Abstract
Whole-cell patch-clamp techniques were used to study the effects of nerve growth factor on voltage-dependent potassium conductance in normal and axotomized identified large cutaneous afferent dorsal root ganglion neurons (48–50 μm diameter) many of which probably give rise to myelinated Aβ fibers. K-currents were isolated by blocking Na- and Ca-currents with appropriate ion replacement and channel blockers. Separation of current components was achieved on the basis of response to variation in conditioning voltage. Cutaneous afferents were labeled by the retrograde marker hydroxy-stilbamide (FluoroGold) which was injected into the skin of the foot. The sciatic nerve was either ligated or crushed with fine forceps five to seven days later. Neurons were dissociated 14–17 days after injury. The cut ends of the sciatic nerves were positioned into polyethylene tubes, which were connected to mini-osmotic pumps filled with either nerve growth factor or sterile saline. Control neurons displayed a prominent sustained K-current and the transient potassium currents “A” and “D”. Nerve ligation, which blocks target reconnection resulted in near 50% reduction of total outward current; isolated sustained K-current and transient A-current were reduced by a comparable amount. Nerve crush, which allows regeneration to peripheral targets and exposure of the regenerating nerve to the distal nerve segment, resulted in a small reduction in sustained K-current but no reduction in transient A-current compared to controls. Levels of transient A-current and sustained K-current were maintained at control levels after nerve growth factor treatment.
These results indicate that the large reduction in transient A-current, and in sustained K-current, observed in cutaneous afferent cell bodies after nerve ligation is prevented by application of nerve growth factor.
Keywords: axotomy, nerve crush, potassium channels, cutaneous afferents, sustained current, transient A-current
Large cutaneous afferent neurons express three distinct classes of potassium current; a dominant sustained (K-current; IK), a fast inactivating (A-current; IA) and a slow inactivating (D-current; ID).11 Following nerve ligation and neuroma formation there is a 50% reduction in K- and A-currents on large cutaneous neurons.9 This reduction in potassium currents has implications for the mechanisms underlying the hyperexcitablilty of these neurons following nerve injury. The rationale is that because of the hyper-polarizing effects of K-current activation, a reduction in these currents could render a neuron more prone to hyper-excitability. Indeed, following nerve injury, dorsal root ganglion (DRG) neurons can become a site of abnormal impulse generation in addition to ectopic impulse activity of the injured axon.5 The better understanding of mechanisms which regulate neuronal K-currents has therapeutic implications, and could aid in the development of strategies to prevent the dysregulation of these channels and to reduce the probability of ectopic impulse generation after nerve injury.
In the present study we examined the effects of nerve growth factor (NGF) applied via osmotic pumps to cut sciatic nerves on the two types of inactivating current and the dominant sustained current in injured large cutaneous afferent DRG neurons. NGF has been demonstrated to modulate Na-currents on these neurons.24 In particular, repriming properties of fast Na-currents and the maintenance of kinetically slow Na-currents3,24 are influenced by NGF which is present in the target zone of these neurons. Our results indicate a selective and large reduction in IK and IA in axotomized cutaneous afferent neurons, which can be restored to control levels after presentation of NGF to a cut nerve. The neurons studied are of a size which suggests that they give rise to myelinated axons, probably including Aβ fibers functionally involved in tactile sensation of the skin. Much work now indicates that abnormal firing properties of Aβ fibers contribute to neuropathic pain either by increased peripheral excitability,5 or by plastic changes in dorsal horn innervation.32 In addition to its modulatory role on Na-currents on cutaneous afferents, these results indicate that NGF has an important role in maintaining K+ channel organization. Part of this work showing that chronic administration of NGF can modulate K-currents in somatic DRG neurons has been presented in abstract form.9
EXPERIMENTAL PROCEDURES
Cell identification and culture techniques
Retrograde labeling with hydroxy-stilbamide (FluoroGold)26 was routinely used to identify cutaneous afferent DRG neurons.16 The FluoroGold (Fluorochrome, Englewood, CO, USA) was mixed in distilled water to give a 2–4% solution, which was then injected into the lateral plantar region which is innervated by the sural nerve. Results are reported from 79 identified adult cutaneous afferent DRG neurons taken from 36 adult female Wistar rats (180–240 g); the animals were exsanguinated under pentobarbital sodium anesthesia (60 mg/kg i.p.). The cutaneous afferent neurons were then identified in vitro by fluorescence on brief exposure of the culture dishes to ultraviolet light. This technique has been shown to reveal cutaneous afferent neurons with distinctly different kinetic and pharmacological properties to those of muscle afferents.22,23 The sciatic nerve was exposed and bilaterally ligated (4–0 silk suture) near the sciatic notch.19
To prevent regeneration to peripheral targets in axotomized neurons, the nerve was sectioned immediately distal to the ligature site; a 10- to 15-mm section of the distal nerve was removed and the distal stump was retracted. While most of the neurons in the L4, L5 DRG are axotomized by this procedure, as many as 30% of the DRG neurons at L4 and L5 may remain unaffected because their axons leave the nerve above the ligature site.15 Osmotic pumps (Alzet 2002; Palo Alto, CA, USA) containing either, 50 μg NGF (mouse NGF; Upstate Biotech, Lake Placid, NY, USA) or, as controls for the neurotrophin treatment, saline. The osmotic pumps were connected via a poly-ethylene tube catheter to a silicone cuff into which the sciatic nerve stump was sutured.13,24 Neurons taken from L4 and L5 on the left, unoperated side of the animals were also used as controls in all cultures examined. Controls consisted of two groups: (a) unoperated sciatic nerve (termed Controls); and (b) saline pumps (termed Saline). The contents of the pumps were delivered to the transected nerve stumps continuously for 14 days. In an attempt to confirm the delivery of the pumps’ contents to the target site, three animals had osmotic pumps containing a 4% FluoroGold solution attached to their sciatic nerve stumps and their DRG neurons at L4 and L5 were excised, sectioned and examined for fluorescence. High staining of the DRGs confirmed the delivery to the ganglia.
