Abstract
Placental growth factor (PlGF) is a member of the vascular endothelial growth factor family and is associated with inflammation and with pathologic angiogenesis. PlGF is released from marrow erythroid cells and serum PlGF concentrations have been reported to distinguish sickle cell patients from healthy controls. We observed that CFU-E from homozygous sickle cell (SS) patients are less sensitive to inhibition by recombinant human (rh) γ interferon (IFN) than those from healthy controls, and the contribution of PlGF to this process was evaluated. At concentrations 10 – 1000 pg/mL, PlGF neither inhibits nor enhances CFU-E colony formation, and there were no differences between the responses of SS patients or healthy controls. rhPlGF 100 pg/mL reversed the inhibitory effects of rhγIFN on CFU-E colony formation. rhPlGF significantly attenuated rhγIFN induction of Fas ligandin an erythroid cell line (HCD57). Both HCD57 cells and CD36+ human marrow cells express Flt-1, a receptor for PlGF. Neutralizing antibody against Flt-1 partially attenuated the IFN-protective effect of rhPlGF, although this effect was not statistically significant. In conclusion, increased PlGF concentrations in the marrow of SS patients may protect erythroid progenitors from cytokine-induced inhibition of colony formation, and may be a mechanism by which erythropoiesis in sickle cell disease is preserved despite concurrent inflammation.
Keywords: Sickle cell anemia, Placental growth factor, Cytokines, Erythropoiesis, Interferon
INTRODUCTION
Although sickle cell anemia is the result of an abnormality in the beta globin chain of hemoglobin, its clinical manifestations cannot be explained solely on that basis. It has become clear over the last several years that inflammation and inflammatory mechanisms contribute to the clinical syndromes associated with sickle cell disease(1–3).
A number of cytokines involved in inflammation suppress erythropoiesis in vitro and in vivo: this is a component of the pathogenesis of the anemia of chronic disease(4–6). However, in sickle cell patients erythropoiesis is preserved despite the active inflammatory state. Placental growth factor (PlGF) is a member of the vascular endothelial growth factor (VEGF) family and is associated with inflammation and with pathologic angiogenesis(7;8). Perelman and colleagues have reported that PlGF is released from marrow erythroid cells and that its circulating concentration is 50% higher in patients with severe sickle cell disease than it is in healthy controls(9). Other investigators found similar results, but only during acute painful crises(10). In studies of the contribution of inflammatory cytokines to the regulation of erythropoiesis in homozygous sickle cell (SS) patients, we measured concentrations of PlGF, interleukin (IL)-1, IL-6, tumor necrosis factor (TNF) and γ interferon (IFN) in marrow aspirates from steady-state, low severity SS patients and healthy volunteer control subjects(11). Marrow aspirate PlGF concentrations were significantly higher in SS patients than in controls; there were no differences in the other cytokines measured. The marrow aspirate plasma concentrations were higher than the concentrations detectable in concurrent serum or plasma specimens from SS patients. When the PlGF concentrations were normalized to marrow erythroid progenitor content, the difference between SS patients and controls persisted, suggesting that the increased marrow PlGF concentrations did not derive from increased erythroid activity, but rather represented an increase per erythropoietic unit(11). In this study, we evaluate the possibility that PlGF interferes with suppression of erythropoiesis by inflammatory mediators.
MATERIALS AND METHODS
Human subject participation
This research was carried out according to the Declaration of Helsinki volunteers under protocols approved by the Institutional Review Boards of the Medical University of South Carolina and the University of Kentucky, and by the Research & Development Committees of the Ralph H. Johnson VA Medical Center (Charleston SC USA) and the Lexington (KY USA) VA Medical Center. After informed consent, 5 mL bone marrow aspirates were collected from paid stable SS patient volunteers and from paid healthy The collected specimens were suspended in heparinized Iscove’s Modified Dulbecco’s Medium.
