Abstract
Pediatric cardiovascular surgeons often encounter patients requiring surgical intervention utilizing foreign materials to repair complex lesions. However, the materials that are commonly used lack growth potential, and long-term results have revealed several material-related failures, such as stenosis, thromboembolization, calcium deposition, and risk of infection. To solve these problems, in particular for children who require the implantation of dynamic material with growth potential, we sought to develop optimal filling materials with biocompatibility and growth potential. Previously, we reported the advantages of tissue-engineered vascular autografts (TEVAs) in animal models and in human clinical applications utilizing autologous cells and biodegradable scaffolds. The key benefits from utilizing such scaffolds is that they degrade in vivo, thereby avoiding the long-term presence of foreign ma-terials, and the seeded cells proliferate and differentiate to construct new tissue.
Recent studies have demonstrated the existence of bone marrow-derived endothelial pro-genitor cells that contribute to vasculogenesis and angiogenesis and the successful endothelialization of artificial grafts using bone marrow cells. We provided evidence that bone marrow cells as a source for seeding onto a biodegradable scaffold are useful and that seeded cells contribute to the histogenesis of TEVAs. Therefore, we applied this technique in clinical trials with good results. In this review article, we provide an overview of our work developing “tissue-engineered blood vessels” created by utilizing autologous mononuclear bone marrow cells.
Congenital Heart Disease: A significant medical problem
Cardiac defects are the most common congenital anomalies. Despite major advances in the treatment of congenital heart disease (CHD), it remains the leading cause of death due to congenital anomalies in the newborn period (http://www.americanheart.org/). Single ventricle anomalies make up one of the largest groups of cardiac anomalies that result in severe disease. Though such anomalies differ structurally, they share a common feature in that only one of the two ventricles is of adequate functional size. Some of the cardiac anomalies that result in single ventricle changes include tricuspid atresia, pulmonary atresia, and hypoplastic left heart syndrome. This group of congenital cardiovascular anomalies results in mixing of deoxygenated blood from the pulmonary circulation and oxygenated blood from the systemic circulation. The circulation of deoxygenated blood through the systemic circulation causes chronic hypoxia and cyanosis. Mixing of blood between the pulmonary and systemic circulation also can cause volume overload, which, if left untreated, can lead to heart failure. Untreated single ventricle cardiac anomalies are associated with 70 percent mortality during the first year of life [1]. The treatment of choice is surgical reconstruction [2,3]. Without surgery, survival of this cohort into adulthood is rare.
Surgical Treatment of CHD: Limitations associated with currently used vascular conduits
Despite the dramatic structural differences in the cardiac defects causing single ventricle physiology, the ultimate plans for staged surgical reconstruction are actually quite similar. The goal of these operations is to separate the pulmonary circulation from the systemic circulation. This eliminates the mixing of systemic and pulmonary blood flow, resulting in improved systemic oxygenation and preventing volume overload, thus normalizing the volume work of the systemic ventricle, thereby preventing heart failure. This is accomplished through a series of staged operations designed to reconstruct the cardiovascular structures so that the single ventricle pumps oxygenated blood through the systemic circulation. The deoxygenated blood is then passively circulated through the pulmonary circulation, where it is oxygenated and returned to the heart. This type of surgical procedure is referred to as a Fontan operation. The Fontan operation has undergone several modifications since it was first reported in 1971 [4]. The most commonly performed modification of the Fontan operation is the extra cardiac total cavopulmonary connection (EC TCPC) (Figure 1). The modified Fontan operation is considered the standard of care for the treatment of patients with single ventricle cardiac anomalies and substantially has improved both the quality and long-term survival of these patients; however, it is still considered a palliative (non-curative) procedure with significant morbidity and mortality [2,3]. One important cause of morbidity and mortality in patients requiring the Fontan operation is the conduit used to connect the inferior vena cava to the right pulmonary artery when native tissue cannot be used [5]. When Fontan and Kirklin reviewed the late outcome of an early cohort of patients surviving the Fontan procedure, they concluded that much of the late morbidity could be attributed to problems associated with conduit use [6]. It is widely accepted that the ideal conduit has not yet been developed [7,8]. Polytetrafluoroethylene (PTFE, or Gore-Tex®) conduits are currently the most widely used vascular grafts for EC TCPC [9]. Use of PTFE essentially has replaced the use of Dacron in most centers. Failure rates for Dacron grafts used for EC TCPC is limited but tends to be worse than PTFE failure rates described in the literature [2]. Homografts also are used as EC TCPC conduits, but to a much more limited extent compared to PTFE [3].
Figure 1.
