1) Introduction
Physiological processes including sleep are regulated in part by humoral substances. Cytokines as signaling molecules are involved in the regulation of many of such processes. Two cytokines, interleukin-1 beta (IL1) and tumor necrosis factor alpha (TNF) are well characterized for their roles in sleep regulation and are the focus of this review. The study of the humoral regulation of sleep began almost 100 years ago with the publication of Ishimori’s [1] and Legendre and Pieron’s [2] work showing that the transfer of cerebrospinal fluid from sleep deprived dogs into normal dogs enhanced sleep in the recipients. In the past century, many have replicated these findings in similar experiments [e.g. 3 and reviewed 4]. Many substances have now been implicated in sleep regulation. These sleep regulatory substances (SRSs) range from low molecular weight substances with short half-lives, e.g. adenosine, nitric oxide (NO), to longer lived peptides such as growth hormone releasing hormone and orexin and proteins including the cytokines.
Many experimental approaches have been used to discover and characterize SRSs [reviewed 4,5,6]. All of these approaches, including the newer methods such as genome wide searches and use of mutant animals, are limited because sleep can not be isolated as an independent variable. All physiological parameters, e.g. body temperature, hormonal levels, respiration rate, urinary output, brain metabolism etc. change with sleep. As a consequence, it is not possible to know, for example, if change in expression of a particular molecule that correlates with sleep or sleep loss, does so in fact as a direct consequence of sleep or of some other variable. Sleep researchers have thus developed lists of criteria that candidate SRSs need to meet before they can be reasonably proposed as being involved in sleep regulation [6,7,8,9,] (List 1). To date, only a few substances have met all these criteria; IL1 and TNF are among them. By way of example, TNF is the only substance for which there is a literature demonstrating that its plasma levels in humans in health and disease correlate with sleep propensity [reviewed 4].
Our knowledge of SRSs has led to unexpected developments in our understanding of sleep mechanisms and brain organization of sleep. In fact, our view of what exactly it is that sleeps has shifted from whole organisms to neural networks such as cortical columns (also called neuronal assemblies or neuronal groups). Further, the fact that all SRSs identified to date play a role in neural plasticity has focused ideas dealing with sleep function on that process. The role that cytokines have played in these developments is discussed herein.
List 1: Criteria for sleep regulatory substances
The SRS should enhance a sleep phenotype, e.g. duration of NREMS or EEG delta wave power.
Inhibition of the SRS should reduce spontaneous sleep.
SRS levels in brain should correlate with sleep propensity.
SRSs should act on sleep regulatory circuits
SRSs levels during pathology should correlate with sleepiness.
Derived from Jouvet [7], Inoue [6], Borbely [8] and Krueger and Obal [9].
2) TNF and IL1 meet all the criteria for SRSs
Systemic or central injection of either TNF or IL1 enhances duration of NREMS and EEG delta wave power during NREMS in every species thus far tested including, rats, mice, rabbits, humans, monkeys, cats and sheep (Criterion 1, List 1) [10, 11, reviewed 4]. After intracerebroventricular (icv) injections of either IL1 or TNF, increases in NREMS manifest within the first hour and depending upon dose, last up to 8-12 hours. The effects on NREMS can be large, e.g. after 600 femtomoles icv IL1 rabbits had about 2 hours of extra NREMS during the first 12 post-injection hours. The effects on REMS are route of administration-, time of day-, and dose-dependent. For instance, low somnogenic doses usually do not alter duration of REMS although high somnogenic doses inhibit REMS. High doses of either IL1 or TNF inhibit sleep; the sleep responses after these high doses resemble the sleep that occurs during severe infectious disease, e.g. sleep episode duration is shortened.
Inhibition of either IL1 or TNF using several different approaches reduces spontaneous NREMS (Criterion 2, List 1). For example, the IL1 receptor antagonist (an endogenous gene product), IL1 and TNF soluble receptors (also endogenous substances), and anti-IL1 or anti-TNF antibodies inhibit NREMS if given to experimental animals. In humans the TNF soluble receptor is a normal constituent of cerebrospinal fluid and inhibits sleep [12] and fatigue [13]. These inhibitors also inhibit the NREMS rebound that normally occurs after sleep deprivation [reviewed 4]. Those findings provide very strong data for the hypothesis that TNF and IL1 play key roles in sleep homeostasis (see Section 6 and Figure 1). Substances that inhibit the production, release or actions of IL1 or TNF also inhibit duration of NREMS [reviewed 4]. For example, glucocorticoids, interleukins 4, 10 and 13, and corticotrophin releasing hormone all inhibit IL1 and TNF and reduce spontaneous NREMS. Another approach to inhibit SRSs is to remove one or more of the genes in its signaling pathway. Knockout mice that lack either the IL1 type I receptor [14] or the TNF 55 kD receptor [15] have less spontaneous sleep than control strains of mice. The results from those studies suggest some independence of the somnogenic actions of IL1 and TNF although these cytokines induce each other in brain in vivo [16]. Thus, the NREMS deficits in the TNF receptor knockout mice occur mostly during the first hours of daylight while the NREMS deficits in the IL1 type I receptor knockout mice occur mostly during the nighttime. Further, the TNF receptor knockout mice exhibit NREMS responses if given IL1 and the IL1 receptor knockout mice do likewise if given TNF.
