Abstract
We report a case of severe diabetic macular edema (DME) that developed after pioglitazone was used by a patient with proliferative diabetic retinopathy. A 30-year-old woman with poorly controlled type 2 diabetes mellitus visited our clinic in 2004. She had moderate pre-proliferative diabetic retinopathy OU. Because of the rapid progression of the diabetic retinopathy, she received pan-retinal photocoagulation in both eyes. Two weeks before using pioglitazone, her visual acuity was 0.9 OD and 0.7 OS. On October 2007, pioglitazone was prescribed by her internist because of poorly controlled blood glucose level. Two weeks later, her body weight increased, and her face became edematous. Her visual acuity decreased to 0.5 OU, and ophthlamoscopy showed severe DME in both eyes. Two weeks after stopping pioglitazone, her visual acuity improved to 0.8 OD and 0.5 OS, but the DME was still severe in the optical coherence tomographic images. Then, one half the usual dose (25 mg) of spironolactone, a diuretic, was given and her macular edema was resolved. Her final visual acuity improved to 0.9 OD and 0.7 OS. We recommend that when a patient taking pioglitazone complains of decreased vision, the physician should promptly consult an ophthalmologist.
Keywords: pioglitazone, diabetic macular edema, spironolactone, optical coherence tomography
Introduction
Pioglitazone, a thiazolidinedione agent, is prescribed to diabetic patients to reduce insulin resistance (Aronoff et al 2000; Herz et al 2003). A recent meta-analysis showed that pioglitazone reduces the risk of cardiovascular events in patients with type 2 diabetes mellitus (DM) (Lincoff et al 2007). Thus, pioglitazone has become one of the major drugs prescribed for the primary care for patients with type 2 DM.
Pioglitazone can cause fluid retention and peripheral edema in diabetic patients (Niemeyer and Janney 2002; Mudaliar et al 2003), and the systematic fluid retention can be manifested as diabetic macular edema (DME) (Perkovich and Meyers 1988; Tokuyama et al 2000). However, one report has indicated the adverse effect of pioglitazone on DME in patients with type 2 DM (Ryan et al 2006). We report a case of severe DME that developed after pioglitazone was used by a patient with proliferative diabetic retinopathy. In this case, the DME resolved with cessation of the pioglitazone and the use of a diuretic.
Case report
A 30-year-old woman visited our clinic for examination of her diabetic retinopathy in 2004. She was a type 2 diabetic and had moderate nonproliferative diabetic retinopathy with a glycosylated hemoglobin A1c (HbA1c) of 12%. No treatment was given, but during the follow-up period her retinopathy worsened and pan-retinal photocoagulation was applied to both eyes; the left eye had pan-retinal photocoagulation in 2004 with 1700 burns, and the right eye had pan-retinal photocoagulation in 2006 with 2000 burns. Although mild vitreous hemorrhage developed in both eyes during the pan-retinal photocoagulation, the retinopathy became stable with proliferative tissues surrounding the optic disc in both eyes (Figures 1 and 2). DME did not develop during and immediately after the pan-retinal photocoagulation in both eyes.
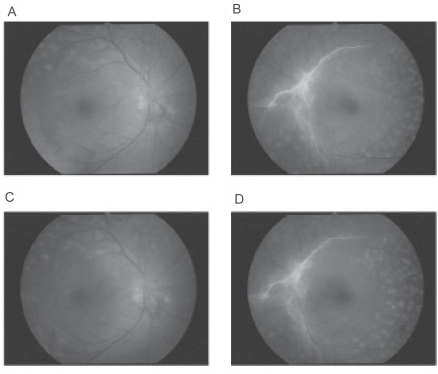
Figure 1.
Fundus photographs before and after pioglitazone treatment. A and B: 4 months before pioglitazone treatment, macula edema is not present in either eye (A shows the right eye, B the left eye). C and D: during pioglitazone, DME is present in both eyes but it is difficult to detect in fundus photographs because the DME is very diffuse (C shows the right eye, D the left eye).
Abbreviations: DME, diabetic macular edema.
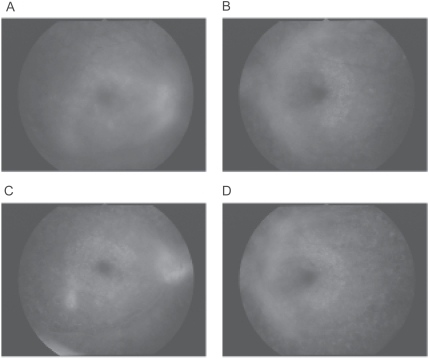
Figure 2.
Fluorescein angiograms before and after pioglitazone treatment. A and B: 6 months before pioglitazone treatment, neovascular abnormalities with no leakage are present in both maculas but the activity is low and no DME is observed (A shows the right eye, B the left eye). C and D: During pioglitazone treatment, the status of proliferative diabetic retinopathy is not changed before and after pioglitazone treatment. DME is difficult to detect in the fluorescein angiograms because it is very diffuse. Optical coherence tomography shows the diffuse DME more clearly (C shows the right eye, D the left eye).