At two to three weeks post-ligation, the adult Wistar rats (180–240 g) were exsanguinated under sodium pentobarbital anesthesia (60 mg/kg; i.p.) and lumbar ganglia (L4, L5) excised and prepared for dissociation and culture (for review, see Refs 16 and 22). Our analysis was limited to relatively large (48–50 μm in diameter) cutaneous afferents.
All experimental procedures conformed to the NIH Guide for Care and Use of Laboratory Animals and the Yale University Animal Care and Use Committee. Every effort was made to minimize animal suffering and to reduce the number of animals used.
Electrophysiological techniques and analysis
To avoid neurite outgrowth, which could cause variations in expressed types and amounts of current, and to circumvent space clamp problems, the neurons were studied 2–8 h after plating. With our culture conditions, 10 h was the maximum time after plating for injured cells before processes start to develop. In uninjured cells this “window” for experiment without processes is much larger. Short-term culture was essential because the ligated neurons sprout neurites more rapidly than controls in culture.20 Coverslips plated with the DRG neurons were rinsed with normal bath solution (see Table 1, E) and placed in a recording chamber on the stage of an inverted phase-contrast microscope (Nikon, Diaphot), and perfused with solution. Recording electrodes were fabricated from thin-walled, single filamented, borosilicate glass tubing (Warner Instrument Corp.), with a micropipette puller (Model P-97, Sutter Instruments), and fire-polished with the use of a Narashige MI 83 microforge, to a resistance of 1–2 MΩ; seal resistances were ≥1 GΩ. All recordings were made at room temperature (21 ± 1°C).
Table 1.
Solutions for voltage-clamp experiments
| Solutions | Intracellular | Extracellular |
|---|---|---|
| I | E | |
| TMA Cl, mM* | 100 | 140 |
| KCl, mM† | 40 | 3 |
| CaCl2, mM† | 0 | 1 |
| MgCl2, mM† | 1 | 1 |
| CdCl2, μM† | 0 | 100 |
| TTX, μM‡ | 0 | 1 |
| HEPES, mM§ | 0 | 10 |
| EGTA, mM†,¶ | 10 | 0 |
| pH | 7.2 | 7.4 |
EGTA and HEPES buffers were prepared with the hydroxide of the main cation; cation concentrations given in the table include contributions from the added hydroxide.
Aldrich Chemical, Milwaukee, WI, USA.
Sigma Chemical, St. Louis, MO, USA.
Calbiochem, La Jolla, CA, USA.
American Bioanalytical, Natick, MA, USA.
RBI Research Biochemicals Incorporated, Natick, MA, USA.
Voltage-clamp recordings were made in the whole-cell patch-clamp configuration14 using an EPC 9 amplifier driven by the “Pulse” program (Heka-Electronik). The neurons were voltage-clamped at −80 mV for all experimental manipulations. Capacity and leakage subtraction were performed using a p/±6 subtraction protocol. Series resistance compensation of between 60 and 75% was routinely employed to reduce any voltage error. Cells in which series resistance compensation was possible but less than 60% were discounted from the study. Cell capacitance was 90 pF ±10 in all groups. The current was sampled at 50 kHz, low-pass filtered (four pole Bessel) at 10 kHz, unless otherwise stated, and initially digitized and stored on computers (Macintosh, Quadra 700 and PowerBook 1400c). Outward potassium currents were elicited by stepping to a conditioning voltage of either −40 mV or −120 mV, from a holding potential of −80 mV, then depolarizing the membrane to −40 mV and on up to +50 mV, in increments of 10 mV (Fig. 1); +50 mV produced the largest peak current in each recording. Activation of the currents was rapid and decay only partial during a 300-ms depolarization pulse. Amplitudes and rates of rise in absolute current increased with increasing depolarization. The compliment of currents that may have been manifest in any of the cells being examined was initially probed using the pulse protocols.
