Abstract
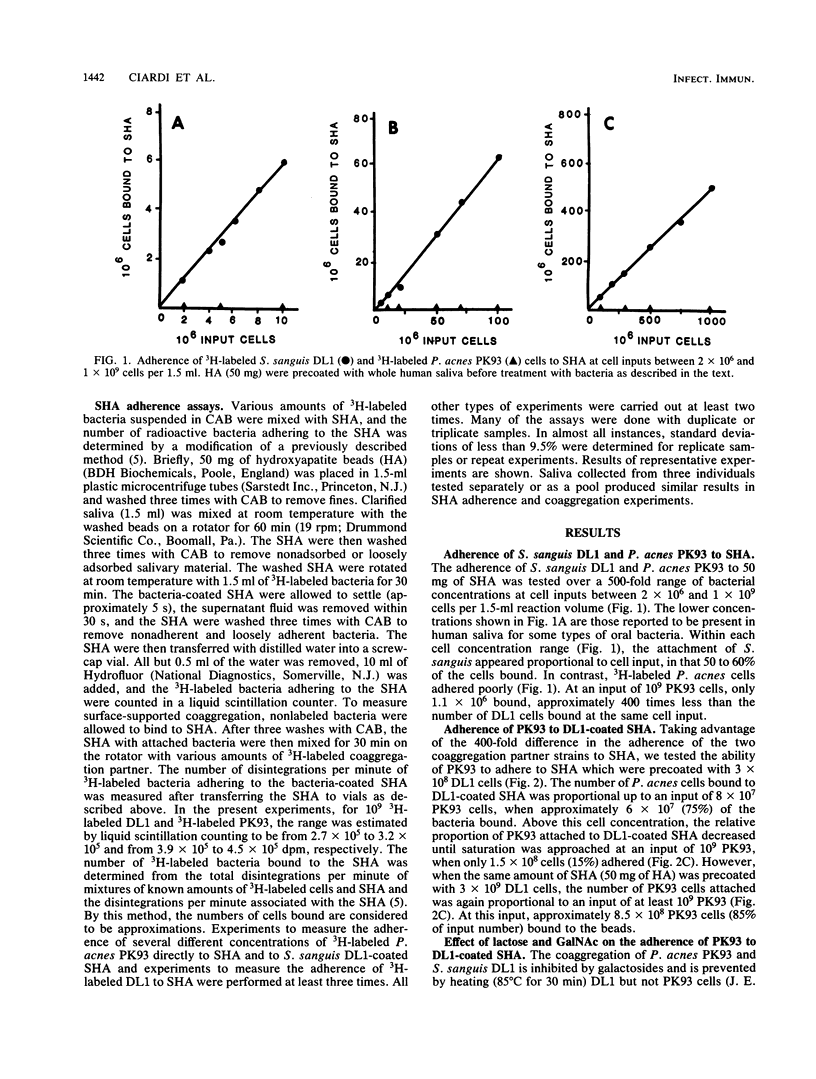
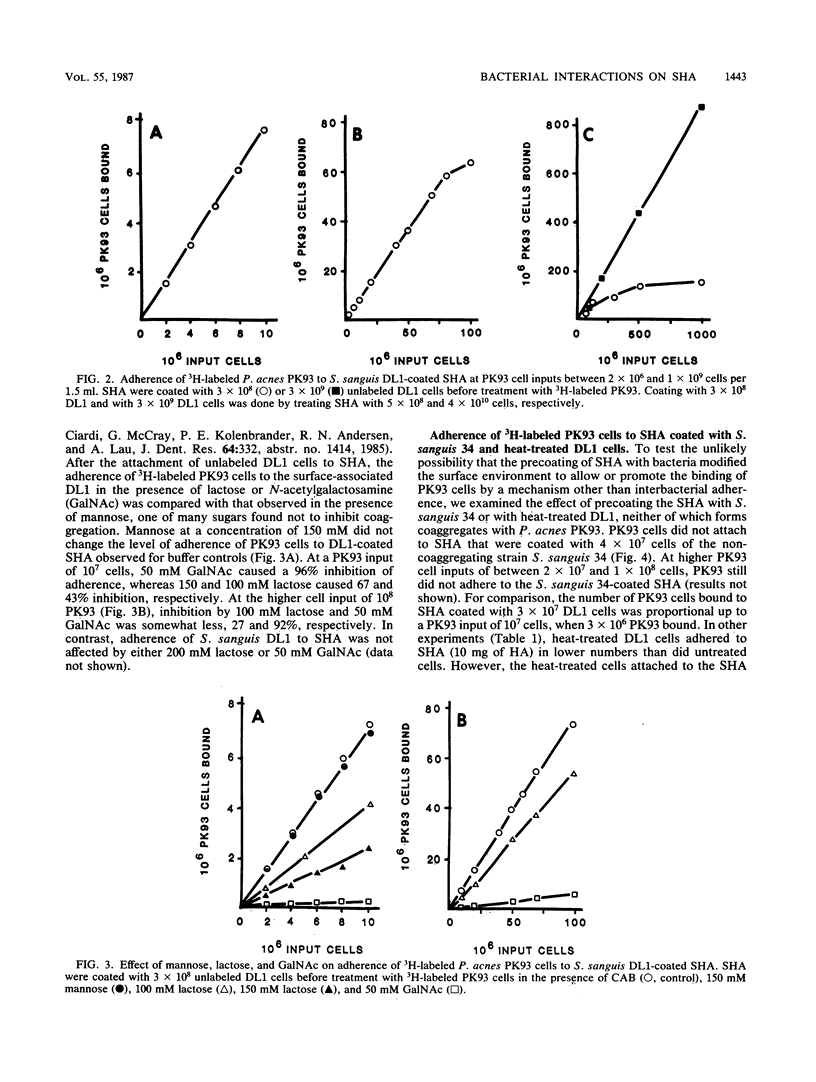
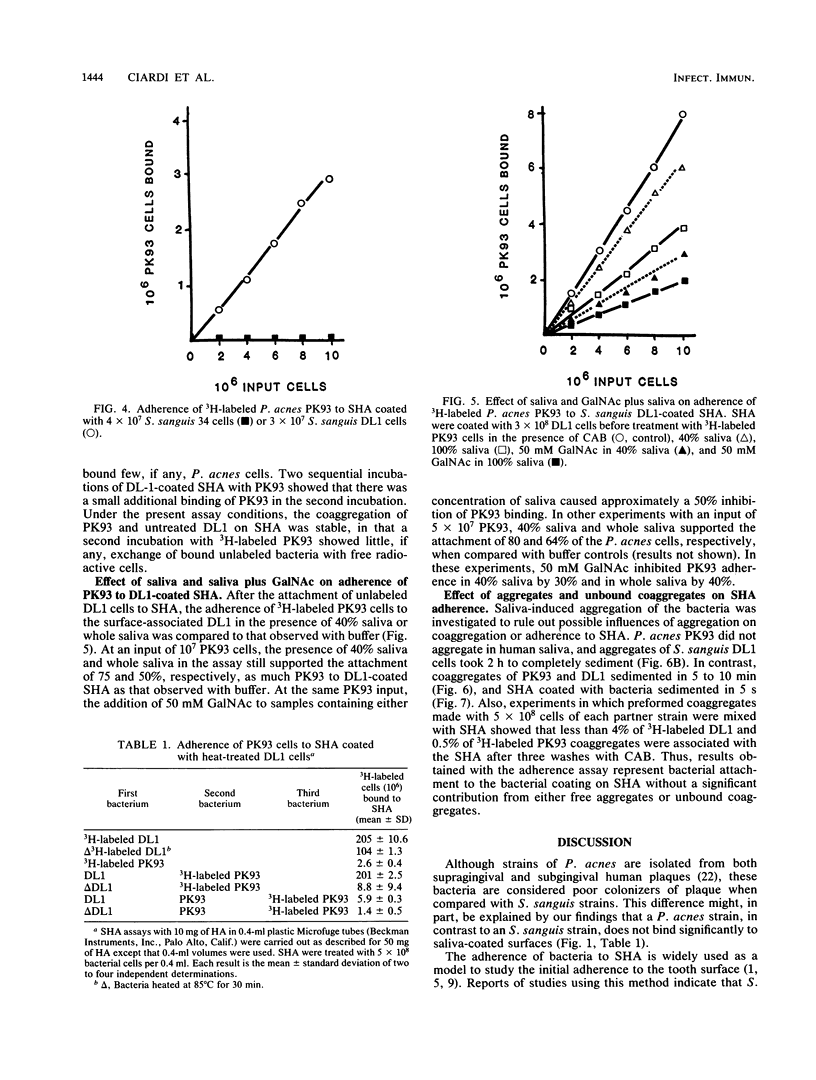
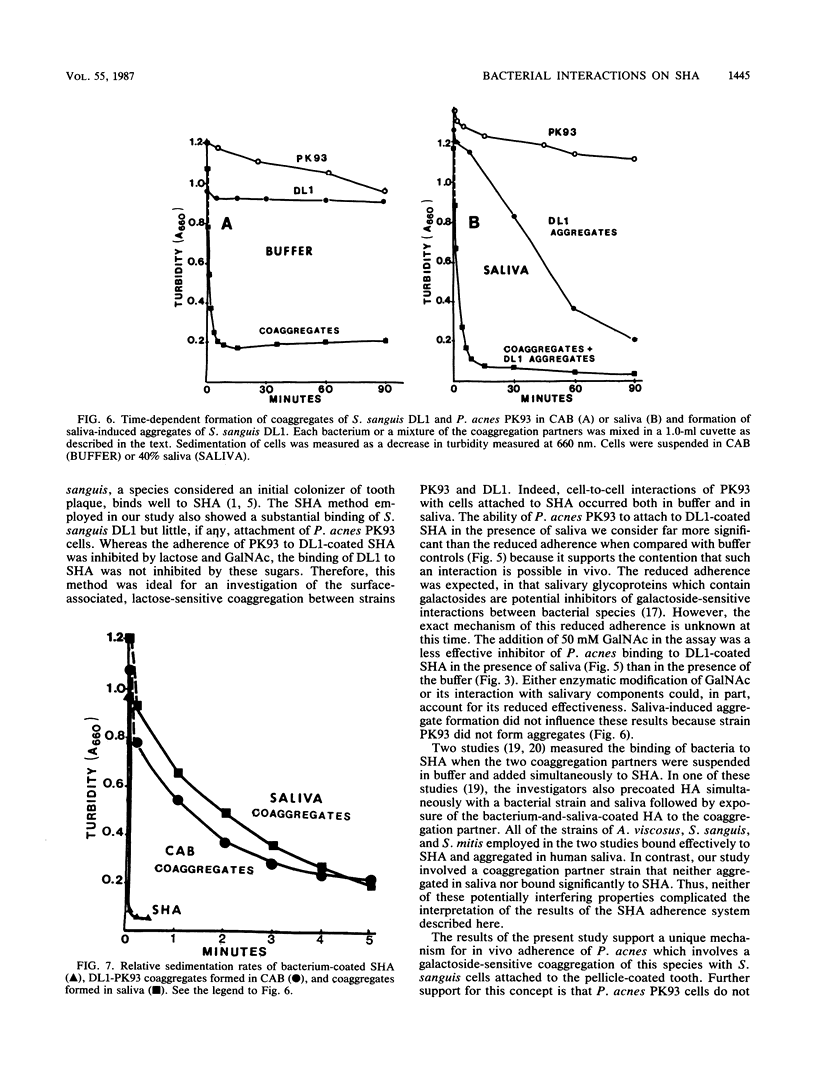
Cell-to-cell interaction (coaggregation) between Propionibacterium acnes PK93 and Streptococcus sanguis DL1 was measured on saliva-coated hydroxyapatite beads (SHA) at bacterial concentrations between 1.3 X 10(6) and 6.7 X 10(8) cells per ml. Four hundredfold more DL1 than PK93 cells adhered to the saliva-coated beads, and the adherence of S. sanguis was proportional to cell input. SHA precoated with 3 X 10(8) DL1 cells bound 75 to 80% of available PK93 cells at all input amounts tested, up to an input of 8 X 10(7) cells. Adherence of PK93 to DL1-coated SHA approached saturation at an input of approximately 10(9) PK93 cells, when 1.5 X 10(8) bound. The coaggregation on SHA occurred either in buffer or saliva and was inhibited by N-acetylgalactosamine and by lactose; the attachment of DL1 to SHA was not inhibited by these sugars. S. sanguis 34 and heat-treated DL1 cells, neither of which form coaggregates with PK93, attached to SHA, but such cells did not bind PK93 cells. The findings of this study indicate that bacteria unable to attach to saliva-coated hydroxyapatite can indeed adhere to such a surface by strong lectin-mediated cell-to-cell interactions with bacteria already attached to the surface.