Abstract
Differential effects of NGF and BDNF on axotomy-induced changes in GABAA-receptor-mediated conductance and sodium currents in cutaneous afferent neurons. J. Neurophysiol. 78: 31–42, 1997. The effects of nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) on injury-induced changes in the electrophysiological properties of adult rat cutaneous afferent dorsal root ganglion (DRG) neurons were examined. Whole cell patch-clamp techniques were used to study γ-aminobutyric acid-A (GABAA)-receptor-mediated conductance, voltage-dependent sodium currents, and action potential waveform in cutaneous afferent neurons (35–60 μm diam) cultured from control and axotomized animals. Cutaneous afferent neurons were identified by retrograde labeling with hydroxy-stilbamidine (Fluoro-gold, a fluorescent retrograde axonal tracer); the sciatic nerve was transected 1 wk after Fluoro-gold injection and L4/L5 DRG neurons were cultured 2–3 wk after axotomy. NGF, BDNF, or Ringer (vehicle) solution was delivered in vivo directly to the transected sciatic nerve stump in axotomized rats via an osmotic pump. Recordings were obtained from neurons 5–24 h after culture. Axotomized neurons from rats treated with vehicle solution displayed a twofold increase in GABA-induced conductance and a prominent reduction in the proportion of neurons expressing action potentials that had inflections on the falling phase. The expression of kinetically slow tetrodotoxin (TTX)-resistant sodium current was markedly reduced and an increased expression of kinetically fast TTX-sensitive current was observed in neurons from vehicle-treated, axotomized rats. Treatment with NGF (0.25 μg/μl at 12 μl/day for 14 days) in axotomized animals resulted in an increase in the proportion of neurons expressing TTX-resistant, slow sodium currents and inflected action potentials, but had no effect on GABA-induced conductance. Treatment with BDNF (0.5 μg/μl at 12 μl/day for 14 days) attenuated the axotomy-induced increase in GABAA-receptor-mediated conductance while minimally affecting action potential waveform. The observed neurotrophin effects occurred independently of cell size changes. These findings indicate a differential regulation of GABAA receptor and sodium channel properties in axotomized rat cutaneous afferent neurons by specific neurotrophic factors.
INTRODUCTION
Peripheral nerve transection results in profound morphological, biochemical, and electrophysiological changes in sensory neurons (Himes and Tessler 1989; Kingery et al. 1988; Wall and Devor 1981; Wells and Vaidya 1989; also see review by Titmus and Faber 1990). It has been hypothesized that decreased retrograde transport of target-derived trophic influences, which normally regulate and maintain the phenotypic differentiation of sensory neurons during development and in the adult animal, contributes to these changes (Bhisitkul et al. 1990; Fitzgerald et al. 1985; Kashiba et al. 1992; Raivich et al. 1991). However, the precise mechanisms that underlie injury-induced changes in neuronal excitability and the relationship between trophic factors and specific changes in the expression of neuronal ion channels and receptors have not been elucidated.
A role has been established for target-derived nerve growth factor (NGF) in the regulation of the electrophysiological phenotype in a subset of cutaneous afferent neurons during development (Ritter et al. 1991). Although the requirement for neurotrophins in the regulation of sensory neuronal function is less clear in the adult animal, it has been suggested that the role of neurotrophins follows an ontogenic shift from mediation of survival and phenotypic differentiation during development to maintenance of the differentiated neuronal phenotype in the mature animal (Carroll et al. 1992; Gorin and Johnson 1980; Lindsay 1988). Studies examining the expression of neurotrophin receptors in adult dorsal root ganglion (DRG) neurons have suggested that TrkA, the high-affinity receptor that binds NGF, is expressed in approximately half of cutaneous afferents, albeit primarily in those of small size. TrkB, the high-affinity receptor that binds brain-derived neurotrophic factor (BDNF), shows a less clear pattern of expression (McMahon et al. 1994). The axons of DRG neurons have been shown to support ongoing retrograde transport of these neurotrophins in the adult animal (DiStefano et al. 1992). However, the role of retrogradely transported neurotrophins in the regulation of the electrophysiological phenotype of adult cutaneous afferent neurons is not fully understood.
