Abstract
The purpose of this study is to understand the processes of adaptation (changes in within-trial postural responses) and habituation (reductions in between-trial postural responses) to visual cues in older and young adults. Of particular interest were responses to sudden increases in optic flow magnitude. The postural sway of 25 healthy young adults and 24 healthy older adults was measured while subjects viewed anterior-posterior 0.4 Hz sinusoidal optic flow for 45 s. Three trials for each of three conditions were performed: 1) constant 12 cm optic flow amplitude (24 cm peak-to-peak), 2) constant 4 cm amplitude (8 cm p-t-p), and 3) a transition in amplitude from 4 to 12 cm. The average power of head sway velocity (Pvel) was calculated for consecutive 5 s intervals during the trial to examine the changes in sway within and between trials. A mixed factor repeated measures ANOVA was performed to examine the effects of subject Group, Trial, and Interval on the Pvel. Pvel was greater in older adults in all conditions (p < 0.001). During the 12 cm constant amplitude trials, within-trial adaptation occurred for all subjects, but there were differences in the between-trial habituation. Pvel of the older adults decreased significantly between all 3 trials, but decreased only between trial 1 and 2 in young adults. While the responses of the young adults to the transition in optic flow from 4 to 12 cm did not significantly change, older adults had an increase in Pvel following the transition, ranging from 6.5 dB for the first trial to 3.4 dB for the third trial. These results show that older adults can habituate to repeated visual perturbation exposures; however, this habituation requires a greater number of exposures than young adults. This suggests aging impacts the ability to quickly modify the relative weighting of the sensory feedback for postural stabilization.
Keywords: habituation, sensory reweighting, elderly, balance
INTRODUCTION
Alterations in the sensory environment occur frequently in everyday life. Examples include walking from a firm to a compliant surface, such as from a hard floor to a carpeted floor (somatosensory perturbation), or walking from a narrow corridor to a large lobby (visual perturbation). The ability to generate appropriate postural responses for the maintenance of upright stance in light of these changing cues is a dynamic process and requires intact central and peripheral sensory and motor systems. Recent studies have concluded that under varying environmental conditions that impact sensed body position and motion (e.g., moving platform or moving scene perturbations), the relative contributions of the sensory systems change; this process is often called ``sensory re-weighting” and is interpreted as evidence of adaptation to the environmental change/perturbation [1–4]. Older adults typically exhibit elevated sway responses to postural perturbations compared with young adults, and their ability to integrate sensory information and generate an appropriate postural response when environmental conditions or sensory cues change rapidly has been shown to be reduced [5–9]. One possible explanation for the reduced ability to adapt to new sensory cues is that older adults take longer to reweight the relative contributions of the sensory inputs.
Although the elevated sway responses to suddenly changing sensory environments have been detailed in older adults, it is not clear how older adults habituate to repeated sensory disturbances. For example, in a study by Teasdale et al., it does not appear that the increase in sway as a result of eye closure changed over the course of three subsequent exposures [5]. In contrast, Sundermier et al. demonstrated that older adults have a reduction in sway during repeated exposure to a moving visual environment, with the greatest reduction occurring after the first trial [10]. Consequently, we wanted to investigate the sway response to visual sensory cues that change suddenly over repeated trials to explore the influence of age on the ability to habituate to sudden visual perturbations. This ability to habituate to sensory perturbations has profound implications in the treatment of balance impairments in older adults.
The primary objective of this study is to clarify the processes of adaptation (changes in within-trial postural responses) and habituation (reductions in between-trial postural responses) to optic flow stimulation in older and young adults. Of particular interest is the impact of age on habituation to sudden changes in optic flow magnitude. Optic flow stimulation, as opposed to other sensory modalities, was chosen for this study because of its important role in providing cues for postural control during stance and locomotion.
METHODS
Subjects
Twenty-five healthy young subjects (14 females, mean age = 27 years, range 21–37 years) and 24 healthy older subjects (13 females, mean age = 70 years, range 60–80 years) completed the study. Within each age group, there was no difference in the age distribution between males and females. The young adult subjects were primarily recruited from the university community, and the older adults were recruited from various community sources by print advertisements. Informed consent approved by the local Institutional Review Board was obtained from each subject before participation.
All participants who participated had normal responses to the following neurological screening tests: 1) Mini-mental State Examination [11] (score greater than 24), 2) cranial nerve and cerebellar function (e.g. normal oculomotor exam, facial muscle movements, rapid alternating movements, finger-to-nose), 3) visual acuity (better than 20/40 acuity, corrected), 4) vibration sense at the lateral malleoli (able to determine onset and termination of 256 Hz vibration), 5) cutaneous pressure sensation (sense 10 g monofilament force), and 6) peripheral vestibular function (unilateral caloric weakness less than 25%, no directional preponderance on rotational tests).
