Abstract
Background: At the Yale University Center for Thoracic Aortic Disease, we have been using our clinical experience and laboratory investigations to shed light on the pathophysiology of thoracic aortic aneurysm (TAA), the clinical behavior of thoracic aortic aneurysm, and the optimal clinical management.
Materials and Methods: The Yale database contains information on 3,000 patients with thoracic aortic aneurysm, with 9,000 patient-years of follow-up and 9,000 imaging studies. Advanced statistical techniques were applied to this information.
Results: Analysis yielded the following Yale-generated observations: (1) TAA is a genetic disease with a predominantly autosomal dominant mode of inheritance; (2) matrix metalloproteinase (MMP) enzymes are activated in the pathogenesis of TAA; (3) wall tension in TAA approaches the tensile limits of aortic tissue at a diameter of 6 cm; (4) by the time a TAA reaches a clinical diameter of 6 cm, 34 percent of affected patients have suffered dissection or rupture; (5) extreme physical exertion or severe emotion often precipitate acute dissection; and (6) single nucleotide polymorphisms (SNPs) and RNA expression profile changes are being identified that predispose a patient to TAA and can serve as biomarkers for screening for this virulent disease.
Conclusions: The “playbook” of TAA is gradually being read, with the help of scientific investigations, positioning practitioners to combat this lethal disease more effectively than ever before.
Imagine if a sports team were able to read the opposing team’s playbook; this would be a boon of major proportions. Aortic diseases represent a lethal opponent for cardiac specialists and for our patients. Over the last 10 years at the Yale Center for Thoracic Aortic Disease, we have made a concerted effort to learn more about the natural history of aortic diseases based on a dataset that includes information on 3,000 patients with 9,000 years of patient follow-up and 9,000 serial imaging studies. This analysis has given us glimpses into the “Playbook” of thoracic aortic diseases; these glimpses have corollaries in terms of the appropriate role and timing of surgical intervention. We will enumerate in this communication some of the specific insights into the natural behavior of thoracic aortic aneurysm that have been gleaned over the last 10 years at the Yale Center for Thoracic Aortic Disease.
We will start with some introductory comments to this disease, before moving on to enumerate and describe our specific glimpses into the “Playbook” of thoracic aortic disease.
Aortic dissection is one of the most catastrophic acute natural events that can befall a human being. The pain of this disorder is often described by those affected as the most severe pain imaginable, eclipsing that of childbirth and kidney stones. It is interesting that nature perceives the pain of dissection as a “splitting” or “tearing” quality, very much apropos of the pathologic process itself. Because acute aortic dissection often masquerades as a heart attack, its true incidence is often underestimated. If a middle-aged or elderly person arrives in the emergency room with acute onset of chest pain, clutches his chest, and promptly dies, he is likely to be signed out as having experienced a “myocardial infarction.” In actual fact, many such presentations represent undiagnosed aortic dissections.
It takes autopsy series to document the true incidence of acute aortic dissection. Such series have indicated that aortic dissection is actually the most common lethal condition affecting the human aorta, more common than the better-appreciated ruptured abdominal aortic aneurysm [1]. Furthermore, with the increasing frequency of 3-D imaging of the human body — including the so-called drive-in computerized tomography (CT) scanners — aortic pathology is being diagnosed more thoroughly.
For all these reasons, acute aortic dissection is a condition of great importance, not only to the surgical specialist but also to the generalist and to other specialists in emergency medicine, radiology, and cardiology, among others.
This article addresses the natural history of aortic dissection. Its surgical treatment will be discussed only in the terms of indications for intervention based on the natural history.
We will discuss the findings from the Yale Center for Thoracic Aortic Disease in terms of specific individual questions that have been answered over the last 10 years.
How fast does the aneurysmal thoracic aorta grow?
When we started our investigations, we found that very little was known about the natural behavior of the aneurysmal thoracic aorta. Although hundreds of articles had been written about how to do aortic operations, very little had been written about when to do them or about how the thoracic aorta behaves.
We began with the fundamental question of how fast the aneurysmal thoracic aorta grows. We found that, although a virulent disease, thoracic aortic aneurysm is an indolent process. The thoracic aorta grows very slowly — at about 0.1 cm per year. The descending aorta grows a bit faster than the ascending (Figure 1). Prior estimates had failed to account for negative observed growth (measurements of any object will vary about a mean) and other statistical complexities, leading to overestimates of actual growth. Dr. John Rizzo of our team developed exponential equations specifically for the purpose of accurately estimating growth rates [2]. In fact, reports of rapid growth of the thoracic aorta are usually reflective of measurement error — specifically, measuring across an oblique portion of the aorta, especially the aortic arch (Figure 2).