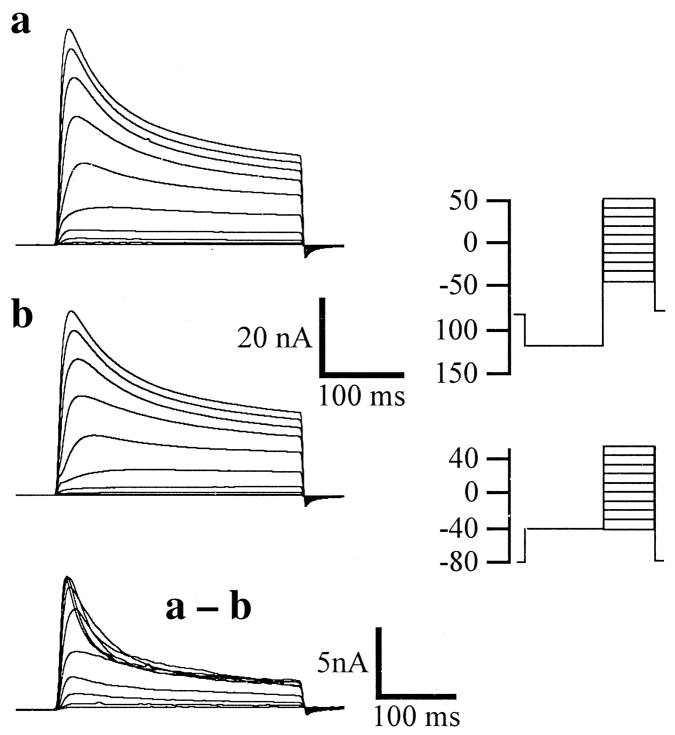
Fig. 1.
Typical recording of a neuron from the control group, i.e. uninjured. The records were obtained by holding the resting potential at −80 mV. Traces in a were recorded following a 500-ms conditioning prepulse potential of −120 mV and those in b using a conditioning prepulse potential of −40 mV. Isolated outward currents were elicited by stepping from −40 mV and on up to +50 mV, in increments of 10 mV (see the pulse protocol representations on the far right of Fig. 1). These pulse protocols were used to obtain the data seen in Figs 1 and 2. Current sensitive to the conditioning voltage was exposed by subtraction of the −40 mV protocol from the −120 mV protocol (as in a – b). Activation of these currents was rapid, and decay only partial, during the 300-ms depolarization pulse. The lower scale bars relate only to the subtraction.
Heka analysis programs (Adams and List), IgorPro (WaveMetrics), and Excel (Microsoft) were used to analyse data. Neurons used in the analysis were those held stable for at least 10 min to facilitate diffusion of the electrode solution into the neuron, thus ensuring more stable and uniform responses. Where necessary, currents were zero-subtracted (d.c. leak current based on the data points corresponding to the first stored segment are subtracted to zero). Exponential fits via the Heka program for Macintosh that uses the root mean square (RMS) deviation between fit and data were applied to estimate inactivation times. The RMS value is used as residual for the simplex fit algorithm (see: Heka Patch Clamp EPC9, Pulse Fit Manual 8.0, 1997). Statistical analysis was by way of a paired two-sample Student’s t-test for mean. A probability level of <0.05 was set for statistical significance.
Solutions
Na+ ions were replaced with tetramethylammoniumchloride (TMA), a nonpermeant ion; tetrodotoxin (TTX) and CdCl2 were used to block Na- and Ca-currents, thus, allowing selective recording of K-current. The standard bath solution contained (in mM): TMA 140, KCl 3, HEPES 10, CaCl2 1, and MgCl2 1 (see Table 1). TTX 1 μM and CdCl2 100 μM were added to block Na- and Ca-currents, respectively. Osmolarity was adjusted to 305–310 mOsmol using glucose, and pH was adjusted to 7.4. To reduce current amplitude and in an attempt to reduce errors caused by series resistance artifacts, TMA was used as the primary non-permeant monovalent cation. Electrodes were filled with (in mM): TMA 100, KCl 40, EGTA 10, MgCl2 1; osmolarity was adjusted to 300–305 mOsmol with glucose, and the pH was adjusted to 7.4. A preliminary investigation showed no detectable effect on outward currents of either 100 μM (n =6), or 200 μM (n =6), CdCl2.
RESULTS
Potassium currents were recorded from relatively large (48–50 μm diameter) cutaneous afferent neurons. These neurons give rise to myelinated axons relating to skin. However, we were unable to distinguish between high- and low-threshold mechanoreceptors. Small nociceptive neurons were not examined. With symmetrical K+ concentrations (40 mM inside and outside) the I–V curve shifted to the right, and the K+ equilibrium potential was close to the predicted reversal potential of 0 mV.11 This is commensurate with K+ being the principal charge carrier.