Equipment
Subjects stood within a full field of view (FOV) display enclosure called the Balance NAVE Automatic Virtual Environment (BNAVE, [12]). The BNAVE consists of three adjacent screens that surround the subject and encompass 180° of the horizontal and 70° of the vertical FOV (Figure 1). The front screen is 1.5 m from the subject, and the side screens are 1.1 m from the subject at its closest point. The optic flow environment was created using custom software and displayed on three EPSON PowerLite 811p Multimedia Projectors, each of which back-projects onto one of three screens. The environment simulated a 2.4 m high ×2.4 m wide room with a textured wall placed 1.5 m in front of the subject. The spatial frequency of the checked pattern was 15 deg at a point lateral to the subject. The flow moved sinusoidally in the anterior-posterior (AP) direction at a frequency of 0.4 Hz.
Figure 1.
Subject standing in full field of view Balance NAVE Automatic Virtual Environment, consisting of three adjacent screens that surround the subject and encompass 180° of the horizontal and 70° of the vertical field of view. Entire visual image moves in anterior-posterior direction.at 0.4 Hz during optic flow stimulation.
Subjects stood on a modified NeuroTest™ (Neurocom, Inc.) posture platform, which measured the ground reaction forces. Center of Pressure was computed from the ground reaction forces. During all of the trials reported in this study, the platform was fixed and level with the ground. The postural sway of each subject was recorded using a six degrees-of-freedom Polhemus Fastrak™ (Polhemus, Inc.) electromagnetic tracking system (accuracy of 0.8 mm). Receivers were placed on a headband and waistbelt worn by each subject.
Procedure
All subjects had viewed constant amplitude optic flow on a previous visit approximately 1 week before, and thus were accustomed to the optic flow environment. Subjects removed their shoes before starting. To prevent injury from falls, a harness attached to an overhead support was used at all times during testing. It was designed to fit comfortably while not interfering with postural movements. If a subject experienced a loss of balance, the trial was stopped and then repeated.
The experiment began with a 45 s baseline trial in which the subject stood still on the force platform, with arms folded across the chest and eyes open, looking straight ahead at a blank screen. The ambient light level was approximately 10 Lux (compared to 40 Lux during the optic flow trials) and the subjects were able to use visual cues for stabilization of body sway. During this trial, the amount of sway that occurred naturally without perturbation (quiet stance) was measured.
This baseline trial was followed by nine experimental trials, each 45 s in duration. The data presented here are part of a larger investigation performed in order to examine the effects of adaptation to sudden changes in the magnitude of optic flow or orientation of the support surface. During Condition 1 (Trials 1 to 3), the amplitude of the 0.4 Hz optic flow was 12 cm (peak-to-peak 24 cm) throughout the duration of the trial. During Condition 2 (Trials 4 to 6), the amplitude was 4 cm (peak-to-peak 8 cm) throughout the duration of the trial. Finally, during Condition 3 (Trials 7 to 9), the amplitude of the optic flow increased from 4 cm to 12 cm at a random time between 22 and 28 s after the beginning of the trial. The transition from 4 to 12 cm occurred so that there was no discontinuity in the A–P translation of the optic flow environment (Figure 2). The amplitude corresponded to the apparent motion of the simulated room. Subjects were not told anything about the characteristics of the optic flow, and thus did not know about the relative amplitude or whether a change in amplitude would occur during each trial. The order of the trials was fixed for several reasons. First, we wanted the subjects to experience the 12 cm amplitude optic flow under steady-state conditions before the transition trials, so that if we found a change in sway response during the transition trials, we could infer that it was due to the transition, and not because they had not experienced that stimulus before. Second, immediately prior to the transition trials, we wanted the subjects to undergo several trials of 4 cm amplitude so that they would not be expecting a change in stimulus amplitude. Each set of three trials was followed by a period of 3 minutes of seated rest.
