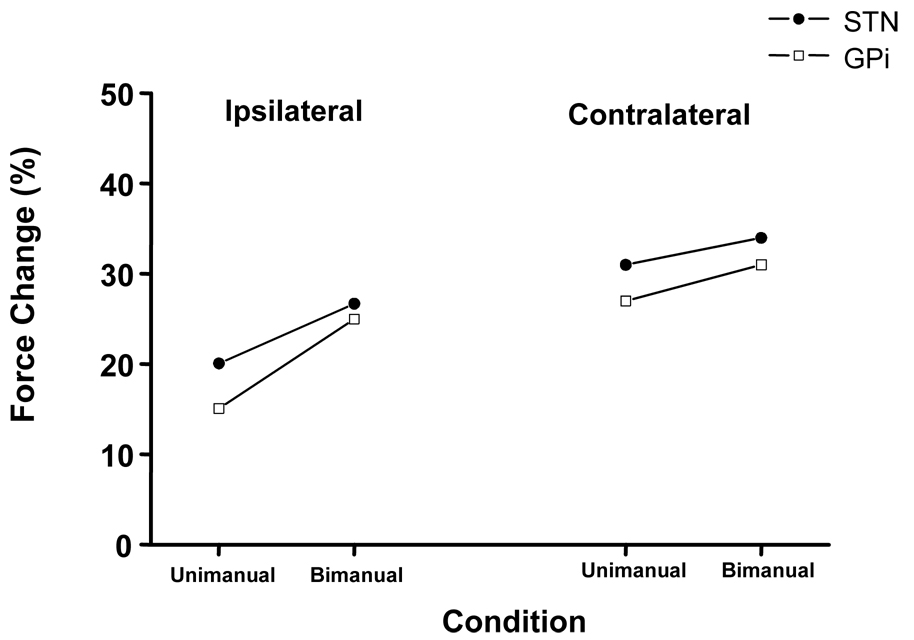
The mean(sd) absolute maximum grip force produced by the ipsilateral and contralateral limbs for both groups while On and Off stimulation are provided in Table 2. The values in Table 2 indicate that maximum grip force amplitude increased for both groups during unimanual and bimanual conditions. The magnitude of change in maximum grip force was assessed by calculating the percent increase in maximum force while Off relative to On stimulation. Figure 2 contains the percent increase in maximum force in both limbs as a result of DBS during unimanual and bimanual conditions. Inspection of Figure 2 indicates, maximum force produced by the ipsilateral limb increased 15% and 20% for the GPi and STN groups respectively (P>0.01). Similarly, contralateral maximum force increased 27% for the GPi and 31% for the STN groups respectively (P>0.001). Results from a paired t-test indicated that contralateral force gains were significantly greater than ipsilateral force gains (P>0.05) for both groups.
Table 2.
Group means (sd) for maximum precision grip force (N) for each limb, across conditions.
| Unimanual Grip - Contralateral limb | Unimanual Grip - Ipsilateral limb | Bimanual Grip - Contralateral limb | Bimanual Grip - Ipsilateral limb | |||||
|---|---|---|---|---|---|---|---|---|
| Off-DBS |
On-DBS |
Off-DBS |
On-DBS |
Off-DBS |
On-DBS |
Off-DBS |
On-DBS |
|
| GPi | 52.8(14.5) | 67.9(17.9) | 49.5(12.2) | 55.9(13.5) | 48.9(12.2) | 64.8(19.2) | 46.6(13.1) | 63.3(18.6) |
| STN | 38.7(17.1) | 50.3(18.7) | 38.1(17.2) | 44.6(15.8) | 35.2(12.0) | 50.1(17.6) | 31.2(14.4) | 43.2(18.7) |
Figure 2.
The average percent change in maximum grip force produced by each limb during unimanual and bimanual testing from Off to On stimulation.
As shown in Figure 2, ipsilateral strength gains were approximately 25% for both groups during bimanual conditions. Strength gains for the ipsilateral limb under bimanual conditions were significantly greater than unimanual conditions (P>0.05 for both groups). Maximum force production in the contralateral limb also increased (P>0.001 for both groups). On average the GPi group increased contralateral maximum force by 31% and the STN group by 34% from Off to On DBS. There was no difference in contralateral strength gains for either group or when data were collapsed across group as a function of unimanual or bimanual conditions.
