SUMMARY AND CONCLUSIONS
The permeability of non-N-methyl-D-aspartate (non-NMDA) glutamate channels to divalent cations and specifically the entry of Ca2+ and subsequent elevations in cytoplasmic and nuclear Ca2+ signals were investigated in cultured neonatal rat retinal ganglion cells using the whole cell patch-clamp technique and Ca2+ imaging with confocal microscopy. In addition, divalent-permeable non-NMDA receptor channels were studied in retinal slices using a Co2+ staining technique.
Using Ca2+ (2.5 mM) as the only permeable cation in the external solution, stimulation with 100 μM kainate produced nondesensitizing, nonselective cation currents with either low or high Ca2+ permeability. Both currents were reversibly blocked by 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX). Neurons with the low divalent-permeable currents (type 1) had reversal potentials of −41.5 ± 4.4 mV (mean ± SD), and neurons with the high divalent-permeable currents (type 2) had reversal potentials of −22.6 ± 5.5 mV. The permeability ratio PCa/PCs was 3.3 for the type 1 currents and 8.5 for the type 2 currents, indicating a 2.5-fold greater permeability to Ca2+ for the type 2 non-NMDA glutamate channels.
Both types of non-NMDA glutamate channels showed relatively little selectivity between Ca2+ and Co2+. The type 1 neurons had a slightly higher permeability to Co2+ than to Ca2+, whereas the type 2 neurons were equally permeable to both divalent cations. The type 2 neurons had a much higher permeability for both divalent cations compared with the type 1 neurons.
Staining for Co2+ uptake through kainate-stimulated non-NMDA glutamate channels in retinal slices provided additional evidence for the presence of the two ganglion cell populations. Activation of the neurons by kainate in conditions isolating the non-NMDA glutamate channel caused differential uptake of Co2+. In contrast, depolarization in the presence of the non-NMDA antagonist CNQX failed to cause Co2+ influx.
Imaging experiments using confocal microscopy showed that kainate stimulation induced an increase in intracellular Ca2+ in both types of retinal ganglion cells, but only the type 2 neurons showed a substantial increase in cytoplasmic and nuclear Ca2+ signals. Kainate-induced Ca2+ signals in the type 2 neurons were almost nine times greater than those of the type 1 neurons.
When intracellular Ca2+ stores were depleted by brief treatment with thapsigargin, kainate-induced Ca2+ signals in the type 1 neurons were unchanged. However, in the type 2 neurons kainate no longer induced large Ca2+ signals in the cytoplasm and nucleus, despite normal influx of Ca2+. After thapsigargin treatment, kainate-induced Ca2+ signals in the type 2 neurons were reduced to the levels of the treated or untreated type 1 neurons. Calcium influx via the type 2 receptor channels therefore appears capable of triggering the release of intracellular Ca2+ stores.
The two types of non-NMDA glutamate channels described here are likely to contribute to a variety of Ca2+-dependent intracellular processes. Glutamate may differentially affect intracellular Ca2+ in retinal ganglion cells. In particular, it appears that Ca2+ entry via the high divalent-permeable type 2 non-NMDA glutamate channel can trigger Ca2+-induced Ca2+ release and thereby amplify intracellular Ca2+ signals.
INTRODUCTION
Changes in intracellular Ca2+ concentration, [Ca2+]i, are important in regulating many cellular functions. In neurons, increased [Ca2+]i can initiate a variety of cellular events from neurosecretion to gene expression. Elevations in [Ca2+]i can arise from Ca2+ influx via voltage-gated Ca2+ channels or receptor-operated Ca2+-permeable ion channels. This influx can in turn release Ca2+ from internal stores.
Studies using confocal fluorescence microscopy have shown changes in nuclear Ca2+ signals in different neuronal types upon depolarization of the cell membrane (Birch et al. 1992; Hernandez-Cruz et al. 1991; Przywara et al. 1991). It has been proposed that altered nuclear Ca2+ levels might be involved in the regulation of gene transcription (Przywara et al. 1991), particularly because increased [Ca2+ ]i has been shown to be a key event in gene expression (Lerea et al. 1992; Morgan and Curran 1986; Zafra et al. 1990). Many studies of gene expression have used depolarization to activate voltage-gated Ca2+ channels, but Ca2+ influx and corresponding increases in gene expression also can occur with activation of either N-methyl-D-aspartate (NMDA) or non-NMDA receptors (Lerea et al. 1992; Zafra et al. 1990). Although non-NMDA receptor activation is known to cause Ca2+ influx indirectly via voltage-gated Ca2+ channels, recent studies have shown that Ca2+ can enter neurons through non-NMDA glutamate channels as well (Gilbertson et al. 1991; Iino et al. 1990), indicating a novel Ca2+ influx pathway that may potentially contribute to the initiation of gene expression.
In a recent in vivo study of gene activation, the non-NMDA glutamate agonist kainate was found to induce the expression of a c-fos-like protein in a subpopulation of ganglion cells in the rabbit retina (Osborne and Barnett 1992). It is unclear whether kainate acted directly or indirectly to activate Ca2+ influx in this study. Distinct protein subunits that can complex to form Ca2+-permeable non-NMDA glutamate channels have been identified in various layers of the rat retina using in situ hybridization (Hamassaki-Britto et al. 1993). Using Ca2+imaging techniques and pharmacological tools, we previously have observed large Ca2+ signals in cultured neonatal rat retinal neurons after stimulation with kainate (Kocsis et al. 1993), indicating Ca2+ influx through non-NMDA glutamate channels. In rat retinal ganglion cells, kainate produces a nondesensitizing current, which has an almost linear current-voltage relationship (Aizenman et al. 1988). This current also has been shown to be nonselective for cations and to be blocked by the non-NMDA antagonist kynureate but not by the NMDA antagonist 2-amino-5-phosphonovalerate (APV). In a subpopulation of salamander bipolar cells, kainate-induced currents have a high Ca2+ permeability (Gilbertson et al., 1991). However, in retinal ganglion cells the presence of Ca2+-permeable non-NMDA receptor channels has only been indicated via Ca2+ imaging techniques (Kocsis et al., 1993).
We are interested in elucidating the mechanisms that lead from activation of cell surface receptors to activation of genomic events (Birch et al. 1992; Kocsis et al. 1993, 1994). Using the whole cell patch-clamp technique, we now report that neonatal rat retinal ganglion cells possess either low Ca2+-permeable (type 1) or high Ca2+-permeable (type 2) nondesensitizing non-NMDA receptor currents. Using Ca2+ imaging with confocal microscopy, we also report that Ca2+ entry via the type 2 receptor channel can induce substantial increases in cytoplasmic and nuclear Ca2+ signals. These signals are shown to be caused not only by Ca2+ influx into the cell, but also by the release of Ca2+ from intracellular stores. The functional properties of these two non-NMDA glutamate receptor types have important implications for potential activation of Ca2+ -mediated gene expression. Some of these results have been published in abstract form (Leinders-Zufall et al. 1994).
METHODS
Freshly dissected retinas from postnatal day 2–3 (P2–P3) neonatal Wistar rats were incubated for 10 min at 37°C in Hanks’ balanced salt solution without Ca2+ and Mg2+ (Gibco) containing 0.25% papain (2× crystallized, Sigma), and 0.04% EDTA (Sigma). Retinas then were mechanically dispersed by trituration through a glass pipette and cells were plated on polyornithine and laminin-coated glass coverslips. The culture medium consisted of 45% Dulbecco’s modified Eagle’s medium (Gibco), 45% F-12 nutrient mixture (Gibco), 10% fetal calf serum and gentamicin (50 μg/ml). Culture medium was replaced every 2–3 days and cells were used for experiments after 3–6 days in culture. Retinal ganglion cells initially were identified by labeling with anti-Thy-1 (kindly provided by Dr. C. J. Barnstable) as previously described (Barnstable and Dräger 1984; Leifer et al. 1984). All cells that had triangular somas > 10μm wide, several short branched processes, and a single long fine process were found to be Thy-1 immunoreactive (data not shown). Cells fitting these morphological criteria exclude small retinal ganglion cells and all amacrine cells (Perry 1981). Retinal ganglion cells also are distinguished from amacrine and bipolar cells by the amplitude of their sodium currents (see Huba et al. 1992; Karschin and Wässle 1990), which are much larger in terms of absolute value and in relation to the outward currents (using K+ instead of Cs+ internally) when compared with amacrine cells. Sodium currents therefore were measured and used in addition to the morphological criteria to unequivocally identify retinal ganglion cells for the imaging and electrophysiological experiments.