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum B., Golub E., Holt S. C., Rosan B. In vitro studies of dental plaque formation: adsorption of oral streptococci to hydroxyaptite. Infect Immun. 1979 Aug;25(2):717–728. doi: 10.1128/iai.25.2.717-728.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladen H., Hageage G., Pollock F., Harr R. Plaque formation in vitro on wires by gram-negative oral microorganisms (Veillonella). Arch Oral Biol. 1970 Feb;15(2):127–133. doi: 10.1016/0003-9969(70)90048-8. [DOI] [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Balcerzak-Raczkowski I. B. Interbacterial aggregation of Actinomyces naeslundii and dental plaque streptococci. J Periodontal Res. 1977 Jan;12(1):11–20. doi: 10.1111/j.1600-0765.1977.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Interbacterial aggregation of plaque bacteria. Arch Oral Biol. 1970 Dec;15(12):1397–1400. doi: 10.1016/0003-9969(70)90031-2. [DOI] [PubMed] [Google Scholar]
- Kelstrup J., Funder-Nielsen T. D. Aggregation of oral streptococci with Fusobacterium and Actinomyces. J Biol Buccale. 1974 Dec;2(4):347–362. [PubMed] [Google Scholar]
- Kolenbrander P. E., Andersen R. N. Cell to cell interactions of Capnocytophaga and Bacteroides species with other oral bacteria and their potential role in development of plaque. J Periodontal Res. 1984 Nov;19(6):564–569. doi: 10.1111/j.1600-0765.1984.tb01315.x. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E., Inouye Y., Holdeman L. V. New Actinomyces and Streptococcus coaggregation groups among human oral isolates from the same site. Infect Immun. 1983 Aug;41(2):501–506. doi: 10.1128/iai.41.2.501-506.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Phucas C. S. Effect of saliva on coaggregation of oral Actinomyces and Streptococcus species. Infect Immun. 1984 May;44(2):228–233. doi: 10.1128/iai.44.2.228-233.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Williams B. L. Lactose-reversible coaggregation between oral actinomycetes and Streptococcus sanguis. Infect Immun. 1981 Jul;33(1):95–102. doi: 10.1128/iai.33.1.95-102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Williams B. L. Prevalence of viridans streptococci exhibiting lactose-inhibitable coaggregation with oral actinomycetes. Infect Immun. 1983 Aug;41(2):449–452. doi: 10.1128/iai.41.2.449-452.