SUMMARY AND CONCLUSIONS
Whole cell patch-clamp recordings were obtained from identified cutaneous and muscle afferent neurons (33-60 μm diam) in dissociated L4 and L5 dorsal root ganglia (DRGs) from normal rats and from rats 2-3 wk after sciatic nerve ligation or crush injury. γ-Aminobutyric acid (GABA)-induced conductance was compared in normal and injured neurons from both functional classes of sensory neurons.
Control cutaneous afferent neurons had a peak GABA-mediated conductance of 287 ± 27 (SE) nS compared with 457 ± 42 nS for control muscle afferent neurons.
An inflection on the downslope of the action potential was observed in 47% of cutaneous afferent neurons compared with 20% of muscle afferent neurons.
After ligation and transection of the sciatic nerve there was no change in the GABA-mediated conductance of muscle afferent neurons or in the action potential waveform (23% inflected). However, the cutaneous afferent neurons displayed a greater than twofold increase in their GABA-mediated conductance and displayed a prominent reduction in the number of neurons with inflected action potentials ( 13% inflected). Input resistance was similar in cutaneous and muscle afferent neurons and decreased after ligation in cutaneous but not muscle afferents. Resting potential averaged from −50 to −56 mV in normal and ligated groups for both cutaneous and muscle afferent neurons.
After crush injury in cutaneous afferent neurons where the transected axons were allowed to regenerate into the distal nerve stump, GABAA-receptor-mediated conductance was elevated compared with controls. However, action potential waveform was not altered by crush injury, suggesting a differential regulation of these two properties in cutaneous afferent neurons.
These data indicate that injury-induced plasticity of GABAA-receptor-mediated conductance and action potential waveform occurs in cutaneous but not muscle afferent DRG neurons. It appears that peripherally derived influences are critical in maintaining the electrophysiological phenotype of cutaneous afferent neurons but not muscle afferent neurons.
INTRODUCTION
Proprioceptive and tactile sensations are subserved by primary afferent neurons that have different peripheral receptors and signal transduction pathways in muscle and tendon and in dermal regions, respectively. Several of the phenotypic properties of these cutaneous and muscle afferent neurons are regulated by peripherally derived trophic influences that differ in their respective target tissues (Davies et al. 1987; Schecterson and Bothwell 1992). Thus the sensory neurons subserving tactile and proprioceptive modalities differ with respect to their electrophysiological (Lewin and McMahon 1991a,b) and biochemical (McMahon et al. 1984, 1989; O’Brien et al. 1989) properties.
Axotomy by nerve ligation provides a model whereby neurons are deprived of the peripheral influences that may mediate and maintain their phenotypic differentiation. Deprivation of peripherally derived trophic influences has been implicated in the alterations in neuropeptide expression (Lindsay and Harmar 1989; Lindsay et al. 1989; Wong and Oblinger 1991), chemosensitivity (Bevan and Winter 1995), and electrophysiological properties (Bhisitkul et al. 1990; Fitzgerald et al. 1985; Kingery et al. 1988; Wall and Devor 1981) observed in sensory neurons after nerve injury. Given that the phenotypic expression of sensory neurons innervating distinct peripheral targets is regulated by different trophic influences (Carroll et al. 1992; DiStefano et al. 1992; Jones et al. 1994; Mu et al. 1993; Ruit et al. 1992), one would expect that muscle afferent and cutaneous afferent neurons might respond differently to axotomy.
We have previously demonstrated that axotomy by nerve ligation increases γ-aminobutyric acid-A (GABAA)-receptor-mediated conductance and alters action potential waveform in medium-sized dorsal root ganglion (DRG) neurons (Oyelese et al. 1995a). In the present study we examine GABA-induced conductance and action potential waveform in identified, size-matched cutaneous and muscle afferent neurons to determine whether our previously observed injury-induced changes are restricted to a particular sensory neuronal functional class. We report here that cutaneous and muscle afferent neurons differ with respect to their GABAA-receptor-mediated conductance and action potential waveform, and in the response of these properties to axotomy. Cutaneous afferent neurons increase their GABAA-receptor-mediated conductance and lose the inflections on the falling phase of their action potential, whereas these properties remain unchanged in muscle afferent neurons.
To better understand the mechanisms regulating the injury-induced electrophysiological changes observed in cutaneous afferent neurons, we additionally axotomized cutaneous afferent neurons with the use of a crush lesion model of nerve injury that permits regeneration of the transected axons into the distal nerve segment and target tissue (Ramon y Cajal 1928). In response to axotomy, the Schwann cells in the distal nerve segment proliferate and increase their synthesis of neurotrophic factors (Funakoshi et al. 1993; Heumann et al. 1987; Meyer et al. 1992), providing a source of endogenous trophic support for regenerating axons. GABA-induced conductance in cutaneous afferent neurons was elevated after crush injury but action potential waveform was unchanged, suggesting that the distal nerve segment provides trophic support for the ion channel organization supporting action potential waveform, but not for the regulation of GABAA receptors.
A portion of this work has been reported in abstract form (Oyelese et al. 1995b).
METHODS
Identification of cutaneous and muscle afferent DRG neurons
DRG somata giving rise to cutaneous and muscle afferent fibers were identified by retrograde labeling with Fluoro-gold (Schmued and Fallon 1986). A 2-4% solution of Fluoro-gold (Fluorochrome, Englewood, CO) mixed in distilled water was prepared and injected into female rats (140-160 g) anesthetized with an intraperitoneal injection of ketamine (40 mg/kg) and xylazine (2.5 mg/kg). Cutaneous afferent neurons were labeled by injection of Fluoro-gold in the lateral plantar region (innervated by the sural nerve) of anesthetized rats (Honmou et al. 1994). To label muscle afferent neurons, an incision was made in the skin of anesthetized rats to expose the gastrocnemius and soleus muscles of the leg. Fluoro-gold (<150 μ1 total) was microinjected at multiple sites directly into the muscle, a layer of silicone grease was applied to the surrounding subcutaneous surface to prevent contamination from seepage, and the wound was sutured closed.
Nerve ligation and crush
Nerve ligation was performed as previously described (Oyelese et al. 1995a) with slight modifications. Briefly, 3-7 days after Fluoro-gold injection into the skin or muscle, the sciatic nerve was exposed and sectioned and the proximal segment was ligated bilaterally and capped with a blind-ending silicone tube to prevent reconnection with the distal segment (Oyelese et al. 1995a). Crush lesions were achieved by surgically exposing the sciatic nerve and compressing for 20-30 s with a pair of fine forceps, which results in transection of the nerve but allows the axons to regenerate into the distal nerve segment (Ramon y Cajal 1928). DRG neuronal cultures from ganglia supplying the sciatic nerve (L4 and L5) were prepared from ligated rats 2-4 wk after nerve injury, and from crush-lesioned rats 3 wk after nerve injury, with the use of previously described methods (Oyelese et al. 1995a). Age-matched uninjured rats were used as controls.
Electrophysiological techniques
Electrophysiological measurements were obtained 5-24 h after plating to minimize changes that might occur in vitro, with the use of previously described techniques (Oyelese et al. 1995a) with the following changes. Briefly, recording electrodes were filled with a solution containing (in mM) 140 KCl, 1 MgCl2, 3 ATP (magnesium salt), 1 CaC12, 11 ethylene glycol-bis (β-aminoethyl ether)-N,N,N’,N’-tetraacetic acid, and 10 N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid, pH 7.2, osmolarity 290-305 mosmol. Voltage-clamp recordings were made with a patch-clamp amplifier (Axopatch ID, Axon Instruments) with the use of a low-gain headstage (feedback resistor: 50 MΩ), (CV-4 0.1/100) to allow measurement of large GABA-induced currents typically encountered in DRG neurons of the size examined in this study. Recordings were low-pass filtered (Bessel filter) at 10 kHz and data were digitized and stored on computer with the use of a commercially available data acquisition system (TL-1 DMA interface and PClamp software, Axon Instruments; sampling rate 50 Hz—5 kHz/channel) and on a VCR with the use of a digitizing unit (Neurocorder DR-484, Neurodata instruments; sampled at 44 kHz). Estimation of access resistance, input resistance, cell capacitance, and GABA-induced conductance was performed as previously described for the low-gain headstage. Independent t-tests assuming unequal variance were used to determine levels of significant difference between groups.