Ionic currents were measured using the whole cell voltage-clamp technique. Patch electrodes were pulled from thick-walled borosilicate glass (A-M Systems) and had a resistance of 4–8 MΩ. Currents were recorded to videotape using an Axoclamp-2A amplifier; for off-line analysis the signals were digitized and stored on an IBM 386 computer using PCLAMP software (Axon Instruments). All solutions were prepared from milli-Q purified water (Millipore). Internal solution contained (mM): 140 CsCl, 20 N-2-hydroxyethylpiperazine- N′-2-ethanesulfonic acid (HEPES), 3 ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′,-tetraacetic acid (EGTA), and 0.661 CaCl2. The pH was adjusted to 7.2 with CsOH and the osmolarity was adjusted to 300 mOsm with sucrose. Normal external solution contained (mM): 140 NaCl, 20 HEPES, 5 KCl, 2.5 CaCl2, and 1 MgCl2. The pH was adjusted to 7.4 with NaOH and the osmolarity was adjusted to 330 mOsm with glucose. External choline solution contained (mM): 145 choline-Cl, 20 HEPES, and either 2.5 CaCl2 or 2.5 CoCl2. The pH was adjusted to 7.4 with N-methyl-D-glucamine and the osmolarity was adjusted to 330 mOsm with glucose. External solutions containing either 100μM kainate or 100μM kainate and 25 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, Cambridge Research Biochemicals) were delivered directly to individual cells via a multibarrel micropipette using a picospritzer (General Valve Corp.). Thapsigargin (Research Biochemicals International) was dissolved in ethanol, and small amounts of stock solution were dispersed in the external medium, yielding a nominal concentration of 400 nM thapsigargin and 0.2% (vol/vol) of solvent. Recordings were made at room temperature. Unless otherwise stated all chemicals were obtained from Sigma.
The apparent permeability ratios PCa/PCs and PCo/PCs were calculated as described by Iino et al. (1990) from the various values of reversal potentials using ion concentrations. The permeability ratios also were estimated, using ionic activities instead of ion concentrations. Ionic activities for Ca2+ and Cs+ were calculated by multiplying their concentrations by their activity coefficients, which were estimated from Shatkay (1968) and Bates et al. (1970).
Cells were loaded with Ca2+ indicator dye by adding fluo-3AM (Molecular Probes) at a final concentration of 5 μM and incubating for 35 min, as described previously (Kocsis et al. 1993). The fluo-3 loaded cells were imaged with a Bio-Rad MRC-500 confocal system and an inverted microscope (Nikon Diaphot) using a ×40, 1.3 numeric aperture objective. An argon ion laser was used for excitation illumination at λ488 nm. Confocal scanning settings (black level, gain, detection aperture, and neutral density filter) were kept the same over the series of experiments. Fluorescence intensities were measured with NIH Image (Wayne Rasband, National Institutes of Health). For all experiments 8–12 images were taken at 3-s intervals before, during, and after kainate application for 4 s with the multibarrel micropipette. The relative increase in fluorescence was calculated by dividing the difference between the image having the peak response during stimulation and the image before stimulation (control image) by the control image.
Cobalt uptake by kainate stimulation was examined in retinal slices using a modification of a method used to identify divalent cation-permeable kainate receptors in cerebellum and hippocampus (Pruss et al. 1991). Whole retinas were dissected rapidly and carefully transferred to petri dishes. The retinas were washed twice in uptake medium containing (in mM): 145 choline-Cl, 20 HEPES; pH adjusted to 7.4 with N-methyl-D-glucamine and osmolarity adjusted to 330 mOsm with glucose. The retinas then were stimulated for 30 min at room temperature in uptake medium supplemented with either 100 μM kainate, 2.5 mM CoCl2, and 100 μM APV or 50 mM KC1, 2.5 mM CoCl2, 100 μM APV, and 100 μM CNQX. After stimulation the retinas were rinsed once in uptake medium, then in uptake medium containing 2 mM EDTA to remove nonspecifically bound Co2+, and again in uptake medium; the intracellular Co2+ was then precipitated with 1.2% (NH4)2S for 5 min. After fixation with ice-cold 4% paraformaldehyde for ≥1 hour, retinas were embedded into 4% agar/phosphate-buffered saline (PBS) solution and cut into 50 or 100 μm slices with a Vibratome. The individual slices were transferred to microscope slides, after which a silver enhancement of the CoS precipitate was performed using the intenSEM kit from Amersham.
Data were analyzed with statistical software (StatView 4.0 and SuperANOVA 1.1, Abacus Software, Berkeley, CA; SAS/STAT, SAS Institute, Cary, NC). The least significant difference (LSD) test was used as a post hoc comparison of the analysis of variance (ANOVA). Results are presented as means ± SD.
RESULTS
Identification of two types of kainate-induced currents in subpopulations of retinal ganglion cells
Kainate (50 and 100 μM) evoked nondesensitizing currents in all dissociated neonatal rat retinal ganglion cells (Fig. 1 A). Currents induced by 100 μM kainate were inward at negative potentials and had a mean value of −514 ± 181 pA (n = 17) at −80 mV. Peak currents measured at several holding potentials were used to generate current-voltage relationships which did not differ from linearity in normal external solution (P = 0.97 – 0.99; Fig. 1 B). The average reversal potential for the kainate-induced currents was 0.93 ± 2.84 mV (n = 5). The responses to 100 μM kainate were completely and reversibly blocked by 25 μM CNQX, a selective antagonist of non-NMDA receptors (Fig. 1C).
FIG. 1.
A: kainate (KA) at a concentration of 100 μM produced nondesensitizing currents as shown at various membrane potentials in normal external solution. B: KA-induced currents are plotted as a function of the membrane potential. Reversal potential was 0 mV. C: effect of the non-N-methyl-D-aspartate (NMDA) antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) on the KA-induced current. In the control situation the retinal ganglion cell was stimulated by 100 μM kainate. Subsequent exposure of the same cell to 100 μM KA and 25 μM CNQX resulted in complete block of the current, which was reversible. The holding potential was −80 mV.
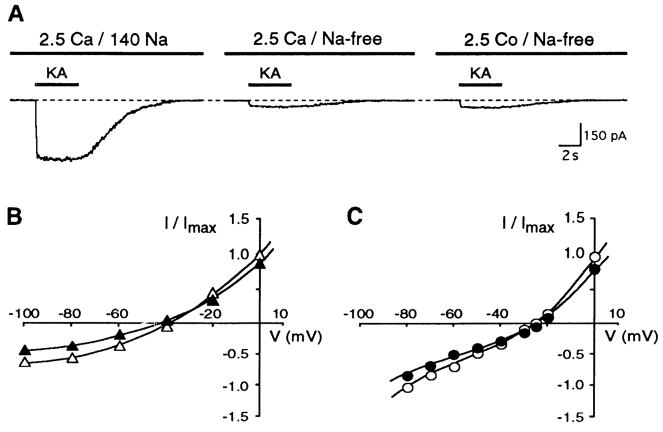
To test the kainate-induced current for Ca2+ permeability, we used a Na+, K+-free solution (choline solution) supplemented with 2.5 mM Ca2+. Under these conditions kainate induced small inward Ca2+ currents at −80 mV that could be blocked completely and reversibly by 25 μM CNQX (Fig. 2A). Inward currents were abolished completely using 145 mM choline with no other cations in the external solution. Comparison of the kainate-induced inward current in the presence and absence of Na+ showed that under normal physiological conditions most of the inward current is carried by an inward Na+ flow.
FIG. 2.