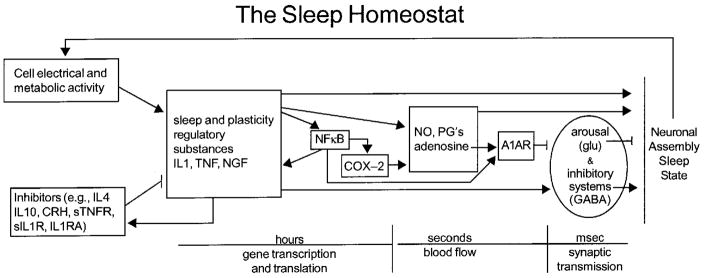
Figure 1. Molecular networks comprise the sleep homeostat.
Sleep regulatory substances (SRSs), including TNF and IL1 and nerve growth factor (NGF), are produced and released in response to neuronal activity and metabolism during wakefulness (see text). SRSs levels are influenced by positive and negative feedback including transcription factors such as NFκB, and cytokines and hormones. Cytokine production and actions involve transcription and translation events and occur over periods of hours or more. As such they offer a mechanism by which the CNS can track sleep/wake history. Their direct somnogenic actions involve labile substances with half lives of seconds such as NO and adenosine. Cytokines and their effector molecules in turn influence excitatory and inhibitor neurotransmitter systems to orchestrate sleep. The sleep homeostat possesses many of the characteristics of biological networks and engineered systems [reviewed 78,79]. It is modular in that several proteins are working in “overlapping co-regulated groups”. The molecular network is robust in that removal of any one of the components does not result in complete sleep loss. The network operates as a recurring circuit element in the sense that multiple molecular networks work in parallel, e.g. each within a different semiautonomous cortical column. Abbreviations, see text and IL-1 RA, IL1 receptor antagonist; sIL1R, soluble IL1 receptor; anti-IL1, anti-IL1 antibodies; CRH, corticotrophin releasing hormone; PGD2, prostaglandin D2; sTNFR, soluble TNF receptor; A1AR, adenosine A1 receptor; COX-2, cyclooxygenase 2; glu, glutamine acid; GABA, gamma amino buteric acid.
Brain levels of either IL1 or TNF or their respective mRNAs vary with sleep propensity (Criterion 3, List 1). Thus for example, IL1 cerebrospinal fluid levels in cats vary with the sleep/wake cycle [17]. Spontaneous brain levels of IL1, TNF, IL1 mRNA and TNF mRNA vary with sleep propensity in rats with highest levels occurring at the onset of daylight hours [reviewed 4]. Rat hypothalamic (a NREMS regulatory network) levels of both IL1 [18] and TNF [19] are highest at the time when spontaneous NREMS duration is greatest. Cerebral cortical levels of IL1 and TNF also vary with the time of day and affect EEG delta power locally (see Section 7). If sleep propensity is enhanced by sleep deprivation, both IL1 mRNA and TNF mRNA levels increase in brain [reviewed 4]. Further, if rats are fed a cafeteria diet their NREMS is enhanced as are their hypothalamic IL1 mRNA levels [20]. Finally, during infectious disease states when sleep in enhanced, brain levels of IL1 and TNF mRNAs are enhanced, e.g. during influenza virus infections in mice [21].
If either IL1 or TNF is microinjected into sleep regulatory circuits, NREMS is enhanced (Criterion 4, List 1). Thus, microinjection of TNF into the anterior hypothalamus is associated with dose-dependent increases in NREMS [22]. In contrast, injection of the TNF soluble receptor into the anterior hypothalamus inhibits spontaneous NREMS. Similarly, an extensive study of IL1-responsive sites indicated that sites near the ventricles and subarachnoid sites near the hypothalamus are associated with enhanced NREMS [23]. Microinjection of either IL1 or TNF into the locus coeruleus [24] or IL1 into the dorsal raphe [25] enhances NREMS. Further, IL1 receptive hypothalamic neurons also are receptive to growth hormone releasing hormone, another well-characterized SRS [reviewed 4], and those neurons are GABAergic. Sleep active hypothalamic neurons firing rates are enhanced by IL1 while wake-active hypothalamic neurons are inhibited [26]. Collectively these data indicate that IL1 and TNF act on sleep regulatory circuits to enhance NREMS. However, both cytokines also have the capacity to act directly on the cerebral cortex to enhance sleep intensity regionally and that suggests that these substances can act throughout the neuraxis to alter state within neuronal assemblies. This view of brain organization of sleep is discussed in Section 7.
Many pathologies with associated changes in sleep propensity also alter cytokines (Criterion 5, List 1). Already mentioned are the changes in hypothalamic cytokines associated with influenza virus in mice. Human studies have greatly enriched the literature relating circulating cytokines to pathology-associated sleepiness. TNF plasma levels are elevated in multiple diseases associated with enhanced sleepiness including patients with AIDS, chronic fatigue, insomnia, myocardial infarct, excessive daytime sleepiness, post-dialysis fatigue, pre-eclampsia, alcoholism, and sleep apnea [reviewed 4]. The TNF polymorphic variant, G-308A, is linked to metabolic syndrome [27] and sleep apnea [28]. Systemic endotoxin, a Gram-negative bacterial cell wall product, enhances sleep and plasma TNF levels in humans [29]. Blood levels of IL1 in humans may also vary with sleep propensity but this literature is not as clear as that for TNF. IL1 plasma levels peak at the onset of sleep [30] and are enhanced during sleep deprivation [31, 32]. Circulating levels of either TNF or IL1 affect sleep via the vagus nerve since vagotomy blocks intraperitoneal TNF [33] or IL1 [34] enhanced NREMS. Systemic injections of either IL1 or TNF enhance brain levels of IL1 and TNF mRNAs [16]. Vagotomy also blocks the intraperitoneal IL1-enhanced hypothalamic IL1 mRNA [35]. Collectively it seems that the sleep disturbances associated with pathology are mediated in part via IL1 and TNF [reviewed 36].