Figure 3 contains the sum of forces produced by each limb under unimanual and bimanual conditions. Unimanual summed force is the summation of maximum force produced by the left and right limb during their respective unimanual maximal efforts. During bimanual efforts the maximum force produced by each limb is summed. Comparisons were made between unimanual and bimanual summations while On and Off stimulation. While patients were Off stimulation (upper plots of Figure 3), a bilateral deficit was present. A bilateral deficit is present when the summed force of the two limbs is greater in the unimanual compared to bimanual conditions (i.e. the sum of force produced by the left and right limbs is greater during separate unimanual efforts compared to simultaneous efforts). While Off stimulation both groups exhibited a significant (P<0.01) decrease in total force producing capabilities in the bimanual condition. During stimulation neither group exhibited a significant bilateral deficit. In fact, the GPi group actually exhibited a bilateral facilitation as summed force during the bimanual trials was 3.3% greater than summed force for the unimanual trials. Patients in the STN group decreased their bilateral deficit to a negligible 1.7%. These differences between groups were not statistically significant.
Figure 3.
The summed force produced by each hand during unimanual (left + right during separate unimanual efforts)and bimanual (left + right during simultaneous efforts)conditions for both groups while off (upper plots) and on (lower plots) stimulation. Negative numbers above bars indicate a bilateral deficit while the positive value represents a bilateral facilitation.
Bimanual Dexterity
In all patients the contralateral limb (which was also their dominant limb) was used to manipulate the upper object while the ipsilateral limb served to stabilize the lower object. Patients selected this pattern on their own as they were only instructed to perform this activity like a typical daily action such as opening a container. The MT for both limbs decreased significantly while On stimulation compared to Off stimulation (P<0.001). While Off DBS average(sd) MT for the ipsilateral (stabilizing) limb was 938(97) and 1121(102) ms for the GPi and STN groups respectively. Ipsilateral MT decreased by nearly 25 (704(89) ms) and 28 percent (807(98) ms) for the GPi and STN groups respectively. The MT for contralateral or manipulating limb decreased, as a result of DBS, approximately 26 (895(106) to 655(88) ms) and 32 (1093(122) to 745(69) ms) percent for the GPi and STN groups respectively.