A: in all retinal ganglion cells, 100 μM KA was able to induce a current in Na+, K+ -free (choline) solution with 2.5 mM Ca2+ as the only permeable cation present. KA-induced Ca2+ current could be reversibly blocked by 25 μM CNQX. Holding potential was −80 mV. B: normalized current-voltage relationships of KA-induced currents from 3 different cells in choline solution only (△) or in choline solution supplemented with 2.5 mM Ca2+ (○ and ●). KA-induced currents in the presence of Ca2+ were classified into two types having a reversal potential either near −41 mV (type 1; ○) or near −22 mV (type 2; ●). Current shown in (A) belonged to a type 2 response. C: The two types of Ca2+ -permeable KA-induced currents in P2–P3 rat retinal ganglion cells did not change between day 3 and 6 in culture. Number of cells shown in parentheses.
The current-voltage relationship, measured in the presence of external choline only, demonstrated a complete outward rectification and a shift in the reversal potential as expected for a cation conductance (Fig. 2B; ▵). When the choline solution was supplemented with 2.5 mM Ca2+, two types of inward Ca2+ currents within the population of retinal ganglion cells were distinguished. The two populations had different reversal potentials and current amplitudes at −80 mV. The current-voltage relationships in 18 out of 36 cells had a slight outward rectification and a reversal potential of −41.5 ± 4.4 mV (Fig. 2B, ○). At −80 mV these kainate-induced Ca2+ currents had a mean value of −50.6 ± 15.4 pA. In the other 18 retinal ganglion cells, the current-voltage relationships showed a double rectification with a reversal potential of −22.6 ± 5.5 mV (Fig. 2B, • ) and currents of −74.3 ± 17.4 pA. Statistical analysis indicated that the two types were significantly distinct (ANOVA: P < 0.001). We refer to the low-permeable and high-permeable kainate responses as type 1 and type 2, respectively, and used a reversal potential of −30 mV as the cut-off between them (see also Fig. 4E). Figure 2A gives an example of a type 2 response and Fig. 3 A shows a type 1 response. The two types of Ca2+-permeable kainate currents did not change between day 3 and 6 of culture (LSD: P = 0.13 – 0.99; Fig. 2C), indicating that they differentiated before this time.
FIG. 4.
Retinal ganglion cells loaded with fluo-3 were imaged using confocal microscopy before and during application of 100,μM K. A while perfused with Na +, K + -free (choline) solution supplemented with 2.5 mM Ca2+. Type 1 retinal ganglion cells imaged before kainate application (A) and at peak fluorescence during kainate application (B) typically responded with a relatively low increase in Ca2+ -induced fluorescence. In contrast, type 2 retinal ganglion cells imaged before KA application (C) and at peak fluorescence during kainate application (D) typically responded with large increases in Ca2+ -induced fluorescence. Scale bar = 10 μm. E: scatterplot of reversal potentials vs. increases in Ca2+ -induced fluorescence for all imaged retinal ganglion cells. Neurons with low divalent cation permeability (type 1); reversal potentials less than −30 mV) are clearly distinguished from neurons with high divalent cation permeability (type 2; reversal potentials greater than −30 mV).
FIG. 3.
A: in retinal ganglion cells, 100 μM KA induced a current in Na+, K+ -free (choline) solution supplemented with 2.5 mM Ca2+ as well as in Na+, K+ -free (choline) solution supplemented with 2.5 mM Co2+ as the only permeable cation present. Holding potential was −80 mV. B: normalized current-voltage relationships of the type 1 KA-induced currents from a single cell in the presence of 2.5 mM Ca2+ (▲) or 2.5 mM Co2+ (△) as the only permeable cation in the external choline solution. C: normalized current-voltage relationships of the type 2 KA-induced currents from a single cell in the presence of 2.5 mM Ca2+ (●) or 2.5 mM Co2+ (○) as the only permeable cation in the external choline solution.
Because cations, which block voltage-gated Ca2+ channels, are able to move through divalent-permeable receptor channels (Iino et al. 1990; Ogura et al. 1990; Pruss et al. 1991), the determination of the reversal potential was repeated with choline solution supplemented with Co2+ instead of Ca2+. Before the reversal potential of the kainate-induced Co2+ current was measured, we determined whether the ganglion cells belonged to the type 1 or type 2 Ca2+ response using Ca2+ in the external solution as described above. The kainate-induced Co2+ peak current was always slightly higher than the Ca2+ peak current (Fig. 3A). Ganglion cells classified as having a type 1 response had Co2+ currents of −52.2 ± 8.2 pA (n = 3) and reversal potentials of −38.5 ± 3.1 mV(n = 3). Ganglion cells classified as having a type 2 response had Co2+ currents of −84.1 ± 21 pA (n = 3) and reversal potentials of −26.5 ± 4.0 mV (n = 3). For both types of cells, the shape of the current-voltage relationship was similar for Co2+ and Ca2+ (Fig. 3, B and C). The two types of Co2+ reversal potentials were not different from their corresponding Ca2+ reversal potentials (LSD: P = 0.63 for type 1 and P = 0.06 for type 2), but they were significantly different from each other (ANOVA: P < 0.001; Fig. 3, B and C). We were able to take advantage of the differential influx of Co2+ to stain the two populations of retinal ganglion cells in the whole retina (see below).
To estimate the permeability of the non-NMDA receptor channels to Ca2+ and to Co2+, the constant field equation was used as described by Iino et al. (1990). The permeability coefficients to Ca2+ and Cs+ (Pca/Pcs) were calculated to be 3.3 ± 0.6 (n = 18) for type 1 and 8.5 ± 2.5 (n= 18) for type 2, revealing a 2.5 times higher permeability of the type 2 non-NMDA receptor channel to Ca2+ (see Table 1). The permeability coefficients for Co2+ and Cs+ (PCo/Pcs) were 3.8 ± 0.5 (n = 3) for the type 1 and 6.8 ± 1.4 (n = 3) for the type 2 responses, revealing a 1.8 times greater permeability of the type 2 non-NMDA receptor channel to Co2+. The permeability ratios for Co2+ relative to Ca2+ were calculated from the shift in the reversal potential measured in the same cell, assuming constant PCs. The relative permeabilities were 1.34 ± 0.30 and 0.94 ± 0.09 for the type 1 and type 2 responses, respectively. Thus the type 1 response was more permeable to Co2+ than to Ca2+ whereas the type 2 response was equally permeable to both divalent cations. However, the type 2 response had a much higher permeability for both divalent cations compared with the type 1 response (LSD: P < 0.05).
TABLE 1.
Apparent permeability ratios for glutamate-gated ion channels
| PCa/Pmono |
||||
|---|---|---|---|---|
| 1 | 2 | Receptor | Tissue | Reference |
| 0.12 | 0.15 | Non-NMDA | Mouse hippocampus and spinal cord | Mayer and Westbrook 1987 |
| <0.18 | Type 1 non-NMDA | Rat hippocampus | Iino et al. 1990 | |
| 2.3 | 5.3 | Type 2 non-NMDA | ||
| 3.2 | Non-NMDA | Salamander bipolar cells | Gilbertson et al. 1991 | |
| 1.4 | Non-NMDA | Fusiform glia cells (~ Bergman glia) | Burnashev et al. 1992a | |
| 3.3 | 7.1 | Type 1 non-NMDA | Rat retinal ganglion cells | Present study |
| 8.5 | 17.5 | Type 2 non-NMDA | ||
| 4.0 | 10.6 | NMDA | Mouse hippocampus and spinal cord | Mayer and Westbrook 1 987 |
| 6.2 | 14.3 | NMDA | Rat hippocampus | Iino et al. 1990 |
The apparent Ca2+/monovalent ion permeability ratios (PCa/Pmono) were calculated from reversal potentials using the constant field equation with either ionic concentrations (1) or ionic activities (2).