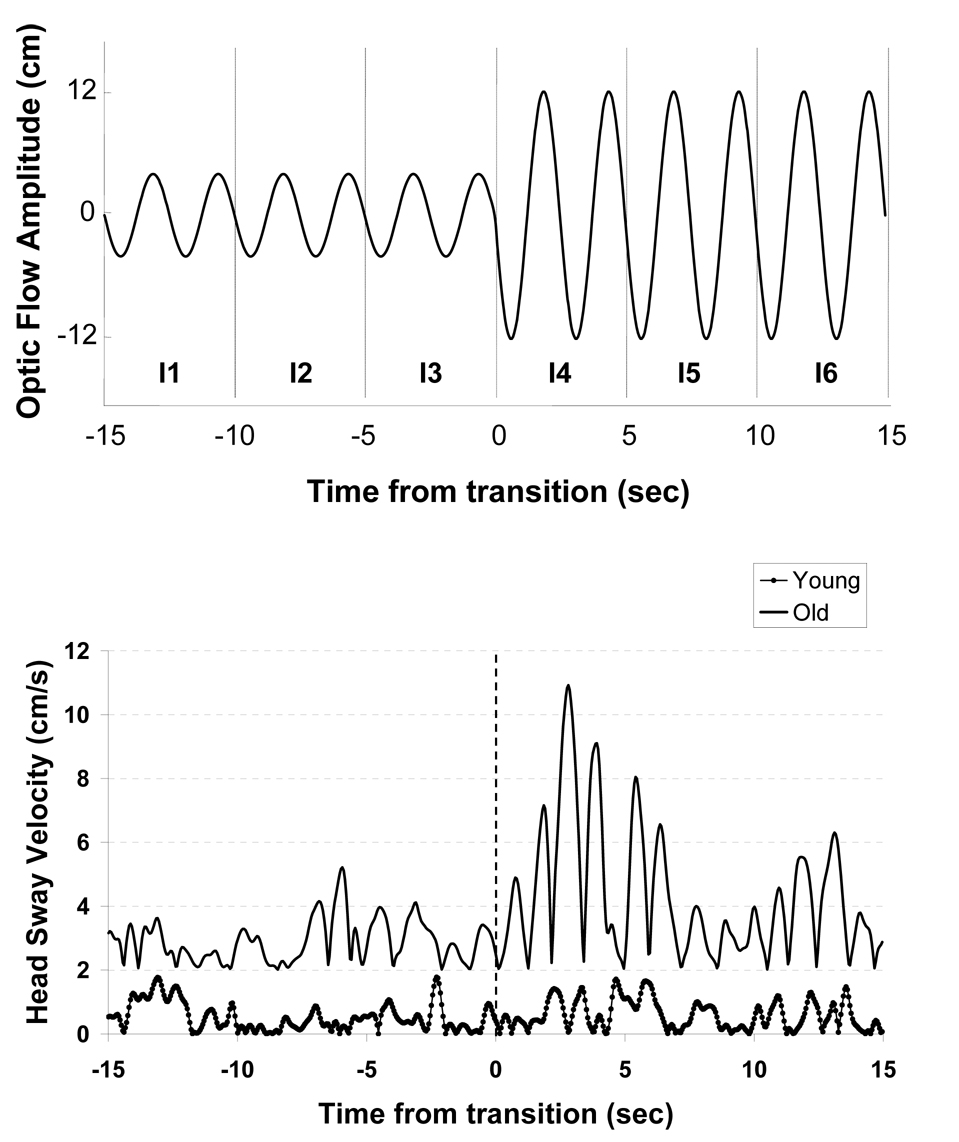
Figure 2.
Top: Amplitude of optic flow stimulus during trials 7 to 9. The power of recorded head sway velocity in response to this stimulus was averaged for each 5 s interval (I1-I6). Bottom: Representative traces of instantaneous velocity of AP head sway obtained from young (dotted line) and older adult (solid line) during trial 7. Observe large increase in sway velocity in older, but not young adult subject.
Data Collection and Analysis
Data from the force platform and electromagnetic tracking system were collected at a sampling rate of 20 Hz using LabVIEW (National Instruments). Data analysis was performed using MATLAB (The MathWorks, Inc.). There were no meaningful differences in the patterns of sway or statistical results when comparing the head, trunk, and center of pressure. Furthermore, no substantial statistical differences occurred when we compared the total power of head sway velocity (less than 2.0 Hz) with the power of head sway velocity limited to a pass band about the optic flow driving frequency (0.3 to 0.5 Hz). Therefore, only the results from the total power of head sway velocity are presented. Because the optic flow moved in the AP direction, the predominant direction of sway was AP. Since 99% of the power of sway is below 1.5 Hz in similar studies conducted in our lab, the head AP position signal for each 45 s trial was filtered with a 2nd order lowpass digital Butterworth filter with cutoff frequency 2 Hz in the forward and backward directions [13]. The resulting signal was then differentiated to obtain the velocity of head movement. Sway velocity distinguishes between older adults who report balance problems from those who do not [14]. The instantaneous power of the velocity was computed by squaring the velocity time series.
One objective was to examine adaptation and habituation during the steady-state optic flow trials (Conditions 1 and 2); consequently, we analyzed the entire length of the trial (45 s). Another objective was to examine the sway response to the change in optic flow magnitude. Analysis was thus limited to the 15 s preceding and 15 s following each transition in Condition 3 (Figure 2). Based on visual inspection of the current data, as well as previous work in which changes in peak sway velocity due to sudden visual perturbations occurred in less than 5 s [13], we further subdivided the velocity power signals into 5 s intervals that formed the basis of the statistical analysis (Figure 2). The average power of head sway velocity (Pvel) was computed for each of the 5 s intervals across all nine trials. Thus, there were nine intervals for Conditions 1 and 2, and six intervals for Condition 3.