Representative waveforms typical of the depolarization-activated K-currents in the control group, i.e. uninjured, are shown in Fig. 1. The neurons were initially held at −80 mV and then stepped to either −120 mV (Fig. 1a), or −40 mV (Fig. 1b), for 500 ms (conditioning prepulse potential). Isolated outward currents were elicited by stepping from the conditioning prepulse potential to −40 mV up to +50 mV in increments of 10 mV (see the pulse protocol representations on the far right of Fig. 1). Current sensitive to the conditioning voltage was exposed by subtraction of the −40 mV protocol from the −120 mV protocol (Fig. 1a–b). Activation of these currents was rapid and decay only partial during the 300-ms step depolarization pulses. In uninjured cells (Fig. 1), fast and slow inactivating currents are observed, the fast-inactivating component being evident, although, as in this example, it can sometimes be contaminated with the slower inactivating component of current (subtraction a – b). However, K-current components can be isolated in these neurons using only conditioning voltage.10,11 Currents which resemble those found here in DRG neurons, “A” “K” and “D”, have been reported by Wu and Barish34 in mouse hippocampal neurons and also in a variety of other species and tissues.1,12,21,28,30 A-current is a fast activating and inactivating potassium current; K-current is a sustained current which is also fast activating but does not decay during the stimulation protocol; D-current activates slower than K-current but then inactivates slowly on reaching a peak. In our experimental paradigm A-current could be totally dissected from the sustained current and K-type elements using two different prepulse voltages (Vc) with identical stimulation pulse protocols (Vp);11 however, this was dependent upon the types of component current expressed in any individual neuron. Thus, Fig. 1a shows composite K-current, Fig. 1b shows currents when transient currents are at least partially inactivated using a different pulse protocol, and the subtraction (a – b) illustrates partially isolated transient (A and D) currents. Previously, we have demonstrated the ability to dissect fast inactivating current, or A-current, from sustained current, or K-current, using solely our voltage protocol. The efficacy of this dissection was confirmed using a pharmacological protocol (6 mM 4-AP) to extinguish the A-current leaving only sustained current (K).11
Axotomy by nerve section and ligation results in a distinct reduction in both K- and A-current.10 Note the reduction in K-currents of a cutaneous afferent neuron after nerve section and attachment to the cut end of the nerve to an osmotic pump containing saline (Fig. 2A) as compared to an unsectioned nerve (Fig. 1). The outward currents elicited by either −120 mV or −40 mV conditioning prepulse were reduced by about half, and the subtraction of these entities was also comparatively reduced. This indicates a reduction in both noninactivating K-current and transient A-current (compare mean peak current of “No Injury” group with “Saline” group, Table 2).
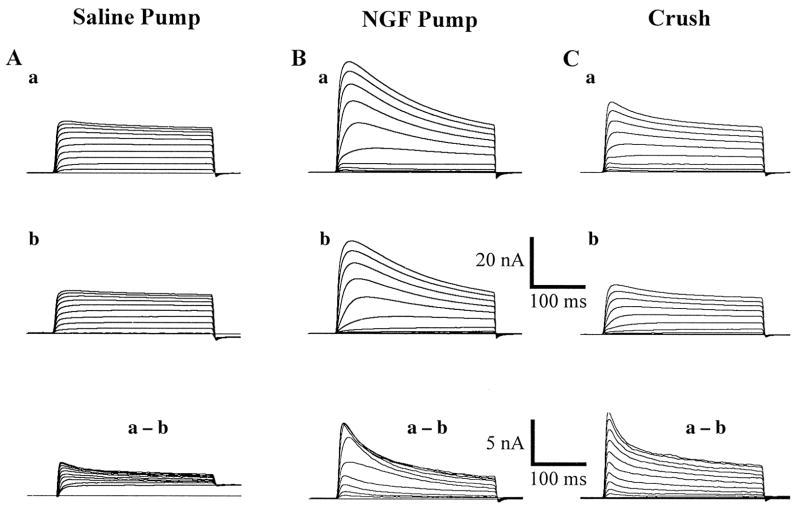
Fig. 2.
Representative waveforms of the depolarization-activated K-currents in crushed, and axotomized, identified cutaneous afferent DRG neurons (48 μm in diameter). Axotomized neurons with both NGF, and saline, pumps. In the NGF treated axotomized neurons (Fig. 2B) peak currents are seen to be much larger in both prepulse conditions compared to axotomized neurons with Ringer pumps (Fig. 2A), and the cells in the crush group (Fig. 2C). Cells closest to the means of all three groups are selected here for demonstration purposes. See also Fig. 3 for a more complete description of the overall population.
Table 2.
