Abstract
The effect of recombinant human interleukin-1a on the survival rate of Std-ddY male mice systemically infected with Pseudomonas aeruginosa 12 or Klebsiella pneumoniae P-5709 was evaluated. In P. aeruginosa infection, interleukin-1a given intramuscularly twice, 3 days and 1 day before inoculation of bacteria, most effectively protected animals from death due to infection. The effect was dose dependent, with a maximum survival rate of 92.5% at 10 micrograms per mouse, while only 8.3% of the control group survived until the end of the observation period. The 50% effective dose of interleukin-1a was 0.261 microgram per mouse. In K. pneumoniae infection, interleukin-1a given intramuscularly twice, simultaneously with and 1 day after the inoculation of bacteria, was most effective. The protective effect of interleukin-1a was again dose dependent and was generally more marked than in P. aeruginosa infection. The 50% effective dose was 0.034 microgram per mouse. In both infections, there was no significant increase in the survival rates of animals injected with human albumin or heat-inactivated interleukin-1a. These observations raise the possibility that human interleukin-1a could serve as a therapeutic tool for patients with bacterial infections.
Full text
PDF
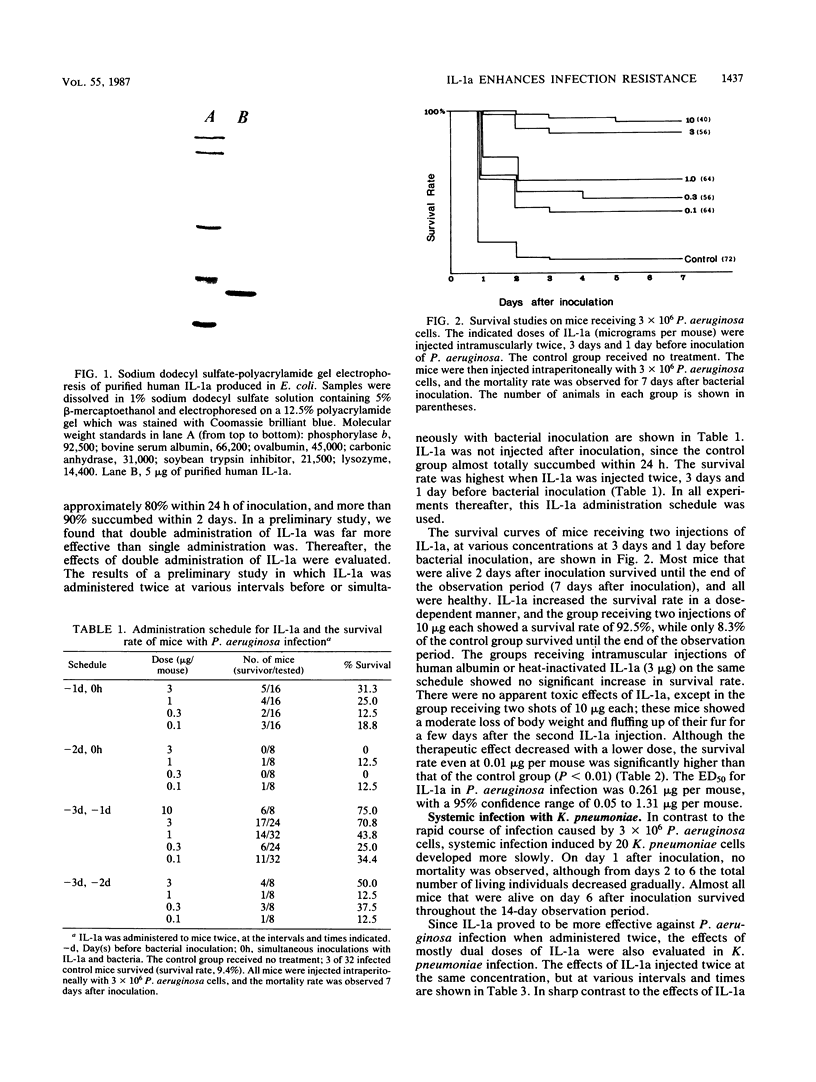
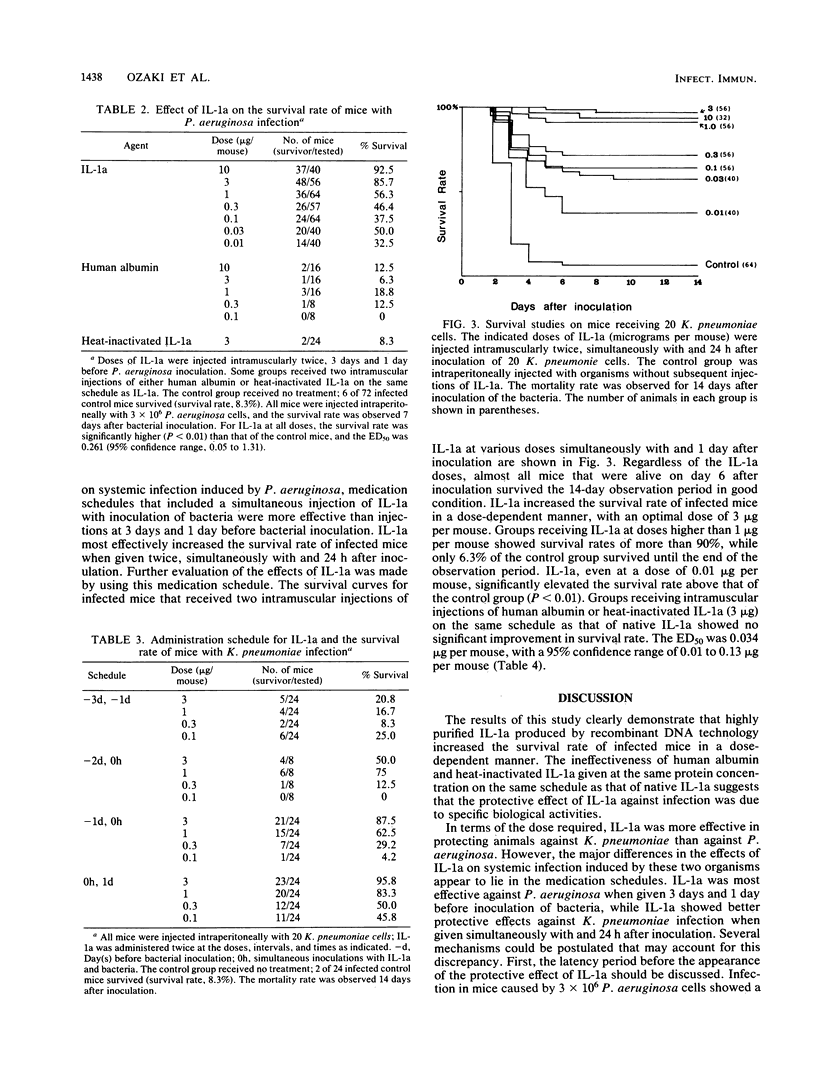


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dinarello C. A., Wolff S. M. Molecular basis of fever in humans. Am J Med. 1982 May;72(5):799–819. doi: 10.1016/0002-9343(82)90548-4. [DOI] [PubMed] [Google Scholar]
- Furutani Y., Notake M., Yamayoshi M., Yamagishi J., Nomura H., Ohue M., Furuta R., Fukui T., Yamada M., Nakamura S. Cloning and characterization of the cDNAs for human and rabbit interleukin-1 precursor. Nucleic Acids Res. 1985 Aug 26;13(16):5869–5882. doi: 10.1093/nar/13.16.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson D. F., Murphy P. A. Demonstration of interleukin 1 activity in apparently homogeneous specimens of the pI 5 form of rabbit endogenous pyrogen. Infect Immun. 1984 Aug;45(2):483–490. doi: 10.1128/iai.45.2.483-490.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampschmidt R. F. Infection, inflammation, and interleukin 1 (IL-1). Lymphokine Res. 1983;2(3):97–102. [PubMed] [Google Scholar]