Reagents/cell lines
rhγIFN was purchased from R&D Systems (Minneapolis MN. USA) rhPlGF was purchased from Research Diagnostics Inc. (Flanders NJ USA). Neutralizing antibody against Flt-1 was purchased from Abcam (Cambridge MA USA). For western blot analysis, antibodies were purchased from Alpha Diagnostics (San Antonio TX USA; anti-Flt-1) and Upstate Technology (Lake Placid NY USA; anti-Fas ligand) The HCD57 murine erythroleukemia cell line was a generous gift from Dr. Stephen Sawyer, Department of Pharmacology and Toxicology, Medical College of Virginia/Virginia Commonwealth University, Richmond VA USA. HepG2 hepatoma cells were a generous gift of Dr. Kenneth Ain, Lexington VA Medical Center and the University of Kentucky.
Culture of erythroid colony forming units (CFU-E)
After collection, marrow cells were depleted of bony spicules, red cells, and granulocytes by centrifugation over Ficoll-Hypaque. The remaining light density mononuclear (LDMN) cells were then cultured for CFU-E at a concentration of 105 cells/mL in 25% fetal calf serum (FCS), deionized bovine serum albumin (BSA), and recombinant human (rh) erythropoietin (EPO) 1 U/mL, with the compounds under investigation (rhPlGF, rhγIFN, anti-flt1) in 0.2 mL plasma clots in 48-well plastic culture plates for 7 days in a humidified 95% CO2 air/5%incubator. The clots were then removed from wells, fixed on glass slides with 5% glutaraldehyde, and stained with hematoxylin-benzidine(12). CFU-E were then identified according to established criteria(13). In order to compare the results of different experiments, colony formation was expressed as a percentage of control (CFU-E colony formation at rhEpo 1 U/mL).
RESULTS
Marrow CFU-E from SS patients are less sensitive to inhibition by rhγIFN than CFU-E from healthy controls
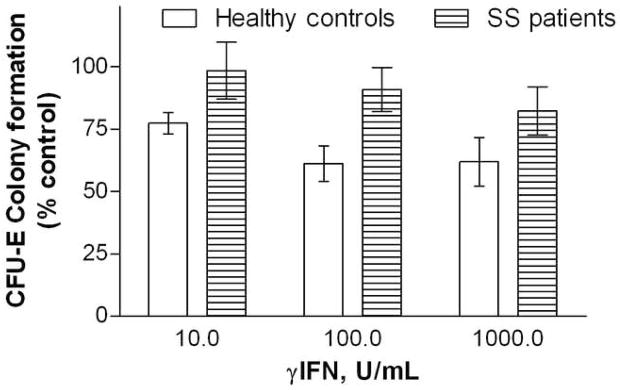
Marrow LDMN cells from stable SS patients and healthy volunteers were cultured with rhγIFN 0–1000 U/mL. CFU-E colony formation by marrow cells from SS patients was significantly less susceptible to inhibition by rhγIFN than was colony formation by marrow cells from healthy controls (p = 0.004; ANOVA). This effect was most pronounced at lower rhγIFN concentrations (Figure 1). CFU-E colony formation under control conditions did not differ significantly between the subject subsets.
Figure 1. Effects of rhγIFN on CFU-E colony formation by marrow LDMN cells from SS patients and healthy volunteers.
Bars represent mean ± SEM. Control CFU-E 498 ± 193/105 LDMN cells for healthy volunteers (4 experiments) and 534± 183/105 LDMN cells for SS patients (3 experiments).