Figure 1.
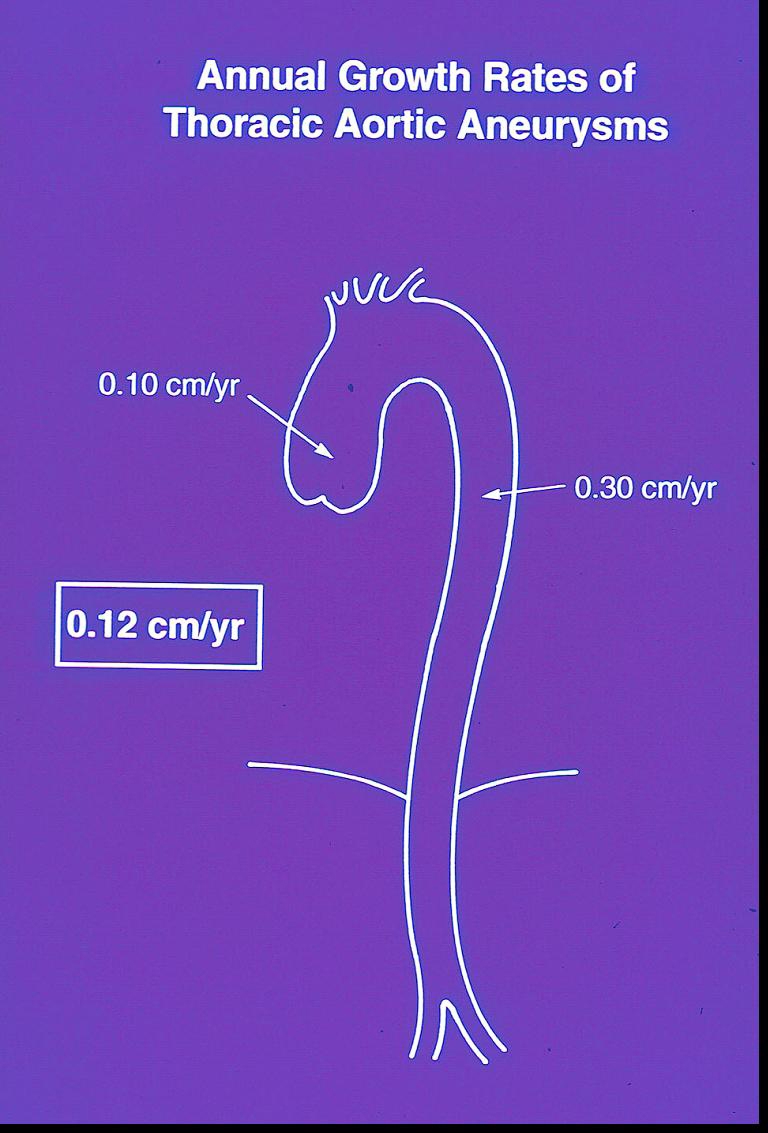
Growth rate of the aneurysmal thoracic aorta. This is a virulent, but indolent common process.
Figure 2.
Measurement error from measuring across an oblique portion of the aorta: spurious-dotted lines; true solid lines.
The only condition we have encountered in which the thoracic aorta truly grows rapidly in a short time is when there has been an intercurrent aortic dissection; this should be suspected in a circumstance in which the diameter of the aorta truly has grown rapidly.
At what size does the aorta dissect or rupture? When should preemptive surgery be performed?
The poor prognosis after realized dissection indicates how critically important it is to intervene before aortic dissection occurs. Our detailed studies of the natural history of the enlarged aorta have defined criteria that predict when dissection and rupture are likely to occur in an enlarged thoracic aorta.
We started by analyzing the lifetime risk of rupture or dissection. Analysis revealed sharp “hinge points” in the aortic size at which rupture or dissection occur. These hinge points occur at 6 cm in the ascending aorta and 7 cm in the descending aorta (Figure 3). It is important to intervene before the aorta reaches these hinge point dimensions. Specifically, as seen in the figure, an individual with thoracic aortic aneurysm incurs a 34 percent risk of rupture or dissection by the time that his ascending aorta reaches a diameter of 6 cm. As seen in the figure, the descending aorta, for inexplicable reasons, does not rupture until a larger dimension.