A mixed-factor repeated measures analysis of variance (ANOVA) was conducted separately for each set of three trials. The independent variables were subject group (GROUP: young, older), trial repetition (TRIAL: 1, 2, 3) and the 5 s time interval of the analyzed data (INTERVAL: 1 to 9 for Conditions 1 and 2, 1 to 6 for Condition 3). The Pvel values were log-transformed and expressed in dB (10*log(power of sway velocity), reference value of 1 cm2/s2), to stabilize the variance. Analyses included main effects, two-way and three-way interactions. Post-hoc analyses included tests of linear contrast as well as pairwise comparisons using Sidak’s adjustment for multiple comparisons. Within the older adult group, correlation analysis was used to determine if there was any relationship between age (in years) and Pvel during the first steady-state optic flow trial and change in Pvel after the optic flow amplitude increased. A significance level of α = 0.05 was used.
RESULTS
Baseline Characteristics
Young and older adults differed significantly in their baseline functional balance performance (p = 0.003). During quiet stance with eyes open on a fixed platform, older adults had greater Pvel (−1.1 + SEM 0.7 dB) than young adults (−3.8 + SEM 0.6 dB).
Steady-state optic flow
During the first three optic flow trials using 12 cm optic flow, young and older subjects swayed in response to the stimulus, indicated by values above the quiet stance (QS) Pvel (Figure 3). The ANOVA demonstrated that older adults swayed more in response to the optic flow compared to the young subjects (Group, F1,47 = 15.3, p < 0.001). Pvel was significantly reduced in each successive trial (Trial, F2,94 = 49.3, p < 0.001). A significant interaction between Group X Trial was also observed (F2,94 = 8.5, p = 0.002). Post hoc analysis revealed that a significant reduction in Pvel occurred between trials 1 and 2, and between trials 2 and 3 in older adults; in young adults, a significant drop occurred only after trial 1. There was also a significant linear decrease in Pvel over time within trials (Interval, F8,376 = 23.9, p < 0.001). This decrease depended on sequence of trials (Trial X Interval, F16,752 = 2.3, p=0.011) because there was a greater reduction in sway velocity during the course of Trial 1 compared with Trials 2 and 3.
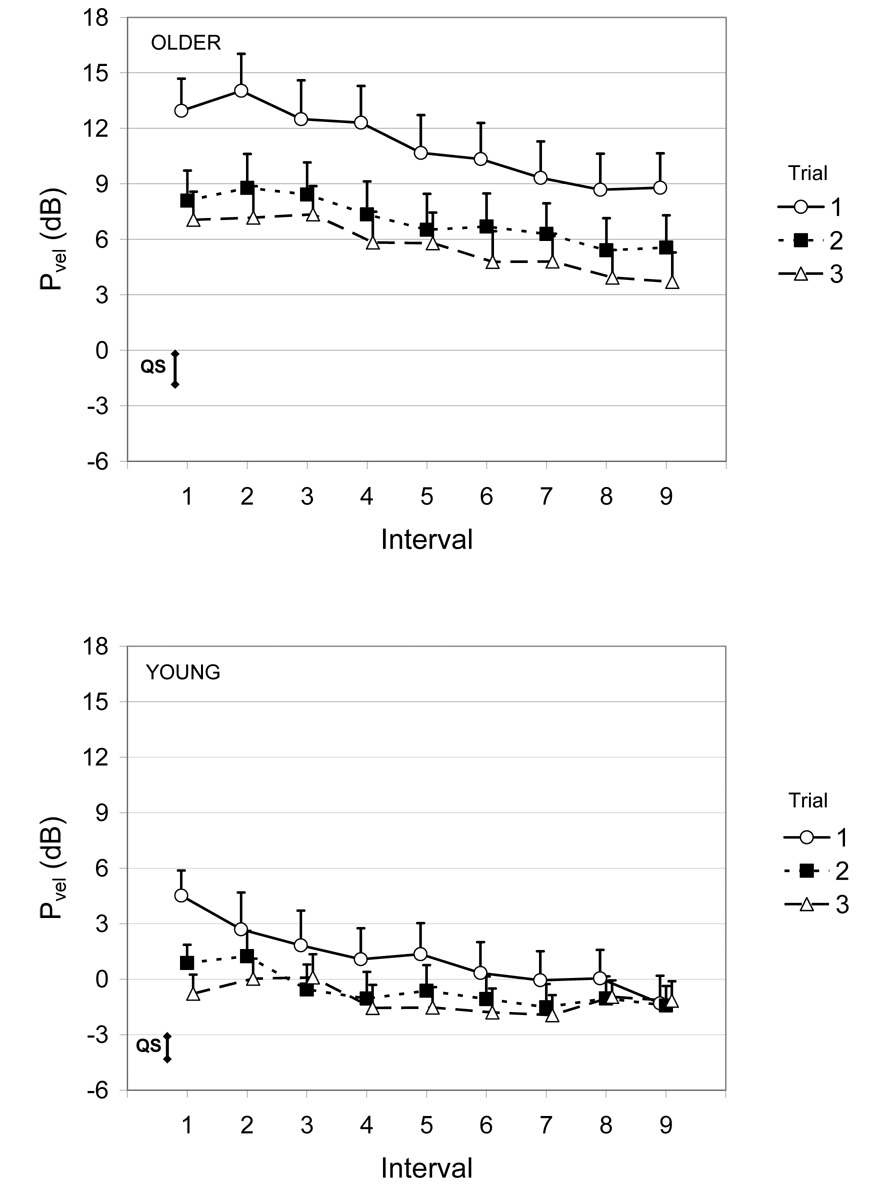
Figure 3.