rhPlGF decreases sensitivity of CFU-E colony formation to inhibition by rhγIFN
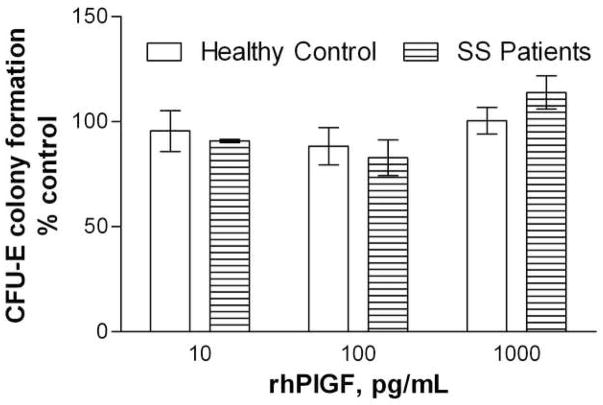
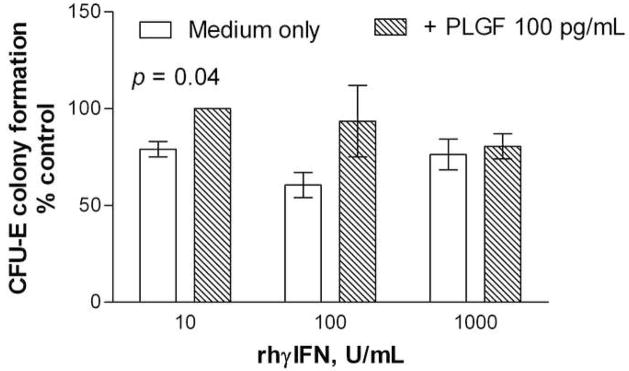
LDMN marrow cells from SS patients and healthy volunteers were cultured with PlGF at concentrations of 10 – 1000 pg/mL, in order to evaluate any effects of PlGF on erythropoiesis in the absence of cytokines other than Epo. Neither inhibition nor enhancement of CFU-E colony formation were observed, and there were no differences observed between the responses to PlGF of progenitors from SS patients or from healthy volunteers (Figure 2). In order to identify any potential contribution of PlGF to this decreased sensitivity to IFN inhibition observed with SS marrow, the effect of PlGF 100 pg/mL on CFU-E colony formation by LDMN cells from healthy volunteers in the presence of rhγIFN was evaluated. PlGF 100 pg/mL reversed the inhibitory effects of rhγIFN on CFU-E colony formation by LDMN cells from healthy volunteers (p = 0.04; ANOVA). This effect was most apparent at lower rhγIFN concentrations, similar to the results shown in Figure 1 (Figure 3).
Figure 2. Effects of rhPlGF on CFU-E colony formation by marrow LDMN cells from SS patients and healthy volunteers.
Bars represent mean ± SEM. Control CFU-E 431 ± 123/105 LDMN cells for healthy volunteers (3 experiments) and 329 ± 130/105 LDMN cells for SS patients (2 experiments).
Figure 3. Effects of rhPlGF on rhγIFN suppression of CFU-E colony formation by marrow LDMN cells from healthy volunteers.
Bars represent mean ± SEM. Control CFU-E 335 ± 255/105 LDMN cells (2 experiments).
rhPlGF decreases induction of Fas ligand by rhγIFN
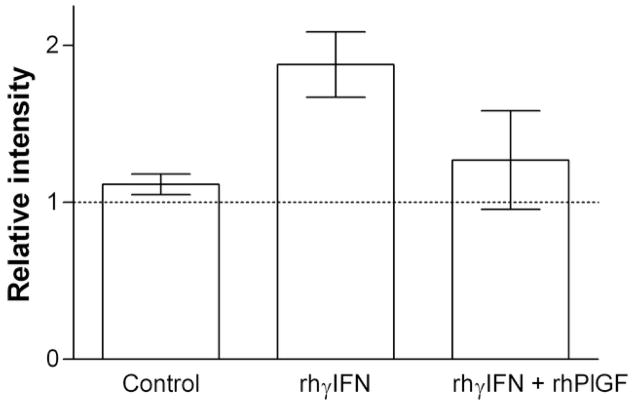
Erythroid sensitivity to rhγIFN has been shown to be a Fas-dependent process(14). The HCD57 erythroleukemia cell line was used to evaluate the mechanism of this protective effect. Concurrent exposure to rhPlGF 100 pg/mL significantly attenuated induction of Fas ligand by rhγIFN in HCD57 cells (Figure 4).
Figure 4. Effects of rhPlGF on induction of Fas ligand by rhγIFN in HCD57 erythroleukemia cells.
Bars represent mean ± SEM of 4 experiments. Dashed line represents control value (1.0; no exposure to rhPlGF or rhγIFN).