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama K., Gibbons R. J. Inhibition of lactose-reversible adherence between Actinomyces viscosus and oral streptococci by salivary components. Caries Res. 1984;18(3):193–200. doi: 10.1159/000260765. [DOI] [PubMed] [Google Scholar]
- Komiyama K., Gibbons R. J. Interbacterial adherence between Actinomyces viscosus and strains of Streptococcus pyogenes, Streptococcus agalactiae, and Pseudomonas aeruginosa. Infect Immun. 1984 Apr;44(1):86–90. doi: 10.1128/iai.44.1.86-90.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K., Paul A. Role of bacterial interactions in the colonization of oral surfaces of Actinomyces viscosus. Infect Immun. 1980 Jul;29(1):83–90. doi: 10.1128/iai.29.1.83-90.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Bloomquist C. G., Germaine G. R. Effect of bacterial aggregation on the adherence of oral streptococci to hydroxyapatite. Infect Immun. 1981 Mar;31(3):935–941. doi: 10.1128/iai.31.3.935-941.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Smibert R. M., Hash D. E., Burmeister J. A., Ranney R. R. Bacteriology of severe periodontitis in young adult humans. Infect Immun. 1982 Dec;38(3):1137–1148. doi: 10.1128/iai.38.3.1137-1148.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]