Summary of the mean peak currents (nA); with corresponding subtracts, and mean cell capacitance for each group
| Cell size (μ) | Imax −120 mV | Imax −40 mV | Total (max) voltage- sensitive current subtract | Imin −120 mV | Imin −40 mV | Total (min) voltage- sensitive current subtract | Total no. cells in each group | Mean pF cell capacity | |
|---|---|---|---|---|---|---|---|---|---|
| No injury | 50 ±0.19 | 46.69 ±4.78 | 39.52 ±4.09 | 7.17 | 28.41 ±3.03 | 24.09 ± 2.32 | 4.33 | n =13 | 88.07 ± 2.9 |
| Saline pump | 49 ± 0.28 | 21.65 ± 1.4 | 17.04 ± 1.18 | 4.61 | 16.33 ± 1.16 | 13.66 ± 1.14 | 2.67 | n =14 | 87.87 ± 3.12 |
| Crush | 49 ± 0.23 | 30.03 ± 2.42 | 22.61 ± 1.69 | 7.42 | 20.84 ± 1.75 | 17.23 ± 1.29 | 3.61 | n =12 | 91.8 ± 2.61 |
| NGF pump | 49 ± 0.11 | 46.47 ± 5.31 | 36.60 ± 4.04 | 9.87 | 31.01 ± 3.72 | 26.39 ± 3.33 | 4.61 | n =16 | 91.54 ± 3.38 |
Data are shown as mean ± S.E.M.
Imax is the mean peak current value, and Imin is the mean lowest current value, obtained during the stimulation phase of the pulse protocol. The table compares the neurons of the three experimental groups with those of the control/uninjured group. All data were obtained using the two pulse protocols described in Fig. 1.
Figure 2 compares waveforms of the depolarization-activated K-currents in axotomized (A) neurons exposed to either saline or NGF through an osmotic pump (B), and neurons which have had their corresponding sciatic nerve crushed (C). Axotomized neurons, which have been presented with NGF (Fig. 2B), show peak currents which are much larger in both prepulse conditions (−120 mV and −40 mV) compared with axotomized neurons with pumps filled only with a saline solution (Fig. 2A); the cells in the crushed group showed a partial reduction in peak current (Fig. 2C; Table 2). The families of traces in the NGF group (Fig. 2B) closely approximate those of the control group, an example of which can be seen in Fig. 1. Thus, when NGF was applied to the end of the sectioned nerve for 14 days (Fig. 2B) the currents were indistinguishable from control nerves (Table 2). Moreover, following nerve crush when the nerve is axotomized but allowed to re-establish target connection, there is a modest reduction in sustained current, but no significant change in A-current. These results are presented graphically in Fig. 3 and numerically in Table 2.
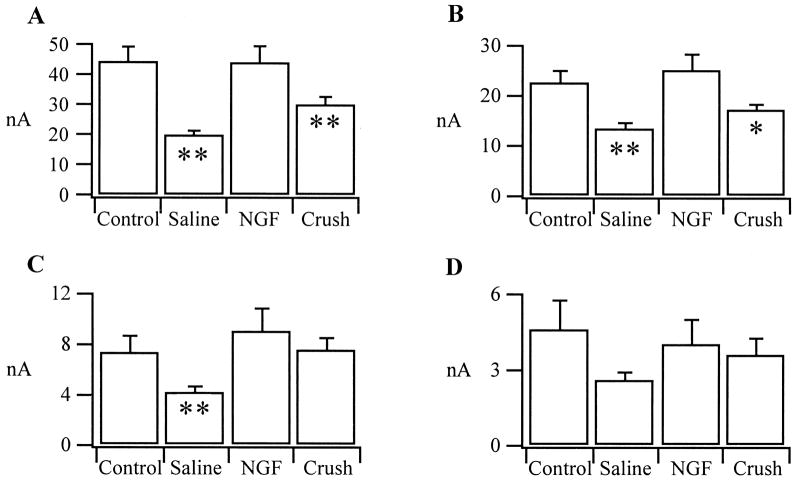
Fig. 3.
Charts showing means with S.E. bars for each group of neurons. (A) Maximum peak current; peak current being composed of all three potassium currents. The mean peak current value for the NGF group of cells after axotomy is almost identical with that of the control/uninjured mean peak current value. Statistically, the NGF group does not differ from controls, whereas, both the saline pump and crush groups are significantly different from the control/uninjured group (**P < 0.02) after axotomy, implying protection by NGF. IK, or maximum mean sustained current (B) in the NGF group is not significantly different from controls. However, as was expected, IK (sustained current) in the saline pump group is significantly reduced (**P < 0.02) after axotomy compared to the control/uninjured group. IK in the crushed neuron group is also significantly different from the control/uninjured group being reduced after axotomy (*P < 0.05). In C only IA (the fast inactivating current) in the saline group is seen to be significantly reduced (**P < 0.02) after axotomy. Mean A-current, or IA, in the NGF and crushed neuron groups approximate the control/uninjured values for this current after axotomy suggesting a recovery of the fast inactivating current to something near normal. D demonstrates no significant difference for the slow inactivating, or D-current, between the controls/uninjured and the three experimental groups after 14 days axotomy.