HCD57 and marrow LDMN cells express a PlGF receptor
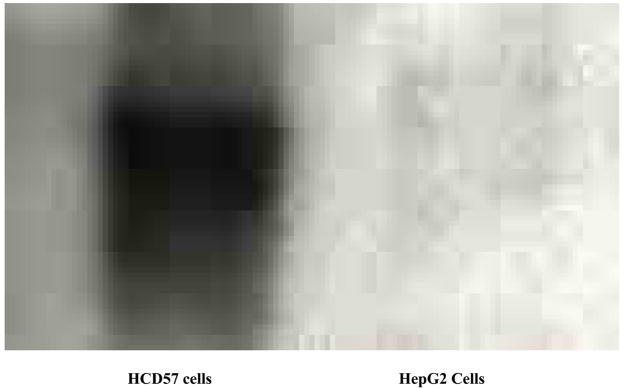
PlGF exerts its effects through members of the VEGF family of receptors, particularly fms-like tyrosine kinase (Flt) -1(15;16). Western blot analysis was performed on HCD57 cells and demonstrated the presence of Flt-1. HepG2 hepatoma cells, used as a negative control, expressed Flt-1 at a minimal level (Figure 5A). Bone marrow LDMN cells from healthy volunteers were separated into CD36+ and CD36− fractions using immunomagnetic beads. Greater than 95% of CFU-E were found in the CD36+ fraction (data not shown). CD36 + LDMN marrow cells expressed Flt-1 protein strongly while CD36- fraction had no apparent Flt-1 expression (Figure 5B).
Figure 5. Detection of Flt-1 protein in HCD57 cells and marrow cells.
A: HCD57 erythroleukemia cells and HepG2 hepatoma cells (negative control); B: CD36± and CD36− LDMN marrow cells.
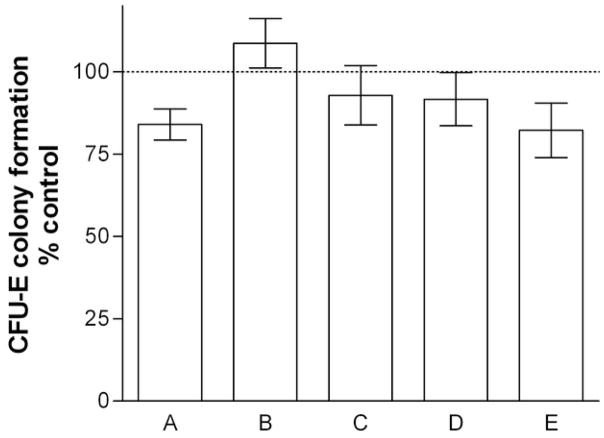
Neutralizing antibody against Flt-1 reduces rhPlGF attenuation of rhγIFN suppression of colony formation
The presence of Flt-1 in bone marrow LDMN cells does not necessarily prove that this is implicated in effects attributed to PlGF. In order to provide stronger and specific support for a potential role of PlGF and the Flt-1 receptor in attenuation of hematosuppressive effects of inflammatory cytokines, marrow LDMN cells from healthy volunteers were cultured for CFU-E in the presence and absence of rhPlGF, rhγIFN, and neutralizing antibody against Flt-1. Neutralizing antibody against Flt-1 decreased the ability of rhPlGF to correct inhibition of CFU-E colony formation by rhγIFN in all experiments, although this difference did not attain statistical significance (Figure 6).
Figure 6. Effect of anti-Flt-1 on rhPlGF attenuation of inhibition of CFU-E colony formation by rhγIFN.
Bars represent mean ± SEM of 5 experiments. Dashed line represents control value (100%; no exposure to rhPlGF, rhγIFN, or anti-Flt-1). A: rhγIFN 100 U/mL; B: rhPlGF 100 pg/mL; C: rhγIFN + rhPlGF; D. anti-flt-1 10 μg/mL; E: rhγIFN + rhPlGF + anti-Flt-1. Control CFU-E 495 ± 161/105 LDMN cells. Results in Columns A and E are significantly different from control values (p < 0.05; paired t-test), however, columns C and E do not significantly differ from each other.