Previous work has demonstrated an increase in γ-aminobutyric acid-A (GABAA)-receptor-mediated conductance, a loss of kinetically slow sodium current, and decreased occurrence of inflected action potential (APs) in cutaneous afferent neurons after axotomy (Oyelese and Kocsis 1996; Rizzo et al. 1995). The present study was undertaken to determine whether or not these biophysical changes are mediated by deprivation of target-derived neurotrophins after axotomy. We examined whether or not in vivo application of exogenous NGF and BDNF to the proximal stump of the transected sciatic nerve could prevent the injury-induced changes in GABAA-receptor-mediated conductance, sodium current expression, or AP waveform in cutaneous afferent neurons. The results indicate that exogenous NGF limits the injury-induced loss of slow sodium current and inflected APs in cutaneous afferent neurons but has no effect on GABAA-receptor-mediated conductance. In contrast, BDNF reduces the injury-induced increase in GABAA-receptor-mediated conductance but has a minimal effect on AP waveform.
A portion of this work has appeared in abstract form (Oyelese et al. 1995b).
METHODS
Identification of cutaneous afferent DRG neurons and surgical techniques
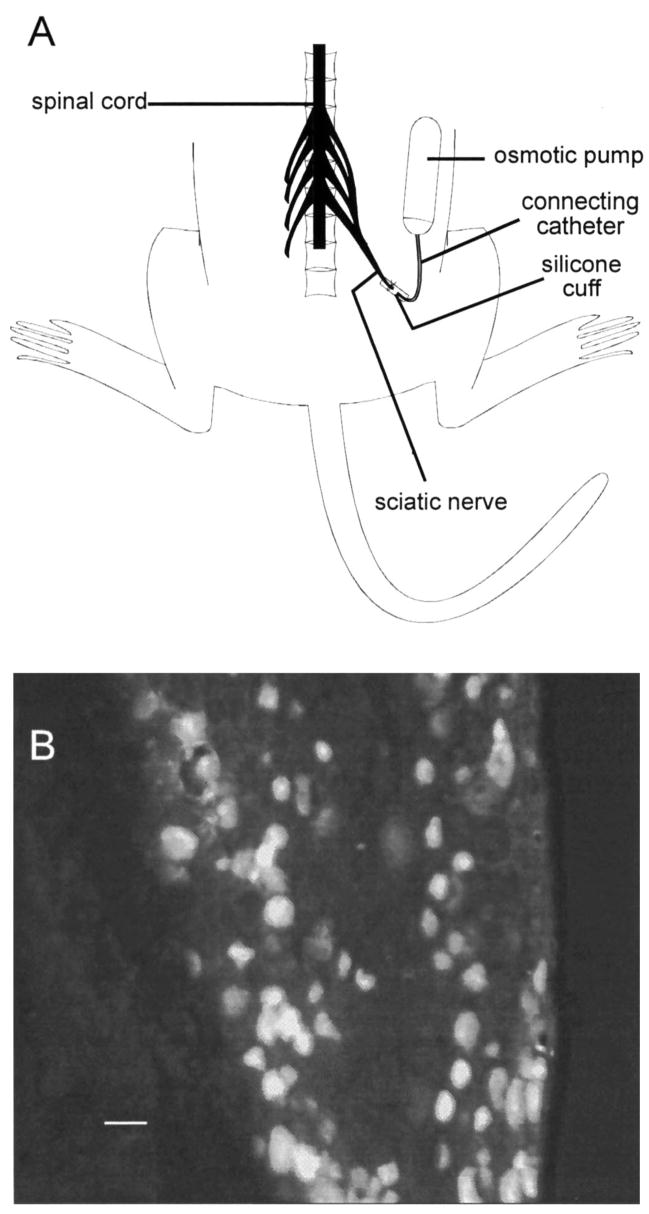
DRG somata giving rise to cutaneous afferent fibers were identified by retrograde labeling with hydroxy-stilbamidine (Fluoro-gold; Honmou et al. 1994; Oyelese and Kocsis 1996; Schmued and Fallon 1986). A 2–4% solution of Fluoro-gold (Fluorochrome, Englewood, CO) mixed in distilled water was prepared and injected into female rats (140–160 g) anesthetized with an intraperitoneal injection of ketamine (40 mg/kg) and xylazine (2.5 mg/kg). Cutaneous afferent neurons were labeled by intradermal injections of Fluoro-gold in the lateral plantar region (innervated by the sural nerve) of anesthetized rats (Honmou et al. 1994) and were identified in vitro by fluorescence on brief exposure of the culture dish to ultraviolet light. Nerve ligation procedures were performed as previously described (Oyelese et al. 1995a), with slight modifications for implanting the osmotic pumps. Briefly, 50 μg NGF (mouse NGF; Upstate Biotech, Lake Placid, NY) or 100 μg BDNF (recombinant human; courtesy of Regeneron, Tarrytown, NY) was dissolved in 200 μl Ringer solution and injected into osmotic pumps (Alzet 2002; Alza, Palo Alto, CA) with a delivery rate of 0.5 μl/h. The sciatic nerve on the right side was exposed in the upper thigh region of anesthetized animals and was ligated and transected, and the pumps were connected via a polyethylene tubing catheter to a silicone cuff into which the ligated sciatic nerve stump was sutured to prevent nerve regeneration (Fitzgerald et al. 1985) (Fig. 1A). As a control for neurotrophin treatment, osmotic pumps filled with Ringer (vehicle) solution were implanted on the left side in neurotrophin-treated animals