3) Downstream mechanisms of IL1 and TNF enhanced sleep
IL1 and TNF affect many other molecules that in turn affect sleep. Nuclear factor kappa B (NFκB) and c-Fos (AP-1) are transcription factors that are activated by IL1 and TNF [reviewed 4, 36]. These transcription factors promote production of IL1 and TNF and many other substances implicated in sleep regulation including many other cytokines(see Section 5), the adenosine A1 receptor (A1AR) (Figure 1), cyclooxygenase-2, and the GHRH receptor. NFκB is activated within the hypothalamus and cortex by sleep deprivation [37,38]. Adenosine also elicits NFκB nuclear translocation in basal forebrain slices via the A1AR [39]. An inhibitor of NFκB inhibits NREMS [40]. IL1 and TNF also affect many small molecules with short half lives that are involved in sleep regulation including NO, adenosine and prostaglandins [e.g. 41 and reviewed 4]. For example, inhibition of NO synthase blocks IL1-induced NREMS responses [42]. Cytokines also interact with multiple neurotransmitters involved in sleep regulation including GABA, norepinephrin, serotonin and acetylcholine [reviewed 4]. The exact somnogenic biochemical pathways affected by cytokines likely depend upon circumstances such as time-of-day, waking activity, pathology, etc, although it seems clear that known SRSs work in concert with each other to affect sleep. An exciting new development, discussed in Section 7, is that these biochemical events alter state within cortical columns.
4) Upstream mechanisms
Within brain, a major stimulus for IL1 and TNF production and release is neuronal activity. This seems to occur by two separate mechanisms. First, ATP is co-released with neurotransmitters [reviewed 43]. ATP in turn induces IL1 [44] and TNF [45] release from glia via P2X receptors [reviewed 46]. A second mechanism involves afferent activity-enhanced IL1 and TNF expression. Thus, within rat somatosensory cortical columns (also called barrels) receiving facial whisker afferent projections, if their corresponding whisker is stimulated repeatedly, TNF immunoreactivity within the barrel receiving the excess afferent input is enhanced while in adjacent barrels the enhanced immunoreactivity is not observed [47]. How these two pools of neural activity-dependent cytokines interact to affect sleep remains unstudied. It is worthwhile to emphasize that ATP levels are affected by metabolism and neural activity and in turn affect extracellular levels of adenosine and cytokines thereby providing direct links between neural activity, metabolism and sleep regulation.
5) Other cytokines in sleep regulation
The regulation of the brain cytokine network is not understood. Nevertheless, a variety of cytokines and cytokine-associated substances have been shown to alter sleep. Several of these such as the IL1 and TNF soluble receptors were mentioned above. Cytokine-associated substances such as the IL1 receptor antagonist and several anti-somnogenic substances, such as IL4, IL10, IL13, transforming growth factor beta, inhibit spontaneous NREMS. In contrast, other cytokines such as IL6, IL18, acidic fibroblast growth factor, interferon gamma, nerve growth factor, brain-derived neurotrophic factor, glia-derived neurotrophic factor and others, promote NREMS [reviewed 4]. There are some cytokines that apparently do not affect sleep, at least under the conditions tested; they include interferon beta and basic fibroblast growth factor. The cytokine network is characterized by redundancy, positive feedback loops, self-stimulation and many other complexities; most of it remains to be studied within the context of sleep.
6) Cytokines and sleep homeostasis
The brain has the ability to keep track of its sleep/wake history, often over periods of days or more. Prolonged bouts of wakefulness are followed by sleep rebound, sometimes over multiple subsequent sleep periods. Sleep rebound is characterized by increased time spent in sleep and increased sleep intensity as defined by amplitudes of EEG delta waves. Sleep homeostasis is a defining characteristic of sleep and its mechanisms likely involve the production and release of SRSs including IL1 and TNF (Figure 1). Thus, injection of exogenous IL1 or TNF induces a NREMS that resembles sleep after sleep loss in that its duration and intensity is greater. Further, if either IL1 or TNF is inhibited during sleep loss, the expected subsequent sleep rebound is greatly attenuated [reviewed 4]. These latter findings coupled with the evidence presented in Section 2 strongly implicate IL1 and TNF in sleep homeostasis.
7) Brain organization of sleep; cytokine involvement in cortical column state
Sleep researchers have yet to reach consensus as to exactly what it is that’s sleeps. This problem has the potential to confuse discussions of sleep regulation. For instance, traditionally sleep was considered a whole animal phenomenon; either the subject was asleep or awake. However, it is now clear that some marine mammals exhibit unihemispheric sleep. Further, some characteristics of sleep such as EEG delta wave activity, metabolism and blood flow manifest regionally depending upon prior waking activity in those regions. In addition, a fundamental meta finding within sleep research is that regardless of where a lesion in brain may occur, if the subjects survive, they sleep. This strongly indicates that sleep is an intrinsic property of any viable neuronal network and, contrary to the prevailing sleep regulatory paradigm, that sleep regulatory circuits do not impose sleep on the brain [reviewed 48].
These considerations led us to propose that sleep is a fundamental property of neural networks [49]. It is possible that individual cells may sleep but if one entertains this hypothesis definitional problems are confronted; e.g. is a silent neuron, or a bursting neuron, asleep? Most likely not because such characteristics can be found in a variety of conditions not associated with sleep. There also seems to be little chance of causally connecting activity of a single neuron to state beyond correlation of firing rates. The positing of a brain organization level at which sleep emerges allows falsifiable hypotheses to be made at the appropriate level of organization. By way of analogy, to study the heat capacity, osmotic properties, vapor pressure, or taste of water one does not study H or O; these emergent properties are the result of combining H and O and are fundamentally not predictable from our current knowledge of H or O. To relate our hypothesis to our past work with cytokines we framed it within a biochemical mechanistic causal proposal (List 2). There is now considerable evidence for the hypothesis and it is discussed in this section.