Maximum mean peak current of the saline pump group (n =14), where neurons were presented with saline through an osmotic pump, is significantly smaller (P < 0.02) than control/uninjured cells, as was the maximum peak current of neurons in the crush group (P < 0.02) (n =12). The maximum peak current is shown in Fig. 3A, peak current being composed of all three potassium currents. In Fig. 3B maximum mean sustained current, or IK, (the mean of all the peaks of the sustained currents obtained through subtraction, then lumped to give a mean value) the NGF group is not significantly different from controls, again suggesting a protective mechanism of NGF. However, IK in the saline pump group is significantly reduced (P < 0.02) after axotomy compared to the control/uninjured group adding weight to our hypothesis that NGF can either restore or protect potassium currents. IK in the crushed neuron group (Fig. 3B) is also significantly different from the control/uninjured group (P < 0.05), being reduced after axotomy.
No difference was observed in levels of A- or D-current between the controls and cells which had either nerve section with NGF treatment, or nerve crush (Fig. 3C, D). In Fig. 3C only IA in the saline pump group is seen to be significantly reduced (P < 0.02) after axotomy. Mean A-current, or IA, in the NGF and crushed neuron groups approximate the control/uninjured values for this current after axotomy suggesting a recovery to near normal levels. ID, or D-current, shows no significant difference between the control/uninjured group and the three experimental groups after 14 days axotomy (Fig. 3D).
DISCUSSION
Potassium currents have an important role in the regulation of neuronal excitability. Computer simulations predict that a reduction in K+ channel density after nerve injury could contribute to sensory neuronal hyperexcitability.6 Application of pharmacological K+ channel antagonists to demyelinated rat sensory axons32,33 and to sites of ectopic afferent discharge facilitates ectopic firing.5,7 Moreover, injection of these agents into nerve end neuromas in humans provokes intense pain.3 Characterization of K-currents in axotomized DRG cutaneous afferents is therefore important for better understanding the mechanisms of neuronal hyperexcitability.
Following nerve ligation there is a large and selective reduction in overall K-current and more specifically in IK and IA in axotomized cutaneous afferent neurons;10 these currents are reduced by about 50%. The neurons showing these changes were of a size suggestive of their giving rise to myelinated Aβ fibers, and being functionally involved in tactile sensation of the skin. The reported large reduction in the K-current could contribute to the injury-induced increase in excitability in these neurons by reducing the ability of the neuron to hyperpolarize. In this regard, one might predict that the action potential of the axotomized DRG neuron might broaden because of the reduction in IA; indeed, Stebbing et al.29 showed DRG action potentials recorded in vivo broadened after nerve section. Because NGF can restore K-current after injury, this broadening may not occur and normal spike waveform might be restored in the cell body.
Recent work demonstrates the importance of NGF in the regulation of certain Na-currents in DRG neurons24 and their subunits,8 but little is known about the role of neurotrophin regulation of K-currents. However, in PC-12 cells regulation of the KV 2.1 channel protein by NGF occurs at both the translational and post-translational levels.27 Recently, in situ hybridization studies on DRG neurons indicate reduced K+ channel mRNA expression after nerve injury.17 The reduction in K-current in axotomized cutaneous afferent neurons and the protective effect of NGF strongly argue for a role of this neurotrophin in the regulation of K-current on cutaneous afferent neurons.
The nerve crush procedure used in this study results in transection of the axons but they regenerate back to the periphery and re-establish functional connections.18 Moreover, the Schwann cells in the distal degenerating nerve segment begin to express both NGF and P75 NGF receptors.31 Following nerve crush we observed a reduction in both Peak current and K-current with no detectable reduction in A-current. This supports our hypothesis that NGF may be important in the maintenance of appropriate K-current levels, given that following nerve crush the regenerating axons have greater access to endogenous NGF than following nerve transection.
Recent studies on axotomized DRG neurons indicate that these injured neurons display an increase in sub-threshold membrane oscillations which can give rise to ectopic impulse discharge.2 These oscillations are a few millivolts in amplitude and a millisecond or two in duration. While they are dependent upon Na-current, it has been suggested that they may be modulated by K-current. Reduction in K-current as reported here after axotomy, could lower the threshold for the membrane oscillations and thus increase ectopic impulse firing.
The prevention of the large reduction in overall K-current of cutaneous afferent neurons by NGF, indicates that the two dominant ionic currents responsible for action potential electrogenesis in these neurons, Na-and K-currents, are both regulated by NGF. Limitation of endogenous NGF to the neuron by axotomy results in reorganization of these currents on the axotomized neuron. After nerve transection kinetically slow Na-current (PN3/SNS1) is reduced,25 the kinetically fast Na-current shows more rapid repriming,4 and overall outward K-current is reduced to about half. The more rapid repriming of the residual Na-current and the reduced K-current could both contribute to enhanced hyperexcitability of the injured neuron as described above. Prevention of these changes by local application of NGF could, therefore, limit abnormal ectopic firing associated with peripheral nerve injury.
Acknowledgments
We thank Ms Hui-Fen Mi for the preparation of neuronal cultures. This work was supported in part by the Medical Research Service of the Department of Veterans Affairs and by the NIH (NS-10174, NS-06208).