or bilaterally in untreated animals. The contents of the pumps were continuously delivered to the transected sciatic nerve stumps for 14 days. To ensure that there was delivery of the pumps’ contents to the DRG, pumps in one animal were filled with 4% Fluoro-gold and the L4 and L5 DRGs were excised, sectioned, and examined for fluorescence. The high staining levels of the DRG with Fluoro-gold confirmed delivery of the pumps’ contents to the ganglia (Fig. 1B). Two to three weeks after surgery, the animals were killed, the L4 and L5 DRGs were excised and dissociated, and the neurons were maintained in short-term culture with the use of previously described methods (Oyelese et al. 1995a). Age-matched unoperated rats were used as controls. In summary, the sciatic nerve was transected 1 wk after injection of Fluoro-gold into distal cutaneous fields and the animals were killed 2–3 wk later, at which time the DRGs were removed and maintained in culture for 5–24 h. Electrophysiological studies were performed only on neurons showing fluorescence on exposure to ultraviolet light; thus all neurons examined in the control and experimental groups of this study were cutaneous afferent neurons identified by retrograde labeling with Fluoro-gold.
FIG. 1.
Delivery of contents of osmotic pump implants to dorsal root ganglion (DRG). A: schematic showing osmotic pump and connecting catheter used to deliver nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and vehicle solution directly to transected sciatic nerve stump. Pumps containing NGF or BDNF were implanted on right side of animal. Pumps containing Ringer (vehicle) solution were implanted on left side in treated animals or bilaterally in untreated animals as a control for neurotrophin treatment (see METHODS). B: to ensure that pumps’ contents were delivered to DRG, 1 pump was filled with hydroxy-stilbamidine (Fluoro-gold, 4% in vehicle solution), DRG was removed and sectioned, and delivery was confirmed by fluorescence in DRG neurons (scale bar = 50 μm).
Electrophysiological techniques
Electrophysiological measurements were obtained 5–24 h after plating to minimize changes such as neurite extension that might occur in vitro; aneuritic neurons were selected to reduce space-clamp problems. Neurons plated on glass coverslips were placed in a recording chamber on the stage of an inverted microscope (Nikon) and superfused with a modified Krebs’ solution [composition (in mM): 124 NaCl, 3.0 KCl, 2.0 CaCl2, 2.0 MgCl2, 1.3 NaH2PO4, 26 NaHCO3, and 10 dextrose, pH 7.4] at room temperature (20–25°C) at a rate of 3–5 ml/min. GABA (100 μM) was mixed in the above solution and applied to individual cells by pressure microejection (Picospritzer 2, General Valve) with the use of a micropipette positioned near the neuron. Trypan blue (0.4%) was added to the solution to allow visualization and ensure uniform delivery of GABA over the entire cell surface.