Figure 3.
Depiction of “hinge points” for lifetime natural history complications at various sizes of the aorta. The y-axis lists the probability of complication; complication refers to rupture or dissection. The x-axis shows aneurysm size. A. The ascending aorta. B. The descending aorta. Reproduced from Coady MA, Rizzo JA, Hammond GL, et al. What is the appropriate size criterion for resection of thoracic aortic aneurysm? J Thorac Cardiovasc Surg. 1997;113:476–91.
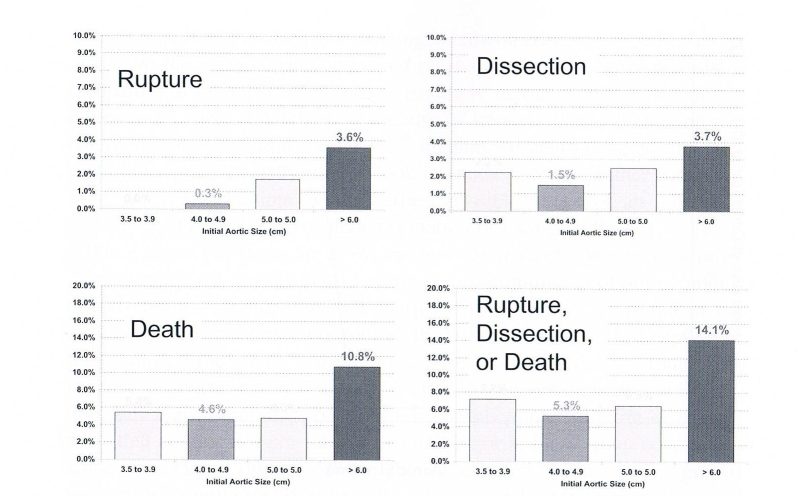
To calculate yearly growth rates required even more robust data, which have now become available in our database. This analysis reveals that the incidence of rupture, dissection, or death increases in a roughly linear fashion as the aorta grows, reaching maximal levels at an aortic dimension of 6 cm (Figure 4). There is something special about the dimension of 6 cm, which we shall see is important mechanically as well as clinically.
Figure 4.
Yearly rates of rupture, dissection, or death related to aortic size. Reproduced from Elefteriades JA. Beating a sudden killer. Sci Am. 2005.
These data have permitted our formulating evidence-based guidelines for surgical intervention for thoracic aortic aneurysms (Figure 5). These guidelines have been widely adopted. The simple fact is that the vast majority of ruptures, dissections, and aneurysm-related deaths can be prevented by preemptive extirpation of the aneurysmal thoracic aorta before the critical dimension of 6 cm is reached.
Figure 5.

Guidelines for surgical intervention.
It is well-known that dissection can occur in patients with Marfan’s disease whose aortic diameter is quite small. For this reason, we have decreased the intervention criterion for Marfan’s patients to lower levels, namely 5.0 cm for the ascending aorta and 6.0 cm for the descending aorta. We consider patients without Marfan’s disease but with a family pattern of aortic disease just as vulnerable as Marfan’s patients, so we use these lower criteria for patients with a positive family history as well. We also use a 5.0 cm criterion for patients with bicuspid aortic valve, whose aortas we have shown to be biologically deficient in terms of the balance of MMPs and their restraining tissue inhibitors of matrix metalloproteinases or TIMPs [3].
It should be emphasized that these criteria apply to asymptomatic aneurysms. Symptomatic aneurysms should be resected regardless of size. Symptoms are a harbinger of aortic rupture or dissection and must not be ignored. However, symptoms are rare in this disease; only about 5 percent of patients are symptomatic before an acute aortic event occurs. For the other 95 percent of patients, the first symptom is often death. Ascending aneurysms can produce retrosternal pain that is not exertional in nature, and descending aneurysms can produce inter-scapular back pain. Attention to these symptoms literally can be lifesaving.