Abbreviations
- DRG
dorsal root ganglion
- EGTA
ethylene glycol-bis (β-aminoethyl ether)-N, N, N′, N′-tetraaceticacid
- HEPES
N-2-hydroxy-ethylpiperazine-N′-2-ethanesulfonic acid
- NGF
nerve growth factor
- RMS
root mean square
- TMA
tetramethylammoniumchloride
- TTX
tetrodotoxin
References
- 1.Albert JL, Nerbonne JM. Calcium-independent depolarization-activated potassium currents in superior collicus-projecting rat visual cortical neurons. J Neurophysiol. 1995;73(6):2163–2178. doi: 10.1152/jn.1995.73.6.2163. [DOI] [PubMed] [Google Scholar]
- 2.Amir R, Michaelis M, Devor M. Membrane potential oscillations in dorsal root ganglion neurons: role in normal electrogenesis and neuropathic pain. J Neurosci. 1999;19:8589–8596. doi: 10.1523/JNEUROSCI.19-19-08589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chabal C, Jacobson L, Burchiel K. Pain responses to perineuromal injection of normal saline, gallamine, and lidocaine in humans. Pain. 1989;36:321–325. doi: 10.1016/0304-3959(89)90091-2. [DOI] [PubMed] [Google Scholar]
- 4.Cummins TR, Waxman SG. Downregulation of tetrodotoxin-resistant sodium currents and upregulation of a rapidly repriming tetrodotoxinsensitive sodium current in small spinal sensory neurons after nerve injury. J Neurosci. 1997;17:3503–3514. doi: 10.1523/JNEUROSCI.17-10-03503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devor M. Potassium channels moderate ectopic excitability of nerve end neuromas in rats. Neurosci Lett. 1983;40:181–186. doi: 10.1016/0304-3940(83)90299-9. [DOI] [PubMed] [Google Scholar]
- 6.Devor M. The pathophysiology of damaged peripheral nerve. In: Wall PD, Melzack R, editors. Texbook of Pain. Churchill Livingston; London: 1994. pp. 79–100. [Google Scholar]
- 7.Devor M, Govrin-Lippmann R. Axoplasmic transport block reduces ectopic impulse generation in injured peripheral nerves. Pain. 1983;16:73–86. doi: 10.1016/0304-3959(83)90087-8. [DOI] [PubMed] [Google Scholar]
- 8.Dib-Hajj SD, Black JA, Cummins TR, Kenney AM, Kocsis JD, Waxman SG. Rescue of a-SNS sodium channel expression in small dorsal root ganglion neurons following axotomy by in vivo administration of nerve growth factor. J Neurophysiol. 1998:79. doi: 10.1152/jn.1998.79.5.2668. [DOI] [PubMed] [Google Scholar]
- 9.Everill B, Kocsis JD. Effects of nerve growth factor on potassium conductance after nerve injury in adult cutaneous afferent dorsal root ganglion neurons. Neurosci Soc Abstr. 1998b;24:1332. doi: 10.1016/s0306-4522(00)00263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everill B, Kocsis JD. Reduction in potassium currents in identified cutaneous afferent DRG neurons after axotomy. J Neurophysiol. 1999;82:700–708. doi: 10.1152/jn.1999.82.2.700. [DOI] [PubMed] [Google Scholar]
- 11.Everill B, Rizzo MA, Kocsis JD. Morphologically identified large cutaneous afferent DRG neurons express three different potassium currents in varying proportions. J Neurophysiol. 1998;79:1814–1824. doi: 10.1152/jn.1998.79.4.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ficker E, Heinemann U. Slow and fast transient potassium currents in cultured rat hippocampal cells. J Physiol, Lond. 1992;445:431–455. doi: 10.1113/jphysiol.1992.sp018932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzgerald M, Wall PD, Goedert M, Emerson PC. Nerve growth factor counteracts the neurophysiological and neurochemical effects of chronic sciatic nerve section. Brain Res. 1985;332:131–141. doi: 10.1016/0006-8993(85)90396-8. [DOI] [PubMed] [Google Scholar]
- 14.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 15.Himes BT, Tessler A. Death of some dorsal root ganglion neurons and plasticity of others following sciatic nerve section in adults and neonatal rats. J comp Neurol. 1989;284:215–230. doi: 10.1002/cne.902840206. [DOI] [PubMed] [Google Scholar]
- 16.Honmou O, Utzschneider DA, Rizzo MA, Bowe CM, Waxman SG, Kocsis JD. Delayed depolarization and slow sodium currents in cutaneous afferents. J Neurophysiol. 1994;71:1627–1637. doi: 10.1152/jn.1994.71.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishikawa K, Tanaka M, Black JA, Waxman SG. Changes in expression of voltage-gated potassium channels in dorsal root ganglion neurons following axotomy. Muscle Nerve. 1999;22:502–507. doi: 10.1002/(sici)1097-4598(199904)22:4<502::aid-mus12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 18.Kenney AM, Kocsis JD. Peripheral axotomy induces long-term c-jun amino-terminal kinase-1 activation and activator protein-1 binding activity by c-Jun and junD in adult rat dorsal root ganglia in vivo. J Neurosci. 1998;18(4):1318–1328. doi: 10.1523/JNEUROSCI.18-04-01318.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kocsis JD, Preston RJ, Targ EF. Retrograde impulse activity and horseradish peroxide tracing of nerve fibers entering neuroma studied in vitro. Expl Neurol. 1984;85:400–412. doi: 10.1016/0014-4886(84)90150-x. [DOI] [PubMed] [Google Scholar]