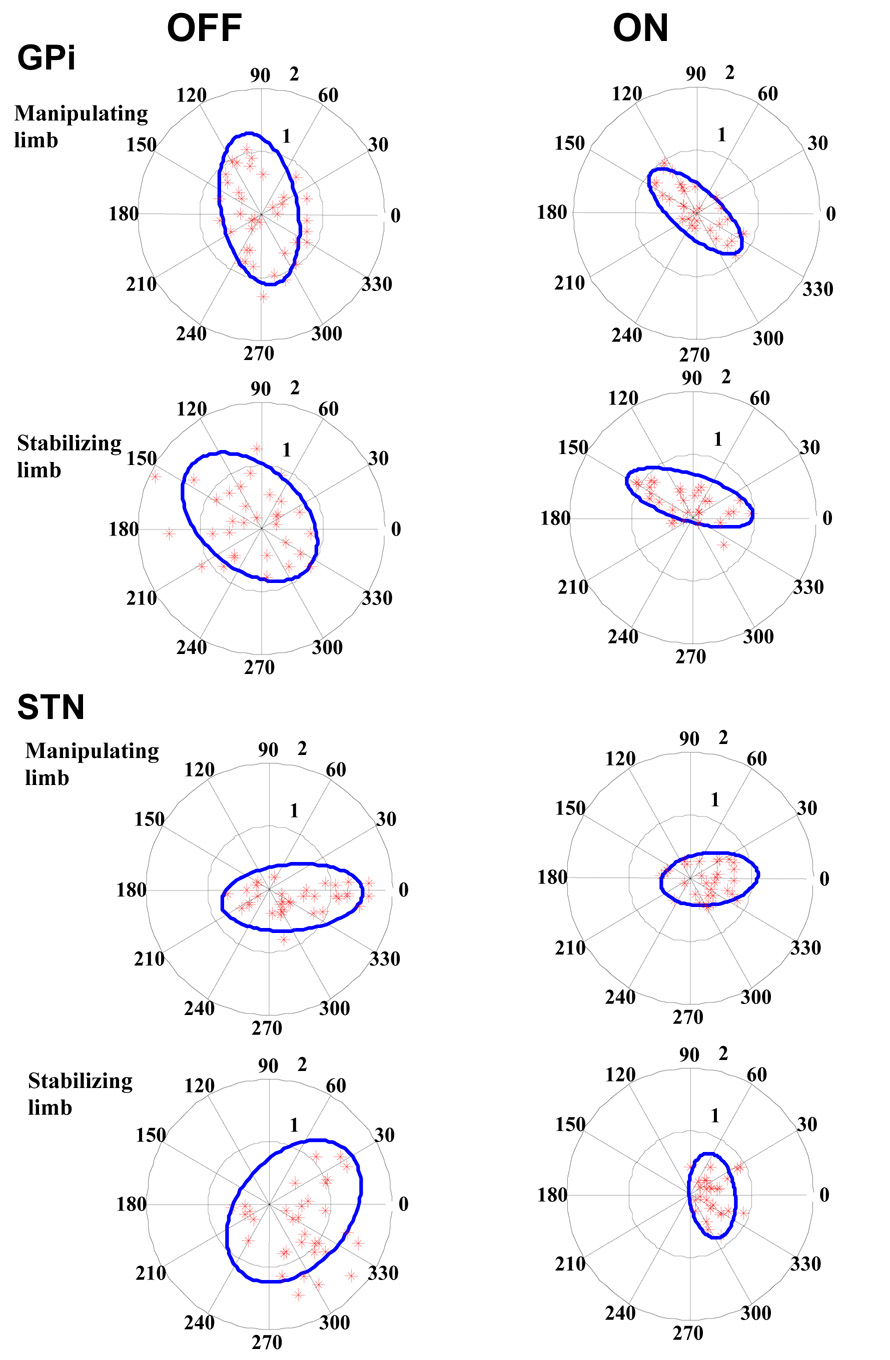
Individual patient COP data for both groups and both limbs while On and Off DBS are provided in Figure 4 (upper plots=GPi; lower plots=STN). While Off DBS patients COP was highly variable as it was dispersed across the surface of the transducers. With stimulation patients’ tended to grasp the transducers in a more consistent position (e.g. nearer the center of the objects). The area of the ellipse encompassing 95 percent of the COP data was significantly reduced for both limbs in both groups of patients (P>0.001) as a result of stimulation. For the GPi group while Off DBS, the area for the manipulating and stabilizing limbs was 3.57 cm2 and 4.65 cm2 respectively; during unilateral DBS area decreased to 1.48 cm2 and 1.53 cm2 for the two limbs. Subthalamic DBS resulted in a decrease in COP area; Off DBS area was 2.99 cm2 and 3.89 cm2 for the manipulating and stabilizing limbs respectively. During DBS area for the manipulating and stabilizing limbs decreased to 1.03 cm2 and .81 cm2 respectively.
Figure 4.
Center of pressure (COP) values for all bimanual dexterity trials for both groups while Off and On DBS. The object area, outer circle of radius 2 cm, is divided into sectors of 30 degrees (dotted lines) in counter clock wise direction. The inner circle (gray dotted line) indicates the sub area of 1 cm radius. COP data is represented by stars and the solid ellipse defines the area of the spread to encompass 95 percent of the data.
Discussion
The current study is the first to systematically examine the long-term effects of unilateral GPi or STN DBS on the force producing capabilities of both limbs under unimanual and bimanual motor conditions. Overall, the results indicate that unilateral GPi or STN DBS is effective in improving overall motor function, maximum force production and the control and specification of grasping forces in both limbs and these effects persist at least one year after DBS surgery. Stimulation produced greater clinical gains in motor function and strength for the contralateral limb; significant improvements were also observed in the ipsilateral limb. The data indicate that ipsilateral function is enhanced under conditions in which both limbs are performing a task. Improved motor function for both limbs, under unimanual and bimanual task conditions, as a result of unilateral stimulation provides a compelling argument that unilateral DBS may provide sufficient symptom relief for those patients with asymmetric motor symptoms.