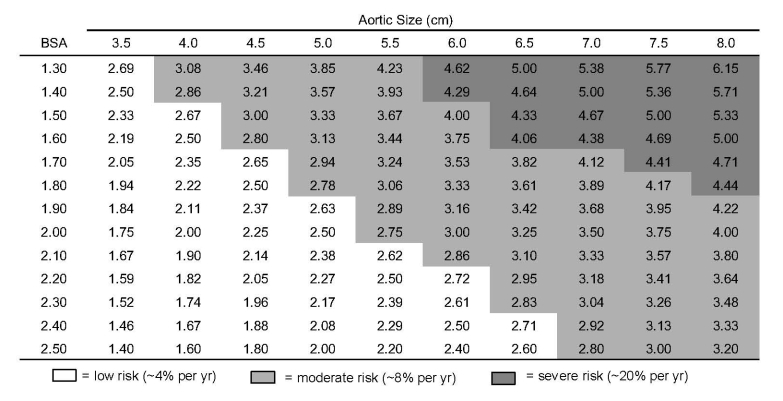
Now, it may be questioned whether one set of size criteria for surgical intervention can be applied to all individuals, regardless of body size. In fact, it is indeed appropriate to use body size corrections, especially for very small or very large individuals. Our data now has become so robust that we have been able to determine appropriate criteria for interventions based on an “aortic size index,” which takes into account both aneurysm size and the patient’s body surface area (Figure 6).
Figure 6.
“Aortic size index” nomogram. Reproduced from Davies RR, Gallo A, Coady MA, et al. Novel measurement of relative aortic size predicts rupture of thoracic aortic aneurysms. Ann Thorac Surg. 2006;81:169-177.
What causes thoracic aortic aneurysm? Is this a genetic disease?
Marfan’s syndrome, based on any of a large number of mutations on the fibrillin gene, is a well-known cause of aortic dissection. Patients with Marfan’s disease, if denied surgical intervention, run a 50 percent risk of developing aortic dissection during their lifetime. Marfan’s disease accounts for about 5 percent of all cases of aortic dissection. A small number of other collagen vascular diseases (including Ehler-Danlos syndrome) cause an even smaller percentage of aortic dissections. As is well-known, the condition of cystic medial necrosis provides the anatomic backdrop in which the dissecting process can occur. Figure 7 provides a classic example of this pathologic entity. One can easily picture how a tear in the endothelium can permit blood under pressure to enter one of the intramedial lacunae and how such pressurized entry can split the aortic wall and propagate distally. Cystic medial necrosis is a nonspecific degenerative condition of the media that can occur from one of the collagen vascular syndromes like Marfan’s disease, from the effects of chronic hypertension, or from the aging process itself.
Figure 7.

Classic cystic medial necrosis. Note the dropout of medial cell bodies and the cystic spaces within the media.
Although the medial degeneration may take decades to develop, the process of dissection itself is literally instantaneous: one moment the aorta is whole, and a split second later, it is dissected. We have visualized this process in an animal model of experimental aortic dissection by direct aortic examination and by echography in our laboratories [4]. By the naked eye and by echo, it is possible to see the dissection extend from the proximal descending aorta, where we induce an intimal tear, along the entire length of the aorta. By producing the intimal tear and then inducing severe iatrogenic hypertension (BP 300 mmHg after epinephrine injection), we can reliably produce these aortic phenomena and evaluate various surgical treatments.
Marfan’s disease has been elucidated for decades. However, Marfan’s disease explains only about 5 percent of known thoracic aortic aneruysm. The question arises whether genetic abnormalities explain some of the remaining 95 percent of patients with thoracic aortic aneurysm. The answer is a resounding “yes.” The Yale database has allowed us to recognize that many patients with aortic dissection do not fit any acknowledged syndrome of collagen vascular disease. In fact, Marfan’s disease and the similar disorders account for only the “tip of the iceberg” of thoracic aortic aneurysm and dissection. Our studies have indicated a strong genetic component in patients with thoracic aortic aneurysm and dissection among those patients without Marfan’s disease. Our construction of nearly 500 family trees of patients with thoracic aortic aneurysm or dissection has indicated that 21 percent of our probands have at least one family member with a known aneurysm somewhere in the arterial tree [5,6]. The patients with positive family trees showed a higher rate of growth of their aortas and presentation with clinical disease at an earlier age, two findings that strongly support the presence of an inherent genetic defect of the aortic wall. Figure 8 shows the positive family trees from among our first 100 family trees. As the figure shows, the predominant method of inheritance is autosomal dominant, but other genetic patterns also are expressed. The true rate of inheritance is likely much higher than shown, as many family members may harbor aneurysms without being aware of their presence.