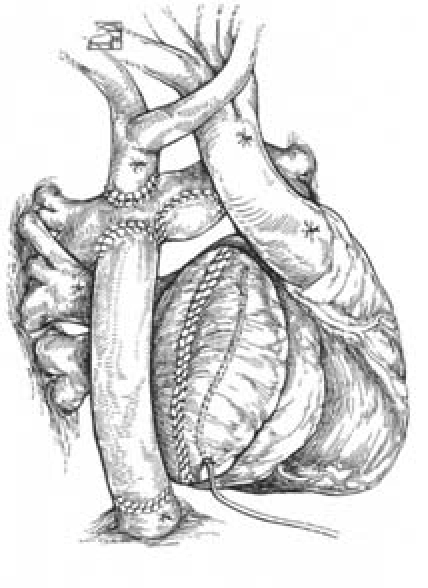
Schematic Diagram of EC TCPC. In this modified Fontan operation, venous return from the lower half of the body is passively returned to the pulmonary artery by anastomosing the IVC to the pulmonary artery using a conduit. Blood is then oxygenated in the pulmonary circulation and returned to the single ventricle, where it is pumped through the systemic circulation.
While data describing the long-term graft failure rates for conduits used for EC TCPC is limited, long-term data regarding use of both valved and unvalved conduits for other similar congenital heart operations such as the Rastelli operation (right ventricle to pulmonary artery conduit) are widely available and are poor [10]. Late problems include conduit degeneration with progressive obstruction, lack of growth potential, potential for infection, and potential for thromboembolic complications. Both synthetic and biological conduits are used for these operations. PTFE and other synthetic conduits such as Dacron lack growth potential necessitating re-operation when a patient outgrows his vascular graft. Synthetic conduits are a significant cause of thromboembolic complication due to the large area of synthetic material in contact with blood that causes activation of the coagulation cascade [3]. Other clinically available conduits, including biological grafts such as homografts and heterografts, are associated with significantly lower thromboembolic complication rates compared to synthetic grafts; however, they, too, lack growth potential and unfortunately have poor durability due to their propensity for accelerated calcific degradation and secondary graft failure [11,12,13]. These grafts tend to become stenotic and calcify. This process seems to be immune mediated and more aggressive in younger patients [14]. It is basically assumed that all such conduits eventually will need to be replaced [15]. Re-operations are associated with significant morbidity and mortality with early post-operative mortality rates around 5 percent in the best centers [10]. Early and mid-term results for these grafts are variable with five-year patency rates between 65 and 90 percent. Long-term data demonstrating graft failure rates between 70 and 100 percent at 10 to 15 years have been reported [11,13,16]. Primary determinants of graft failure include size (with an increased rate of failure in grafts less than 18 mm with another significant drop off below 15 mm) and re-operation (with primary grafts performing better than replacement grafts) [16]. The best long-term results have been obtained when autologous tissue has been used for or incorporated into the conduit with long-term patency rates exceeding 80 percent [15].
Development of the autologous tissue-engineered vascular graft
Autografts, conduits created from autologous tissue, have better long-term effectiveness than any synthetic or biological conduit currently available for use in pediatric cardiovascular surgical applications [10,15,17]. Unfortunately, autografts are limited in supply, necessitating the use of synthetic or biological conduits in most cases [16]. Use of synthetic or biological vascular grafts result in increased graft failure rates and increased morbidity and mortality rates when compared to similar operations performed using autologous tissue [13,15]. Tissue engineering offers a strategy for constructing autologous grafts and thereby increasing the pool of potential autografts. Using the classical tissue engineering paradigm, autologous cells can be seeded onto a biodegradable tubular scaffold. The scaffold provides sites for cell attachment and space for neotissue formation [18]. The resulting neotissue can be used for reconstructive surgical applications such as creation of a vascular graft for use in pediatric cardiothoracic operations [17]. Extensive large animal studies have demonstrated the feasibility of using tissue engineering methodology to construct a conduit for use as large caliber grafts in the venous or pulmonary circulation [17,19,20,21,22].
In the mid 1990s, we performed a series of experiments applying the classical tissue engineering paradigm toward the development of a neotissue for use in reconstructive cardiovascular surgical applications. During this time, we demonstrated the feasibility of creating a tissue-engineered vascular graft for use in congenital heart surgery [17].