Kainate stimulation increases cytoplasmic and nuclear Ca2+ signals
The method of loading Ca2+ indicator dye into cells can influence the measurement of nuclear Ca2+ signals (Rand et al. 1994a,b). In the present study, retinal ganglion cells were loaded using the nondisruptive, acetoxymethylester (AM) form of fluo-3. Confocal measurements of kainate-induced Ca2+ signals were made for each retinal ganglion cell before patch clamping to avoid prestimulating the neurons with the patch pipette. When we attempted to combine imaging and patch-clamp techniques, the cells appeared to be activated at the moment of breaking the membrane to voltage clamp the cell. Even though we were careful to preset the amplifier to the average resting potential (−50 mV) to avoid activation of voltage-sensitive Ca2+ channels and buffered the intracellular solution to 10 nM Ca2+, imaging the neurons at the moment of membrane disruption indicated that an influx of Ca2+ occurred in most of the cells. This resulted in elevated nuclear Ca2+ signals at baseline that lasted for many minutes. When the neurons then were stimulated with kainate, only small increases in the already elevated Ca2+ signals occurred. By imaging the neurons before disturbing them with the patch pipette we were able to avoid this problem.
After loading retinal neurons with fluo-3AM, retinal ganglion cells had low Ca2+ signals at rest (Fig. 4, A and C). Upon 4 s of kainate stimulation Ca2+ signals rose rapidly, peaked ~10 s later, and then recovered over a period of ~40 s to the original baseline. Kainate induced an increase in intracellular Ca2+ signals in both types of retinal ganglion cells, but only those with type 2 kainate-induced currents showed a substantial increase in cytoplasmic and nuclear Ca2+ signals (Fig. 4, B and D).
Confocal microscopy measurements of fluo-3-loaded retinal ganglion cells confirmed the results showing Ca2+ permeation through kainate-gated channels, and showed a dramatic difference in the effect of Ca2+ influx through the two types of non-NMDA channels. Retinal ganglion cells were imaged before and during application of 100 μM kainate while they were perfused with choline solution containing 2.5 mM Ca2+. All imaged neurons subsequently had their reversal potentials determined. Figure 4, A–D, shows two retinal ganglion cells before kainate application and at peak fluorescence during kainate application. Two types of Ca2+ responses were observed. One population of retinal ganglion cells had a relatively low increase in fluorescence (as shown in Fig. 4, A and B), whereas the other had a substantial increase in cytoplasmic and nuclear Ca2+ signals upon kainate stimulation (as shown in Fig. 4, C and D). These two types of elevated Ca2+ signals were exclusively associated with the presence of the type 1 or type 2 kainate-induced Ca2+ currents (Fig. 4E). Neurons with fluorescence increases >50% fit the classification of type 2 neurons, and were clearly distinct from the type 1 neurons. To verify this, a discriminant analysis was used to test the hypothesis that there were two different groups (as opposed to a continuum of responses) based on reversal potentials and fluorescence changes; the result was significant at P < 0.02 (χ2 = 10.33), with 100% concordance between tested and predicted group membership.
The imaged neurons had reversal potentials of −39.6 ± 2.6 mV(n = 8) for the type 1 and −22.0 + 5.3 mV(n = 9) for the type 2 responses. The type 1 ganglion cells had a relatively low increase in fluorescence, whereas the type 2 cells showed a considerable increase in Ca2+ signals (Fig. 5A). The range of increases in fluorescence was 0.9–38% for type 1 and 83–207% for type 2 responding cells. The two types were very different in their Ca2+ -induced fluorescence (ANOVA: P < 0.001). The enhancement in Ca2+ signals for type 2 ganglion cells was almost nine times higher than the type 1 ganglion cells. Glutamate might therefore differentially affect [Ca2+]i in retinal ganglion cells via these two types of non-NMDA-gated ion channels.
FIG. 5.
Both type 1 and type 2 retinal ganglion cells responded with an increase in Ca2+ signals upon kainate stimulation. A: retinal ganglion cells having high Ca2+ -permeable type 2 currents with reversal potentials near −22 mV had a considerably greater increase in Ca2+ -induced fluorescence compared with retinal ganglion cells having low Ca2+ -permeable type 1 currents with reversal potentials near −41 mV. B: nuclear Ca2+ signals exceeded those of the cytoplasm, and neurons with type 2 responses had much larger Ca2+ signals than those with type 1 responses. C: after thapsigargin treatment, type 1 and type 2 retinal ganglion cells could not be differentiated by their increase in fluorescence upon kainate stimulation. D: after thapsigargin treatment, nuclear Ca2+ signals still exceeded those of the cytoplasm, but neurons with type 2 responses had Ca2+ signals that were reduced to the same level as neurons with type 1 responses. Thapsigargin treatment did not affect the magnitude of KA-induced Ca2+ signals in type 1 neurons. The dashed lines indicate the mean reversal potentials of the 2 types.
In both types of retinal ganglion cells, nuclear Ca2+ signals exceeded those of the cytoplasm upon stimulation with kainate (Fig. 5B). The ratio of nuclear to cytoplasmic Ca2+ signals was 1.50 ± 0.66 (n = 5) for type 1 neurons and 1.60 ± 0.30 (n = 6) for type 2 neurons. However, the type 2 neurons had much larger Ca2+ signals in both intracellular compartments.
The kainate-induced Ca2+ signals may have been due to Ca2+ entering the cell and triggering the release of Ca2+ from intracellular stores. To investigate possible amplification of the signals by Ca2+ -induced Ca2+ release (CICR), 400 nM thapsigargin was applied to the bath for ≥ 10 min. Thapsigargin specifically inhibits the family of Ca2+ -ATP-ases that move Ca2+ into the lumen of the sarcoplasmic or endoplasmic reticulum, and consequently depletes intracellular Ca2+ stores (Thastrup et al. 1990). We used the same imaging and electrophysiological procedures as described above and compared the thapsigargin-treated neurons to the previously described ganglion cells (control). Fluorescence signals in the cytoplasm and nucleus did not noticeably change during thapsigargin treatment and remained approximately equal in the two compartments. Figure 6 shows control and thapsigargin-treated retinal ganglion cells at peak fluorescence during kainate application. After thapsigargin treatment, only type 2 ganglion cells had a substantially lower increase in Ca2+ signals upon kainate stimulation (LSD: P < 0.001). For all thapsigargin-treated type 1, ganglion cells the stimulation-induced fluorescence was not significantly different from the control type 1 ganglion cells (LSD: P = 0.63; Fig. 5A and C). Notably, the increase in fluorescence of the type 2 ganglion cells was only 11–90% instead of 83–207% and did not differ from the small increase in fluorescence of the type 1 ganglion cells (LSD: P = 0.27; Fig. 5C). The reduced increase in Ca2+ signals in type 2 ganglion cells after thapsigargin treatment was not due to an inhibition of the kainate current. Comparison of the kainate-induced Ca2+ currents in the absence and presence of thapsigargin revealed no change in the peak currents (LSD: P = 0.17 and 0.52 for type 1 and type 2, respectively). The reversal potentials also did not differ from their corresponding values in the control situation (LSD: P = 0.36 and 0.91 for type 1 and type 2, respectively). The relatively large Ca2+ currents through the type 2 receptor channel therefore appeared to trigger CICR and lead to an amplification of the intracellular Ca2+ signals.
FIG. 6.
Thapsigargin-treated retinal ganglion cells loaded with fluo-3 were imaged using confocal microscopy during application of 100 μM KA in Na+, K+-free (choline) solution supplemented with 2.5 mM Ca2+. A type 1 retinal ganglion cell imaged at peak fluorescence during K A application before (A) and duringthapsigargin treatment (B) responded with approximately the same low increase in Ca2+-induced fluorescence. A type 2 retinal ganglion cell imaged at peak fluorescence during KA application responded with a large increase in Ca2+-induced fluorescence before treatment with thapsigargin (C), and a small increase in Ca2+-induced fluorescence after exposure to thapsigargin (D). Scale bar = 10 μm.
After thapsigargin treatment, nuclear Ca2+ signals still exceeded those of the cytoplasm in both types of neurons upon kainate stimulation (Fig. 5D). The increase for the Ca2+ signals in the nucleus relative to the cytoplasm was 1.26 ± 0.15 (n = 5) for the type 1 neurons and 1.15 ±0.07 (n = 4) for the type 2 neurons. However, the type 2 neurons no longer had much larger Ca2+ signals in both intracellular compartments. Nuclear-to-cytoplasmic fluorescence ratios for control and thapsigargin-treated cells upon kainate stimulation were not significantly different irrespective of conductance type (LSD: P = 0.07–0.68).