Abbreviations: DME, diabetic macular edema.
Her best corrected visual acuity was 0.9 OD and 0.7 OS 2 weeks before using pioglitazone, and DME was not present before the pioglitazone treatment (Figures 1, 2, 3A). She had been prescribed 3 drugs for controlling the hyperglycemia before taking pioglitazone. No drugs other than pioglitazone were prescribed after the cessation of pioglitazone.
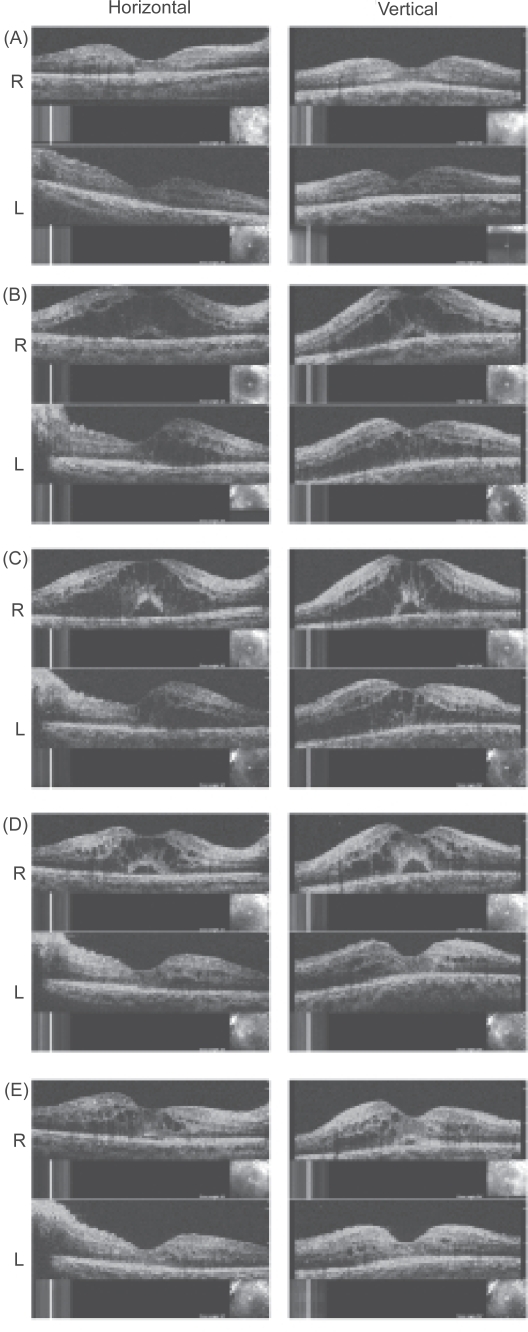
Figure 3.
OCTs before and after pioglitazone treatment. A: 3 months before pioglitazone treatment, macular edema is not present in both eyes. B: 2 weeks after pioglitazone, severe diabetic macular edema can be seen in both eyes. C: 6weeks after pioglitazone, DME is worse and a serous retinal detachment is present in the right eye. D: 2 weeks after cessation of pioglitazone, visual acuity has improved but severe DME is still present in the OCT images of the right eye. E: 2 months later after receiving a half-dose of spironolactone, DME is significantly reduced in both eyes.
Abbreviations: DME, diabetic macular edema; OCT, optical coherence tomogram.
Two weeks after taking pioglitazone, she visited our clinic. Her face was edematous, and she had gained weight (Table 1). Optical coherence tomography (OCT) showed that she had severe DME in both eyes, and a serous retinal detachment in the right eye (Figure 3B). Her visual acuity was decreased to 0.5 OU. We recommended that she ask her primary physician to stop the pioglitazone; however, pioglitazone could not be stopped because there had been no reports in Japan of any adverse effect of pioglitazone on developing DME and the HbA1c level was still high.
Table 1.
Clinical course of systemic condition
| Before pioglitazone treatment | During pioglitazone treatment | After pioglitazone treatment | |
|---|---|---|---|
| HbA1c (%) | 8–16 (large deviation) | 11.1 | 11.1 |
| Body weight (kg) | 65 ± 2 | 70 | 62 |
| BUN (mg/dL) | 8.0–20.0 | 12.3 | 9 |
| Cre (mg/dL) | 0.3–0.9 No renal dysfunction | 0.39 No renal dysfunction | 0.42 No renal dysfunction |
Before pioglitazone treatment, HbA1c levels showed large deviation. However, no renal dysfunction has been observed during the follow-up period (4 years). Body weight was significantly increased during pioglitazone treatment.
Abbreviations: BUN; blood urea nitrogen, Cre; creatinine.