Figure 8.

Among the first 100 family pedigrees that we constructed, these 21 showed a family pattern for thoracic aortic aneurysm. Autosomal dominant inheritance predominates, but other patterns are discernable as well.
Our most recent analysis indicates that the location of the aneurysm in the proband has a strong impact on the site at which family members develop their aneurysms [6]. Specifically, probands with ascending aortic aneurysm have family members with predominantly ascending aortic aneurysms (Figure 9). However, probands with descending thoracic aneurysms most commonly have family members with abdominal aortic aneurysms. This analysis fits a conception that aneurysm disease divides itself into two entities at the ligamentum arteriosum: above the ligament is one disease and below the ligament another.
Figure 9.

Note how the location of the proband aneurysms influences the location of aneurysms in the family members. Note that probands with ascending aneurysms have family members with ascending aneurysm, whereas probands with descending thoracic aneurysm are more likely to have family members with abdominal aortic aneurysm. See text. [6]
We have undertaken intensive efforts aimed toward identifying the specific genetic aberrations that underlie these family transmissions. We studied 30,000 RNA expression patterns in the blood of patients with thoracic aortic aneurysm and compared them with control patients. We found that a 31-SNP panel could discriminate quite well between patients with and without aneurysm — from a blood test alone [7] (Figure 10). This “RNA Signature” is more than 80 percent accurate in determining from a blood test alone whether the patient has an aneurysm. We hope that this may eventuate in a screening test for family members or even the general public. This level of accuracy far exceeds that of the PSA test used for prostate cancer.
Figure 10.
The RNA Signature test for thoracic aortic aneurysm. In the hierarchical cluster diagram on the left, each vertical line represents a patient (red are aneurysm patients, and blue are controls), and each horizontal line represents an RNA. In the grid, green indicates underexpression and red indicates overexpression. Note in the diagram on the left how the overexpression and underexpression cluster, depending on phenotype. In the figure on the right, note that if all the reds were together and all the blues were together, the test would have been 100 percent accurate. As it turns out, the overall accuracy was over 82 percent.
In collaboration with colleagues at Celera Diagnostics in California, we have also performed genome-wide scans for SNPs associated with thoracic aortic aneurysm and dissection. We have studied more than 500 Yale patients and their spousal controls in this manner. Results are encouraging, with several SNPs strongly predicting the aneurysm disease. Replication studies from our European collections sites are currently underway.
How is the thoracic aorta degraded in aneurysm diseases?
The pathophysiology of aneurysm formation is complex, involving, among other factors, inflammation, proteolysis, and disturbed survival and function of smooth muscle cells in the aortic wall [8].
We have looked specifically at the profiles of proteolytic enzymes in the aortic wall of aneurysm patients and compared these profiles to those of normal individuals. Work at Yale has implicated the MMP (matrix metalloproteinase) enzymes in the wall of the aorta as a prime agent of deleterious change in aneurysm and dissection disease. We have found a marked elevation of the proteolytic enzmes (MMPs) and a marked depression of the inhibitory enzymes (TIMPs) [9] (Figure 11). Thus, in aneurysm patients, the balance is shifted strongly toward increased proteolysis — indicating an enzymatic attack on the fibrillin and collagen that form the structural basis of the aortic wall.
Figure 11.

Note the elevation of MMP-1 and MMP-9 in the aneurysm patients. Note in the inset photograph how terribly thin this ascending aorta has become, permitting a ruler to be read right through the aortic wall. This is not a safe situation.
These lytic enzymes have a hand in destroying the aortic wall, so that, in an extreme case, it can become as thin and translucent as seen in the inset in Figure 11. No one would want an aorta so thin as that seen in the picture restraining the force of the bloodstream.
What mechanical forces play on the dilated aorta?
We have performed a detailed engineering analysis on the aortic wall in patients with ascending aortic aneursyms. We have done this by epiaortic echocardiography at the time of aortic surgery. The full spectrum of engineering calculations of the mechanical properties of the dilated aorta can be determined via measurement of six independent variables: aortic pressure in systole and diastole, aortic diameter in systole and diastole, and aortic wall thickness in systole and diastole. These measurements have shown that as the aorta enlarges, distensibility of the aortic wall falls, so that by about 6 cm size, the aorta becomes a rigid tube [10]. The end result is that because the aneurysmal aortic wall cannot “stretch” in systole, all the force of cardiac contraction is translated into wall stress.