The initial scaffold was constructed from off-the-shelf biodegradable polymers originally designed for other biomedical applications. While creating a tissue-engineered vascular graft with this scaffold was feasible, the design of the PGA scaffold was not optimal due to the relatively stiff nature of the PGA fibers that resulted in poor compliance match and poor surgical handling qualities. The hybrid polymeric scaffold fabricated from either polyglycolic or polylactic acid fiber-based mesh coated with a 50:50 copolymer of L-lactide and ɛ-caprolactone (PCLA/PGA or PCLA/PLA) was specifically designed for use as a biodegradable scaffold for creating a tissue engineered vascular graft. The hybrid scaffolds are more elastic than the PGA scaffold, resulting in an improved compliance match between the vessel and the conduit and with better surgical handling characteristics [19].
In our initial studies, vascular cells were isolated from autologous vessel biopsies. This methodology was problematic for many reasons. First, it required an invasive procedure (biopsy) in addition to the need for a substantial period of time in order to expand the cells in culture that limited its clinical utility. As our experience grew, we also began to recognize the inherent difficulty in obtaining healthy autologous cells from diseased donors. Finally, use of cell culture resulted in an increased risk of contamination of the cultured cells. In an attempt to identify an alternative autologous cell source that could be obtained with minimal manipulation and without the use of cell culture, we explored the use of autologous cells obtained from bone marrow aspirate [21].
We further refined our method in order to remove platelets and red blood cells and eliminate the inherent thrombogenic risk associated with use of these cell types by performing density centrifugation and isolating the mononuclear fraction of the bone marrow. This technique had the added benefit of providing a source of autologous serum that could be used instead of media to bathe the seeded scaffold during the brief period of time the cells were in contact with the scaffold ex vivo [22].
Tissue-Engineered Vascular Autograft (TEVA) Clinical Trial
We reported the first clinical use of a TEVA for use as an extracardiac total cavopulmonary connection (EC TCPC) conduit [23,24,25]. This study was performed under an Institutional Review Board (IRB) approved protocol at Tokyo Women’s Hospital, Tokyo, Japan. To date, 23 tissue-engineered vascular grafts have been implanted as conduits for EC TCPC with follow-up through six years [21,24] (Table). The tissue-engineered vascular grafts functioned well without evidence of aneurismal change or graft rupture. There have been three graft-related complications: the development of significant stenosis in a smaller diameter (< 18 mm) conduit that required and was successfully treated with angioplasty. There were no reported thromboembolic or hemorrhagic complications or infectious complications. No graft has had to be replaced. Serial imaging has demonstrated the growth potential of these grafts. These data support the overall feasibility and safety of this technology (Figure 2). We have received IRB approval and have applied for FDA approval for the first clinical trial investigating the use of TEVA in the United States.
Figure 2.
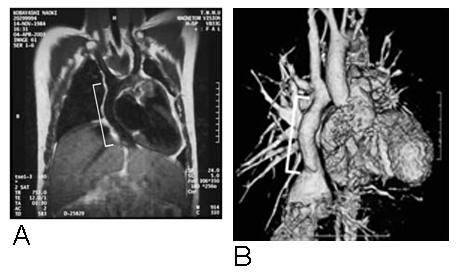
A. Magnetic resonance image 9 months after implantation of TCPC graft. White line indicates location of tissue-engineered conduit. B. 3-D CT angiogram of tissue-engineered EC TCPC one year after implantation.
Conclusion
Successful translation of cardiovascular tissue engineering principles from the bench to the clinic through the development of an improved venous conduit for repair of congenital heart defects also could be utilized to create improved vascular grafts for a variety of other cardiovascular surgical applications. The need for improved vascular grafts for angioaccess for patients requiring hemodialysis for treatment of end stage renal disease affects more than 375,000 patients in the United States alone. Currently used vascular grafts require endovascular interventions or replacement within two years of implantation. Similarly, clinical results associated with use of synthetic small caliber vascular grafts for the treatment of both cardiovascular and peripheral arterial disease are so poor as to prohibit their use in most circumstances. It is estimated that creation of an improved small caliber arterial graft would affect the lives of more than 100,000 patients per year in the United States. These patients require life- or limb-saving operations that suffer from the same limitations due to inadequate vascular grafts as congenital heart operations. Using the same basic tissue engineering methodologies described in this proposal, we could continue to expand upon our current work and begin to address these critical health issues.
Tissue engineering holds great promise for the advancement of all fields of medicine. The ability to create tissue from an individual’s own cells that can be used to replace tissues that are diseased, destroyed, or congenitally absent without the risk of rejections and improved biocompatibility dramatically could improve our ability to treat a variety of the most serious medical problems affecting our society.