- Kampschmidt R. F., Pulliam L. A. Stimulation of antimicrobial activity in the rat with leukocytic endogenous mediator. J Reticuloendothel Soc. 1975 Mar;17(3):162–169. [PubMed] [Google Scholar]
- Keenan R. A., Moldawer L. L., Yang R. D., Kawamura I., Blackburn G. L., Bistrian B. R. An altered response by peripheral leukocytes to synthesize or release leukocyte endogenous mediator in critically ill, protein-malnourished patients. J Lab Clin Med. 1982 Dec;100(6):844–857. [PubMed] [Google Scholar]
- Klempner M. S., Dinarello C. A., Gallin J. I. Human leukocytic pyrogen induces release of specific granule contents from human neutrophils. J Clin Invest. 1978 May;61(5):1330–1336. doi: 10.1172/JCI109050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky P. E., Thompson P. A., Rosenwasser L. J., Dinarello C. A. The role of interleukin 1 in human B cell activation: inhibition of B cell proliferation and the generation of immunoglobulin-secreting cells by an antibody against human leukocytic pyrogen. J Immunol. 1983 Jun;130(6):2708–2714. [PubMed] [Google Scholar]
- Luger T. A., Charon J. A., Colot M., Micksche M., Oppenheim J. J. Chemotactic properties of partially purified human epidermal cell-derived thymocyte-activating factor (ETAF) for polymorphonuclear and mononuclear cells. J Immunol. 1983 Aug;131(2):816–820. [PubMed] [Google Scholar]
- Murphy P. A., Simon P. L., Willoughby W. F. Endogenous pyrogens made by rabbit peritoneal exudate cells are identical with lymphocyte-activating factors made by rabbit alveolar macrophages. J Immunol. 1980 May;124(5):2498–2501. [PubMed] [Google Scholar]
- Ozaki Y., Ohashi T., Niwa Y. Oxygen radical production by neutrophils from patients with bacterial infection and rheumatoid arthritis. Measurement of hydrogen peroxide may most accurately represent enhancement of oxygen radical production during infection. Inflammation. 1986 Jun;10(2):119–130. doi: 10.1007/BF00915994. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Baltz M. L. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol. 1983;34:141–212. doi: 10.1016/s0065-2776(08)60379-x. [DOI] [PubMed] [Google Scholar]
- Ramadori G., Sipe J. D., Dinarello C. A., Mizel S. B., Colten H. R. Pretranslational modulation of acute phase hepatic protein synthesis by murine recombinant interleukin 1 (IL-1) and purified human IL-1. J Exp Med. 1985 Sep 1;162(3):930–942. doi: 10.1084/jem.162.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]