Kainate-induced cobalt uptake in retinal slices
Fresh whole retinas were stimulated with kainate in conditions that isolated Co2+ influx via the non-NMDA receptor channels to determine if the two populations of retinal ganglion cells having high and low divalent-permeable channels were present in the intact retina. This was done because the channel properties of the two populations observed in vitro might have been altered by the techniques of dissociation and culture. Because the type 2 response had a much higher permeability to Co2+ than the type 1 response, we attempted to see a difference in Co2+ staining in cells within the ganglion cell layer of the whole retina.
Because of the possibility of glutamate release in the whole retina, all Co2+ staining experiments were carried out in the presence of the NMDA antagonist APV to prevent Co2+ entry through NMDA glutamate channels. As a control, whole retinas were depolarized with 50 mM K+ in the presence of the kainate antagonist CNQX, to ensure that Co2+ influx did not occur through voltage-gated Ca2+ channels during the period of kainate stimulation (30 min). Depolarization failed to induce Co2+ uptake under these conditions (Fig. 7A). Cobalt uptake during kainate stimulation in the presence of APV therefore would be expected to be caused by influx through divalent-permeable non-NMDA receptor channels.
FIG. 7.
Cobalt uptake in radial sections from P3 rat retina induced by (A) depolarization using 50 mM KCl in the presence of 100 μM CNQX or (B) stimulation with 100 μM kainate. Both conditions used 145 mM choline solution supplemented with 2.5 mM Co2+ and included 100 μM 2-amino-5-phosphonovalerate to prevent activation of NMDA receptor channels. Cobalt staining was detected only in retinal slices stimulated by K.A, as indicated by the appearance of brown precipitate in the cell bodies. The ganglion cell layer shows lighter (arrow) and darker stained cells (arrowhead). GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; ONL, outer nuclear layer.
When 100 μM kainate was used to stimulate the retinas of P3 animals, many retinal neurons took up Co2+, indicated by a brown precipitate in the cell bodies (Fig. 7B). The darkest-stained cells were found in the ganglion cell layer and in the part of the inner nuclear layer that borders the inner plexiform layer. The staining in the ganglion cell layer was heterogeneous, and either dark or lightly-stained cells were observed (Fig. 7B). Differential staining always was observed when retinas were stimulated for ≤30 min, but after 45 min saturated staining occurred. These results indicate that Co2+ influx was responsible for the differential staining. There was also no discernible edge artifact; as can be seen in Fig. 7B, cells near the inner plexiform layer were differentially stained as well as cells at the edge of the ganglion cell layer. High-power magnification (not shown) revealed that cells with almost no staining were not empty of cytoplasmic material but only less-darkly stained, and dark-stained neurons were single cells, not overlapping cells. Only a few neurons at the edge of the tissue appeared to be washed out during the staining procedure, and they were easily identified at high-power magnification.
Although we cannot exclude the possibility that some of the darkly stained cells in the ganglion cell layer are displaced amacrine cells, the size of the cells and their location within the ganglion cell layer indicate that they are likely to be ganglion cells and not displaced amacrine cells. Displaced amacrine cells have been found in the ganglion cell layer of adult rat retina (Perry 1981}. However, at postnatal day 3, 70–85% of the cells in the ganglion cell layer are retinal ganglion cells (Perry et al. 1983). Furthermore, displaced amacrine cells at P3 are located predominantly at the border of the ganglion cell layer and the inner plexiform layer. The likelihood of amacrine cells accounting for the differential staining in the ganglion cell layer at P3 therefore seems remote. The heterogeneous staining of the retinal ganglion cell layer supports the idea that the two types of retinal ganglion cell responses in vitro were not a characteristic of the acute dissociation.
The dark-stained cells at the inner border of the inner nuclear layer (Fig. 7B) are likely to be amacrine cells, and they appear to have the high Ca2+-permeable non-NMDA glutamate channel. Cells in the outer nuclear layer of the rat retinal slices also were stained and appear to be rod photoreceptors. The intensity of the staining indicates that they too may have Ca2+-permeable non-NMDA glutamate channels at this point in development. Although not clearly shown in Fig. 7B, we frequently have observed darkly stained cells at regular distances along the outer border of the inner nuclear layer, proximal to the not-yet-formed outer plexiform layer. These may be horizontal cells, which previously have been shown to have Ca2+-permeable non-NMDA glutamate channels (Linn and Christensen 1992).
DISCUSSION
Two populations of retinal ganglion cells were identified by the presence of kainate-induced currents with either a low (type 1) or a high (type 2) divalent cation permeability. The type 2 kainate-gated ion channels had a higher permeability to Ca2+ than the type 1 kainate-gated ion channels. The presence of these two populations of ganglion cells in the retina was supported by a Co2+ staining technique modified from Pruss et al. (1991).
In both types of ganglion cells, Ca2+ signals were elevated after stimulation with kainate. In ganglion cells possessing the type 1 response there was only a slight increase in intracellular Ca2+ signals. The larger Ca2+ currents mediated by the type 2 receptor ion channels induced a dramatic elevation in cytoplasmic and nuclear Ca2+ signals. A substantial portion of these cytoplasmic and nuclear Ca2+ signals was due to the release of Ca2+ from intracellular stores. These results establish a direct relationship between kainate-induced currents and elevated nuclear Ca2+ signals in retinal ganglion cells.
Divalent cation-permeable ion channels activated by kainate
On the basis of ligand affinity, non-NMDA receptors are classified as α-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) receptors and kainate receptors (Keinänen et al. 1990; Seeburg 1993; Sommer and Seeburg 1992). The family of AMPA receptors comprises four different protein subunits, GluR1 to GluR4, which when assembled show nondesensitizing currents to kainate stimulation (Keinänen et al. 1990). In contrast, high-affinity kainate receptor ion channel complexes are formed from protein subunits GluR5 to GluR7 as well as KA1 and KA2, and show desensitizing currents upon kainate activation (Herb et al. 1992). In addition, AMPA can activate receptors formed by particular combinations of the high-affinity kainate receptor subunits (see Seeburg 1993; Sommer and Seeburg 1992). The concentration of kainate used in the present study was likely sufficient to activate both types of non-NMDA glutamate channels. The kainate-induced currents in our experiments showed no desensitization, indicating that they belong to the family of AMPA receptors. Consistent with these data, Hamassaki-Britto et al. (1993) found that there was more labeling for protein subunits of the AMPA receptor family in the ganglion cell layer than for the subunits of the high-affinity kainate receptor.
The Ca2+ permeability and the current-voltage relationship of recombinant non-NMDA glutamate channels is dominated by the presence of the GluR2 subunit (Burnashev et al. 1992b; Hollmann et al. 1991; Nakanishi et al. 1990; Verdoorn et al. 1991). In the presence of physiological external solution, recombinant AMPA glutamate channels coassembled with GluR2 protein subunits show either linear or simple outward rectification and lose their Ca2+ permeability. In the present study, both types of non-NMDA receptor currents had linear current-voltage relationships in normal external solution, implying that the retinal ganglion cell receptors have GluR2 subunits. We therefore would expect the retinal ganglion cells to have low Ca2+ permeability; in contrast, we found that they had relatively large kainate-induced Ca2+ currents. Hamassaki-Britto et al. (1993) reported that patches of cells within the rat retinal ganglion cell layer were labeled heavily by the GluR2 subunit probe. Our results suggest that this subpopulation of retinal ganglion cells might possess the low Ca2+-permeable type 1 response, whereas the remainder of the ganglion cells might underexpress or lack the GluR2 subunit, conferring the high Ca2+ permeability of the type 2 response. In fact, a study using recombinant ion channel subunit expression has shown that a greater amount of GluR2 coinjected for heteromeric ion channel expression decreases the Ca2+ permeability of the resultant receptor ion channel (Hollmann et al. 1991).