List 2: Sleep Mechanisms
There is activity-dependent production of sleep regulatory substances (SRSs)
Activity-dependent SRSs act locally on nearby neurons to change their electrical/receptive properties and thereby alter the input-output relationships of the networks within which they are found.
Altered input-output relationships within neuronal assemblies reflect functional state changes of the assemblies.
Synchrony of state between semi-autonomous neural assemblies occurs because they are loosely connected via neural projections and humoral substances.
Sleep regulatory circuits coordinate neuronal assembly functional state changes into organism sleep.
There is cell activity-dependent expression of cytokines in brain (Step 1; List 2). This is well known for cytokines such as NGF and BDNF [reviewed 50] but is less studied for IL1 and TNF. Conditions such as kindling, sleep deprivation, or extracellular glutamate enhance brain IL1 or TNF suggesting that excessive activity or excitatory stimuli are responsible [51–54]. Extracellular ATP, co-released with neurotransmitters, induces IL1 and TNF release from glia as mentioned in Section 4. Preliminary data from our laboratory indicate that within cerebral cortical neurons or glia TNF is enhanced if afferent neuronal activity into the specific column is enhanced [47]. Collectively, such data strongly suggest that cytokine expression in neurons/glia is activity-dependent. The activity-dependent cytokines act on neurons to change their electrical and responsive properties (Step 2, List 2). For some cytokines such as NGF and BDNF this is well known. For IL1 and TNF it is also studied but within the context of the fever literature [reviewed 55]. For instance, IL1 or TNF alter hypothalamic neuron sensitivity to temperature. From another literature, we know that TNF up-regulates while IL1 down-regulates AMPA receptor expression in neurons (see Section 8) and that changed populations of AMPA receptors will alter neuronal response patterns. From yet another literature we know that IL1 receptors on hypothalamic neurons co-localize with growth hormone releasing hormone receptors on GABAergic cells [56]. IL1 enhances presynaptic release of GABA in hypothalamic cells [57]. We also know that IL1 enhances hypothalamic sleep-active neurons while inhibiting wake-active neurons [26]. There is thus ample evidence indicating that cytokines act on neurons to change their electrical properties.
These cytokine-induced altered neuronal properties affect sleep phenotype. Thus if either IL1 [58] or TNF [59] is applied to the cortex unilaterally there is a dose- and state-dependent increase in EEG delta power on the side receiving the cytokine. The increases occur during NREMS but not during REMS or waking and are confined to the ½–4 Hz frequency band. Further, if TNF expression is unilaterally inhibited using a small-interfering RNA within the cortex, there is a reduction of EEG delta power unilaterally [60]. Finally, if rats are deprived of sleep and pretreated with a TNF soluble receptor or an IL1 soluble receptor the enhanced EEG delta wave power that occurs during subsequent NREMS is attenuated [58,59]. Collectively, these data suggest that TNF and IL1 are produced in response to activity and act locally on networks to change input-output properties resulting in a regionally more intense sleep or if inhibited a regionally less intense NREMS.
There is now direct evidence that neuronal assemblies oscillate between two or more functional states and one of these states is induced by TNF and is sleep-like in character (Step 3, List 2). If cortical columns are probed with afferent stimulation and subsequent amplitudes of evoked potentials are measured, different functional states can be determined [61]. One of those states correlates with whole animal sleep and the probability of entering that state is dependent upon prior afferent input to the column and past state status. Excessive afferent input to a cortical column increases the likelihood that the column will enter the sleep-like state. Similarly, the longer the column is in a wake-like state, the higher the probability that later it will enter the sleep-like state. These properties of cortical column sleep-like states are also properties of whole animal sleep. Further, cortical column state affects behavior. If rats are trained to lick in response to stimulation of a whisker, the error rate is higher if the stimulated whisker’s cortical column is in the sleep-like state than if it is in the wake-like state [62]. Finally, localized injection of TNF onto cortical columns induces the sleep-like state in the affected columns [63]. Collectively, these data suggest that sleep is a fundamental property of neuronal assemblies.
During organism sleep and wake most of the columns are in their respective sleep- and wake-like states suggesting synchrony of state between columns (Step 4, List 2). Columns are topographically organized and in general the closer a column is to another the more tightly are the two linked by neural and humoral connections. Because they are linked, it is likely from a theoretical view that they will functionally synchronize with each other [64].
Cortical columns are also connected to subcortical sleep regulatory circuits (Step 5, List 2). In fact, unilateral injection of either TNF or IL1 onto the cerebral cortex activates reticular thalamic neurons as determined by fos expression [65,66]. Further, prefrontal cortical neurons, ventral lateral preoptic neurons and medial preoptic neurons are also activated by IL1 [66]. These data suggest that the status of cortical column state could be relayed to these NREMS regulatory networks. It is also possible that these regulatory networks are thus involved in coordinating whole animal sleep using cortical column state status information. Thus in this view sleep is; a) dependent on prior cellular activity, b) initiated at the cortical column level, c) a self-organized state being coordinated between columns and being a statistical property of the number of columns is the sleep-like state, and d) it is refined and timed into whole animal sleep by sleep regulatory networks. Each of these is a falsifiable hypothesis.