GABA-ACTIVATED CURRENT RECORDINGS
Whole cell voltage-clamp recordings were obtained from identified cutaneous DRG afferent neurons with the use of the patch-clamp technique (Hamill et al. 1981). Recording electrodes (1–2 MΩ) were fabricated from thin-walled, single-filament borosilicate glass tubing (World Precision Instruments) with a micropipette puller (Sutter Instruments, Model P-80/PC). Electrodes were filled with a solution containing (in mM) 140 KCl, 1 MgCl2, 3 ATP (Mg salt), 1 CaCl2, 11 ethylene glycol-bis (β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA, resulting in an intracellular Ca2+ concentration of 10−9 mM), and 10 N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), pH 7.2, osmolarity 290–305 mosM. Seal resistances were ≥2 GΩ. Voltage-clamp recordings were made with a patch-clamp amplifier (Axopatch 1D, Axon Instruments) with the use of a low-gain headstage (feedback resistor: 50 MΩ), (CV-4 0.1/100) to allow measurement of large GABA-induced currents typically encountered in DRG neurons of the size examined in this study. Recordings were low-pass filtered (Bessel filter) at 10 kHz and data were digitized and stored on computer with the use of a commercially available data acquisition system (TL-1 DMA interface, and pClamp software, Axon Instruments; sampling rate 50 Hz–5 kHz/channel) and on a videocasette recorder with the use of a digitizing unit (Neurocorder DR-484, Neurodata instruments; sampled at 44 kHz). Estimation of access resistance, input resistance, cell capacitance, and GABA-induced conductance was performed as previously described for the low-gain headstage (Oyelese et al. 1995a). Independent t-tests assuming unequal variance were used to determine levels of significant difference between groups.
AP RECORDINGS
AP data were obtained in current-clamp mode. A series of hyperpolarizing and depolarizing current pulses 10 ms in duration from −0.2 or −0.4 nA with 12 incremental step pulses ranging from 0.2 to 0.4 nA was used to elicit APs in each neuron. AP width (at 50% of spike height) was analyzed for each neuron. Pharmacological testing on APs elicited in cutaneous afferent neurons was performed with the use of the solutions listed in Table 1.
TABLE 1.
Solutions used in pharmcological testing of action potential waveforms
| Solution
|
|||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| NaCl | 140 | 140 | 140 | 140 | 140 | 0 | 0 |
| KCl | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| HEPES | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Dextrose | 15–20 | 15–20 | 15–20 | 15–20 | 15–20 | 15–20 | 15–20 |
| CaCl2 | 2 | 2 | 2 | 2 | 0 | 0 | 10 |
| MgCl2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| CdCl2 | 0 | 0.1 | 0 | 0.1 | 0 | 0 | 0 |
| TTX | 0 | 0 | 2 × 10−4 | 2 × 10−4 | 0 | 0 | 0 |
| EGTA | 0 | 0 | 0 | 0 | 5 | 0 | 0 |
| Choline chloride | 0 | 0 | 0 | 0 | 0 | 140 | 140 |
All values are in mM. Solution 1 is normal solution. HEPES, N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; TTX, tetrodotoxin; EGTA, ethylene glycol-bis (β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid.
VOLTAGE-DEPENDENT NA+ CURRENT RECORDINGS
Solutions were designed to reduce the driving force for Na+ ion and to block voltage-dependent K+ and Ca2+ currents (Rizzo et al. 1994). Tetramethylammonium (TMA+) served as the primary nonpermeant monovalent cation in place of external Na + to reduce current amplitude and therefore errors caused by series resistance artifact. The bath solution consisted of (in mM) 20 NaCl, 110 tetramethylammonium chloride, 1 MgCl2, 1 CaCl2, 0.1 CdCl2, and 10 HEPES-NaOH, titrated to pH 7.4. Recording pipettes (1.2–3.8 MΩ) were filled with (in mM) 140 CsCl or 70 Cs2SO4 + 3 Na2SO4, 2 MgCl2, 1 EGTA, and 10 HEPES-CsOH, titrated to pH 7.2. Internal Cs + salts were used to minimize current through voltage-dependent K + channels. External 0.1 mM Cd2+ was used throughout the voltage-clamp recordings to block current through voltage-activated Ca2+ channels. Additional details of the voltage-clamp recording techniques are found in Honmou et al. (1994). P/4 pulse protocols (Bezanilla and Armstrong 1977) were applied at 1-s intervals to test potentials indicated in the figure legends. Generally, four sweeps were averaged at each test pulse.