In most neurons, non-NMDA glutamate channels have linear or outwardly rectifying current-voltage relationships and exhibit low permeability to Ca2+ (Mayer and Westbrook 1987), but in some neurons and glia cells the non-NMDA receptor channels are highly permeable to Ca2+ (Burnashev et al. 1992a; Gilbertson et al. 1991; Iino et al. 1990). Table 1 shows reported Ca2+/monovalent ion permeability ratios (PCa/Pmono) of NMDA and non-NMDA glutamate channels in various tissues, as well as those found in the present study. In hippocampal neurons, two types of non-NMDA receptors have been observed. Type 1 receptors have a low permeability ratio and exhibit a linear current-voltage relationship, whereas the type 2 receptors have a high permeability ratio and exhibit a strong inward rectifying current-voltage relationship (Iino et al. 1990). In contrast, non-NMDA receptors in salamander bipolar cells exhibit a high permeability ratio and show a linear current-voltage relationship (Gilbertson et al. 1991). The two types of non-NMDA receptors reported here resemble those of bipolar cells, in that they are Ca2+ permeable and have linear current-voltage relationships. The type 1 receptor described in the present study has a permeability ratio in the same range as that previously reported for non-NMDA receptors with high permeability to Ca2+ (Table 1). The type 2 receptor had a much higher permeability to Ca2+ and even exceeded the permeability ratios reported for the NMDA receptor channel (Table 1). The high Ca2+ permeability of the two types of non-NMDA glutamate channels indicates that they may contribute to a variety of Ca2+-dependent processes in the retina. Although we used a maximal or near-maximal dose of kainate in the present study (see Murphy et al. 1987; Ozawa et al. 1991), it is possible that differential agonist sensitivity might account for the two types of divalent-permeable responses.
In contrast to voltage-gated Ca2+ channels, Ca2+-permeable non-NMDA receptor channels have been reported to be permeable to a wide range of other divalent cations including Ba2+, Mg2+, Sr2+, and Mn2+ (Burnashev et al. 1992b; Hollmann et al. 1991; Iino et al. 1990) and have been shown indirectly to be permeable to Co2+ (Pruss et al. 1991). Our results demonstrate that the non-NMDA receptor is highly permeable to Co2+ in retinal ganglion cells. The permeability sequence of the type 1 and type 2 responses was Co2+ > Ca2+ and Co2+ = Ca2+, respectively. Because the type 2 Co2+ permeability was almost two times greater than the type 1 Co2+ permeability, we used a Co2+ staining technique to identify the two populations of retinal ganglion cells in retinal slices, supporting the electrophysiological and Ca2+ imaging data. The existence of physiologically different populations of retinal ganglion cells has been implicated by measurements of cGMP-gated cation channels in a subpopulation of these cells (Ahmad et al. 1994) and by measurements of glutamate currents in retinal whole mount preparations (Röhrig and Grantyn 1993).
Apart from heterogeneous Co2+ staining in the ganglion cell layer, the inner third of the inner nuclear layer also was stained heavily by Co2+. This is the same region that has strong labeling for GluR3 and GluR4 subunit probes (Hamassaki-Britto et al. 1993), supporting the presence of high divalent-permeable AMPA receptors in a subpopulation of bipolar cells (Gilbertson et al. 1991) and possibly amacrine cells. In addition, Hamassaki-Britto et al. (1993) report that GluR3 and GluR4 labeling appeared to be concentrated in some cell bodies in the outer third of the inner nuclear layer, where horizontal cells are located, and Ca2+ permeable non-NMDA glutamate channels have been reported in horizontal cells of the fish retina (Linn and Christensen 1992). In the present study, scattered cell bodies in this region had strong Co2+ staining, supporting the presence of high Ca2+-permeable receptor ion channels in a subset of horizontal cells.
Increased Ca2+ signals after kainate stimulation
Both types of retinal ganglion cells responded to kainate with elevations in cytoplasmic and nuclear Ca2+, but the type 2 neurons had substantially greater Ca2+ signals. There are several possible explanations for the different magnitudes of the Ca2+ signals. First, they might be exclusively due to the amount of Ca2+ influx. Alternatively, because Ca2+ can cause the release of Ca2+ from intracellular stores (Holliday et al. 1991; Lipscombe et al. 1988), the amount of Ca2+ influx might trigger or modulate the amount of Ca2+ released from intracellular stores. To assay the possible contribution of CICR to the Ca2+ signals, we treated neurons with thapsigargin, which depletes intracellular Ca2+ stores (Thastrup et al. 1990). This manipulation allowed us to generally distinguish Ca2+ influx from the combination of Ca2+ influx and subsequent CICR. After thapsigargin treatment, the Ca2+ signals of type 2 ganglion cells were diminished to the level of the type 1 ganglion cells. These results indicate that Ca2+ entry via the type 2 non-NMDA glutamate channels evoked CICR. The latency to the peak of the kainate-induced Ca2+ signals in untreated neurons (~6 s after the end of stimulation) also indicates the release of Ca2+ from intracellular stores. In retinal horizontal cells of the rabbit retina (Linn and Christensen 1992) and in spinal neurons of Xenopus embryos (Holliday et al. 1991), Ca2+ entry has been reported to be amplified by CICR. In rat retinal neurons, caffeine pretreatment decreased intracellular Ca2+ signals (probably by depletion of Ca2+ stores) and removal of extracellular Ca2+ greatly reduced kainate-induced intracellular Ca2+ signals (Kocsis et al. 1993). The kainate-induced intracellular Ca2+ signals therefore appear to be dependent on Ca2+ influx and subsequent amplification via CICR. Interestingly, in the present study the kainate-induced Ca2+ signals of the type I ganglion cells did not change after thapsigargin treatment. These results suggest that either Ca2+ entry in the type 1 neurons is not sufficient to activate CICR or that these neurons do not have large accessible Ca2+ stores. Regarding the latter possibility, depolarization caused Ca2+ signals of the same magnitude as the type 2 neurons (data not shown), indicating that the type 1 neurons have similar Ca2+ stores.
Although it has been suggested that spatially distinct sites of Ca2+ entry (Lerea and McNamara 1993) or the mode of Ca2+ entry (Bading et al. 1993) might differentially affect transcriptional activation, it seems likely that the amount of Ca2+ entry also might modulate activation of a subset of cellular events. The two Ca2+-permeable non-NMDA glutamate channels described here therefore may confer different physiological and genomic responses to glutamate stimulation depending on the differential expression and composition of GluR protein subunits. Burnashev et al. (1992b) have suggested that the assembly of several protein subunits into a functional ion channel may depend on their respective mRNA levels in the cell and may generate a range of different whole cell current-voltage relationships and divalent permeabilities. Our observations of two distinct populations of Ca2+-permeable non-NMDA receptors indicate a more restricted expression or assembly of GluR protein subunits for retinal ganglion cells.
Nuclear Ca2+ signals in retinal ganglion cells
Several studies of neurons using AM Ca2+ indicator dyes and confocal microscopy have found that nuclear Ca2+ signals increase more than those in the cytoplasm upon depolarization (Birch et al. 1992; Hernandez-Cruz et al. 1991; Holliday et al. 1991; Przywara et al. 1991). AM Ca2+-indicator dyes are trapped metabolically by cells, loading them in a nondisruptive manner. A study using micropipette loading of Ca2+ indicator dye in neurons has suggested that depolarization-induced Ca2+ signals are not higher in the nucleus compared with the cytoplasm (Al-Mohanna et al. 1994). However, we have found recently that micropipette dye loading can elevate baseline nuclear Ca2+ signals (Rand et al. 1994a, b), confounding measurements of [Ca2+]i relative to baseline. In the present study, we initially found that patch clamping the retinal ganglion cells raised their intracellular Ca2+ signals, particularly in the nucleus, and it was therefore necessary to image kainate-induced Ca2+ signals in individual neurons before mechanically perturbing them with the patch pipette.