Average power of head sway velocity (Pvel) for the older (top) and young adults (bottom) during steady-state 12 cm optic flow (Condition 1, Trials 1 to 3). Error bars are the standard error of the mean. For reference, the vertical bar labeled “QS” is the mean + SEM of the Pvel obtained during the baseline quiet stance trial. Reference level for dB scale is 1 cm2/s2.
During trials 4–6 which used 4 cm optic flow (Figure 4), the Pvel was greatly reduced compared with the 12 cm optic flow trials (F1,47 = 73.7, p < 0.001). The ANOVA determined that older adults had greater Pvel compared with young adults (Group, F1,47 = 12.2, p < 0.001). A significant Trial effect (F2,94 = 15.8, p < 0.001) showed that Pvel was greater in trial 4 compared with trials 5 and 6, but there was no difference between trials 5 and 6. A significant linear trend for the Interval main effect (F8,376 = 9.6, p = 0.002) was found. Further examination of a significant Trial X Interval interaction (F16,752 = 3.8, p < 0.001) revealed that the significant Interval main effect is likely to be caused by values of Pvel that were significantly greater in the first interval compared with subsequent intervals of trial 4, but not greater than later intervals in trials 5 and 6. In addition, the reduction in sway from interval 1 to interval 2 was larger in older adults compared with young adults (Group X Interval, F8,376 = 2.7, p = 0.019)
Figure 4.
Average power of head sway velocity (Pvel) for the older (top) and young adults (bottom) during steady-state 4 cm optic flow (Condition 2, Trials 4 to 6). Error bars are the standard error of the mean. For reference, the vertical bar labeled “QS” is the mean + SEM of the Pvel obtained during the baseline quiet stance trial. Reference level for dB scale is 1 cm2/s2.
Transitions in optic flow exposure
Representative traces of the head sway responses to the increase in optic flow magnitude for an older and young adult subject are shown in Figure 2. During the trials in which optic flow amplitude increased suddenly from 4 cm to 12 cm (trials 7–9), increases in Pvel following the transition were observed, particularly for the older subjects (Figure 5). ANOVA indicated that older adults had greater Pvel than young adults (Group, F1,47 = 15.4, p < 0.001). Post-hoc analyses showed that older, but not young adult subjects had significantly increased sway velocity subsequent to the transition (Group X Interval, F5,235 = 6.0, p = 0.003). Furthermore, this increase depended on the trial order (Group X Trial X Interval, F10,470 = 4.1, p < 0.001). For example, when looking solely at the change in Pvel from interval 3 to 4 in the older adults, the amount of increase declined from trial 7 (change = 6.5 dB) to trial 9 (change = 3.4 dB). .
Figure 5.
Average power of head sway velocity (Pvel) for the older (top) and young adults (bottom) during an increase in optic flow amplitude from 4 to 12 cm (Condition 3, Trials 7 to 9). Transition occurred between Intervals 3 and 4. Error bars are the standard error of the mean. For reference, the vertical bar labeled “QS” is the mean + SEM of the Pvel obtained during the baseline quiet stance trial. Reference level for dB scale is 1 cm2/s2.
Sway variation in older adults
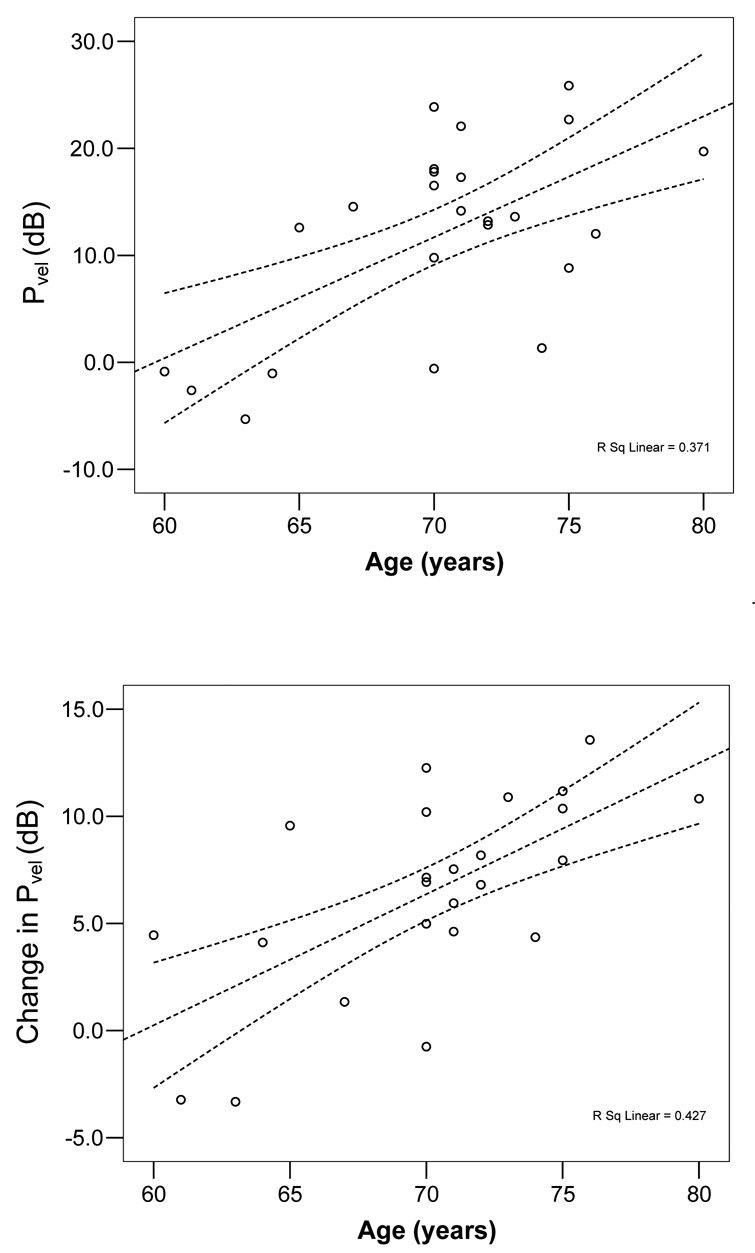
To further explore the age-related adaptation in sway in the older adults, we performed correlation analysis of age with several of the key sway measures. Within the older adult group, the average Pvel obtained during trial 1 increased as the age of subjects advanced (Figure 6, r = 0.61, p = 0.002). When the adaptation was the greatest, age did not have an effect on the magnitude of the decrease in Pvel from the beginning to the end of trial 1 (r = 0.16, p = 0.46). Unexpectedly, the oldest subjects showed the greatest habituation (i.e. reduction in Pvel) from trial 1 to 2 (r = 0.57, p = 0.003). This effect was no longer seen after controlling for the greater average Pvel in Trial 1 (p = 0.08). Finally, the amount of increase in Pvel during the sudden increase in optic flow amplitude during the first transition trial was dependent upon age (Figure 6, r = 0.65, p < 0.001). The systematic variation in these variables with age did not occur in the young adult group.