Action potential data were obtained in current-clamp mode (sampling rate 100 kHz/channel). A series of hyperpolarizing and depolarizing current pulses 10 ms in duration from −0.2 or −0.4 nA with 12 incremental step pulses ranging from 0.2 to 0.4 nA was used to elicit action potentials in each neuron. Action potential duration (at 50% of spike height) was analyzed for each neuron.
RESULTS
Passive properties of normal and injured sensory neurons
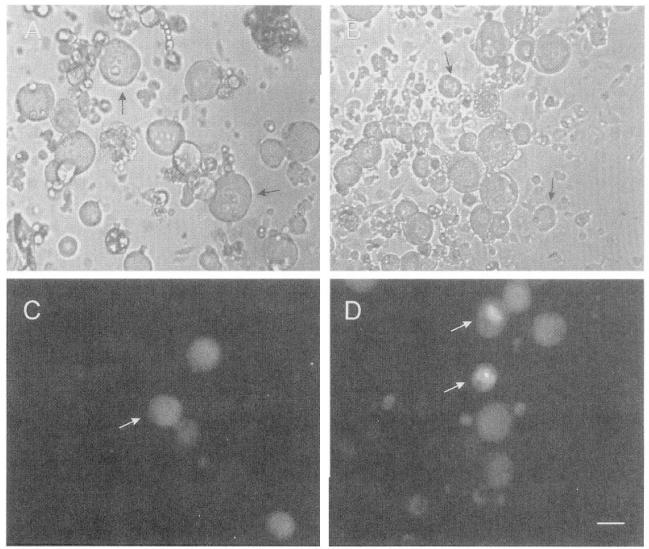
Cutaneous and muscle afferent neurons were identified by retrograde labeling with Fluoro-gold (Fig. 1) and neurons exhibiting a high degree of fluorescence were selected for study. Considerable overlap in the soma diameters of cutaneous (Fig. 1, A and C) and muscle afferent (Fig. 1, B and D) neurons was observed; however, labeled neurons with large soma diameters (>50 μm) were encountered more frequently in cultures of muscle afferent neurons than cutaneous afferent neurons. To eliminate errors that might arise from comparing electrophysiological properties that vary with neuronal size, an effort was made to select size-matched cutaneous and muscle afferent neurons ranging from 33 to 60 μm for study. This neuronal size range corresponds to neurons giving rise to myelinated axons conducting in the Aα/β- and Aδ-ranges (Harper and Lawson 1985). Resting potential was measured in all neurons studied as soon as adequate access was attained in the whole cell configuration and was occasionally observed to steadily depolarize by ∼5 mV over the course of a 15-min recording. Resting potential in normal and injured cutaneous and muscle afferent neurons was essentially similar, but slightly more negative in normal muscle afferents than in cutaneous afferents (−56 ± 0.8 mV, mean ± SE, vs. −53 ± 0.9 mV; Table 1). In response to crush or ligation injury, resting potential in cutaneous afferents did not change (Table 1), but ligated muscle afferents had a more positive resting potential compared with controls (−56 ± 0.8 mV vs. −50 ± 0.8 mV; Table 1). Input resistance of normal cutaneous and muscle afferent neurons was similar (92 ± 6.9 MΩ vs. 100 ± 10.4 MΩ Table 1). After crush injury to cutaneous afferents, no change in input resistance occurred (Table 1), but ligation injury produced a decrease in input resistance in cutaneous afferents (92 ± 6.9 MΩ vs. 68 ± 7.1 MΩ; Table 1). Ligation did not change input resistance in muscle afferents (Table 1). Whole cell capacitance and neuronal diameter were greater in crush-lesioned cutaneous afferents compared with control (88 ± 3.5 pF in controls and 88 ± 3.27 pF in ligated neurons vs. 101 ± 3.9 pF in crush-lesioned neurons; Table 1) but were similar in all other groups studied (Table 1).
Fig. 1.
Retrograde labeling of cutaneous and muscle afferent dorsal root ganglion (DRG) neurons. Respective brightfield and fluorescence images of cutaneous afferent (A and C) and muscle afferent (B and D) DRG neurons retrogradely labeled with Fluoro-gold. White arrows in C and D: examples of cells meeting criteria for size and fluorescence intensity. Black arrows in A and B: examples of cells not showing fluorescence. Calibration bar (D): 30 μm.
Table 1.
Differential effect of axotomy on spike waveform and GABA conductance in cutaneous and muscle afferent DRG neurons
| Cutaneous |
Muscle |
||||
|---|---|---|---|---|---|
| Control | Ligated | Crush | Control | Ligated | |
| Cell size, μm | 43 ± 0.6 | 44 ± 0.8 | 46 ± 0.7† | 45 ± 0.7 | 43 ± 0.6 |
| Cell cap, pF | 88 ± 3.50 | 88 ± 3.27 | 101 ± 3.86†‡ | 90 ± 3.50 | 85 ± 4.11 |
| GGABA, nS | 287 ± 27 | 656 ± 56* | 529 ± 48* | 457 ± 42* | 519 ± 56* |
| GGABA/cap, nS/pF | 3.53 ± 0.36 | 7.69 ± 0.64* | 5.49 ± 0.58*† | 5.42 ± 0.51* | 6.39 ± 0.70* |
| Input resistance, MΩ | 92 ± 6.9 | 68 ± 7.1* | 74 ± 10.9 | 100 ± 10.4 | 86 ± 10.1 |
| Resting potential, mV | −53 ± 0.9 | −56 ± 0.8 | − 56 ± 0.9 | − 56 ± 0.8‡ | − 56 ± 0.8*§ |
| AP duration, ms | 1.09 ± 0.11 | 0.92 ± 0.06 | 1.04 ± 0.17 | 0.68 ± 0.05* | 0.90 ± 0.07§ |
| AP% inflected | 47% | 13% | 48% | 20% | 23% |
| AP inflected | |||||
| GGABA, nS | 125 ± 18 | 299 ± 26* | 326 ± 45* | 239 ± 45‡ | 315 ± 89 |
| GGABA/cap, nS/pF | 1.18 ± 0.13 | 2.87 ± 0.75* | 2.72 ± 0.31* | 1.89 ± 0.29‡ | 3.94 ± 1.49 |
| AP noninflected | |||||
| GGABA, nS | 429 ± 29 | 728 ± 62* | 705 ± 60* | 513 ± 47 | 578 ± 64 |
| GGABA/cap, nS/pF | 5.58 ± 0.39 | 8.63 ± 0.67* | 7.88 ± 0.73* | 6.32 ± 0.55 | 7.10 ± 0.76 |
Values, except for percentages, are means ± SE. For cutaneous afferent neurons, n values were as follows: control, 58; ligated, 46–48; crush, 41–42. The respective values for action potential (AP) inflected were 27, 6, and 19; for AP noninflected, they were 31, 40, and 22. For muscle afferent neurons, n values were as follows: control, 46–49; ligated, 39–40. Respective AP inflected and AP noninflected values were 10 and 31 for controls and 9 and 31 for ligated. GABA, γ-aminobutyric acid; DRG, dorsal root ganglion; cap, capacitance, GGABA, GABA conductance.
P < 0.01 for comparisons with normal cutaneous afferents.
P < 0.05 for comparisons between ligated and crush-lesioned cutaneous afferents.
P < 0.05 for comparisons with normal cutaneous afferents.
P < 0.01 for comparisons between normal and ligated muscle afferents.