8) A neuro-connectivity function for sleep; cytokine involvement
Sleep as a subject of neurobiology is unusual because its function has not been experimentally defined. Its importance is illustrated by the facts that during sleep one does not reproduce, eat, drink or socialize and one is subject to predation. These are high evolutionary costs to overcome by what ever the beneficial effects of sleep are. So what could be so important to the brain to allow such a disadvantaged state to persist? There are many theories of sleep function positing that the answer is neural connectivity [reviewed 48,49]. In this review, we focus on just two, our own [48,49] and that of Kavanau’s [67] because the logic of the two is similar and both are derived in part from the earlier proposal of Roffwarg [68]. The central idea of both theories was the recognition that use-dependent-driven changes in synaptic efficacy and connectivity would lead to dysfunction unless there were processes to stabilize synaptic networks that are constantly being modified by activity. This process is now termed synaptic scaling. Synaptic scaling serves to regulate Hebbian plasticity; thus an increase in network activity causes a slow compensatory decrease in excitatory synaptic efficacy whereas, a decrease in network activity enhances excitatory synaptic strength [69]. The stabilization mechanism proposed by us was SRS-induced changes in local electrical properties as described in Section 7. The mechanism proposed by Kavanau was intrinsic spontaneous electrical activity. These mechanisms are not mutually exclusive and both are scaling mechanisms. More recent sleep-connectivity theories have also invoked synaptic scaling, e.g. [70].
Of importance to this review is that TNF is involved in synaptic scaling. Thus TNF promotes AMPA receptor expression and enhances cytosolic Ca++ levels [71]. This TNF action is physiological because an inhibitor of TNF inhibits AMPA-induced postsynaptic potentials [72] and AMPA-induced changes in cytosolic Ca++ [71]. A TNF siRNA applied to the cortex inhibits gluR1 mRNA levels [60]; gluR1 is a subunit of the AMPA receptor. AMPA receptors are involved in EEG synchronization [73] and synaptic plasticity [74]. More recently direct evidence for the involvement of TNF in synaptic scaling was described [75]. Finally, IL1 may also affect AMPA receptor expression [76]. AMPA receptors in layer V are involved in downscaling during NREMS [77]. Collectively these data suggest a cytokine-dependent mechanism for the reconfiguration of synaptic weights during NREMS. If confirmed and expanded by further studies we will have an experimentally verified function for sleep.
9) Conclusion
IL1 and TNF are well characterized SRSs. They form part of the sleep homeostat. Their release is enhanced by neuronal activity via ATP and in turn, IL1 and TNF activate nuclear factor kappa B, adenosine, and NO downstream mechanisms. The sleep homeostat is thus closely linked to cerebral metabolism and blood flow. Our knowledge of cytokine sleep mechanisms has led to a view of brain organization of sleep positing that sleep is a local property of neural networks being initiated, for example, within cortical columns. Cortical columns oscillate between functional states; the sleep-like state of cortical columns is promoted by TNF. Further, because TNF is involved in glutamanergic AMPA receptor expression and in synaptic scaling mechanisms, cytokine sleep mechanisms provide additional support for the hypothesis that sleep serves a synaptic-connectivity function.
Acknowledgments
This work was supported by the National Institutes of Health grant numbers NS27250, NS25378, NS31453, HD36520, MH71830 and a Sleep Research Society Chris J. Gilin Young Investigators award.
References
- 1.Ishimori K. True cause of sleep – A hypnogenic substance as evidenced in the brain of sleep-deprived animals. Tokyo Igakkai Zasshi. 1909;23:429. [Google Scholar]
- 2.Legendre R, Piéron H. Recherches sur le besoin de sommeil consécutif á une veille prlongée. Z Allg Physiol. 1913;14:235–262. [Google Scholar]
- 3.Miller TB, Goodrich CA, Pappenheimer JR. Sleep-promoting effects of cerebrospinal fluid from sleep-deprived goats. Proc Natl Acad Sci USA. 1967;58:513–517. doi: 10.1073/pnas.58.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obal F, Jr, Krueger JM. Biochemical regulation of sleep. Front Biosci. 2003;8:520–550. doi: 10.2741/1033. [DOI] [PubMed] [Google Scholar]
- 5.Borbély AA, Tobler I. Endogenous sleep-promoting substances and sleep regulation. Physiol Rev. 1989;69:605–670. doi: 10.1152/physrev.1989.69.2.605. [DOI] [PubMed] [Google Scholar]
- 6.Inoué S. Biology of sleep substances. Boca Raton, Florida: CRC Press, Inc.; 1989. [Google Scholar]
- 7.Jouvet M. Neuromediateurs et facteurs hypnogenes. Rev Neurol (Paris) 1984;140:389–400. [PubMed] [Google Scholar]
- 8.Borbely AA, Tobler I. The search for an endogenous “sleep substance. Trends Pharmacol Sci. 1980;1:356–358. [Google Scholar]