Figure 6.
Average power of head sway velocity (Pvel) for during trial 1 (top) and change in Pvel after the increase in optic flow magnitude in trial 7 (bottom), plotted as a function of age for the older adult group. Least-squares fit line and 95% confidence interval are displayed with dashed lines. Reference level for dB scale is 1 cm2/s2.
DISCUSSION
Steady-state optic flow
Habituation to repeated exposures of constant 12 cm optic flow (Condition 1) was more rapid in young adults compared to older adults. The older and young adults displayed significantly greater magnitude of Pvel compared with baseline, consistent with previous research showing that postural sway increases while viewing optic flow, and this increase was greater in older adults [10, 15–17]. Habituation to the optic flow stimulus occurred, as quantified by the reduction in Pvel across trials, consistent with previous findings [4, 10, 13, 18–20]. However,trial-to-trial habituation was more rapid in younger adults. There was no difference in Pvel between trials 2 and 3 in young adults, suggesting that they had fully habituated to the optic flow by trial 2. On the other hand, in older adults, the Pvel during trial 3 was significantly less than in trial 2, indicating an ongoing process of habituation that may or may not have completed after three trials. Due to a concern of fatigue, the number of trials for each condition was limited to 3. Future experiments using a greater number of trials may be able to characterize how many trials are needed for older subjects to completely habituate.
One interpretation of our findings is that the adaptation within-trials and habituation between-trials may be explained by a relative decrease in the contribution of visual feedback gain. In terms of ``sensory re-weighting” postural control models, [1, 2] this relative decrease may be accomplished by an absolute decrease in visual gain, by an increase in vestibular and/or proprioceptive gain, or a combination of an increase in these gains with a concurrent decrease in visual gain. While our protocol does not enable us to determine absolute gain changes in each of the sensory feedback paths, in each scenario outlined, the relative weight of the visual feedback is reduced compared with the proprioceptive and graviceptive feedback. Furthermore, differences between older and young adults may indicate that older adults reduce the relative visual feedback gain at a slower rate than young adults, reflecting changes in central sensory integration with age.
The major effect seen during the second set of steady-state optic flow trials at 4 cm was a substantial reduction in sway velocity during the first 10 seconds of trial 4. It is possible that the reduction was affected by changes in the sensory reweighting or perhaps a change in the central set. Horak et al. observed larger than normal automatic postural responses when a small platform perturbation was preceded by a series of large perturbations [21]. Similarly in this study, subjects may have had elevated sway in interval 1 of trial 4 because they were expecting a larger optic flow stimulus. Overall, some habituation occurred between trials 4 and 5 in both young and older adults. Further evidence of habituation was not seen after trial 5 because subjects were generating sway velocities comparable to baseline conditions.