GABAA-receptor-mediated conductance in normal cutaneous and muscle afferent DRG neurons
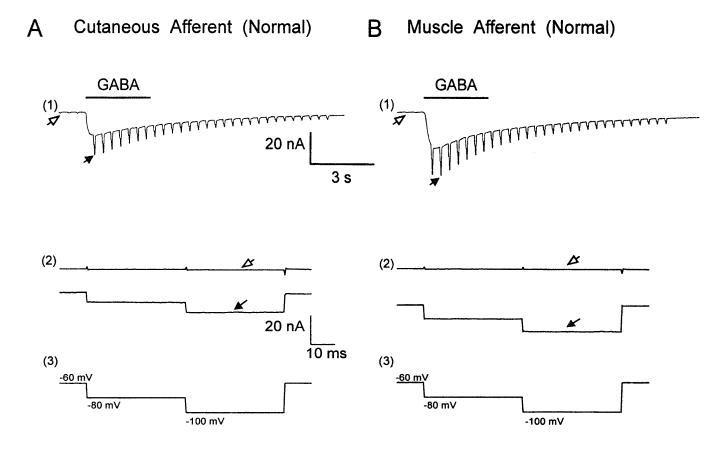
Pressure microejection of GABA (100 μM) for 3 s onto cutaneous and muscle DRG neurons induced a rapidly desensitizing inward current (Fig. 2, A1 and B1) that has previously been shown to result from the activation of a bicuculline-sensitive chloride conductance (Oyelese et al. 1995a; Robertson 1990; White 1990). Cutaneous and muscle afferent neurons were voltage clamped at —60 mV and test pulses (40 ms in duration to —80 and — 100 mV) were applied repeatedly at a rate of 2.5 Hz (voltage-clamp protocol in Fig. 2, A3 and B3). GABA-induced whole cell current was determined as the difference between whole cell current at —60, —80, and —100 mV at rest (Fig. 2, A2 and B2, open arrow) and at the peak response during GABA application (Fig. 2, A2 and B2, solid arrow). GABA-induced slope conductance calculated from plots of GABA-induced whole cell current at the test pulses was compared in identified, normal cutaneous and muscle afferent DRG neurons of similar size and capacitance. GABA-mediated whole cell conductance was larger in muscle afferent neurons (457 ± 42 nS) compared with cutaneous afferent neurons (287 ± 27 nS; Table 1). When normalized for cell size (capacitance), the conductance was similarly greater in muscle afferents compared with cutaneous afferents (5.42 ± 0.58 nS/pF vs. 3.53 ± 0.36 nS/pF; Table 1), indicating that the observed difference was not due to variations in neuronal size.
Fig. 2.
γ-Aminobutyric acid (GABA)-induced whole cell current and conductance in normal cutaneous and muscle afferent DRG neurons. Comparison of GABA-induced whole cell currents in cutaneous afferent (A) and muscle afferent (B) neurons revealed a larger response to GABA in muscle afferents. Neurons were voltage clamped at −60 mV and step pulses (40 ms in duration) to −80 and − 100 mV were applied and repeated at a rate of 2.5 Hz. A1 and B1 : whole cell current at slow time scale showing inward current and conductance increase in response to a 3-s application of GABA (100 μM). A2 and B2: response to individual pulse step at fast time base showing whole cell current before GABA application (open arrows) and at the peak of response to GABA (solid arrows). A3 and B3: voltage-clamp protocol.
Action potential waveform of normal cutaneous and muscle afferent neurons
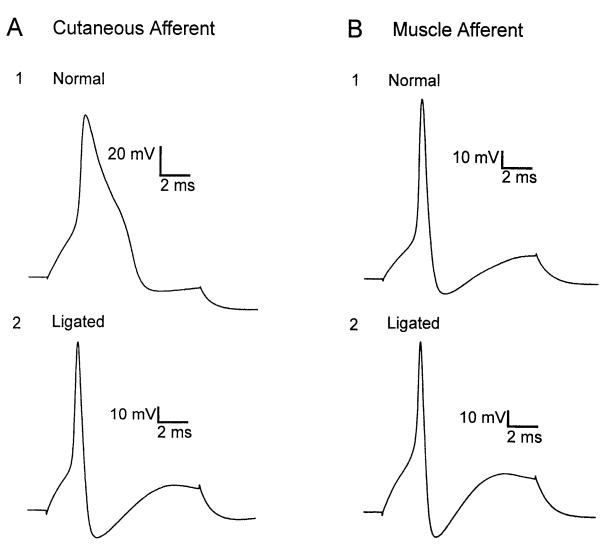
Action potential duration and waveform were studied in normal cutaneous and muscle afferent neurons. Action potentials were elicited by sequential hyperpolarizing and depolarizing pulses in current-clamp mode from a membrane potential of approximately —60 mV. Action potential duration measured at half maximal amplitude was greater in cutaneous afferent neurons (1.09 ± 0.11 ms) compared with muscle afferent neurons (0.68 ± 0.05 ms, Table 1). Approximately one-half of the cutaneous afferent neurons studied displayed varying degrees of inflection on the falling phase of the action potential (Fig. 3A1; Table 1). By comparison, action potentials in most muscle afferent neurons were brief in duration (Fig. 3B1) and only 20% of muscle afferent neurons had inflections on the falling phase (Table 1).
Fig. 3.
Action potential waveform in cutaneous and muscle afferent neurons before and after injury. Action potentials induced by depolarizing current pulse in cutaneous afferent (A) and muscle afferent (B) neurons. A greater proportion of uninjured cutaneous afferent neurons had long-duration action potentials with inflections on the downslope (A1) compared with muscle afferent neurons (B1). After nerve ligation, action potentials elicited from 87% of cutaneous afferent neurons lacked an inflection (A2), whereas action potential waveform in muscle afferent neurons was unchanged (B2).
Neurons with noninflected action potentials had a greater GABAA-receptor-mediated conductance than those with inflected action potentials
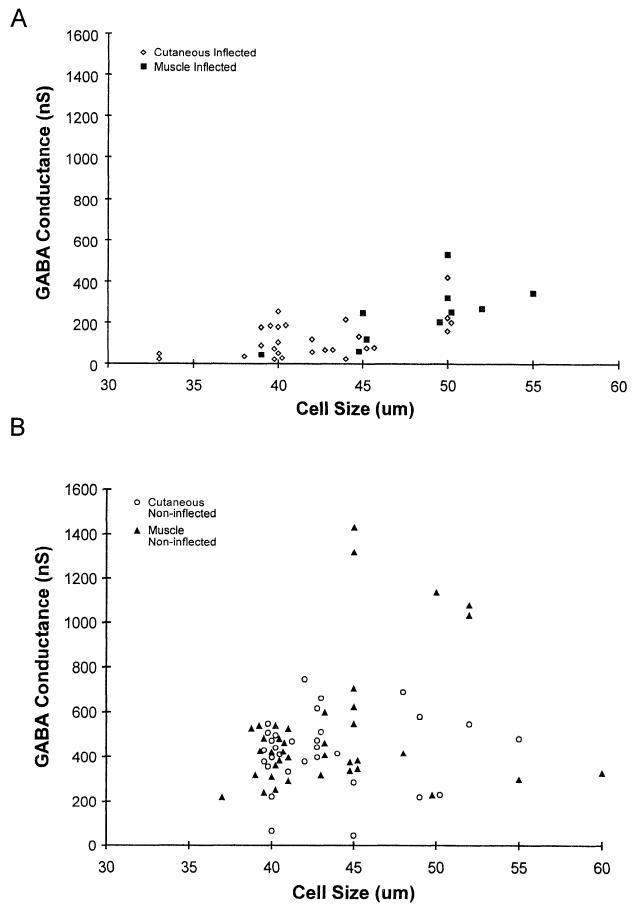
GABAA-receptor-mediated conductance in muscle and cutaneous afferent neurons with action potentials that had inflections on the falling phase was compared with conductance in neurons lacking an inflection. GABA-induced conductance in neurons with inflected action potentials was greater in muscle afferent neurons than in cutaneous afferent neurons (239 ± 45 nS vs. 125 ± 18 nS; Table 1, Fig. 4A). Because the muscle afferents with inflected action potentials were larger than the corresponding cutaneous afferent neurons, GABA conductance normalized for neuronal size (capacitance) was examined and was observed to be greater for muscle afferents than for cutaneous afferents (Table 1). However, no significant difference was found in the GABA-induced conductance of cutaneous and muscle afferent neurons lacking inflected action potentials (429 ± 27 nS vs. 513 ± 47 nS; Table 1, Fig. 4B). In both cutaneous and muscle afferent neuronal groups, neurons lacking inflected action potentials had a greater GABA-induced conductance compared with neurons of the same group expressing inflected action potentials (Table 1). Thus the larger GABA-induced conductance observed in muscle afferents correlated with a greater proportion of muscle afferent neurons expressing noninflected action potentials.