RESULTS
Passive membrane properties of control and vehicle- or neurotrophin-treated neurons
Identified cutaneous afferent DRG neurons ranging in size from 35 to 60 μm diam and corresponding to neurons with myelinated axons (Harper and Lawson 1985) were selected for study. Resting potential, input resistance, cell capacitance, and cell diameter were determined and compared in all neurons in which AP properties and GABA conductance were examined, and are listed in Table 2. Average resting potential in all groups of neurons examined ranged from −53 to −58 mV (Table 2) and was observed to be slightly more negative in axotomized neurons than in uninjured controls. No changes in cell diameter or capacitance were observed in axotomized neurons in which the transected sciatic nerve stump was perfused with vehicle solution. However, NGF treatment increased both neuronal diameter and cell capacitance compared with uninjured controls and vehicle-treated neurons (Table 2). Neuronal diameter, but not capacitance, was decreased in the BDNF-treated neurons examined, compared with vehicle-treated neurons. Input resistance was similar in all groups studied.
TABLE 2.
Differential effect of NGF and BDNF on spike waveform and GABA conductance in axotomized cutaneous afferent DRG neurons
| Control | Ringer Solution | NGF, 0.25 μg/μl | BDNF, 0.5 μg/μl | |
|---|---|---|---|---|
| Cell size, μm | 43 ± 0.6 (58) | 45 ± 1.05 (24) | 47 ± 0.64* (38) | 42 ± 0.84§ (20) |
| Cell cap, pF | 88 ± 3.50 (58) | 86 ± 4.23 (24) | 117 ± 4.92*† (38) | 79 ± 5.35 (20) |
| GGABA, nS | 287 ± 27 (58) | 605 ± 57* (24) | 618 ± 64* (38) | 365 ± 44† (19) |
| GGABA/cap, nS/pF | 3.53 ± 0.36 (58) | 7.45 ± 0.75* (24) | 5.65 ± 0.67* (38) | 5.17 ± 0.66‡§ (19) |
| RI, MΩ | 92 ± 6.9 (58) | 115 ± 22.6 (24) | 97 ± 9.6 (38) | 107 ± 21.1 (20) |
| RP, mV | −53 ± 0.9 (58) | − 58 ± 0.9* (24) | − 57 ± 0.6* (38) | −57 ± 0.8* (20) |
| AP half-width, ms | 1.09 ± 0.11 (58) | 0.76 ± 0.75* (22) | 1.07 ± 0.07† (38) | 0.97 ± 0.14 (20) |
| AP % inflected | 47 (27/58) | 18 (4/22) | 61 (23/38) | 25 (5/20) |
Values are means ± SE, with number of neurons in parentheses. GABA, γ-aminobutyric acid; DRG, dorsal root ganglion; AP, action potential. Parameters were compared in uninjured control neurons and axotomized neurons from animals in which the transected sciatic nerve was perfused with vehicle solution (Ringer solution), nerve growth factor NGF, 0.25 μg/μl), or brain-derived neurotrophic factor (BDNF, 0.5 μg/μl).
P < 0.01,
P < 0.05 as compared with uninjured cutaneous afferents.
P < 0.01,
P < 0.05 as compared with axotomized, Ringer-solution-treated cutaneous afferents.