- 9.Krueger JM, Obál F., Jr . Sleep Factors. In: Saunders NA, Sullivan CE, editors. Sleep and Breathing. New York: Marcel Dekker, Inc; 1994. pp. 79–112. [Google Scholar]
- 10.Krueger JM, Walter J, Dinarello CA, Wolff SM, Chedid L. Sleep-promoting effects of endogenous pyrogen (interleukin-1) Am J Physiol. 1984;246:R994–R999. doi: 10.1152/ajpregu.1984.246.6.R994. [DOI] [PubMed] [Google Scholar]
- 11.Shoham S, Davenne D, Cady AB, Dinarello CA, Krueger JM. Recombinant tumor necrosis factor and interleukin 1 enhance slow-wave sleep. Am J Physiol. 1987;253:R142–R149. doi: 10.1152/ajpregu.1987.253.1.R142. [DOI] [PubMed] [Google Scholar]
- 12.Vgontzas AN, Zoumakis E, Lin HM, Bixler EO, Trakada G, Chrousos GP. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-a antagonist. J Clin Endocrinol Metab. 2004;89:4409–4413. doi: 10.1210/jc.2003-031929. [DOI] [PubMed] [Google Scholar]
- 13.Franklin CM. Clinical experience with soluble TNF p75 receptor in rheumatoid arthritis. Semin Arthritis Rheum. 1999;29:171–181. doi: 10.1016/s0049-0172(99)80028-6. [DOI] [PubMed] [Google Scholar]
- 14.Fang J, Wang Y, Krueger JM. The effects of interleukin-1 beta on sleep are mediated by the type I receptor. Am J Physiol. 1998;274:R655–R660. doi: 10.1152/ajpregu.1998.274.3.R655. [DOI] [PubMed] [Google Scholar]
- 15.Fang J, Wang Y, Krueger JM. Mice lacking the TNF 55 kD receptor fail to sleep more after TNF alpha treatment. J Neurosci. 1997;17:5949–5955. doi: 10.1523/JNEUROSCI.17-15-05949.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Churchill L, Taishi P, Wang M, et al. Brain distribution of cytokine mRNA induced by systemic administration of interleukin-1 beta or tumor necrosis factor alpha. Brain Res. 2006;1120(1):64–73. doi: 10.1016/j.brainres.2006.08.083. [DOI] [PubMed] [Google Scholar]
- 17.Lue FA, Bail M, Jephthah-Ocholo J, et al. Sleep and cerebrospinal fluid interleukin-1 like activity in the cat. Int J Neurosci. 1988;42:179–183. doi: 10.3109/00207458808991595. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen KT, Deak T, Owens SM, et al. Exposure to acute stress induces brain interleukin-1 beta protein in the rat. J Neurosci. 1998;18:2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Floyd RA, Krueger JM. Diurnal variations of TNF alpha in the rat brain. Neuroreport. 1997;8:915–918. doi: 10.1097/00001756-199703030-00020. [DOI] [PubMed] [Google Scholar]
- 20.Hansen MK, Taishi P, Chen Z, et al. Cafeteria-feeding induces interleukin-1 beta mRNA expression in rat liver and brain. Am J Physiol. 1998;43:R1734–R1739. doi: 10.1152/ajpregu.1998.274.6.R1734. [DOI] [PubMed] [Google Scholar]
- 21.Alt JA, Bohnet S, Taishi P, et al. Influenza virus-induced glucocorticoid and hypothalamic and lung cytokine mRNA responses in dwarf lit/lit mice. Brain Behav Immun. 2005 doi: 10.1016/j.bbi.2005.05.002. PMID 15951155. [DOI] [PubMed] [Google Scholar]
- 22.Kubota T, Li N, Guan Z, et al. Intrapreoptic microinjection of TNF-alpha enhances non-REMS in rats. Brain Res. 2002;932:37–44. doi: 10.1016/s0006-8993(02)02262-x. [DOI] [PubMed] [Google Scholar]
- 23.Terao A, Matsumura H, Saito M. Interleukin-1 induces slow-wave sleep at the prostaglandin D2-sensitive sleep-promoting zone in the rat brain. J Neurosci. 1998;18:6599–6607. doi: 10.1523/JNEUROSCI.18-16-06599.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nistico G, De Sarro G, Rotiroti D. Behavioral and electrocortical spectrum power changes of interleukins and tumor necrosis factor after microinjection into different areas of the brain. In: Smirne S, Francesch M, Ferini-Stambi L, Zucconi M, editors. Sleep, hormones, and immunological system. Milan: Mason; 1992. pp. 11–22. [Google Scholar]
- 25.De Sarro G, Gareri P, Sinopoli VA, et al. Comparative, behavioural and electrocortical effects of tumor necrosis factor-alpha and interleukin-1 microinjected into the locus coeruleus of rat. Life Sci. 1997;60:555–564. doi: 10.1016/s0024-3205(96)00692-3. [DOI] [PubMed] [Google Scholar]
- 26.Alam MN, McGinty D, Bashir T, et al. Interleukin-1 beta modulates state-dependent discharge activity of preoptic area and basal forebrain neurons: Role in sleep regulation. Eur J Neurosci. 2004;20:207–216. doi: 10.1111/j.1460-9568.2004.03469.x. [DOI] [PubMed] [Google Scholar]
- 27.Sookoian SC, Gonzalez C, Pirola CJ. Meta-analysis on the G-308A tumor necrosis factor alpha gene variant and phenotypes associated with the metabolic syndrome. Obes Res. 2005;13:2122–2131. doi: 10.1038/oby.2005.263. [DOI] [PubMed] [Google Scholar]
- 28.Riha RL, Brander P, Vennelle M, et al. Tumour necrosis factor-alpha (-308) gene polymorphism in obstructive sleep apnoea-hypopnoea syndrome. Eur Respir J. 2005;26:673–678. doi: 10.1183/09031936.05.00130804. [DOI] [PubMed] [Google Scholar]
- 29.Mullington J, Korth C, Hermann DM, et al. Dose-dependent effects of endotoxin on human sleep. Am J Physiol. 2000;278:R947–R955. doi: 10.1152/ajpregu.2000.278.4.R947. [DOI] [PubMed] [Google Scholar]