Fig. 4.
Relationship between GABA-induced conductance and cell size in inflected and noninflected cutaneous and muscle afferent neurons. GABA-induced conductance in inflected cutaneous and muscle afferent neurons (A) was lower compared with noninflected cutaneous and muscle afferent neurons (B). Because of extensive overlap, some data points were displaced 0.5-1 μm to permit visualization of individual data points.
Axotomy by nerve ligation increased GABAA-receptor-mediated conductance and altered action potential waveform in cutaneous but not muscle afferent neurons
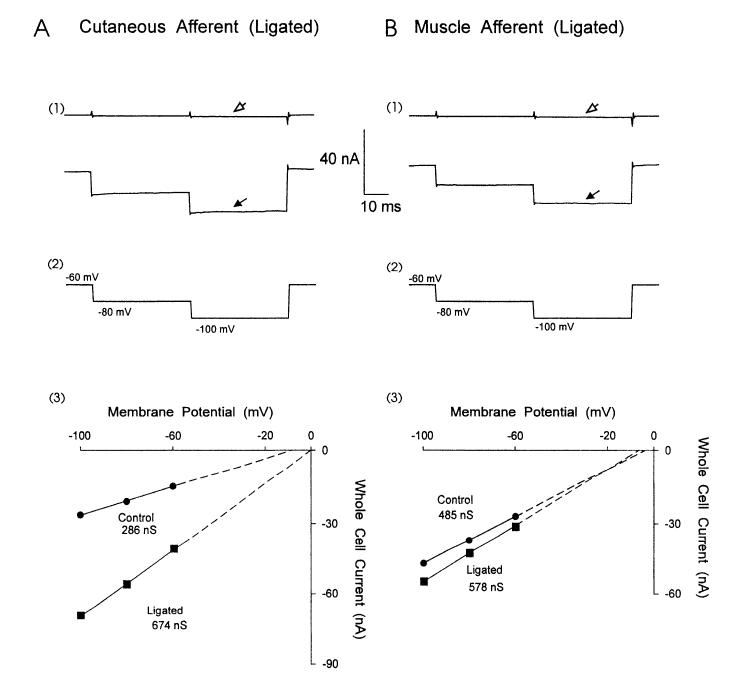
Our method of retrograde labeling of cutaneous and muscle afferent neurons by injection of Fluoro-gold before axotomy and distal to the site of nerve transection allowed us to selectively study identified, axotomized DRG neurons. Two to 4 wk after the sciatic nerve was ligated and cut (ligated neurons), large GABA-induced whole cell currents were observed in cutaneous afferent neurons (Fig. 5A1). Slope conductances derived from whole cell currents of normal (Fig. 2A2) and ligated (Fig. 5A1) cutaneous neurons representive of their means are compared in Fig. 5A3. Ligated cutaneous afferent neurons increased their GABA-induced conductance more than twofold to 656 ± 56 nS (Table 1). This increase remained highly significant when conductance was normalized for neuronal size (Table 1). In addition to the changes in GABA conductance following axotomy, the number of neurons with inflections on the falling phase of their action potentials decreased from 47% to 13% (Table 1). Muscle afferent neurons did not increase their GABA-induced conductance (519 ± 56 nS in ligated neurons vs. 457 ± 42 nS in controls; Table 1) or alter their action potential waveform (23% inflected in ligated neurons vs. 20% in controls) after ligation (Fig. 3B2; Table 1). GABA-induced conductance was larger in ligated cutaneous afferents compared with normal muscle afferents (Table 1). GABAA-receptor-mediated conductance was increased after nerve ligation in cutaneous afferent neurons with inflections on the downslope of their action potentials as well as in those neurons with noninflected action potentials (Table 1).
Fig. 5.
GABA-induced whole cell current in cutaneous and muscle afferent neurons following nerve injury. Whole cell current in cutaneous (A1) and muscle (B1) afferent neurons before GABA (A1 and B1, open arrows) and during peak response to GABA application (A1 and B1, solid arrows) 2-4 wk after nerve ligation. A3 and B3: whole cell GABA-induced currents from the normal cutaneous and muscle neurons in Fig. 1 (●) were compared with currents from the ligated neurons (■) in A1 and B1. Cutaneous but not muscle afferent neurons increased their GABA-induced slope conductance after nerve ligation.
Axotomy by nerve crush enhanced GABAA-receptor-mediated conductances, but did not alter action potential waveform in cutaneous afferent neurons
GABAA-receptor-mediated conductance and action potential waveform were studied in cutaneous neurons 3 wk after a crush lesion that permitted regenerating axons to come in contact with Schwann cells and other elements of the distal nerve segment. The effect of crush injury was not examined in muscle afferent neurons because they displayed changes in response to nerve ligation. As with nerve ligation, GABAA-receptor-mediated conductance in this model of nerve injury was elevated in cutaneous afferent neurons compared with controls (529 ± 48 nS vs. 287 ± 27 nS in controls; Table 1) and was similar to GABA conductance in ligated neurons (Table 1). However, neuronal size as determined by cell diameter and capacitance was greater in crush-lesioned cutaneous neurons compared with either control or ligated neurons (Table 1) and thus, to eliminate increases in GABA-induced conductance attributable to differences in neuronal size, the conductance normalized for capacitance was compared in these groups. Although normalized conductance was greater in crush-lesioned cutaneous neurons compared with controls (Fig. 6), the observed increase was significantly less than that observed in ligated neurons (Fig. 6). Crush injury did not alter the action potential waveform in cutaneous afferents neurons, because the proportion of neurons with inflected action potentials was not different from the proportion in controls (48% vs. 47% in controls; Table 1).
Fig. 6.
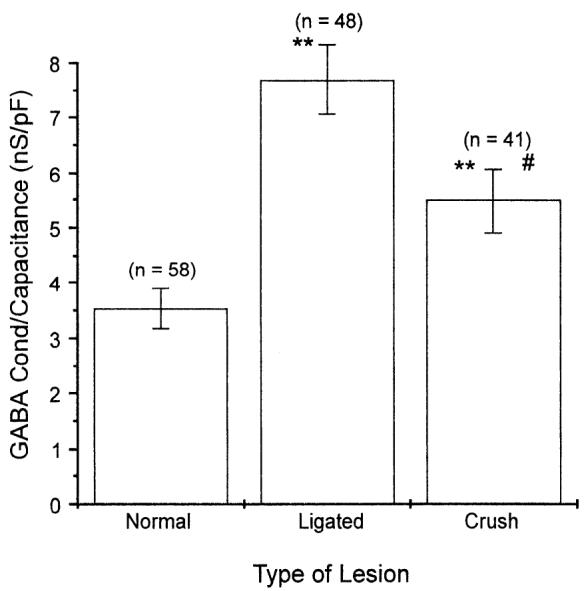
GABA-induced conductance in normal, ligated, and crush-lesioned cutaneous afferent neurons. Ligation and crush lesion resulted in elevated GABA-induced conductance, whereas ligation, but not crush lesion, decreased the proportion of neurons with inflected action potentials (see Table 1). GABA-induced conductance was normalized for neuronal size (conductance/capacitance) because crush-lesioned neurons were larger than control and ligated neurons (Table 1). Although normalized GABA-induced conductance was increased after crush injury, the observed increase was significantly lower than for ligated neurons (means ± SE; n values in parentheses; **P < 0.01 for comparisons with normals, #P < 0.05 for comparisons with ligated neurons).