Table 1. Results of IRB-approved Japanese clinical study (adapted Shinoka, 2005).
| Patients | Age | BW | Diameter of Scaffolds (mm) | Component of Biodegradable Scaffold | Day of Surgery | Duration of Follow-up (years) | Graft/Related Complication | Current status |
| 1 | 2 | 11 | 16 | PCLA/PLA | 2001.9.17 | 5.77 | None | Alive |
| 2 | 1 | 7.5 | 20 | PCLA/PLA | 2001.10.29 | 5.66 | None | Alive |
| 3 | 7 | 18.5 | 18 | PCLA/PLA | 2002.1.25 | 5.42 | None | Alive |
| 4 | 21 | 44.4 | 24 | PCLA/PLA | 2002.3.27 | 5.24 | None | Alive |
| 5 | 4 | 14 | 20 | PCLA/PLA | 2002.6.3 | 5.06 | None | Alive |
| 6 | 12 | 36.7 | 24 | PCLA/PLA | 2002.6.13 | 5.03 | None | Alive |
| 7 | 17 | 46.5 | 24 | PCLA/PLA | 2002.7.1 | 4.98 | None | Alive |
| 8 | 19 | 47 | 22 | PCLA/PLA | 2002.9.25 | 3.57 | None | Late death (heart failure) |
| 9 | 3 | 13.5 | 12 | PCLA/PLA | 2002.10.17 | 4.69 | None | Alive |
| 10 | 2 | 7.5 | 16 | PCLA/PLA | 2002.12.6 | 0.47 | None | Late death (heart failure) |
| 11 | 2 | 11 | 16 | PCLA/PGA | 2003.3.17 | 4.27 | stenosis | Alive PTA* (2005.7.15) |
| 12 | 13 | 23 | 20 | PCLA/PLA | 2002.4.10 | 5.21 | None | Alive |
| 13 | 2 | 9.9 | 16 | PCLA/PGA | 2003.5.21 | 4.09 | None | Alive |
| 14 | 2 | 9.32 | 18 | PCLA/PGA | 2003.6.16 | 4.03 | None | Alive |
| 15 | 2 | 11 | 12 | PCLA/PGA | 2003.6.27 | 3.99 | None | Alive |
| 16 | 2 | 8.67 | 16 | PCLA/PGA | 2003.8.25 | 3.83 | None | Alive |
| 17 | 24 | 51.6 | 18 | PCLA/PGA | 2003.9.25 | 3.75 | None | Alive |
| 18 | 1 | 8.7 | 16 | PCLA/PGA | 2003.10.1 | 3.73 | None | Alive |
| 19 | 11 | 25.5 | 18 | PCLA/PGA | 2003.10.27 | 3.66 | None | Alive |
| 20 | 2 | 11 | 12 | PCLA/PGA | 2003.11.10 | 3.63 | None | Alive |
| 21 | 3 | 10.5 | 16 | PCLA/PGA | 2004.1.27 | 3.41 | None | Alive |
| 22 | 4 | 13 | 18 | PCLA/PGA | 2004.2.1 | 3.40 | None | Alive |
| 23 | 4 | 14.2 | 18 | PCLA/PGA | 2004.3.12 | 3.29 | None | Alive |
*PTA: percutaneous transluminal balloon angioplasty
Abbreviations
- TEVA
tissue-engineered vascular autografts
- CHD
congenital heart disease
- EC TCPC
cavopulmonary connection
- PTFE
polytetrafluoroethylene
- IRB
Institutional Review Board
References
- Samanek M. Children with congenital heart disease: probability of natural survival. Pediatr Cardiol. 1992;13:152–158. doi: 10.1007/BF00793947. [DOI] [PubMed] [Google Scholar]
- Giannico S, Hammad F, Amodeo A, Michielon G, Drago F, Turchetta A, Di Donato R, Sanders SP. Clinical outcome in 193 extracardiac Fontan patients the first 15 years. J Am College Card. 2006;47(10):2065–2073. doi: 10.1016/j.jacc.2005.12.065. [DOI] [PubMed] [Google Scholar]
- Petrossian E, et al. Early results of extracardiac conduit Fontan operation. J Thorac Cardiovasc Surg. 1999;117:688–696. doi: 10.1016/S0022-5223(99)70288-6. [DOI] [PubMed] [Google Scholar]
- Fontan F, et al. Surgical repair of tricuspid atresia. Thorax. 1971;26:240–248. doi: 10.1136/thx.26.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas RA. Commentary on: Petrossian E, et al. Early results of extracardiac conduit Fontan operation. J Thorac Cardiovasc Surg. 1999;117:688–696. doi: 10.1016/S0022-5223(99)70288-6. [DOI] [PubMed] [Google Scholar]
- Fontan F, et al. Outcome after a “perfect” Fontan operation. Circulation. 1990;81:1520–1536. doi: 10.1161/01.cir.81.5.1520. [DOI] [PubMed] [Google Scholar]
- Conte MS. The ideal small arterial substitute: a search for the Holy Grail? FASEB J. 1998;12:43–45. doi: 10.1096/fasebj.12.1.43. [DOI] [PubMed] [Google Scholar]