Regardless of whether nuclear Ca2+ signals are greater than cytoplasmic Ca2+ signals, Ca2+ consistently has been shown to increase in both intracellular compartments upon stimulation (Al-Mohanna et al. 1994; Birch et al. 1992; Hernandez-Cruz et al. 1991; Holliday et al. 1991; Przywara et al. 1991). Observations of stimulation-induced Ca2+ transients imply that a critical level of [ Ca2+ ]i must occur in either the cytoplasm, nucleus, or both before transcription of a subset of genes, given that increased [Ca2+]i has been shown to be important for various types of gene expression (Lerea et al. 1992; Morgan and Curran 1986; Zafra et al. 1990). In the present study, fluo-3 AM showed that nuclear Ca2+ signals always increased more than those in the cytoplasm upon kainate stimulation. Independent of thapsigargin treatment or type of retinal ganglion cell response, nuclear-to-cytoplasmic Ca2+ signal ratios were relatively constant, despite the fact that control type 2 neurons had signals almost nine times greater than type 1 neurons or thapsigargin-treated neurons. This is noteworthy because it indicates that the processes underlying differential signals in the cytoplasm and nucleus might be independent of CICR.
Physiological function of Ca2+-permeable non-NMDA receptors
In the retina glutamate is regarded as a major excitatory neurotransmitter for NMDA and non-NMDA glutamate channels. Although NMDA receptors are abundant in the inner retina, they mediate only a minor portion of the light-driven input to ganglion cells in rabbit retina (Massey and Miller 1990). Therefore, it is likely that signal transmission from bipolar cells to ganglion cells is carried primarily by non-NMDA receptors. Activation of non-NMDA receptors may be involved in c-fos expression in the ganglion cell layer. Under a physiological light/dark cycle, c-fos mRNA levels in a subpopulation of retinal ganglion cells increased transiently for 30 min immediately after the onset of the light period (Yoshida et al. 1993). In addition, an intraocular injection of kainate induced the expression of c-fos-like protein in a subpopulation of ganglion cells in the rabbit retina (Osborne and Barnett 1992). These reports support the involvement of non-NMDA receptors in gene expression in the retinal ganglion cell layer.
In the present study, extracellular sodium was absent to isolate Ca2+ influx via the non-NMDA glutamate channel. Although these conditions were not physiological, we have shown previously that Ca2+ influx and amplification occur with kainate stimulation in the presence of extracellular sodium and that removal of extracellular Ca2+ greatly reduces kainate-induced intracellular Ca2+ signals (Kocsis et al. 1993). The contribution of Ca2+ influx via the non-NMDA glutamate channel in normal extracellular solution also has been directly measured in septal neurons having similar linear conductance properties (Schneggenburger et al. 1993). This study found that the fractional contribution of Ca2+ to the currents was relatively large, consistent with the results reported here.
We have shown that there are two populations of rat retinal ganglion cells with distinct types of divalent cation-permeable non-NMDA glutamate channels. The two types of non-NMDA responses produce different levels of intracellular Ca2+ signals and might differentially modulate Ca2+-dependent gene expression. In type 2 retinal ganglion cells, one pathway for Ca2+ signaling appears to be CICR upon Ca2+ influx via the non-NMDA receptor ion channel resulting in high cytoplasmic and nuclear Ca2+ signals. In type 1 retinal ganglion cells, we did not find evidence for activation of CICR via this pathway. Although Ca2+ influx was smaller in the type 1 than the type 2 retinal ganglion cells, it was still able to elevate intracellular Ca2+ signals. Calcium influx through the non-NMDA receptor in retinal ganglion cells, and particularly the type 2 non-NMDA receptor channel, therefore might contribute to a variety of Ca2+-dependent processes in the retina, analogous to what has been suggested for the NMDA receptor in the hippocampus (Bliss and Collingridge 1993; Goodman and Shatz 1993). In this regard, the role of Ca2+ influx through non-NMDA glutamate channels may have been underestimated previously (see Schneggenburger et al. 1993).
Acknowledgments
We thank H. F. Mi for technical assistance in staining the retina slices, Dr. Frank Zufall for discussion and helpful comments on the manuscript, and Dr. Colin J. Barnstable for kindly supplying Thy-1 antibody and for help with the anatomy of the Co2+-stained retinal slices.
This investigation was supported by the Medical Research Service, Department of Veterans Affairs, and the National Institutes of Health.
References
- Ahmad I, Leinders-Zufall T, Kocsis JD, Shepherd GM, Zufall F, Barnstable CJ. Retinal ganglion cells express a cGMP-gated cation conductance activatable by nitric oxide donors. Neuron. 1994;12:155–165. doi: 10.1016/0896-6273(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Aizenman E, Frosch MP, Lipton SA. Responses mediated by excitatory amino acid receptors in solitary retinal ganglion cells from rat. J Physiol Lond. 1988;396:75–91. doi: 10.1113/jphysiol.1988.sp016951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mohanna FA, Caddy KWT, Bolsover SR. The nucleus is insulated from large cytosolic calcium ion changes. Nature Lond. 1994;357:745–750. doi: 10.1038/367745a0. [DOI] [PubMed] [Google Scholar]
- Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science Wash DC. 1993;260:181–186. doi: 10.1126/science.8097060. [DOI] [PubMed] [Google Scholar]
- Barnstable CJ, Dräger UC. Thy-1 antigen: a ganglion cell specific marker in rodent retina. Neuroscience. 1984;11:847–855. doi: 10.1016/0306-4522(84)90195-7. [DOI] [PubMed] [Google Scholar]
- Bates RG, Staples BR, Robinson RA. Ionic hydration and single ion activities in unassociated chlorides at high ionic strengths. An Chem. 1970;42:867–871. [Google Scholar]
- Birch BD, Eng DL, Kocsis JD. Intranuclear Ca2+ transients during neurite regeneration of an adult mammalian neuron. Proc Natl Acad Sci USA. 1992;89:7978–7982. doi: 10.1073/pnas.89.17.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature Lond. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Khodorova A, Jonas P, Helm PJ, Wisden W, Monyer H, Seeburg PH, sakmann B. Calcium-permeable AMPA-kainate receptors in fusiform cerebellar glial cells. Science Wash DC. 1992a;256:1566–1570. doi: 10.1126/science.1317970. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992b;8:189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- Gilbertson TA, Scobey R, Wilson M. Permeation of calcium ions through non-NMDA glutamate channels in retinal bipolar cells. Science Wash DC. 1991;251:1613–1615. doi: 10.1126/science.1849316. [DOI] [PubMed] [Google Scholar]
- Goodman CS, Shatz CJ. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell/Neuron. 1993;10(Suppl):77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- Hamassaki-Britto DE, Hermans-Borgmeyer I, Heinemann S, Hughes TE. Expression of glutamate receptor genes in the mammalian retina: The localization of GluRl through GluR7 mRNAs. J Neurosci. 1993;13:1888–1898. doi: 10.1523/JNEUROSCI.13-05-01888.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herb A, Burnashev N, Werner P, Sakmann B, Wisden W, Seeburg PH. The KA-2 subunit of excitatory amino acid receptors shows widespread expression in brain and forms ion channels with distantly related subunits. Neuron. 1992;8:775–785. doi: 10.1016/0896-6273(92)90098-x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Cruz A, Sala F, Adams PR. Subcellular calcium transients visualized by confocal microscopy in a voltage-clamped vertebrate neuron. Science Wash DC. 1990:858–862. doi: 10.1126/science.2154851. [DOI] [PubMed] [Google Scholar]