Transitions in optic flow magnitude
Habituation to the sudden perturbations in optic flow was more rapid in young adults compared to older adults. Following the sudden increase in optic flow amplitude, the sway responses were starkly different between the two groups. In trials 7 and 8, Pvel of the older adults increased to values comparable to those observed during the final interval of the earlier trial 3. In contrast, Pvel of the young adults response for the later trials was much less than the value computed at the end of trial 3. Furthermore, Pvel of young subjects during interval 4 was not significantly different from the quiet stance sway. Consequently, in response to the increased optic flow amplitude, the findings of increased sway for older adults, and lack thereof for young adults, indicate that the older adults were using visual feedback to a much greater extent than young adults.
Sway variation in older adults
As a group, older subjects generated greater sway head velocity than young subjects. Moreover, within the older adult group, the average sway velocity and the transient sway response was larger for the oldest subjects. Given that the subjects were screened for peripheral sensory disorders, these findings suggest that changes in the postural responses observed in this experiment may be partly explained by age-related changes in central processing of the sensory input. One area that will require further exploration is why no association was found between age and the magnitude of adaptation during the steady-state trials.
The accumulation of evidence in the current and previous studies supports the hypothesis that re-weighting of the sensory feedback is slower in older adults. For instance, Teasdale et al. studied the postural sway responses in older and younger adults subjected to successively reduced and augmented visual sensory conditions [5]. The older subjects responded with increased sway when visual information was both removed and reinserted, in contrast to the increase experienced by the young subjects only when visual information was removed. Similarly, Hay et al. using intermittent tendon vibration to the ankle dorsi- and plantarflexors, found that older adults had greater sway responses immediately after starting and ending the vibration input, whereas young adults generated increased sway only after the vibration commenced [7]. Simoneau et al. found that suddenly changing a subject’s focal point through the use of elevator-like doors resulted in two to three times more center of pressure range and speed in the older subjects than the younger subjects [6].
There have been conflicting reports of the amount of habituation that can occur in healthy older adults as a result of repeated sensory perturbations. In the current study, from trial 7 to 9, there was successive reduction in the magnitude of change in Pvel that occurred as a result of the transition in optic flow. Our data are consistent with the findings of Sundermier et al. who observed a continued reduction in postural sway in response to successive forward translations in the visual field, with most adaptation occurring between trials 1 and 2 [10]. On the other hand, the data of Teasdale et al. do not show any habituation in postural sway of young or older adults in response to repeated episodes of eye closure and opening [5]. These results may reflect differences in central processing and integration of sensory information when a sensory channel is active and being perturbed, as in our study, versus when a channel is intentionally disengaged, as in the Teasdale et al. study when eyes were closed.
Increased velocity of sway has been implicated as a risk factor for decreased mobility [22], fear of falling [23], and falling [24]. This study provides evidence that sudden changes in the visual input can result in increased head sway velocity in older adults compared with young adults. This may be a contributing factor to greater postural instability seen in older adults, and is consistent with previous findings showing that older adults tend to have more difficulty adapting to sudden sensory or environmental changes affecting balance or gait.
Acknowledgments
This research was supported by funding from the National Institutes of Health (K25 AG001049, P30 AG024827, P30 DC005205) and the Eye and Ear Foundation. We are grateful for the assistance of Prof. Larry F. Hodges, Sabarish V. Babu, and Jeffrey Jacobson for development of the optic flow software and hardware.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Peterka RJ. Sensorimotor integration in human postural control. Journal of Neurophysiology. 2002;88:1097–1118. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- 2.Peterka RJ, Loughlin PJ. Dynamic regulation of sensorimotor integration in human postural control. Journal of Neurophysiology. 2004;91:410–423. doi: 10.1152/jn.00516.2003. [DOI] [PubMed] [Google Scholar]