DISCUSSION
In the present study, utilizing a method of retrograde labeling with Fluoro-gold to identify cutaneous and muscle afferent DRG neurons, we compared their electrophysiological properties and determined their responses to axotomy. The results indicate that GABAA-receptor-mediated conductance and action potential waveform differed in muscle and cutaneous afferent DRG neurons, and we identified injury-induced changes in these properties in cutaneous afferent neurons that were not observed in muscle afferent neurons.
GABAA-receptor-mediated conductance
GABAA-receptor-mediated conductance in sensory neurons may vary with receptor subunit composition (Burt and Kamatchi 1991; Mathews et al. 1994; Sigel et al. 1990; Verdoorn et al. 1990) or increase with cell size (Oyelese et al. 1995a). Heterogeneity in the sensitivity of GABAA receptors in DRG neurons has been observed (White 1992), and studies suggest a difference in the distribution or composition of GABAA receptors in Aδ- versus C primary afferent neurons (Desarmenien et al. 1984). However, GABA-mediated conductance was larger in muscle versus cutaneous afferent neurons even though the neurons studied were similar in size. These findings suggest that a higher GABAA receptor density or a difference in subunit composition exists in muscle afferent neurons, resulting in a greater conductance.
Action potential waveform
A difference was observed in the action potential waveforms of cutaneous and muscle afferent neurons. Approximately half of the cutaneous afferent neurons studied had an inflection on the downslope of the action potentials, whereas action potentials in 80% of muscle afferent neurons lacked an inflection. Action potentials in adult mammalian sensory neurons are morphologically heterogenous and the precise ionic mechanisms underlying the inflected action potential waveform are unknown. Although inflected and noninflected soma action potentials in mammalian DRG neurons have been shown to be sodium dependent and to occur in the absence of calcium in some studies (McLean et al. 1988), calcium dependence has been demonstrated for the inflected action potential of mouse DRG neurons by other investigators (Ransom and Holz 1977; Yoshida et al. 1978). The electrophysiological properties of sensory neurons, including action potential waveform, vary with the type of peripheral receptor innervated (Koerber et al. 1988; Rose et al. 1986; Traub and Mendell 1988). Cutaneous afferent neurons (4450 μm) express a kinetically slow, tetrodotoxin (TTX)-resistant sodium current either singularly or in combination with a fast, TTX-sensitive sodium current (Honmou et al. 1994). The slow TTX-resistant current has been postulated to contribute to the broader, inflected action potential waveform particular to that population of neurons in the adult rat. However, a recent study in which a TTX-resistant sodium channel was cloned localizes the expression of this channel to small-diameter DRG neurons (Akopian et al. 1996).
Changes in GABAA-receptor-mediated conductance and action potential waveform following axotomy
The increase in GABA-mediated conductance and altered action potential waveform in cutaneous afferents could be the result of altered transport of receptors or ion channels (Kitchener et al. 1994; Oblinger and Lasek 1988) in response to injury. It is of interest that presynaptic inhibition of primary afferent terminals in the spinal cord by primary afferent depolarization is mediated in part by activation of axonal GABAA receptors (Eccles et al. 1963; also see Levy 1977 for a review). Primary afferent depolarization has been observed to disappear or show marked reduction after axotomy by nerve section or crush (Horch and Lisney 1981). Decreased GABAA-receptor-mediated depolarization and sensitivity have also been observed in dorsal roots after nerve ligation (Bhisitkul et al. 1990; Kingery et al. 1988). Given that we observe an increase in the somatic GABA conductance in cutaneous afferent neurons, it is possible that axotomy results in a reduction in GABAA receptor transport down the axons with increased proximal incorporation into the somatic membrane. However, alterations in gene expression induced by injury (Hayes et al. 1992; Leah et al. 1991; Wu et al. 1994) resulting in the synthesis of altered species of GABAA receptors and sodium channels with different biophysical properties may also explain these injury-induced responses. Indeed, axotomized DRG neurons display a different pattern of sodium channel mRNA expression following injury, with a reexpression of type III sodium channel mRNA, which is expressed at high levels embryonically but undetectable in normal adult neurons (Waxman et al. 1994). However, it is not known whether the type III sodium channel is associated with fast or slow sodium currents. After nerve ligation, there is the attenuation or loss of slow, TTX-resistant sodium current expression in cutaneous afferents, and a fast TTX-sensitive sodium current predominates (Rizzo et al. 1995). These changes in sodium channel expression may contribute to the altered action potential waveform observed in the present study.
Regulation of injury-induced changes
During development, the survival and differentiation of DRG neurons are mediated by trophic influences derived from peripheral and central target tissues (reviewed in Johnson et al. 1986). Although most adult DRG neurons are no longer dependent on target-derived trophic influences for survival (Lindsay 1988), some still require trophic support to maintain their differentiated phenotype (Lindsay and Harmar 1989; Lindsay et al. 1989). Our nerve ligation paradigm represents a system in which DRG neurons are deprived of their peripheral target-derived trophic support and is therefore convenient for studying the role of the target tissue-derived influences in maintaining the phenotypic expression of adult sensory neurons.
In response to nerve ligation, the biochemical and electrophysiological properties of the surviving neurons may be altered (Anand et al. 1991; Barbut et al. 1981; McGregor et al. 1984; Oblinger and Lasek 1988; Wall and Devor 1981). Nerve ligation alters neuropeptide expression in DRG neurons, with substance P levels declining to a minimum by 2 wk postinjury (Barbut et al. 1981). If the nerve is crushed permitting the transected axons to regenerate into the distal nerve segment, no decrease in substance P levels occurs (Baranowski et al. 1993; Barbut et al. 1981). Supplying the transected nerve with exogenous nerve growth factor has also been shown to rescue substance P expression and reduce injury-induced changes in some electrophysiological properties such as primary afferent depolarization occurring after axotomy (Fitzgerald et al. 1985; Verge et al. 1995), suggesting that these properties may be regulated by neurotrophins in the adult. In addition, neuropeptide expression in axotomized cutaneous or muscle afferent neurons regenerating into inappropriate targets is altered to resemble that of the neurons normally innervating those targets (McMahon et al. 1989). Thus peripherally derived trophic influences available from target tissue with intact innervation or from the distal nerve segment after nerve injury may play a role in maintaining the phenotypic expression of adult sensory neurons.
The regulation of electrophysiological properties may not be entirely target specified in muscle afferent neurons. Certain electrophysiological properties (e.g., conduction velocity) are unchanged in axons of acutely axotomized muscle afferent neurons (Johnson and Munson 1991), and regeneration of acutely or chronically axotomized muscle afferents into cutaneous targets while providing functional rescue does not result in a respecification of electrophysiological properties to match cutaneous targets (Johnson and Munson 1991; Johnson et al. 1995). It thus appears that the expression of at least some of the electrophysiological properties of muscle afferent neurons is intrinsically regulated independently of the peripheral target innervated, which may partially explain why muscle afferents did not alter their electrophysiological properties after axotomy in the present study.