- 30.Moldofsky H, Lue FA, Eisen J, et al. The relationship of interleukin-I and immune functions to sleep in humans. Psychosom Med. 1986;48:309–318. doi: 10.1097/00006842-198605000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Hohagen F, Timmer J, Weyerbrock A, et al. Cytokine production during sleep and wakefulness and its relationship to cortisol in healthy humans. Neuropsychobiology. 1993;28:9–16. doi: 10.1159/000118993. [DOI] [PubMed] [Google Scholar]
- 32.Uthgenannt D, Schoolmann D, Pietrowsky R, et al. Effects of sleep on the production of cytokines in humans. Psychosom Med. 1995;57:97–104. doi: 10.1097/00006842-199503000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Kubota T, Fang J, Guan Z, et al. Vagotomy attenuates tumor necrosis factor-alpha-induced sleep and EEG delta-activity in rats. Am J Physiol. 2001;280:R1213–R1220. doi: 10.1152/ajpregu.2001.280.4.R1213. [DOI] [PubMed] [Google Scholar]
- 34.Hansen MK, Krueger JM. Subdiaphragmatic vagotomy blocks the sleep and fever-promoting effects of interleukin-1 beta. Am J Physiol. 1997;273:R1246–R1253. doi: 10.1152/ajpregu.1997.273.4.R1246. [DOI] [PubMed] [Google Scholar]
- 35.Hansen MK, Taishi P, Chen Z, et al. Vagotomy blocks the induction of interleukin-1 beta mRNA in the brain of rats in response to systemic interleukin-1 beta. J Neurosci. 1998;18:2247–2253. doi: 10.1523/JNEUROSCI.18-06-02247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Majde JA, Krueger JM. Links between the innate immune system and sleep. J Allergy Clin Immunol. 2005;116:1188–1198. doi: 10.1016/j.jaci.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Brandt JA, Churchill L, Rehman A, et al. Sleep-deprivation increases activation of nuclear factor kappa B in lateral hypothalamic cells. Brain Res. 2004;1004:91–97. doi: 10.1016/j.brainres.2003.11.079. [DOI] [PubMed] [Google Scholar]
- 38.Chen Z, Gardi J, Kushikata T, et al. Nuclear factor kappa B-like activity increases in murine cerebral cortex after sleep deprivation. Am J Physiol. 1999;276:R1812–R1818. doi: 10.1152/ajpregu.1999.276.6.R1812. [DOI] [PubMed] [Google Scholar]
- 39.Basheer R, Rainnie DG, Porkka-Heiskanen T, et al. Adenosine, prolonged wakefulness, and A1-activated NF-κB DNA binding in the basal forebrain of the rat. Neuroscience. 2001;104:731–739. doi: 10.1016/s0306-4522(01)00111-7. [DOI] [PubMed] [Google Scholar]
- 40.Kubota T, Kushikata T, Fang J, et al. Nuclear factor kappa B (NFκB) inhibitor peptide inhibits spontaneous and interleukin-1β-induced sleep. Am J Physiol. 2000;279:R404–R413. doi: 10.1152/ajpregu.2000.279.2.R404. [DOI] [PubMed] [Google Scholar]
- 41.Luk WP, Zhang Y, White TD, et al. Adenosine: a mediator of interleukin-1 beta-induced hippocampal synaptic inhibition. J Neurosci. 1999;19:4238–4244. doi: 10.1523/JNEUROSCI.19-11-04238.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kapás L, Shibata M, Kimura M, et al. Inhibition of nitric oxide synthesis suppresses sleep in rabbits. Am J Physiol. 1994;266:R151–R157. doi: 10.1152/ajpregu.1994.266.1.R151. [DOI] [PubMed] [Google Scholar]
- 43.Farber K, Kettenmann H. Purinergic signaling and microglia. Pflugers Arch – Eur J Physiol. 2006;452:615–621. doi: 10.1007/s00424-006-0064-7. [DOI] [PubMed] [Google Scholar]
- 44.Bianco F, Pravettoni E, Colombo A, et al. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol. 2005;174(11):7268–7277. doi: 10.4049/jimmunol.174.11.7268. [DOI] [PubMed] [Google Scholar]
- 45.Hide I, Tanaka M, Inoue A, et al. Extracellular ATP triggers tumor necrosis factor-alpha release from rat microglia. J Neurochem. 2000;75(3):965–972. doi: 10.1046/j.1471-4159.2000.0750965.x. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki T, Hide I, Ido K, et al. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci. 2004;24(1):1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fix C, Churchill L, Hall S, et al. The number of tumor necrosis factor a-immunoreactive cells increases in layer IV of the barrel field in response to whisker deflection in rats. Sleep. 2006;29:A11. [Google Scholar]
- 48.Krueger JM, Obal F., Jr Sleep function. Frontiers in Biosci. 2003;8:511–519. doi: 10.2741/1031. [DOI] [PubMed] [Google Scholar]
- 49.Krueger JM, Obal F., Jr A neuronal group theory of sleep function. J Sleep Res. 1993;2:63–69. doi: 10.1111/j.1365-2869.1993.tb00064.x. [DOI] [PubMed] [Google Scholar]
- 50.Schinder AF, Poo M-M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000;23:639–645. doi: 10.1016/s0166-2236(00)01672-6. [DOI] [PubMed] [Google Scholar]
- 51.De Bock F, Dornand J, Rondouin G. Release of TNF alpha in the rat hippocampus following epileptic seizures and excitotoxic neuronal damage. Neuroreport. 1996;7:1125–1129. doi: 10.1097/00001756-199604260-00004. [DOI] [PubMed] [Google Scholar]
- 52.De A, Krueger JM, Simasko SM. Glutamate induces expression and release of tumor necrosis factor alpha in cultured hypothalamic cells. Brain Res. 2005;1053:54–61. doi: 10.1016/j.brainres.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 53.Schneider H, Pitossi F, Balschun D, et al. A neuromodulatory role of interleukin-1 beta in the hippocampus. Proc Natl Acad Sci USA. 1998;95:7778–7783. doi: 10.1073/pnas.95.13.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yi PL, Tsai CH, Lin JG, et al. Kindling stimuli delivered at different times in the sleep-wake cycle. Sleep. 2004;27:203–212. doi: 10.1093/sleep/27.2.203. [DOI] [PubMed] [Google Scholar]