- 3.Oie KS, Kiemel T, Jeka JJ. Multisensory fusion: Simultaneous re-weighting of vision and touch for the control of human posture. Cognitive Brain Research. 2002;14:164–176. doi: 10.1016/s0926-6410(02)00071-x. [DOI] [PubMed] [Google Scholar]
- 4.Bronstein AM. Suppression of visually evoked postural responses. Experimental Brain Research. 1986;63:655–658. doi: 10.1007/BF00237488. [DOI] [PubMed] [Google Scholar]
- 5.Teasdale N, Stelmach GE, Breunig A, Meeuwsen HJ. Age differences in visual sensory integration. Experimental Brain Research. 1991;85:691–696. doi: 10.1007/BF00231755. [DOI] [PubMed] [Google Scholar]
- 6.Simoneau M, Teasdale N, Bourdin C, Bard C, Fleury M, Nougier V. Aging and postural control: Postural perturbations caused by changing the visual anchor. Journal of the American Geriatrics Society. 1999;47:235–240. doi: 10.1111/j.1532-5415.1999.tb04584.x. [DOI] [PubMed] [Google Scholar]
- 7.Hay L, Bard C, Fleury M, Teasdale N. Availability of visual and proprioceptive afferent messages and postural control in elderly adults. Experimental Brain Research. 1996;108:129–139. doi: 10.1007/BF00242910. [DOI] [PubMed] [Google Scholar]
- 8.Peterka RJ, Black FO. Age-related changes in human posture control: Sensory organization tests. Journal of Vestibular Research. 1990;1:73–85. [PubMed] [Google Scholar]
- 9.Woollacott MH, Shumway-Cook A, Nashner LM. Aging and posture control: Changes in sensory organization and muscular coordination. International Journal of Aging & Human Development. 1986;23:97–114. doi: 10.2190/VXN3-N3RT-54JB-X16X. [DOI] [PubMed] [Google Scholar]
- 10.Sundermier L, Woollacott MH, Jensen JL, Moore S. Postural sensitivity to visual flow in aging adults with and without balance problems. Journals of Gerontology: Series A-Biological Sciences & Medical Sciences. 1996;51:M45–M52. doi: 10.1093/gerona/51a.2.m45. [DOI] [PubMed] [Google Scholar]
- 11.Folstein M, Folstein S PM. "Mini-mental state": A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson J, Redfern MS, Furman JF, Whitney SL, Sparto PJ, Wilson JB, Hodges LF. Proceedings of the ACM symposium on virtual reality software and technology. ACM Press; 2001. Balance nave: A virtual reality facility for research and rehabilitation of balance disorders; pp. 103–109. [Google Scholar]
- 13.Mahboobin A, Loughlin PJ, Redfern MS, Sparto PJ. Sensory re-weighting in human postural control during moving-scene perturbations. Experimental Brain Research. 2005;167:260–267. doi: 10.1007/s00221-005-0053-7. [DOI] [PubMed] [Google Scholar]
- 14.Baloh RW, Spain S, Socotch TM, Jacobson KM, Bell T. Posturography and balance problems in older people. Journal of the American Geriatrics Society. 1995;43:638–644. doi: 10.1111/j.1532-5415.1995.tb07198.x. [DOI] [PubMed] [Google Scholar]
- 15.Ring C, Matthews R, Nayak US, Isaacs B. Visual push: A sensitive measure of dynamic balance in man. Archives of Physical Medicine & Rehabilitation. 1988;69:256–260. [PubMed] [Google Scholar]
- 16.Borger LL, Whitney SL, Redfern MS, Furman JM. The influence of dynamic visual environments on postural sway in the elderly. Journal of Vestibular Research. 1999;9:197–205. [PubMed] [Google Scholar]
- 17.Sparto PJ, Furman JF, Redfern MS. Head sway response to optic flow: Effect of age is more important than the presence of unilateral vestibular hypofunction. Journal of Vestibular Research. 2006 In press. [PMC free article] [PubMed] [Google Scholar]
- 18.Bronstein AM, Hood JD, Gresty MA, Panagi C. Visual control of balance in cerebellar and parkinsonian syndromes. Brain. 1990;113:767–779. doi: 10.1093/brain/113.3.767. [DOI] [PubMed] [Google Scholar]
- 19.Hansen PD, Woollacott MH, Debu B. Postural responses to changing task conditions. Experimental Brain Research. 1988;73:627–636. doi: 10.1007/BF00406622. [DOI] [PubMed] [Google Scholar]
- 20.Timmann D, Horak FB. Prediction and set-dependent scaling of early postural responses in cerebellar patients. Brain. 1997;120:327–337. doi: 10.1093/brain/120.2.327. [DOI] [PubMed] [Google Scholar]
- 21.Horak FB, Diener HC, Nashner LM. Influence of central set on human postural responses. Journal of Neurophysiology. 1989;62:841–853. doi: 10.1152/jn.1989.62.4.841. [DOI] [PubMed] [Google Scholar]
- 22.Baloh RW, Corona S, Jacobson KM, Enrietto JA, Bell T. A prospective study of posturography in normal older people. Journal of the American Geriatrics Society. 1998;46:438–443. doi: 10.1111/j.1532-5415.1998.tb02463.x. [DOI] [PubMed] [Google Scholar]
- 23.Baloh RW, Fife TD, Zwerling L, Socotch T, Jacobson K, Bell T, Beykirch K. Comparison of static and dynamic posturography in young and older normal people. Journal of the American Geriatrics Society. 1994;42:405–412. doi: 10.1111/j.1532-5415.1994.tb07489.x. [DOI] [PubMed] [Google Scholar]
- 24.Fernie GR, Gryfe CI, Holliday PJ, Llewellyn A. The relationship of postural sway in standing to the incidence of falls in geriatric subjects. Age & Ageing. 1982;11:11–16. doi: 10.1093/ageing/11.1.11. [DOI] [PubMed] [Google Scholar]