In our study, a crush lesion still resulted in an increase (albeit attenuated) in GABAA-receptor-mediated conductance in cutaneous afferent neurons whereas the action potential waveform remained unchanged, suggesting that these two properties of cutaneous afferent neurons are regulated by distinct mechanisms. In response to axotomy, distal nerve segment synthesis of nerve growth factor (Heumann et al. 1987; Raivich and Kreutzberg 1987; Taniuchi et al. 1988) and brain-derived neurotrophic factor mRNA (Funakoshi et al. 1993; Meyer et al. 1992) is elevated, with brain-derived neurotrophic factor mRNA displaying a slower time course of elevation (Meyer et al. 1992). In contrast with axotomy by nerve ligation, which prevents target reinnervation and promotes neuroma formation, axons transected by a crush lesion can regenerate into the distal nerve segment and thus be influenced by the increased neurotrophin levels in the distal nerve. It is thus possible that the action potential waveform in adult cutaneous afferent neurons is strongly regulated by one or both of these neurotrophins whereas the expression of GABAA receptors is not.
The expression of inflected, TTX-resistant action potentials in cutaneous afferent neurons innervating high-threshold mechanoreceptors is regulated by nerve growth factor during development (Lewin et al. 1992; Ritter and Mendell 1992). Exogenous nerve growth factor has been observed to influence sodium channel expression (D’Arcangelo et al. 1993; Fanger et al. 1995; Kalman et al. 1990; Rudy et al. 1987; Toledo-Arai et al. 1995) and increase the expression of TTX-resistant sodium channels in PC12 cells (Rudy et al. 1987) and DRG neurons in vitro (Aguayo and White 1992). Axotomy decreases the expression of slow TTX-resistant sodium currents in cutaneous afferent neurons (Rizzo et al. 1995), and DRG neurons maintained in longterm culture in the absence of nerve growth factor do not express TTX-resistant action potentials (Aguayo et al. 1991). Thus a decrease in the retrograde transport of target-derived nerve growth factor and other neurotrophic factors following axotomy may contribute to the decrease in expression of inflected action potentials observed in axotomized cutaneous afferent neurons in the present study. Muscle afferents, on the other hand, are typically dependent on neurotropin-3 (NT-3) and not nerve growth factor for survival (Ernfors et al. 1994; Hory-Lee et al. 1993; Klein et al. 1994; McMahon et al. 1994). Motor neurons and peripheral muscle tissue serve as a source of NT-3 for muscle afferent neurons during development (Schecterson and Bothwell 1992). It would be of interest to determine whether or not motor neurons maintain this influence in the adult, especially after axotomy.
Our data suggest that peripherally derived influences are important in maintaining certain electrophysiological properties of cutaneous afferent neurons, but not muscle afferent neurons, in adult rats. Further work employing different regeneration paradigms in which a varying degree of sciatic nerve regeneration is permitted or in which trophic influences are replenished by supplying the axotomized sciatic nerve and DRG with exogenous neurotrophins would be necessary to elucidate the mechanisms involved in the regulation of these injury-induced changes.
Acknowledgments
We are grateful to H.-F. Mi for preparing the DRG neuronal cultures, B. Toftness for computer assistance, and Dr. Marco Rizzo for critical comments on the manuscript.
This work was supported in part by the Medical Research Service of the Department of Veterans Affairs and by National Institute of Neurological Disorders and Stroke Grant NS-10174.
REFERENCES
- Aguayo LG, Weight FF, White G. TTX-sensitive action potentials and excitability of adult rat sensory neurons in serum- and exogenous nerve growth factor-free medium. Neurosci. Lett. 1991;121:88–92. doi: 10.1016/0304-3940(91)90656-e. [DOI] [PubMed] [Google Scholar]
- Aguayo LG, White G. Effects of nerve growth factor on TTX-and capsaicin-sensitivity in adult rat sensory neurons. Brain Res. 1992;570:61–67. doi: 10.1016/0006-8993(92)90564-p. [DOI] [PubMed] [Google Scholar]
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage gated channel expressed by sensory neurons. Nature Lond. 1996;379:257–262. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- Anand P, Ghatei MA, Christofides ND, Blank MA, McGregor GP, Morrison JFB, Scaravilli F, Bloom SR. Differential neuropeptide expression after visceral and somatic nerve injury in the cat and rat. Neurosci. Lett. 1991;128:57–60. doi: 10.1016/0304-3940(91)90759-m. [DOI] [PubMed] [Google Scholar]
- Baranowski AP, Priestly JV, McMahon S. Substance P in cutaneous primary sensory neurons—A comparison of models of nerve injury that allow varying degrees of regeneration. Neuroscience. 1993;55:1025–1036. doi: 10.1016/0306-4522(93)90316-8. [DOI] [PubMed] [Google Scholar]
- Barbut D, Polak JM, Wall PD. Substance P in spinal cord dorsal horn decreases following peripheral nerve injury. Brain Res. 1981;205:289–298. doi: 10.1016/0006-8993(81)90340-1. [DOI] [PubMed] [Google Scholar]
- Bevan S, Winter J. Nerve growth factor differentially regulates the chemosensitivity of adult rat cultured sensory neurons. J. Neurosci. 1995;15:4918–4926. doi: 10.1523/JNEUROSCI.15-07-04918.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhisitkul RB, Kocsis JD, Gordon TR, Waxman SG. Trophic influence of the distal nerve segment on GABAA receptor expression in axotomized adult sensory neurons. Exp. Neural. 1990;109:273–278. doi: 10.1016/s0014-4886(05)80017-2. [DOI] [PubMed] [Google Scholar]
- Burt DR, Kamatchi GL. GABAA, receptor subtypes: from pharmacology to molecular biology. FASEB J. 1991;5:2916–2923. doi: 10.1096/fasebj.5.14.1661244. [DOI] [PubMed] [Google Scholar]
- Carroll SL, Silos-Santiago I, Frese SE, Ruit KG, Milbrandt J, Snider WD. Dorsal root ganglion neurons expressing trk are selectively sensitive to NGF deprivation in utero. Neuron. 1992;9:779–788. doi: 10.1016/0896-6273(92)90040-k. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Paradiso K, Shepherd D, Brehm P, Halegoua S, Mandel G. Neuronal growth factor regulation of two different sodium channel types through distinct signal transduction pathways. J. Cell Biol. 1993;122:915–921. doi: 10.1083/jcb.122.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AM, Bandtlow C, Heumann R, Korsching S, Rohrer H, Thoenen H. Timing and site of nerve growth factor synthesis in developing skin in relation to innervation and expression of the receptor. Nature Lond. 1987;326:353–358. doi: 10.1038/326353a0. [DOI] [PubMed] [Google Scholar]
- Desarmenien M, Santangelo F, Loeffler J-P, Feltz P. Comparative study of GABA-mediated depolarizations of lumbar Aδ and C primary afferent neurones of the rat. Exp. Brain Res. 1984;54:521–528. doi: 10.1007/BF00235477. [DOI] [PubMed] [Google Scholar]
- DiStefano PS, Friedman B, Radziejewski C, Alexander C, Boland P, Schick CM, Lindsay RM, Wiegand SJ. The neurotrophins BDNF, NT-3 and NGF display distinct patterns of retrograde axonal transport in peripheral and central neurons. Neuron. 1992;8:983–993. doi: 10.1016/0896-6273(92)90213-w. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Schmidt R, Willis WD. Pharmacological studies on presynaptic inhibition. J. Physiol. Lond. 1963;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Lee K, Kucera J, Jaenisch R. Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell. 1994;77:503–512. doi: 10.1016/0092-8674(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Fanger GR, Jones JR, Maue RA. Differential regulation of neuronal sodium channel expression by endogenous and exogenous tyrosine kinase receptors expressed in rat pheochromocytoma cells. J. Neurosci. 1995;15:202–213. doi: 10.1523/JNEUROSCI.15-01-00202.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M, Wall PD, Goedert M, Emson PC. Nerve growth factor counteracts the neurophysiological and neurochemical effects of chronic sciatic nerve section. Brain Res. 1985;332:131–141. doi: 10.1016/0006-8993(85)90396-8. [DOI] [PubMed] [Google Scholar]