- Holliday J, Adams RJ, Seinowski TJ, Spitzer NC. Calcium-induced release of calcium regulates differentiation of cultured spinal neurons. Neuron. 1991;7:787–796. doi: 10.1016/0896-6273(91)90281-4. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Hartley M, Heinemann S. Ca2+ permeability of KA-AMPA-gated glutamate receptor channels depends on subunit composition. Science Wash DC. 1991:851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- Huba R, Schneider H, Hofmann HD. Voltage-gated currents of putative GABAergic amacrine cells in primary cultures and in retinal slice preparations. Brain Res. 1992;577:10–18. doi: 10.1016/0006-8993(92)90531-d. [DOI] [PubMed] [Google Scholar]
- Iino M, Ozawa S, Tsuzuki K. Permeation of calcium through excitatory amino acid receptor channels in cultured rat hippocampal neurones. J Physiol Lond. 1990;424:151–165. doi: 10.1113/jphysiol.1990.sp018060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karschin A, Wässle H. Voltage- and transmitter-gated currents in isolated rod bipolar cells of rat retina. J Neurophysiol. 1990;63:860–876. doi: 10.1152/jn.1990.63.4.860. [DOI] [PubMed] [Google Scholar]
- Keinänen K, Wisden W, Sommer B, Werner P, Herb A, Verdoorn TA, Sakmann B, Seeburg PH. A family of AMPA-selective glutamate receptors. Science Wash DC. 1990;249:556–560. doi: 10.1126/science.2166337. [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Rand MN, Chen B, Waxman SG, Pourcho R. Kainate elicits elevated nuclear calcium signals in retinal neurons via calcium-induced calcium release. BrainRes. 1993;616:273–282. doi: 10.1016/0006-8993(93)90218-c. [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Rand MN, Lankford KL, Waxman SG. Intracellular calcium mobilization and neurite outgrowth in mammalian neurons. J Neurobiol. 1994;25:252–264. doi: 10.1002/neu.480250306. [DOI] [PubMed] [Google Scholar]
- Leifer D, Lipton SA, Barnstable CJ, Masland RH. Monoclonal antibody to Thy-1 enhances regeneration of processes by rat retinal ganglion cells in culture. Science Wash DC. 1984;224:303–306. doi: 10.1126/science.6143400. [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T, Rand MN, Waxman SG, Kocsis JD. Nuclear Ca2+ signals in rat retinal ganglion cells: Ca2+ entry through the kainate receptor initiates calcium-induced calcium release. Pfluegers Arch. 1994;426(Suppl):R30. [Google Scholar]
- Lerea LS, Butler LS, McNamara JO. NMDA and non-NMDA receptor-mediated increase of c-fos mRNA in dentate gyrus neurons involves calcium influx via different routes. J Neurosci. 1992;12:2973–2981. doi: 10.1523/JNEUROSCI.12-08-02973.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerea LS, McNamara JO. Ionotropic glutamate receptor subtypes activate c-fos transcription by distinct calcium requiring intracellular signaling pathways. Neuron. 1993;10:31–41. doi: 10.1016/0896-6273(93)90239-n. [DOI] [PubMed] [Google Scholar]
- Linn CP, Christensen BN. Excitatory amino acid regulation of intracellular Ca2+ in isolated catfish cone horizontal cells measured under voltage-and concentration-clamp conditions. J Neurosci. 1992;12:2156–2164. doi: 10.1523/JNEUROSCI.12-06-02156.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscombe D, Madison DV, Poente M, Reuter H, Tsien RW, Tsien RY. Imaging of cytosolic Ca2+ transients arising from Ca2+ stores and Ca2+ channels in sympathetic neurons. Neuron. 1988;1:355–365. doi: 10.1016/0896-6273(88)90185-7. [DOI] [PubMed] [Google Scholar]
- Massey SC, Miller RF. N-methyl-D-aspartate receptors of ganglion cells in rabbit retina. J Neurophysiol. 1990;63:16–30. doi: 10.1152/jn.1990.63.1.16. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol Lond. 1987;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Role of ion Flux in the control of expression. Nature Lond. 1986;322:552–555. doi: 10.1038/322552a0. [DOI] [PubMed] [Google Scholar]
- Murphy SN, Thayer SA, Miller RJ. The effects of excitatory amino acids on intracellular calcium in single mouse striatal neurons in vitro. J Neurosci. 1987:4145–4158. doi: 10.1523/JNEUROSCI.07-12-04145.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi N, Shneider NA, Axel R. A family of glutamate receptor genes: Evidence for the formation of heteromultimeric receptors with distinct channel properties. Neuron. 1990;5:569–581. doi: 10.1016/0896-6273(90)90212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura A, Akita K, Kudo Y. Non-NMDA receptor mediates cytoplasmic Ca2+ elevation in cultured hippocampal neurones. Neurosci Res. 1990;9:103–113. doi: 10.1016/0168-0102(90)90026-b. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Barnett NL. An intraocular injection of kainate induces expression of c-fos-like protein and activation of protein kinase C (α) in specific rabbit retinal neurones. Mol Brain Res. 1992;15:108–112. doi: 10.1016/0169-328x(92)90157-7. [DOI] [PubMed] [Google Scholar]
- Ozawa S, Iino M, Tsuzuki K. Two types of kainate response in cultured rat hippocampal neurons. J Neurophysiol. 1991;66:2–11. doi: 10.1152/jn.1991.66.1.2. [DOI] [PubMed] [Google Scholar]
- Perry VH. Evidence for an Amacrine cell system in the ganglion cell layer of rat retina. Neuroscience. 1981;6:931–944. doi: 10.1016/0306-4522(81)90174-3. [DOI] [PubMed] [Google Scholar]
- Perry VH, Henderson Z, Linden R. Postnatal changes in retinal ganglion cell and optic axon populations in the pigmented rat. J Comp Neurol. 1983;219:356–368. doi: 10.1002/cne.902190309. [DOI] [PubMed] [Google Scholar]
- Pruss RM, Akeson RL, Racke MM, Wilburn JL. Agonist-activated cobalt uptake identifies divalent cation-permeable kainate receptors on neurons and glial cells. Neuron. 1991;7:509–518. doi: 10.1016/0896-6273(91)90302-g. [DOI] [PubMed] [Google Scholar]
- Przywara DA, Bhave SV, Bhave A, Wakade TD, Wakade AR. Stimulated rise in neuronal calcium is faster and greater in the nucleus than the cytosol. FASEB J. 1991;5:217–222. doi: 10.1096/fasebj.5.2.2004666. [DOI] [PubMed] [Google Scholar]
- Rand MN, Leinders-Zufall T, Agulian S, Kocsis JD. Calcium signals in neurons. Nature Lond. 1994a;371:291–292. doi: 10.1038/371291b0. [DOI] [PubMed] [Google Scholar]
- Rand MN, Leinders-Zufall T, Agulian S, Kocsis JD. Micropipette loading of fluo-3 can elevate baseline nuclear calcium signals. Soc Neurosci Abstr. 1994b;20 [Google Scholar]
- Röhrig B, Grantyn R. Rat retinal ganglion cells express Ca2+-permeable non-NMDA glutamate receptors during the period of histogenetic cell death. Neurosci Lett. 1993;153:32–36. doi: 10.1016/0304-3940(93)90070-2. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Zhou Z, Konnerth A, Neher E. Fractional contribution of calcium to the cation current through glutamate receptor channels. Neuron. 1993;11:133–143. doi: 10.1016/0896-6273(93)90277-x. [DOI] [PubMed] [Google Scholar]
- Seeburg PH. The molecular biology of mammalian glutamate receptor channels. Trends Neuron. 1993;16:359–365. doi: 10.1016/0166-2236(93)90093-2. [DOI] [PubMed] [Google Scholar]
- Shatkay A. Individual activity of calcium ions in pure solutions of CaCl2 and in mixtures. Biophys J. 1968;8:912–919. doi: 10.1016/S0006-3495(68)86528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer B, Seeburg PH. Glutamate receptor channels: novel properties and new clones. Trends Pharmacol Sci. 1992;13:291–296. doi: 10.1016/0165-6147(92)90088-n. [DOI] [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drøbak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc Natl Acad Sci. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoorn TA, Burnashev N, Monyer H, Seeburg PH, Sakmann B. Structural determinants of ion flow through recombinant glutamate receptor channels. Science Wash DC. 1991;252:1715–1718. doi: 10.1126/science.1710829. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Kawamura K, Imaki J. Differential expression of c-fos mRNA in rat retinal cells: regulation by light/dark cycle. Neuron. 1993;10:1049–1054. doi: 10.1016/0896-6273(93)90053-t. [DOI] [PubMed] [Google Scholar]
- Zafra F, Hengerer B, Leibrock J, Thoenen H, Lindholm D. Activity dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. EMBO J. 1990;9:3545–3550. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]