Ionic mechanisms underlying inflected and noninflected APs
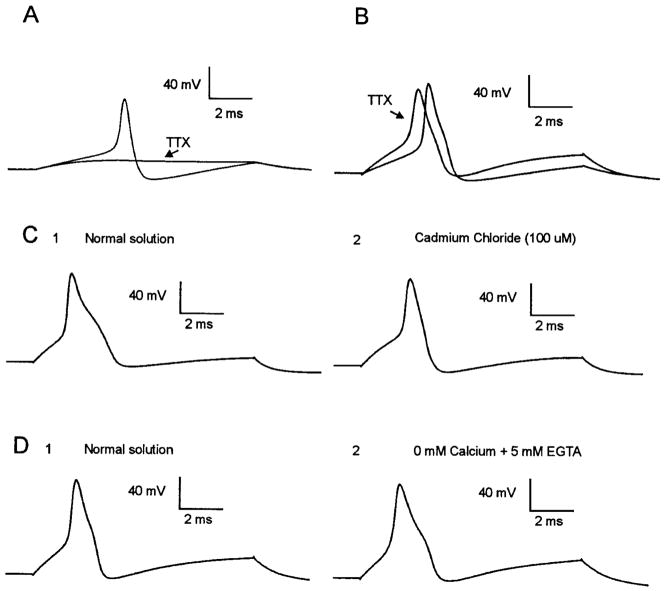
Pharmacological testing of inflected and noninflected APs in a portion of the cells was performed with the use of the solutions listed in Table 1 to elucidate the ionic currents contributing to the AP inflection in cutaneous afferent neurons. Inflected APs recorded in cutaneous afferent DRG neurons were observed to be insensitive to 200 nM tetrodotoxin (TTX; n = 5; solution 3; Fig. 2A), whereas noninflected APs were abolished in the presence of 200 nM TTX (n = 4; Fig. 2B). In some neurons with inflected APs, injection of a larger amount of current was necessary to elicit spikes in the presence of TTX, suggesting an increase in AP threshold. Injection of as much as 9 nA of current in noninflected neurons failed to elicit spikes in the presence of TTX (Fig. 2A). Inflections of APs in neurons with TTX-resistant spikes were observed to persist in calcium-free external solution with 5 mM EGTA (n = 6; solution 5; Fig. 2, D1 and D2); however, the inflections were reversibly abolished by 100 μM cadmium chloride (n = 19; solution 2; Fig. 2, C1 and C2).
FIG. 2.
Ionic components of inflected and noninflected action potentials (APs). APs of cutaneous afferent neurons were examined in solutions listed in Table 1. Non-inflected APs were sensitive to tetrodotoxin (TTX; 200 nM) even when current injection was as great as 9 nA (A). Inflected APs persisted in presence of TTX, but sometimes showed increased threshold (B). Inflection on downslope of TTX-resistant APs was reversibly abolished by 100 μM CdCl2 (C1 and C2). Testing of another neuron revealed that inflection persisted when cells were perfused in calcium-free external solution with 5 mM ethylene glycol-bis (β-aminoethyl ether)-N,N, N′,N′-tetraacetic acid (EGTA) in perfusate (D1 and D2).
NGF prevented the injury-induced changes in AP waveform
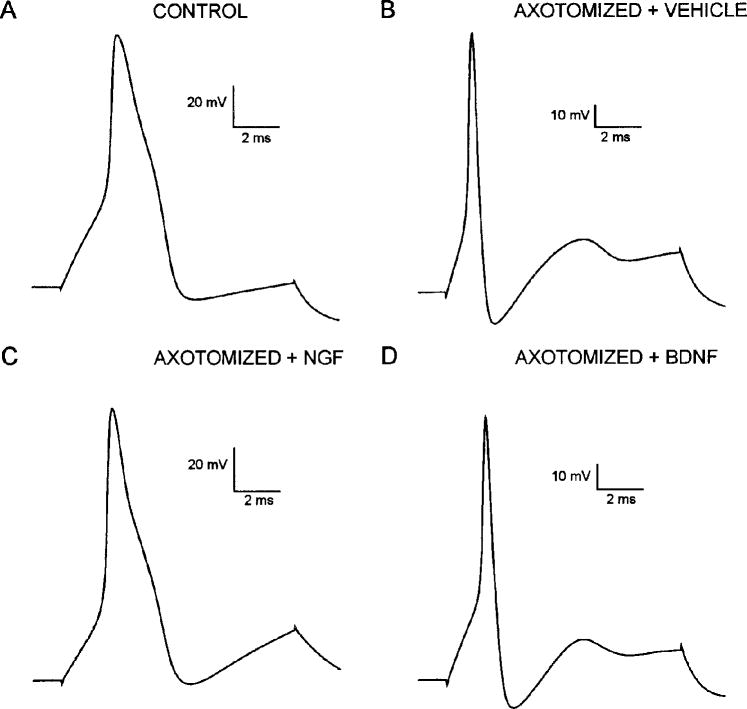