- Kakisis JD, et al. Artificial blood vessel: the Holy Grail of peripheral vascular surgery. J Vasc Surg. 2005;41:349–354. doi: 10.1016/j.jvs.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Petrossian E, et al. The extracardiac conduit Fontan operation using minimal approach extracorporeal circulation: Early and midterm outcomes. J Thorac Cardiovasc Surg. 2006;132:1054–1063. doi: 10.1016/j.jtcvs.2006.05.066. [DOI] [PubMed] [Google Scholar]
- Dearani JA, et al. Late follow-up of 1095 patients undergoing operation for complex congenital heart disease utilizing pulmonary ventricle to pulmonary artery conduits. Ann Thorac Surg. 2003;75:399–411. doi: 10.1016/s0003-4975(02)04547-2. [DOI] [PubMed] [Google Scholar]
- Stark J. The use of valved conduits in pediatric cardiac surgery. Pediatr Cardiol. 1998;19:282–288. doi: 10.1007/s002469900311. [DOI] [PubMed] [Google Scholar]
- Cleveland DC, et al. Failure of cryopreserved homograft valved conduits in the pulmonary circulation. Circulation. 1992;86(suppl II):II150–II153. [PubMed] [Google Scholar]
- Jonas R, et al. Long-term follow-up of patients with synthetic right heart conduits. Circulation. 1985;72(suppl II):II77–II83. [PubMed] [Google Scholar]
- Karamlou T, et al. Oversizing pulmonary homograft conduits does not significantly decrease allograft failure in children. Eur J Cardiothorac Surg. 2005;27:548–553. doi: 10.1016/j.ejcts.2004.12.054. [DOI] [PubMed] [Google Scholar]
- Bermudez CA, et al. Late results of the peel operation for replacement of failing ex-tracardiac conduits. Ann Thorac Surg. 2004;77:881–888. doi: 10.1016/j.athoracsur.2003.08.029. [DOI] [PubMed] [Google Scholar]
- Homann M, et al. Reconstruction of the RVOT with valved biological conduits: 25 years experience with allografts and xenografts. Eur J Cardiothorac Surg. 2000;17:624–630. doi: 10.1016/s1010-7940(00)00414-0. [DOI] [PubMed] [Google Scholar]
- Shinoka T, et al. Creation of viable pulmonary artery autografts through tissue engineering. J Thorac Cardiovasc Surg. 1998;115:536–546. doi: 10.1016/S0022-5223(98)70315-0. [DOI] [PubMed] [Google Scholar]
- Langer R, et al. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- Watanabe M, et al. Tissue engineered vascular autograft: inferior vena cava replacement in a dog model. Tissue Eng. 2001;7(4):429–439. doi: 10.1089/10763270152436481. [DOI] [PubMed] [Google Scholar]
- Matsumura G, et al. Successful application of tissue engineered vascular autografts: clinical experience. Biomaterials. 2003;24:2303–2308. doi: 10.1016/s0142-9612(03)00043-7. [DOI] [PubMed] [Google Scholar]
- Matsumura G, et al. First evidence that bone marrow cells contribute to the construction of tissue engineered vascular autografts in vivo. Circulation. 2003;108:1729–1734. doi: 10.1161/01.CIR.0000092165.32213.61. [DOI] [PubMed] [Google Scholar]
- Matsumura G, et al. Evaluation of tissue-engineered vascular autografts. Tissue Eng. 2006;12:1–9. doi: 10.1089/ten.2006.12.3075. [DOI] [PubMed] [Google Scholar]
- Shinoka T, et al. Transplantation of a tissue engineered pulmonary artery. New Engl J Med. 2001;344(7):532–533. doi: 10.1056/NEJM200102153440717. [DOI] [PubMed] [Google Scholar]
- Shinoka T, et al. Midterm clinical result of tissue engineered vascular auto-grafts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129:1330–1338. doi: 10.1016/j.jtcvs.2004.12.047. [DOI] [PubMed] [Google Scholar]
- Naito Y, et al. Successful clinical application of tissue engineered graft for extracar-diac Fontan operation. J Thorac Cardiovasc Surg. 2003;125:419–420. doi: 10.1067/mtc.2003.134. [DOI] [PubMed] [Google Scholar]