DISCUSSION
Inflammation inhibits erythropoiesis: this inhibition can be mediated through direct or indirect cytokine effects on erythroid progenitors(5;17), through induced alterations in iron availability(18), or through impairment of the erythropoietin response to anemia(19;20), and are typically manifested by an appropriately low reticulocyte production(6). Inflammation appears to be common in SS disease and a significant contributor to its clinical phenotype; however erythropoietic activity in these patients, whether characterized by reticulocyte production or markers like serum soluble transferrin receptor expression, is well preserved and typically increased(21–23). This would imply two possible mechanisms, either or both of which may be involved: erythroid progenitors in SS disease may be intrinsically resistant to suppression by inflammatory mediators, or there may exist an altered hematopoietic environment creating conditions that decrease sensitivity to inhibitory mediators.
PlGF may potentially contribute to either or both of these possibilities. It is a cytokine that appears to distinguish SS patients from healthy controls(9); it is produced by erythroid cells(9); and it is measurable in marrow aspirate plasma at higher concentrations than are seen in concurrent serum or plasma samples(11). The experiments in this report demonstrate that CFU-E colony formation from SS patients is relatively resistant to inhibition by rhγIFN, and that the addition of rhPlGF to cell cultures can confer this resistance phenotype on CFU-E from the marrow of healthy control subjects. These findings are consistent with the possibility that cytokine resistance reflects increased PlGF in the marrow environment, or that increased production of PlGF by erythroid precursors confers cytokine resistance as an autocrine “feedback” phenomenon. Resistance to inhibition by rhγIFN is not an expected consequence of marrow CFU-E in inflammatory states associated with anemia: in an earlier study, marrow LDMN CFU-E from anemic HIV patients were found to be as sensitive to inhibition by rhγIFN as marrow CFU-E from healthy controls(24).
PlGF has been reported to induce an anti-apoptotic phenotype in endothelial cells(25), and related members of the VEGF family appear to regulate early hematopoiesis(26–29). rhγIFN exerts direct inhibitory effects on CFU-E(5). Demonstration of Flt-1 receptor protein expression in CD36+ marrow cells, and the finding that neutralization of the Flt-1 receptor prevents the γIFN-protective effects of rhPlGF, do not necessarily indicate that PlGF also acts directly on CFU-E; it is possible that it exerts its effects through some indirect mediator released by another CD36+ cell type present in marrow, or by promoting cell-cell interaction in the bone marrow. An example of this latter possibility is the proposal that PlGF and other VEGF family members support erythropoiesis by enhancing erythroid cell interaction with marrow macrophages (the so-called “nurse cells”).(30)
Neutralizing a receptor for PlGF partially attenuates this cytokine resistance phenotype, although this effect does not attain statistical significance. This may reflect the involvement of other potential receptors for PlGF: although Flt-1 (also called VEGF receptor-1) appears to be the major PlGF receptors, other receptors for PlGF have been described (31;32).
In summary, PlGF protects erythroid progenitors from cytokine-induced apoptosis and inhibition of colony formation. Increased PlGF concentrations in the marrow of stable SS patients may be a mechanism by which erythropoiesis in sickle cell disease is preserved despite concurrent inflammation.
Acknowledgments
Supported by funds from the Medical Research Service, US Department of Veterans Affairs, and by grant HL69418 from the US National Institutes of Health
The authors thank Dr. Chris Y. Brunson and Ms. Thelma Galliard of the Medical University of South Carolina Sickle Cell Program for invaluable assistance in identifying SS patients for participation; Mr. Thomas W. Fleury, Medical University of South Carolina, for his participation in the early stages of this project; and Ms. Erin Law, University of Kentucky, for her participation in the project’s later stages.