Unilateral stimulation of either site improved strength, movement speed and grasping force control/consistency of digit placement on the objects. Previous studies have reported unilateral GPi[10,16] stimulation leads to significant short-term (three months or less) improvements in UPDRS Motor scores of advanced PD patients. However, these same studies report that after approximately one year unilateral pallidal stimulation is no longer effective in the treatment of PD symptoms; symptoms are similar or in some cases worse than preoperative levels. The clinical and biomechanical data from the GPi patients examined in the present study are not consistent with previous findings. Differences in experimental design and precision of outcome measures may account for these disparate findings, namely the duration between turning the stimulator Off and the evaluation of the patient and the use of biomechanical methods to assess functional dexterity. The interval between turning the patient’s DBS system Off and evaluation was not specified in the previous reports[10,16]. The time-course in which the reappearance of Parkinson’s symptoms occurs is an important issue in accurately determining the effects of stimulation. Temperli and colleagues[17] reported a progressive worsening of symptoms in STN patients up to approximately two hours. Based on these data they recommended that patients be Off stimulation for at least three hours in order to estimate the clinical effects of STN stimulation[17]. Insufficient time between clinical assessments while On and Off stimulation will lead to an underestimation of the beneficial effects of stimulation as there is a progressive worsening of symptoms over time. Because our patients were Off for three hours, the current data likely provide a more accurate assessment of the effects of unilateral GPi and STN stimulation on both limbs. The use of a functional manual dexterity task provides an opportunity to objectively and precisely quantify specific movement parameters during the performance of a task that replicates those performed on a daily basis[18]. Clinical measures such as the UPDRS Motor Exam do not offer these same opportunities.
Previous studies have shown that a bilateral deficit exists between unimanual and bimanual maximal efforts[19]. A bilateral deficit occurs when the summed force of each limb under bimanual conditions is less than the summed force of each limb under unimanual conditions. The effects of PD and DBS on bimanual force production have not been examined previously. In the current study, while Off stimulation the GPi group had a bilateral deficit of seven percent (e.g. summed force produced by each hand during bimanual conditions was seven percent less than summed force of each hand during unimanual conditions) and the STN group had a bilateral deficit of 15.6 percent. A potential mechanism underlying bilateral deficit is reduced neural drive during simultaneous contractions of homologous muscles of the two limbs[20]. Reduced cortical activity is well documented in PD[21,22] and is integrated into models of basal ganglia function[23,24]. The presence of a bilateral deficit while Off stimulation is consistent with the notion that decreased motor cortical activity associated with PD may be contributing to the inability to fully activate the muscles of each limb simultaneously.
Unilateral stimulation essentially eliminated the bilateral deficit for both groups. The GPi group actually exhibited a bilateral facilitation of 3.3 percent, while the STN reduced their deficit to just 1.7 percent. Unilateral DBS also improved bimanual dexterity as the consistency of digit placement on the objects being manipulated was significantly reduced. The reduction/elimination of the bilateral deficit and improved motor function during stimulation suggests DBS results in an activation of motor cortical areas, possibly via the supplementary motor area (SMA).
Despite the fact that the basal ganglia consist of multiple parallel pathways projecting to each of the motor cortical areas[25], impaired movement related cortical activity in PD is particularly pronounced in the SMA[26]. The abnormal processing of phasic cortical input by the basal ganglia and transmission of this altered output to the SMA or other cortical areas is likely to disrupt the normal firing patterns of movement related cortical neurons. Alterations in the function of this circuitry are likely responsible for the bilateral deficit observed in PD patients. The fact that stimulation enhanced bimanual force production and dexterity, for both groups, suggests that it may be blocking the abnormal patterns of activity transmitted from the basal ganglia to the SMA or stimulation may produce higher ordered activity in the basal ganglia that reduces the disruption in cortical signal transmission[27].