- 20.Lankford KL, Waxman SG, Kocsis JD. Mechanisms of enhancement of neurite regeneration: rat DRG neurite arborization in vitro after a conditioning sciatic nerve lesion in vivo. J comp Neurol. 1998;391:11–29. doi: 10.1002/(sici)1096-9861(19980202)391:1<11::aid-cne2>3.0.co;2-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez-Padron M, Ferrus A. Presynaptic recordings from Drosophilia: correlatin of macroscopic and single-channel K+ currents. J Neurosci. 1997;17:3412–3424. doi: 10.1523/JNEUROSCI.17-10-03412.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oyelese AA, Eng DL, Richerson GB, Kocsis JD. Enhancement of GABAA receptor-mediated conductances induced by nerve injury in a subclass of sensory neurons. J Neurophysiol. 1995;74:673–683. doi: 10.1152/jn.1995.74.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oyelese AA, Kocsis JD. GABAA receptor-mediated conductance and action potential waveform in cutaneous and muscle afferent neurons of the adult rat: differential expression and response to nerve injury. J Neurophysiol. 1996;76:2383–2392. doi: 10.1152/jn.1996.76.4.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oyelese AA, Rizzo MA, Waxman SG, Kocsis JD. Differential effects of NGF and BDNF on axotomy-induced changes in GABAA-receptor-mediated conductance and sodium currents in cutaneous afferent neurons. J Neurophysiol. 1997;78:31–42. doi: 10.1152/jn.1997.78.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rizzo MA, Kocsis JD, Waxman SG. Selective loss of slow enhancement of fast Na+ currents in cutaneous afferent DRG neurons following axotomy. Neurobiol Dis. 1995;2:87–96. doi: 10.1006/nbdi.1995.0009. [DOI] [PubMed] [Google Scholar]
- 26.Schmued LC, Fallon JH. Fluoro-gold: a new fluorescent retrograde axonal tracer with numerous unique properties. Brain Res. 1986;377:147–154. doi: 10.1016/0006-8993(86)91199-6. [DOI] [PubMed] [Google Scholar]
- 27.Sharma N, D’Arcangelo G, Kleinklaus A, Halegoua S, Trimmer JS. Nerve growth factor regulates the abundance and distribution of K+ channels in PC12 cells. J Cell Biol. 1993;123:1835–1843. doi: 10.1083/jcb.123.6.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stansfeld CE, Marsh SJ, Halliwell JV, Brown DA. 4-Aminopyridine and dendrotoxin induce repetitive firing in rat visceral sensory neurons by blocking a slowly inactivating outward current. Neurosci Lett. 1986;64:299–304. doi: 10.1016/0304-3940(86)90345-9. [DOI] [PubMed] [Google Scholar]
- 29.Stebbing M, Eschenfelder S, Habler H, Acosta M, Janig W, McLachlan E. Changes in the action potential in sensory neurones after peripheral axotomy in vivo. NeuroReport. 1999;10:201–206. doi: 10.1097/00001756-199902050-00001. [DOI] [PubMed] [Google Scholar]
- 30.Strong PN. Potassium channel toxins. Pharmac Ther. 1990;46:137–162. doi: 10.1016/0163-7258(90)90040-9. [DOI] [PubMed] [Google Scholar]
- 31.Taniuchi M, Clark HB, Schweitzer JB, Johnson EM. Expression of nerve growth factor receptors by Schwann cells of axotomized peripheral nerves: ultrastructure location, supression by axonal contact and binding properties. J Neurosci. 1988;8:664–681. doi: 10.1523/JNEUROSCI.08-02-00664.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Targ EF, Kocsis JD. 4-Aminopyridine leads to restoration of conduction in demyelinated rat sciatic nerve. Brain Res. 1985;328:358–361. doi: 10.1016/0006-8993(85)91049-2. [DOI] [PubMed] [Google Scholar]
- 33.Targ EF, Kocsis JD. Action potential characteristics of demyelinated rat sciatic nerve following application of 4-aminopyridine. Brain Res. 1986;363:1–9. doi: 10.1016/0006-8993(86)90652-9. [DOI] [PubMed] [Google Scholar]
- 34.Wu R-L, Barish ME. Two pharmacologically and kinetically distinct transient potassium currents in cultured embryonic mouse hippocampal neurons. [Accepted 26 May 2000];J Neurosci. 1992 12(6):2235–2246. doi: 10.1523/JNEUROSCI.12-06-02235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]