The correlate of this is that the enlarged aorta demonstrates a very high wall tension. These findings are shown in Figures 12a and b. The dotted line in Figure 12b represents the ultimate strength of human aorta. As seen in Figure 12b, during episodes of moderate hypertension, part of everyday life, an individual with a 6 cm aorta will “flirt” with the ultimate bursting strength of human aortic tissue.
Figure 12.
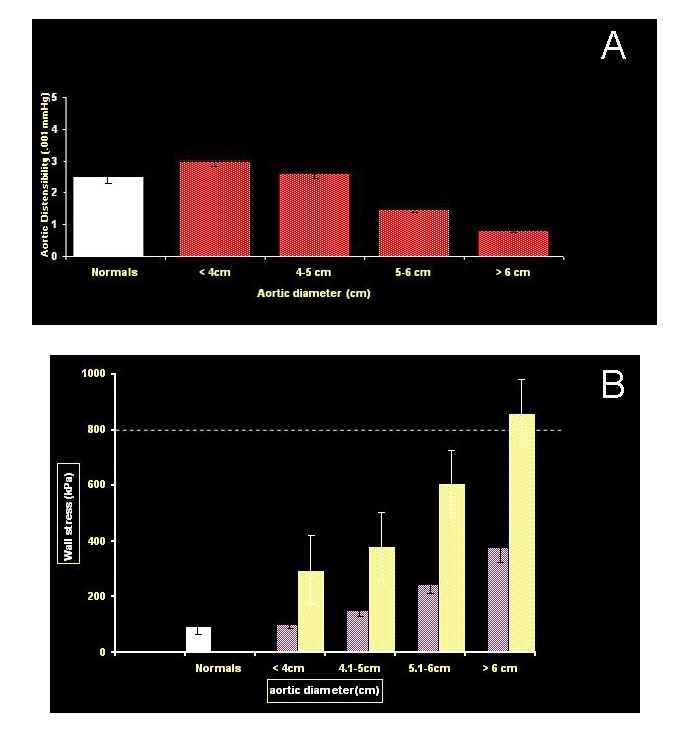
A. Distensibility values in normal aortas and aortic aneurysms of different diameters. Distensibility of ascending aortic aneurysms decreases rapidly as diameter increases, to very low values at dimensions greater than 6 cm. B. Exponential relationship between wall stress and aneurysm size in ascending aortic aneurysms. The dark columns represent a blood pressure of 100 mmHg, and the light columns represent a blood pressure of 200 mmHg. The lines at 800 kPa represents the maximum tensile strength of the human aorta.
Please note how beautifully the engineering data dovetail with the clinical data. It is at 6 cm that the mechanical properties of the aneurysmal aorta deteriorate markedly. It is at precisely 6 cm that the malignant behaviors of the dilated human aorta — rupture and dissection — commonly become manifest.
How does acute aortic dissection pick a date and time to occur?
How does aortic dissection pick a specific time to occur? How is it that dissection picks October 10, 2008, at 5:03 p.m. (the moment that this sentence is being written) to occur? The author formerly believed that the occurrence of dissection in a susceptible patient was random. He does not think so any longer.
It is interesting that aortic dissection has long been known to occur in circadian and diurnal patterns, with a preponderance of instances in the winter months and in the early morning hours. The reasons behind these patterns are unknown, but these characteristics correlate with the season and time of day when blood pressure is known to be highest.
Acute aortic dissection also can occur in pregnancy, threatening both mother and fetus. The dissection usually occurs peripartum, during delivery or shortly thereafter. These are times when blood volume, cardiac output, blood pressure, and, of course, physical straining are high. Also, bicuspid aortic valve, a very common congenital condition (1 percent to 2 percent of the general population) predisposes to aortic dissection. One in 20 patients with a bicuspid aortic valve will develop aortic dissection. Of note, dissection usually occurs before the onset of symptomatic aortic stenosis in these patients. Aortic coarctation also predisposes strongly to aortic dissection. In the present era, cocaine use has emerged as an increasingly frequent cause of aortic dissection.
Studies at Yale have added another dimension to our understanding of the timing of aortic dissection in susceptible individuals. Specifically, we have found that extreme exertion or emotion may precipitate acute aortic dissection [11].