Activation studies using PET have sought to identify the effects of GPi and STN DBS on motor cortical areas. Pallidotomy has been shown to result in an increase in movement related cortical activity in the SMA[28]; similar finding have been reported in patients with GPi DBS[29]. Davis and colleagues[29] reported an increase in rCBF levels in the SMA and other motor cortical areas during effective stimulation. Two PET studies have shown that STN DBS leads to increased activation centered around the SMA (ipsilateral to stimulation and contralateral to movement) during internally generated movements (e.g. similar to the current paradigm)[30,31]. Improved SMA function in the hemisphere ipsilateral to stimulation site is most likely the mechanism responsible for enhanced bimanual functioning observed in the current study. The extensive interhemispheric connections between the SMA[32] and ipsilateral projections from the SMA to striatum[33] provide anatomical support for the notion that improved SMA functioning leads to improvements in motor function in the ipsilateral and contralateral limbs. The importance of the SMA in the control and planning of bimanual activities[22] may explain why ipsilateral motor function of PD patients during DBS is enhanced during bimanual activities.
Long-term improvements in motor function with unilateral stimulation of the STN are consistent with a recent report[6]. Persistent improvements in motor function with unilateral stimulation of GPi differ with earlier reports[10,16]. As discussed previously, differences in methodology, namely the duration between turning the stimulator Off and performing the clinical evaluation and/or lead location and precision of movement assessment, are possible explanations for the disparate findings. One limitation of a maximum force production paradigm is that patient motivation and fatigue could impact effort level. While patients were consistent in their maximum force produced within conditions, it is acknowledged that in order to achieve the patients’ true maximum force, twitch interpolation techniques are required.
Additional studies are necessary to determine if a unilateral staged implantation strategy may be more favored over immediate bilateral implantation, especially for those patients with asymmetric symptoms, older patients or those with mild preexisting cognitive impairments. We are currently comparing the effects of unilateral to bilateral DBS on upper and lower extremity motor function using additional biomechanical measures and paradigms that require the precise control of grasping forces in both limbs simultaneously.
Acknowledgements
This study supported by NIH AG20797-01, NS037959, and the American Academy of Neurology Foundation.
Abbreviations
- ANOVA
analysis of variance
- DBS
deep brain stimulation
- GPi
globus pallidus pars interna
- STN
subthalamic nucleus
- SMA
supplementary motor area
- UPDRS
Unified Parkinson’s Disease Rating Scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rodriguez-Oroz MC, Gorospe A, Guridi J, Ramos E, Linazasoro G, Rodriguez-Palmero M, Obeso JA. Bilateral deep brain stimulation of the subthalamic nucleus in Parkinson's disease. Neurology. 2000;55:S45–S51. [PubMed] [Google Scholar]
- 2.Benabid AL, Pollak P, Gross C, Hoffmann D, Benazzouz A, Gao DM, Laurent A, Gentil M, Perret J. Acute and long-term effects of subthalamic nucleus stimulation in Parkinson's disease. Stereotact.Funct.Neurosurg. 1994;62:76–84. doi: 10.1159/000098600. [DOI] [PubMed] [Google Scholar]