- Funakoshi H, Frisén J, Barbany G, Timmusk T, Zachrisson O, Verge VMK, Persson H. Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J. Cell Biol. 1993;123:455–465. doi: 10.1083/jcb.123.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill 0P, Neher ME, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pfluegers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J. Physiol. Lond. 1985;359:31–46. doi: 10.1113/jphysiol.1985.sp015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes RC, Wiley RG, Armstrong DM. Induction of nerve growth factor receptor (p75NGFr) mRNA within hypoglossal motoneurons following axonal injury. Mol. Brain Res. 1992;15:291–297. doi: 10.1016/0169-328x(92)90120-z. [DOI] [PubMed] [Google Scholar]
- Heumann R, Korsching S, Bandtlow C, Thoenen H. Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection. J. Cell Biol. 1987;104:1623–1631. doi: 10.1083/jcb.104.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honmou O, Utzschneider DA, Rizzo MA, Bowe CM, Waxman SG, Kocsis JD. Delayed depolarization and slow sodium currents in cutaneous afferents. J. Neurophysiol. 1994;71:1627–1637. doi: 10.1152/jn.1994.71.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horch KW, Lisney SJW. Changes in primary afferent depolarization of sensory neurons during peripheral nerve regeneration in the cat. J. Physiol. Lond. 1981;313:287–299. doi: 10.1113/jphysiol.1981.sp013665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hory-Lee F, Russel M, Lindsay RM, Frank E. Neurotrophin 3 supports the survival of developing muscle sensory neurons in culture. Proc. Natl. Acad. Sci. USA. 1993;90:2613–2617. doi: 10.1073/pnas.90.7.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EM, Jr., Rich KM, Yip HK. The role of NGF in sensory neurons in vivo. Trends Neurosci. 1986;9:33–37. [Google Scholar]
- Johnson RD, Munson JB. Regenerating sprouts of axotomized cat muscle afferents express characteristic firing patterns to mechanical stimulation. J. Neurophysiol. 1991;66:2155–2158. doi: 10.1152/jn.1991.66.6.2155. [DOI] [PubMed] [Google Scholar]
- Johnson RD, Taylor JS, Mendell LM, Munson JB. Rescue of motoneuron and muscle afferent functions in cats by regeneration into skin. I. Properties of afferents. J. Neurophysiol. 1995;73:65l–661. doi: 10.1152/jn.1995.73.2.651. [DOI] [PubMed] [Google Scholar]
- Jones KR, Fariñas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman D, Wong B, Horvai AE, Cline MJ, O’Lague PH. Nerve growth factor acts through CAMP-dependent protein kinase to increase the number of sodium channels in PC12 cells. Neuron. 1990;2:355–366. doi: 10.1016/0896-6273(90)90048-k. [DOI] [PubMed] [Google Scholar]
- Kingery WS, Fields RD, Kocsis JD. Diminished dorsal root GABA sensitivity following chronic peripheral nerve injury. Exp. Neural. 1988;100:478–490. doi: 10.1016/0014-4886(88)90033-7. [DOI] [PubMed] [Google Scholar]
- Kitchener PD, Wilson P, Snow PJ. Sciatic axotomy compromises axonal transport of transganglionic tracer BSI-B4 from the central terminals of C fibre afferents. Neurosci. Lett. 1994;166:121–125. doi: 10.1016/0304-3940(94)90466-9. [DOI] [PubMed] [Google Scholar]
- Klein R, Silos-Santiago I, Smeyne RJ, Lira SA, Brambilla R, Bryant S, Zhang L, Snider WD, Barbacid M. Disruption of the neurotrophin-3 receptor gene trkC eliminates Ia muscle afferents and results in abnormal movements. Nature Lond. 1994;368:249–251. doi: 10.1038/368249a0. [DOI] [PubMed] [Google Scholar]
- Koerber HR, Druzinsky RE, Mendell LM. Properties of somata of spinal dorsal root ganglion cells differ according to peripheral receptor innervated. J. Neurophysiol. 1988;60:1584–1596. doi: 10.1152/jn.1988.60.5.1584. [DOI] [PubMed] [Google Scholar]
- Leah JD, Herdegen T, Bravo R. Selective expression of Jun proteins following axotomy and axonal transport block in peripheral nerves in the rat: evidence for a role in the regeneration process. Brain Res. 1991;566:198–207. doi: 10.1016/0006-8993(91)91699-2. [DOI] [PubMed] [Google Scholar]
- Levy RA. The role of GABA in primary afferent depolarization. Prog. Neurobiol. 1977;9:211–267. doi: 10.1016/0301-0082(77)90002-8. [DOI] [PubMed] [Google Scholar]
- Lewin GR, McMahon SB. Physiological properties of primary sensory neurons appropriately and inappropriately innervating skin in the adult rat. J. Neurophysiol. 1991a;66:1205–1217. doi: 10.1152/jn.1991.66.4.1205. [DOI] [PubMed] [Google Scholar]
- Lewin GR, McMahon SB. Physiological properties of primary sensory neurons appropriately and inappropriately innervating skeletal muscle in the adult rat. J. Neurophysiol. 1991b;66:1218–1231. doi: 10.1152/jn.1991.66.4.1218. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Ritter AM, Mendell LM. On the role of nerve growth factor in the development of myelinated nociceptors. J. Neurosci. 1992;12:1896–1905. doi: 10.1523/JNEUROSCI.12-05-01896.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay RM. Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurons. J.Neurosci. 1988;8:2394–2405. doi: 10.1523/JNEUROSCI.08-07-02394.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay RM, Harmak AJ. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature Lond. 1989;337:362–364. doi: 10.1038/337362a0. [DOI] [PubMed] [Google Scholar]
- Lindsay RM, Lockett C, Steknberg J, Winter J. Neuropeptide expression in cultures of adult sensory neurons: modulation of substance P and calcitonin gene-related peptide levels by nerve growth factor. Neuroscience. 1989;33:53–65. doi: 10.1016/0306-4522(89)90310-2. [DOI] [PubMed] [Google Scholar]
- Mathews GC, Bolos-Sy AM, Holland KD, Isenberg KE, Covey DF, Ferrendelli JA, Rothman SM. Developmental alteration in GABAA receptor structure and physiological properties in cultured cerebellar granule neurons. Neuron. 1994;13:149–158. doi: 10.1016/0896-6273(94)90465-0. [DOI] [PubMed] [Google Scholar]
- McGregor GP, Gibson SJ, Sabate IM, Blank MA, Christofides ND, Wall PD, Polak JM, Bloom SR. Effect of peripheral nerve section and nerve crush on spinal cord neuropeptides in the rat; increased VIP and PHI in the dorsal horn. Neuroscience. 1984;13:207–216. doi: 10.1016/0306-4522(84)90270-7. [DOI] [PubMed] [Google Scholar]
- McLean MJ, Bennett PB, Thomas RM. Subtypes of dorsal root ganglion neurons based on different inward currents as measured by whole-cell voltage clamp. Mol. Cell. Biochem. 1988;80:95–107. doi: 10.1007/BF00231008. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Armanini MP, Ling LH, Phillips HS. Expression and coexpression of Trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron. 1994;12:1161–1171. doi: 10.1016/0896-6273(94)90323-9. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Lewin GR, Anand P, Ghatei MA, Bloom SR. Quantitative analysis of peptide levels and neurogenic extravasation following regeneration of afferents to appropriate and inappropriate targets. Neuroscience. 1989;33:67–73. doi: 10.1016/0306-4522(89)90311-4. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Sykova E, Wall PD, Woolf CJ, Gibson SJ. Neurogenic extravasation and substance P levels are low in muscle as compared to skin in the rat hindlimb. Neurosci. Lett. 1984;52:235–240. doi: 10.1016/0304-3940(84)90167-8. [DOI] [PubMed] [Google Scholar]