Let us presume that a patient is rendered susceptible to aortic dissection due to his genetic make up and then consider what factors or forces might precipitate dissection at one particular moment in time.
A few years ago, we noticed a cluster of healthy young weight lifters who had presented to Yale with acute ascending aortic dissection and required urgent surgery. We reported this cluster in JAMA [12]. We did a biomechanical study on ourselves and found that, during severe weight lifting, we reached blood pressures approaching or exceeding 300 mmHg (Figure 13). These are levels of hypertension simply not seen in any type of cardiac care, be it in the coronary care unit or in the cardiothoracic surgical unit. We hypothetized that extreme elevation of blood pressure during lifting was a factor in the cluster of dissections we had treated in weight lifters. After publication of our original report, we received cases from around the country. We did a follow-up report enumerating 31 similar cases of acute aortic dissection in the setting of weight lifting or other severe straining activity [13]. All of these dissections occurred in young men with previously unknown moderate aortic enlargement (4 to 5 cm). We recommended routine screening of all athletes embarking on weight lifting or other heavy athletic activity. We saw this as the only means to protect these young athletes from needless death. The Olympic Committee now requires an echocardiogram for every athlete competing in the Olympics.
Figure 13.

Note the marked elevation in blood pressure for these three scientists as they lift various percentages of their body weight in the bench press. JAE represents the author.
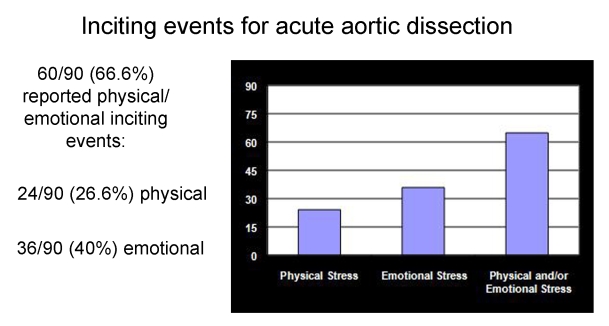
We subsequently did another study in which we contacted each patient or family member we had treated for acute aortic dissection at Yale University. This investigation implicated emotion as well as exertion as an etiologic factor in the acute onset of aortic dissection. Specifically, we found that a majority of patients could recall a specific episode of severe emotional upset or extreme exertion at the time of their dissection. (Dissection is so painful (see above) that most patients and family members remember exactly what they were doing at the moment of onset of pain.) (Figure 14). We conclude that the extreme rise in blood pressure from the emotion or exertion is the immediate precipitating trigger for dissection in a susceptible individual.
Figure 14.
Emotional or exertional events immediately preceding the onset of the pain of acute aortic dissection.
Synthesis
These many studies, looking at aneurysm and dissection from multiple viewpoints — clinical, biologic, genetic (Mendelian and molecular), mechanical, epidemiological — permit formulation of the following schema for the onset of acute aortic dissection in a specific individual (Figure 15).
Figure 15.

Overall schematic understanding of how aortic dissection picks a specific time to occur. See text.
The susceptibility to aortic aneurysm and dissection is set from birth by genetics. The aorta is destroyed over time, at least in part by excess proteolyisis by the MMPs. The aorta enlarges as its wall is damaged. As the aorta enlarges, the mechanical properties deteriorate, with loss of distensibility and imposition of excess wall tension. An acute hypertensive event supervenes — usually emotional or exertional — and exceeds the tensile limit of the aortic wall, producing an acute aortic dissection.
Conclusion
In considering the natural history of aortic dissection, it is clear that this beast is a fierce one. Once it occurs, the impact on both short-term and long-term survival is severe. Although advances in imaging techniques and surgical sciences have succeeded to some extent in taming this disorder, it continues to exert a significant impact on the human race. Once the aorta has been rent into two layers, despite the most talented surgical efforts, the outlook for that aorta — and, consequently, for the patient — will be irrevocably impaired.
The best hope resides in predicting and preventing aortic dissection so it will not occur. Natural history investigations have clarified criterion levels for preemptive intervention on the basis of aortic size. If the genetic studies underway bear fruit, it may be possible to predict the diathesis for dissection from a simple blood test [7]. Understanding the genetic underpinnings of dissection disease may, in the future, permit conventional therapies aimed at strengthening the abnormal structural proteins coded by those genes. Even specific genetic remedies can be envisioned for the future.