- 3.Volkmann J, Allert N, Voges J, Sturm V, Schnitzler A, Freund HJ. Long-term results of bilateral pallidal stimulation in Parkinson's disease. Ann Neurol. 2004;55:871–875. doi: 10.1002/ana.20091. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Oroz MC, Obeso JA, Lang AE, Houeto JL, Pollak P, Rehncrona S, Kulisevsky J, Albanese A, Volkmann J, Hariz MI, Quinn NP, Speelman JD, Guridi J, Zamarbide I, Gironell A, Molet J, Pascual-Sedano B, Pidoux B, Bonnet AM, Agid Y, Xie J, Benabid AL, Lozano AM, Saint-Cyr J, Romito L, Contarino MF, Scerrati M, Fraix V, Van Blercom N. Bilateral deep brain stimulation in Parkinson's disease: a multicentre study with 4 years follow-up. Brain. 2005;128:2240–2249. doi: 10.1093/brain/awh571. [DOI] [PubMed] [Google Scholar]
- 5.Schupbach WM, Chastan N, Welter ML, Houeto JL, Mesnage V, Bonnet AM, Czernecki V, Maltete D, Hartmann A, Mallet L, Pidoux B, Dormont D, Navarro S, Cornu P, Mallet A, Agid Y. Stimulation of the subthalamic nucleus in Parkinson's disease: a 5 year follow up. J Neurol Neurosurg Psychiatry. 2005;76:1640–1644. doi: 10.1136/jnnp.2005.063206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Germano IM, Gracies JM, Weisz DJ, Tse W, Koller WC, Olanow CW. Unilateral stimulation of the subthalamic nucleus in Parkinson disease: a double-blind 12- month evaluation study. J Neurosurg. 2004;101:36–42. doi: 10.3171/jns.2004.101.1.0036. [DOI] [PubMed] [Google Scholar]
- 7.Kumar R, Lozano AM, Sime E, Halket E, Lang AE. Comparative effects of unilateral and bilateral subthalamic nucleus deep brain stimulation. Neurology. 1999;53:561–566. doi: 10.1212/wnl.53.3.561. [DOI] [PubMed] [Google Scholar]
- 8.Saint-Cyr JA, Trepanier LL, Kumar R, Lozano AM, Lang AE. Neuropsychological consequences of chronic bilateral stimulation of the subthalamic nucleus in Parkinson's disease. Brain. 2000;123(Pt 10):2091–2108. doi: 10.1093/brain/123.10.2091. [DOI] [PubMed] [Google Scholar]
- 9.Vitek JL, Bakay RA, Freeman A, Evatt M, Green J, McDonald W, Haber M, Barnhart H, Wahlay N, Triche S, Mewes K, Chockkan V, Zhang JY, DeLong MR. Randomized trial of pallidotomy versus medical therapy for Parkinson's disease. Ann Neurol. 2003;53:558–569. doi: 10.1002/ana.10517. [DOI] [PubMed] [Google Scholar]
- 10.Visser-Vandewalle V, van der Linden C, Temel Y, Nieman F, Celik H, Beuls E. Long-term motor effect of unilateral pallidal stimulation in 26 patients with advanced Parkinson disease. J Neurosurg. 2003;99:701–707. doi: 10.3171/jns.2003.99.4.0701. [DOI] [PubMed] [Google Scholar]
- 11.Skidmore FM, Rodriguez RL, Fernandez HH, Goodman WK, Foote KD, Okun MS. Lessons learned in deep brain stimulation for movement and neuropsychiatric disorders. CNS Spectr. 2006;11:521–536. doi: 10.1017/s1092852900013559. [DOI] [PubMed] [Google Scholar]
- 12.Trepanier LL, Kumar R, Lozano AM, Lang AE, Saint-Cyr JA. Neuropsychological outcome of GPi pallidotomy and GPi or STN deep brain stimulation in Parkinson's disease. Brain Cogn. 2000;42:324–347. doi: 10.1006/brcg.1999.1108. [DOI] [PubMed] [Google Scholar]
- 13.Fields JA, Troster AI, Wilkinson SB, Pahwa R, Koller WC. Cognitive outcome following staged bilateral pallidal stimulation for the treatment of Parkinson's disease. Clin Neurol Neurosurg. 1999;101:182–188. doi: 10.1016/s0303-8467(99)00044-x. [DOI] [PubMed] [Google Scholar]
- 14.Starr PA, Vitek JL, Bakay RA. Ablative surgery and deep brain stimulation for Parkinson's disease. Neurosurgery. 1998;43:989–1013. doi: 10.1097/00006123-199811000-00001. discussion 1013–1015. [DOI] [PubMed] [Google Scholar]
- 15.Langston JW, Widner H, Goetz CG, Brooks D, Fahn S, Freeman T, Watts R. Core assessment program for intracerebral transplantations (CAPIT) Mov Disord. 1992;7:2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- 16.Visser-Vandewalle V, Temel Y, van der Linden C, Ackermans L, Beuls E. Deep brain stimulation in movement disorders. The applications reconsidered. Acta Neurol Belg. 2004;104:33–36. [PubMed] [Google Scholar]