- Meyer M, Matsuoka I, Wetmore C, Olson L, Thoenen H. Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J. Cell Biol. 1992;119:45–54. doi: 10.1083/jcb.119.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X, Silos-Santiago I, Carroll SL, Snider WD. Neurotrophin receptor genes are expressed in distinct patterns in developing dorsal root ganglia. J. Neurosci. 1993;13:4029–4041. doi: 10.1523/JNEUROSCI.13-09-04029.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oblinger MM, Lasek RJ. Axotomy-induced alterations in the synthesis and transport of neurofilaments and microtubules in dorsal root ganglion cells. J. Neurosci. 1988;8:1747–1758. doi: 10.1523/JNEUROSCI.08-05-01747.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CO, Woolf CJ, Fitzgerald M, Lindsay RM, Molander C. Differences in the chemical expression of rat primary afferent neurons which innervate skin, muscle or joint. Neuroscience. 1989;32:493–502. doi: 10.1016/0306-4522(89)90096-1. [DOI] [PubMed] [Google Scholar]
- Oyelese AA, Eng DL, Richerson GB, Kocsis JD. Enhancement of GABA, receptor-mediated conductances induced by nerve injury in a subclass of sensory neurons. J. Neurophysiol. 1995a;74:673–683. doi: 10.1152/jn.1995.74.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyelese AA, Rizzo MA, Waxman SG, Kocsis JD. Differential effect of neurotrophins on injury-induced plasticity of GABAA receptors and sodium currents in cutaneous afferent DRG neurons. Sot. Neurosci. Abstr. 1995b;21:1055. [Google Scholar]
- Raivich G, Kreutzberg GW. Expression of growth factor receptors in injured nervous tissue. 1. Axotomy leads to a shift in the cellular distribution of specific β-nerve growth factor binding in the injured and regenerating PNS. J. NeurocytoZ. 1987;16:689–700. doi: 10.1007/BF01637660. [DOI] [PubMed] [Google Scholar]
- y Cajal S. Ramon. Vol. 1. Oxford Univ. Press; London: 1928. Degeneration and Regeneration of the Nervous System. [Google Scholar]
- Ransom RB, Holz RW. Ionic determinants of excitability in cultured mouse dorsal root ganglion and spinal cord cells. Brain Res. 1977;136:445–453. doi: 10.1016/0006-8993(77)90069-5. [DOI] [PubMed] [Google Scholar]
- Ritter AM, Mendell LM. Soma membrane properties of physiologically identified sensory neurons in the rat: effects of nerve growth factor. J. Neurophysiol. 1992;68:2033–2041. doi: 10.1152/jn.1992.68.6.2033. [DOI] [PubMed] [Google Scholar]
- Rizzo MA, Kocsis JD, Waxman SG. Selective loss of slow and enhancement of fast Na+ currents in cutaneous afferent DRG neurons following axotomy. Neurobiol. Dis. 1995;2:87–96. doi: 10.1006/nbdi.1995.0009. [DOI] [PubMed] [Google Scholar]
- Robertson B. Characteristics of GABA-activated chloride channels in mammalian dorsal root ganglion neurones. J. Physiol. Lond. 1989;411:285–300. doi: 10.1113/jphysiol.1989.sp017574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose RD, Koerber HR, Sedivec MJ, Mendell LM. Somal action potential duration differs in identified primary afferents. Neurosci. Lett. 1986;63:259–264. doi: 10.1016/0304-3940(86)90366-6. [DOI] [PubMed] [Google Scholar]
- Rudy B, Kirschenbaum B, Rukenstein A, Greene LA. Nerve growth factor increases the number of functional Na channels and induces TTX-resistant Na channels in PC1 2 pheochromocytoma cells. J. Neurosci. 1987;7:1613–1625. doi: 10.1523/JNEUROSCI.07-06-01613.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruit KG, Elliot JL, Osborne PA, Yan Q, Snider WD. Selective dependence of mammalian dorsal root ganglion neurons on nerve growth factor during embryonic development. Neuron. 1992;8:573–587. doi: 10.1016/0896-6273(92)90284-k. [DOI] [PubMed] [Google Scholar]
- Schecterson LC, Bothwell B. Novel roles for neurotrophins are suggested by BDNF and NT-3 mRNA expression in developing neurons. Neuron. 1992;9:449–463. doi: 10.1016/0896-6273(92)90183-e. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Fallon JH. Fluoro-gold: a new fluorescent retrograde axonal tracer with numerous unique properties. Brain Res. 1986;377:147–154. doi: 10.1016/0006-8993(86)91199-6. [DOI] [PubMed] [Google Scholar]
- Sigel E, Baur R, Trube G, Möhler H, Malherbe P. The effect of subunit composition of rat brain GABAA receptors on channel function. Neuron. 1990;5:703–711. doi: 10.1016/0896-6273(90)90224-4. [DOI] [PubMed] [Google Scholar]
- Taniuchi M, Clark HB, Schweitzer JB, Johnson EM., Jr. Expression of nerve growth factor receptors by Schwann cells of axotomized peripheral nerves: ultrastructural location, suppression by axonal contact and binding properties. J. Neurosci. 1988;8:664–681. doi: 10.1523/JNEUROSCI.08-02-00664.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Arai JJ, Brehm P, Halegoua S, Mandel G. A single pulse of nerve growth factor triggers long-term neuronal excitability through sodium channel gene induction. Neuron. 1995;14:607–611. doi: 10.1016/0896-6273(95)90317-8. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Mendell LM. The spinal projections of individual identified A-δ- and C-fibers. J. Neurophysiol. 1988;59:41–55. doi: 10.1152/jn.1988.59.1.41. [DOI] [PubMed] [Google Scholar]
- Verdoorn TA, Draguhn A, Ymer S, Seeburg PH, Sakmann B. Functional properties of recombinant rat GABAA, receptors depend upon subunit composition. Neuron. 1990;4:919–928. doi: 10.1016/0896-6273(90)90145-6. [DOI] [PubMed] [Google Scholar]
- Verge VMK, Richardson PM, Weisenfeld-Hallin Z, Hökfelt T. Differential effect on nerve growth factor on neuropeptide expression in vivo: a novel role in peptide suppression in adult sensory neurons. J. Neurosci. 1995;15:208l–2096. doi: 10.1523/JNEUROSCI.15-03-02081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall PD, Devor M. The effect of peripheral nerve injury on dorsal root potentials and on transmission of afferent signals into the spinal cord. Bruin Res. 1981;209:95–111. doi: 10.1016/0006-8993(81)91174-4. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Kocsis JD, Black JA. Type III sodium channel mRNA is expressed in embryonic but not adult spinal sensory neurons, and is reexpressed following axotomy. J. Neurophysiol. 1994;72:466–470. doi: 10.1152/jn.1994.72.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White G. GABAA-receptor-activated current in dorsal root ganglion neurons freshly isolated from adult rats. J. Neurophysiol. 1990;64:57–63. doi: 10.1152/jn.1990.64.1.57. [DOI] [PubMed] [Google Scholar]
- White G. Heterogeneity in EC50 and nH of GABAA, receptors on dorsal root ganglion neurons freshly isolated from adult rats. Bruin Res. 1992;585:56–62. doi: 10.1016/0006-8993(92)91190-p. [DOI] [PubMed] [Google Scholar]
- Wong J, Oblinger MM. NGF rescues substance P expression but not neurofilament or tubulin gene expression in axotomized sensory neurons. J. Neurosci. 1991;11:543–552. doi: 10.1523/JNEUROSCI.11-02-00543.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Toma JG, Chan H, Smith R, Miller F. Disruption of fast axonal transport in vivo leads to alterations in Schwann cell gene expression. Dev. Biol. 1994;163:423–439. doi: 10.1006/dbio.1994.1159. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Matsuda Y, Samejima A. Tetrodotoxin-resistant sodium and calcium components of action potentials in dorsal root ganglion cells of the adult mouse. J. Neurophysiol. 1978;41:1096–1106. doi: 10.1152/jn.1978.41.5.1096. [DOI] [PubMed] [Google Scholar]