Footnotes
Presented in part at the Southern Clinical Research Meeting, Atlanta GA, March 4, 2006.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Platt OS. Sickle cell anemia as an inflammatory disease. Journal of Clinical Investigation. 2000;106:337–8. doi: 10.1172/JCI10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaul DS, Hebbel RP. Hypoxia/reoxygenation causes inflammatory response in transgenic sickle mice but not in normal mice. Journal of Clinical Investigation. 2000;106:411–20. doi: 10.1172/JCI9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller ST, Sleeper LA, Pegelow CH, Enos LE, Wang WC, Weiner SJ, et al. Prediction of adverse outcomes in children with sickle cell disease. New England Journal of Medicine. 2000;342:83–9. doi: 10.1056/NEJM200001133420203. [DOI] [PubMed] [Google Scholar]
- 4.Means RT, Dessypris EN, Krantz SB. Inhibition of human colony-forming units erythroid by tumor necrosis factor requires accessory cells. Journal of Clinical Investigation. 1990;86:538–41. doi: 10.1172/JCI114741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Means RT, Dessypris EN, Krantz SB. Inhibition of human erythroid colony-forming units by interleukin-1 is mediated by gamma interferon. Journal of Cellular Physiology. 1992;150:59–64. doi: 10.1002/jcp.1041500109. [DOI] [PubMed] [Google Scholar]
- 6.Means RT. Recent developments in the anemia of chronic disease. Current Hematology Reports. 2003;2:116–21. [PubMed] [Google Scholar]
- 7.Oura H, Bertoncini J, Velasco P, Brown LF, Carmeliet P, Detmar M. A critical role of placental growth factor in the induction of inflammation and edema formation. Blood. 2004;101:560–7. doi: 10.1182/blood-2002-05-1516. [DOI] [PubMed] [Google Scholar]
- 8.Vercellotti GM. PlGF: a link between inflammation and angiogenesis in sickle disease. Blood. 2003;102:1153. [Google Scholar]
- 9.Perelman N, Selvaraj SK, Batra S, Luck LR, Edreich-Epstein A, Coates TD, et al. Placenta growth factor activates monocytes and correlates with sickle cell disease activity. Blood. 2003;102:1506–14. doi: 10.1182/blood-2002-11-3422. [DOI] [PubMed] [Google Scholar]
- 10.Duits AJ, Rodriguez T, Schnog JJ. Serum levels of angiogenic factors indicate a pro-angiogenic state in adults with sickle cell disease. Br J Haematol. 2006 Jul;134(1):116–9. doi: 10.1111/j.1365-2141.2006.06103.x. [DOI] [PubMed] [Google Scholar]
- 11.Dallalio G, Brunson CY, Means RT., Jr Cytokine concentrations in bone marrow of stable sickle cell anemia patients. J Invest Med. 2007 Mar;55(2):69–74. doi: 10.2310/6650.2007.06029. [DOI] [PubMed] [Google Scholar]
- 12.McLeod DL, Shreeve MM, Axelrad AA. Improved plasma culture system for production of erytrocytic colonies in vitro: quantitative assay method for CFU-E. Blood. 1974;44:517–34. [PubMed] [Google Scholar]
- 13.Eaves AC, Eaves CJ. Erythropoiesis in culture. Clinics in Haematology. 1984;13:371–91. [PubMed] [Google Scholar]
- 14.Dai CH, Price JO, Brunner T, Krantz SB. Fas ligand is present in human erythroid colony-forming cells and interacts with Fas induced by interferon (gamma) to produce erythroid cell apoptosis. Blood. 1998;91:1235–42. [PubMed] [Google Scholar]
- 15.Luttun A, Tjwa M, Moons L, Wu Y, ngelillo-Scherrer A, Liao F, et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med. 2002 Aug;8(8):831–40. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- 16.Bae DG, Kim TD, Li G, Yoon WH, Chae CB. Anti-flt1 peptide, a vascular endothelial growth factor receptor 1-specific hexapeptide, inhibits tumor growth and metastasis. Clin Cancer Res. 2005 Apr 1;11(7):2651–61. doi: 10.1158/1078-0432.CCR-04-1564. [DOI] [PubMed] [Google Scholar]