One hundred years ago, Sir William Osler indicated that “there is no disease more conducive to clinical humility than aneurysm of the aorta.” Osler’s statement is still true; aneurysm is a humbling disease. However, due to advances made at Yale, as well as other centers, the balance is shifting in favor of the patients and their doctors, who fight valiantly against the virulent disease of thoracic aortic aneurysm and dissection.
Abbreviations
- TAA
thoracic aortic aneurysm
- MMP
matrix metalloproteinase
- SNPs
single nucleotide polymorphisms
- CT
computerized tomography
- TIMPs
tissue inhibitors of matrix metalloproteinases
References
- Anagnostopoulos CE. Acute Aortic Dissection. Baltimore, MD: University Park Press; 1975. [Google Scholar]
- Rizzo JA, Coady MA, Elefteriades JA. Procedures for estimating growth rates in thoracic aortic aneurysms. J Clin Epidemiol. 1998;51(9):747–754. doi: 10.1016/s0895-4356(98)00050-x. [DOI] [PubMed] [Google Scholar]
- Koullias GJ, Korkolis DP, Ravichandran P, Psyrri A, Hatzaras I, Elefteriades JA. Tissue microarray detection of matrix metalloproteinases, in diseased tricuspid and bicuspid aortic valves with or without pathology of the ascending aorta. Eur J Cardiothor Surg. 2004;26(6):1098–1103. doi: 10.1016/j.ejcts.2004.07.050. [DOI] [PubMed] [Google Scholar]
- Morales DLS, Quin JA, Braxton JH, et al. Experimental confirmation of effectiveness of fenestration in acute aortic dissection. Ann Thorac Surg. 1998;66:1679–1683. doi: 10.1016/s0003-4975(98)00901-1. [DOI] [PubMed] [Google Scholar]
- Coady MA, Davies RB, Roberts M, et al. Familial patterns of thoracic aortic aneurysm. Arch Surg. 1999;134:361–367. doi: 10.1001/archsurg.134.4.361. [DOI] [PubMed] [Google Scholar]
- Albornoz G, Coady MA, Roberts M, Davies RR, Rizzo J, Elefteriades JA. Familial thoracic aortic aneurysms and dissections — Incidence, modes of inheritance, and phenotypic patterns. Ann Thorac Surg. 2006;82(4):1400–1405. doi: 10.1016/j.athoracsur.2006.04.098. [DOI] [PubMed] [Google Scholar]
- Wang Y, Barbacioru CC, Shiffman D, et al. Gene expression signature in peripheral blood detects thoracic aortic aneurysm. PLoS ONE. 2007;2(10):e1050. doi: 10.1371/journal.pone.0001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman AE, LeMaire SA, Thompson RW. Long Term SuppressiveTherapy: Clinical Reality and Future Prospects. In: Elefteriades JA, editor. Acute Aortic Disease. New York: Informa; 2007. [Google Scholar]
- Koullias GJ, Ravichandran P, Korkolis DP, Rimm DL, Elefteriades JA. Increased tissue microarray MMP expression favors proteolysis in thoracic aortic aneurysms and dissections. Ann Thorac Surg. 2004;78(6):2106–2110. doi: 10.1016/j.athoracsur.2004.05.088. [DOI] [PubMed] [Google Scholar]
- Koullias G, Modak RK, Korkolis D, Barash P, Elefteriades JA. Mechanical and elastic properties of the normal and aneurysmal ascending aorta by intraoparative epiaortic echocardiography. J Am Coll Cardiol. 2003;41(6 Suppl II):513a. [Google Scholar]
- Hatzaras IS, Bible JE, Kallias GJ, et al. Role of exertion or emotion as inciting events for acute aortic dissection. Am J Cardiol. 2007;100:1470–1472. doi: 10.1016/j.amjcard.2007.06.039. [DOI] [PubMed] [Google Scholar]
- Elefteriades JA, Hatzaras I, Tranquilli M, et al. Weight lifting and rupture of silent aortic aneurysms. JAMA. 2003;290:2803. doi: 10.1001/jama.290.21.2803. [DOI] [PubMed] [Google Scholar]
- Hatzaras I, Tranquilli M, Coady MA, Barrett PW, Bible J, Elefteriades JA. Weight lifting and aortic dissection: More evidence for a connection. Cardiol. 2006;107(2):103–106. doi: 10.1159/000094530. [DOI] [PubMed] [Google Scholar]