- 55.Shibata M. Hypothalamic neuronal responses to cytokines. Yale J Biol Med. 1990;63:147–156. [PMC free article] [PubMed] [Google Scholar]
- 56.De A, Churchill L, Obal F, Jr, et al. GHRH and IL1beta increase cytoplasmic Ca2+ levels in cultured hypothalamic GABAergic neurons. Brain Res. 2002;949:209–212. doi: 10.1016/s0006-8993(02)03157-8. [DOI] [PubMed] [Google Scholar]
- 57.Tabarean IV, Korn H, Bartfai T. Interleukin-1βinduces hyperpolarization and modulates synaptic inhibition in preoptic and anterior hypothalamic neurons. Cell Neurosci. 2006;4:1685–1695. doi: 10.1016/j.neuroscience.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 58.Yasuda T, Yoshida H, Garcia-Garcia F, et al. Interleukin-1βhas a role in cerebral cortical state dependent electroencephalographic slow-wave activity. Sleep. 2005;28:177–184. doi: 10.1093/sleep/28.2.177. [DOI] [PubMed] [Google Scholar]
- 59.Yoshida H, Peterfi Z, Garcia-Garcia F, et al. State-specific asymmetries in EEG slow wave activity induced by local application of TNF alpha. Brain Res. 2004;1009:129–136. doi: 10.1016/j.brainres.2004.02.055. [DOI] [PubMed] [Google Scholar]
- 60.Kay D, Taishi P, Kruger JM. Local application of short interfering RNA targeting tumor necrosis factor α(TNFα) induces state-specific asymmetries in EEG slow wave activity. Sleep. 2006;29:A360. [Google Scholar]
- 61.Rector DM, Topchiy IA, Carter KM, et al. Local functional state differences between rat cortical columns. Brain Res. 2005;1047:45–55. doi: 10.1016/j.brainres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 62.Walker AJ, Topchiy I, Kouptsov K, et al. ERP differences during conditioned lick response in the rat. Sleep. 2005;28:A15. [Google Scholar]
- 63.Churchill L, Rector D, Yasuda K, et al. Tumor necrosis factor a increases surface evoked potentials in the barrel field by whisker deflection during sleep in rats. Sleep. 2006;29:A12–A13. [Google Scholar]
- 64.Strogatz SH, Stewart I. Coupled oscillators and biological synchronization. Sci Am. 1993;269:102–109. doi: 10.1038/scientificamerican1293-102. [DOI] [PubMed] [Google Scholar]
- 65.Churchill L, Yasuda K, Yasuda T, et al. Unilateral cortical application of tumor necrosis factor alpha induces asymmetry in Fos- and interleukin-1 beta-immunoreactive cells within the corticothalamic projection. Brain Res. 2005;1055:15–24. doi: 10.1016/j.brainres.2005.06.052. [DOI] [PubMed] [Google Scholar]
- 66.Yasuda K, Churchill L, Yasuda T, et al. Unilateral cortical application of interleukin-1β (IL1β) induces asymmetry in Fos- and IL1β-immunoreactivity: Implication for sleep regulation. Brain Res. doi: 10.1016/j.brainres.2006.11.051. In Press. [DOI] [PubMed] [Google Scholar]
- 67.Kavanau JL. Sleep and dynamic stabilization of neural circuitry: A review and synthesis. Behav Brain Res. 1994;63:111–126. doi: 10.1016/0166-4328(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 68.Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152:604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- 69.Abbott LF, Nelson SB. Synaptic plasticity: Taming the beast. Nat Neurosci. 2000;3:1178–1183. doi: 10.1038/81453. [DOI] [PubMed] [Google Scholar]
- 70.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 71.De A, Krueger JM, Simasko SM. Tumor necrosis factor alpha increases cytosolic calcium response AMPA and KCl in primary cultures of rat hippocampal neurons. Brain Res. 2003;981:133–142. doi: 10.1016/s0006-8993(03)02997-4. [DOI] [PubMed] [Google Scholar]
- 72.Beattie EC, Stellwagen D, Morishita W, et al. Control of synaptic strength by glial TNF alpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- 73.Bazhenov M, Timofeev I, Steriade M, et al. Model of thalamocortical slow-wave sleep oscillations and transitions to activated states. J Neurosci. 2002;22:8691–8704. doi: 10.1523/JNEUROSCI.22-19-08691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Ann Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 75.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 76.Lai AY, Swayze RD, El-Husseini A, et al. Interleukin-1 beta modulates AMPA receptor expression and phosphorylation in hippocampal neurons. J Neuroimmunol. 2006;175:97–106. doi: 10.1016/j.jneuroim.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 77.Czarnecki A, Birtoli B, Ulrich D. Cellular mechanisms of burst-firing mediated long-term depression in rat neocortical pyramidal cells. J Physiol. 2006 doi: 10.1113/jphysiol.2006.123588. PMID 17082228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alon U. Biological networks: The tinkerer as an engineer. Science. 2003;301:1866–1867. doi: 10.1126/science.1089072. [DOI] [PubMed] [Google Scholar]
- 79.Laughlin SB, Sejnowski TJ. Communication in neuronal networks. Science. 2003;301:1870–1874. doi: 10.1126/science.1089662. [DOI] [PMC free article] [PubMed] [Google Scholar]