- 17.Means RT, Krantz SB. Inhibition of human erythroid colony-forming units by tumor necrosis factor requires beta interferon. Journal of Clinical Investigation. 1993;91:416–9. doi: 10.1172/JCI116216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganz T. The role of hepcidin in iron sequestration during infections and in the pathogenesis of anemia of chronic disease. Israel Medical Association Journal:Imaj. 2002;4:1043–5. [PubMed] [Google Scholar]
- 19.Baer AN, Dessypris EN, Goldwasser E, Krantz SB. Blunted erythropoietin response to anaemia in rheumatoid arthritis. British Journal of Haematology. 1987;66:559–64. doi: 10.1111/j.1365-2141.1987.tb01344.x. [DOI] [PubMed] [Google Scholar]
- 20.Miller CB, Jones RJ, Piantadosi S, Abeloff MD, Spivak JL. Decreased erythropoietin response in patients with the anemia of cancer. New England Journal of Medicine. 1990;322:1689–92. doi: 10.1056/NEJM199006143222401. [DOI] [PubMed] [Google Scholar]
- 21.Singhal A, Cook JD, Skikne BS, Thomas P, Serjeant B, Serjeant G. The clinical significance of serum transferrin receptor levels in sickle cell disease. British Journal of Haematology. 1993;84:301–4. doi: 10.1111/j.1365-2141.1993.tb03068.x. [DOI] [PubMed] [Google Scholar]
- 22.Tancabelic J, Sheth S, Paik M, Piomelli S. Serum transferrin receptor as a marker of erythropoiesis suppression in patients on chronic transfusion. Am J Hematol. 1999 Feb;60(2):121–5. doi: 10.1002/(sici)1096-8652(199902)60:2<121::aid-ajh6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 23.Ballas SK, Marcolina MJ. Determinants of red cell survival and erythropoietic activity in patients with sickle cell anemia in the steady state. Hemoglobin. 2000 Nov;24(4):277–86. doi: 10.3109/03630260008993134. [DOI] [PubMed] [Google Scholar]
- 24.Dallalio G, North M, Means RT. Inhibition of marrow CFU-E colony formation from human immunodeficiency virus-infected patients by β- and γ-interferon. American Journal of Hematology. 1996;53:118–20. doi: 10.1002/(SICI)1096-8652(199610)53:2<118::AID-AJH10>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 25.Cai J, Ahmad S, Jiang WG, Huang J, Kontos CD, Boulton M, et al. Activation of vascular endothelial growth factor receptor-1 sustains angiogenesis and Bcl-2 expression via the phosphatidylinositol 3-kinase pathway in endothelial cells. Diabetes. 2003 Dec;52(12):2959–68. doi: 10.2337/diabetes.52.12.2959. [DOI] [PubMed] [Google Scholar]
- 26.Cerdan C, Rouleau A, Bhatia M. VEGF-A165 augments erythropoietic development from human embryonic stem cells. Blood. 2004 Apr 1;103(7):2504–12. doi: 10.1182/blood-2003-07-2563. [DOI] [PubMed] [Google Scholar]
- 27.Hattori K, Heissig B, Wu Y, Dias S, Tejada R, Ferris B, et al. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1(+) stem cells from bone-marrow microenvironment. Nat Med. 2002 Aug;8(8):841–9. doi: 10.1038/nm740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin R, Lahlil R, Damert A, Miquerol L, Nagy A, Keller G, et al. SCL interacts with VEGF to suppress apoptosis at the onset of hematopoiesis. Development. 2004 Feb;131(3):693–702. doi: 10.1242/dev.00968. [DOI] [PubMed] [Google Scholar]
- 29.Tam BY, Wei K, Rudge JS, Hoffman J, Holash J, Park SK, et al. VEGF modulates erythropoiesis through regulation of adult hepatic erythropoietin synthesis. Nat Med. 2006 Jun 25; doi: 10.1038/nm1428. [DOI] [PubMed] [Google Scholar]
- 30.Tordjman R, Delaire S, Plout J, Ting S, Gaulard P, Fichelson S, et al. Erythroblasts are a source of angiogenic factors. Blood. 2001;97:1968–74. doi: 10.1182/blood.v97.7.1968. [DOI] [PubMed] [Google Scholar]
- 31.Janer J, Andersson S, Haglund C, Karikoski R, Lassus P. Placental growth factor and vascular endothelial growth factor receptor-2 in human lung development. Pediatrics. 2008 Aug;122(2):340–6. doi: 10.1542/peds.2007-1941. [DOI] [PubMed] [Google Scholar]
- 32.Fischer C, Jonckx B, Mazzone M, Zacchigna S, Loges S, Pattarini L, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007 Nov 2;131(3):463–75. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]