One month later, her DME and serous retinal detachment worsened (Figure 3C). We explained our findings to the primary physician and suggested that if possible, pioglitazone be stopped. He kindly cooperated and agreed to stop the pioglitazone. Two weeks later, her vision improved to 0.8 OD and 0.5 OS. However, the severe DME was still present in the right eye in the OCT images (Figure 3D). We then prescribed 25 mg of spironolactone, a diuretic, which is one half the usual dose for reducing fluid retention and peripheral (facial) edema. Two months later, her body weight was reduced by 8 kg (Table 1), and her DME was significantly reduced in both eyes (Figure 3E). Her final visual acuity returned to 0.9 OD and 0.7 OS.
Discussion
Fluid retention and peripheral edema develop in 10% of patients using pioglitazone (Niemeyer and Janney 2002; Mudaliar et al 2003). Because systematic fluid retention can aggravate DME (Perkovich and Meyers 1988; Tokuyama et al 2000), the vision of patients with DME using pioglitazone should be carefully monitored. Unfortunately in Japan, some physicians prescribing pioglitazone are not aware of the development of DME caused by pioglitazone.
One case has been reported that showed that rosiglitazone (not available in Japan), a thiazolidinedione agent, was associated with vision loss due to DME (Colucciello 2005). In that report, the increase of the dosage from 2 to 8 mg/day caused the development of DME (Colucciello 2005). In our case, the lowest dose (15 mg) of pioglitazone that is usually prescribed caused the DME. These findings suggest that individual differences in sensitivity to the glitazones may exist.
In a recent retrospective study, a worsening of DME after glitazone (pioglitazone and/or rosiglitazone) treatment was estimated to be 1.5% and 2.6% of the cases (Ryan et al 2006). However, the investigators did not measure the visual acuity or evaluate the DME before glitazone treatment. Thus, it could not be determined whether their findings were associated with the glitazone treatment or the natural courses of diabetic retinopathy.
We followed the protocol for the treatment of fluid retention and peripheral edema; first we stopped the use of pioglitazone and if there was not a complete resolution of the edema, we prescribed one half dose of spironolactone (25 mg). Two months after the spironolactone treatment, the DME and peripheral edema were significantly improved.
In conclusion, pioglitazone treatment can lead to the development and aggravation of DME and systemic edema because of fluid retention. Cessation of pioglitazone and prescription of one half dose of spironolactone are useful in resolving the DME. When a patient taking pioglitazone complains of a decrease of vision, physicians should promptly consult an ophthalmologist, and suggest the possibility that pioglitazone is causing the same kind of fluid retention in the macula that it causes systematically.
Acknowledgments
We thank Prof. Duco Hamasaki for editing this manuscript.
Disclosures
None of the authors has any conflicts of interest to disclose.
References
- Aronoff S, Rosenblatt S, Braithwaite S, et al. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6-month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care. 2000;23:1605–11. doi: 10.2337/diacare.23.11.1605. [DOI] [PubMed] [Google Scholar]
- Colucciello M. Vision loss due to macular edema induced by rosiglitazone treatment of diabetes mellitus. Arch Ophthalmol. 2005;123:1273–5. doi: 10.1001/archopht.123.9.1273. [DOI] [PubMed] [Google Scholar]
- Herz M, Johns D, Reviriego J, et al. A randomized, double-blind, placebo-controlled, clinical trial of the effects of pioglitazone on glycemic control and dyslipidemia in oral antihyperglycemic medication-naive patients with type 2 diabetes mellitus. Clin Ther. 2003;25:1074–95. doi: 10.1016/s0149-2918(03)80068-1. [DOI] [PubMed] [Google Scholar]
- Lincoff AM, Wolski K, Nicholls SJ, et al. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA. 2007;298:1180–8. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- Mudaliar S, Chang AR, Henry RR. Thiazolidinediones, peripheral edema, and type 2 diabetes: incidence, pathophysiology, and clinical implications. Endocr Pract. 2003;9:406–16. doi: 10.4158/EP.9.5.406. [DOI] [PubMed] [Google Scholar]
- Niemeyer NV, Janney LM. Thiazolidinedione-induced edema. Pharmacotherapy. 2002;22:924–9. doi: 10.1592/phco.22.11.924.33626. [DOI] [PubMed] [Google Scholar]
- Perkovich BT, Meyers SM. Systemic factors affecting diabetic macular edema. Am J Ophthalmol. 1988;105:211–2. doi: 10.1016/0002-9394(88)90190-0. [DOI] [PubMed] [Google Scholar]
- Ryan EH, Jr, Han DP, Ramsay RC, et al. Diabetic macular edema associated with glitazone use. Retina. 2006;26:562–70. doi: 10.1097/00006982-200605000-00011. [DOI] [PubMed] [Google Scholar]
- Tokuyama T, Ikeda T, Sato K. Effects of haemodialysis on diabetic macular leakage. Br J Ophthalmol. 2000;84:1397–400. doi: 10.1136/bjo.84.12.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]