- 17.Temperli P, Ghika J, Villemure JG, Burkhard PR, Bogousslavsky J, Vingerhoets FJ. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology. 2003;60:78–81. doi: 10.1212/wnl.60.1.78. [DOI] [PubMed] [Google Scholar]
- 18.Kilbreath SL, Heard RC. Frequency of hand use in healthy older persons. Aust J Physiother. 2005;51:119–122. doi: 10.1016/s0004-9514(05)70040-4. [DOI] [PubMed] [Google Scholar]
- 19.Van Dieen JH, Ogita F, De Haan A. Reduced neural drive in bilateral exertions: a performance-limiting factor? Med Sci Sports Exerc. 2003;35:111–118. doi: 10.1097/00005768-200301000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Hakkinen K, Kraemer WJ, Newton RU. Muscle activation and force production during bilateral and unilateral concentric and isometric contractions of the knee extensors in men and women at different ages. Electromyogr Clin Neurophysiol. 1997;37:131–142. [PubMed] [Google Scholar]
- 21.Brooks DJ. Basal ganglia function during normal and Parkinsonian movement - PET activation studies. In: Battistin L, Scarlato G, Caraceni T, Ruggieri S, editors. Parkinson'S Disease. Advances in Neurology. Philadelphia: Lippincott-Raven Pub; 1996. pp. 433–441. [PubMed] [Google Scholar]
- 22.Cunnington R, Bradshaw JL, Iansek R. The Role of the Supplementary motor area in the control of voluntary movement. Human Movement Science. 1996;15:627–647. [Google Scholar]
- 23.Wichmann T, DeLong MR. Pathophysiology of parkinsonian motor abnormalities. Adv.Neurol. 1993;60:53–61. [PubMed] [Google Scholar]
- 24.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 25.Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 26.Brooks DJ, Samuel M. The effects of surgical treatment of Parkinson's disease on brain function: PET findings. Neurology. 2000;55:S52–S59. [PubMed] [Google Scholar]
- 27.Dorval AD, Russo GS, Hashimoto T, Xu W, Vitek JL, Grill WM. Neural Interfaces Workshop. Bethesda, MD: 2005. High Frequency Subthalamic Stimulation Restores Order to Pallidal Firing Patterns; p. 16. [Google Scholar]
- 28.Grafton ST, Waters C, Sutton J, Lew MF, Couldwell W. Pallidotomy increases activity of motor association cortex in Parkinson's disease: a positron emission tomographic study. Ann.Neurol. 1995;37:776–783. doi: 10.1002/ana.410370611. [DOI] [PubMed] [Google Scholar]
- 29.Davis KD, Taub E, Houle S, Lang AE, Dostrovsky JO, Tasker RR, Lozano AM. Globus pallidus stimulation activates the cortical motor system during alleviation of parkinsonian symptoms. Nat Med. 1997;3:671–674. doi: 10.1038/nm0697-671. [DOI] [PubMed] [Google Scholar]
- 30.Limousin P, Greene J, Pollak P, Rothwell J, Benabid AL, Frackowiak R. Changes in cerebral activity pattern due to subthalamic nucleus or internal pallidum stimulation in Parkinson's disease. Ann Neurol. 1997;42:283–291. doi: 10.1002/ana.410420303. [DOI] [PubMed] [Google Scholar]
- 31.Ceballos-Baumann AO, Boecker H, Bartenstein P, von Falkenhayn I, Riescher H, Conrad B, Moringlane JR, Alesch F. A positron emission tomographic study of subthalamic nucleus stimulation in Parkinson disease: enhanced movement-related activity of motor-association cortex and decreased motor cortex resting activity. Arch Neurol. 1999;56:997–1003. doi: 10.1001/archneur.56.8.997. [DOI] [PubMed] [Google Scholar]
- 32.Wiesendanger M, Rouiller EM, Kazennikov O, Perrig S. Is the supplementary motor area a bilaterally organized system? Adv Neurol. 1996;70:85–93. [PubMed] [Google Scholar]
- 33.Lehericy S, Ducros M, Krainik A, Francois C, Van de Moortele PF, Ugurbil K. Kim DS: 3-D diffusion tensor axonal tracking shows distinct SMA and pre-SMA projections to the human striatum. Cereb Cortex. 2004;14:1302–1309. doi: 10.1093/cercor/bhh091. [DOI] [PubMed] [Google Scholar]