The proximal stump of the transected sciatic nerve was supplied in vivo with exogenous NGF (0.25 μg/μl; Verge et al. 1995) or vehicle solution continuously at 0.5 μl/h for 12–14 days. APs of cutaneous neurons from axotomized animals in which the sciatic nerve stump was treated with NGF (Fig. 3C) were compared with APs of neurons from axotomized animals treated with vehicle solution (Fig. 3B) and of neurons from uninjured control animals (Fig. 3A). Uninjured control cutaneous afferent neurons displayed APs that were morphologically heterogeneous with those of approximately half of the neurons (47%; Table 2, Fig. 3A), exhibiting varying degrees of inflection on the downslope of the AP. The proportion of inflected APs in axotomized neurons supplied with vehicle solution decreased to 18% (vs. 47% in uninjured controls, Table 2) and the AP duration significantly decreased [0.76 ± 0.75 ms, mean ± SE (n = 22) vs. 1.09 ± 0.11 ms in uninjured controls (n = 58); P < 0.01]. However, APs in axotomized cutaneous afferent neurons supplied with NGF were similar in duration to those observed in uninjured controls (1.07 ± 0.07 ms in NGF-treated neurons; n = 38, P > 0.05) (Table 2) and ~60% had inflections on the downslope (Table 2, Fig. 3C).
FIG. 3.
AP waveform in control, saline-treated, and neurotrophin-treated neurons after axotomy. APs induced by depolarizing current pulse in control (A) and axotomized neurons after treatment with vehicle solution (B), NGF (C), and BDNF (D). Approximately half of uninjured cutaneous afferent neurons studied had long-duration APs with inflections on downslope (A), whereas 82% of axotomized neurons treated with vehicle solution had short-duration APs lacking this inflection (B). After NGF treatment, 60% of neurons expressed inflected APs (C) and 75% of BDNF-treated neurons lacked inflected APs (D).
NGF did not prevent injury-induced changes in GABAA-receptor-mediated conductance
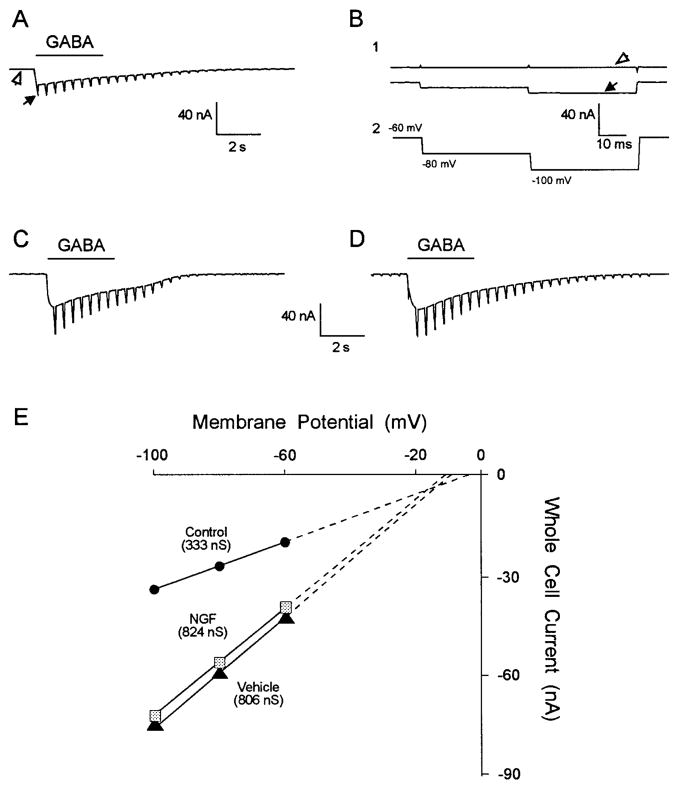
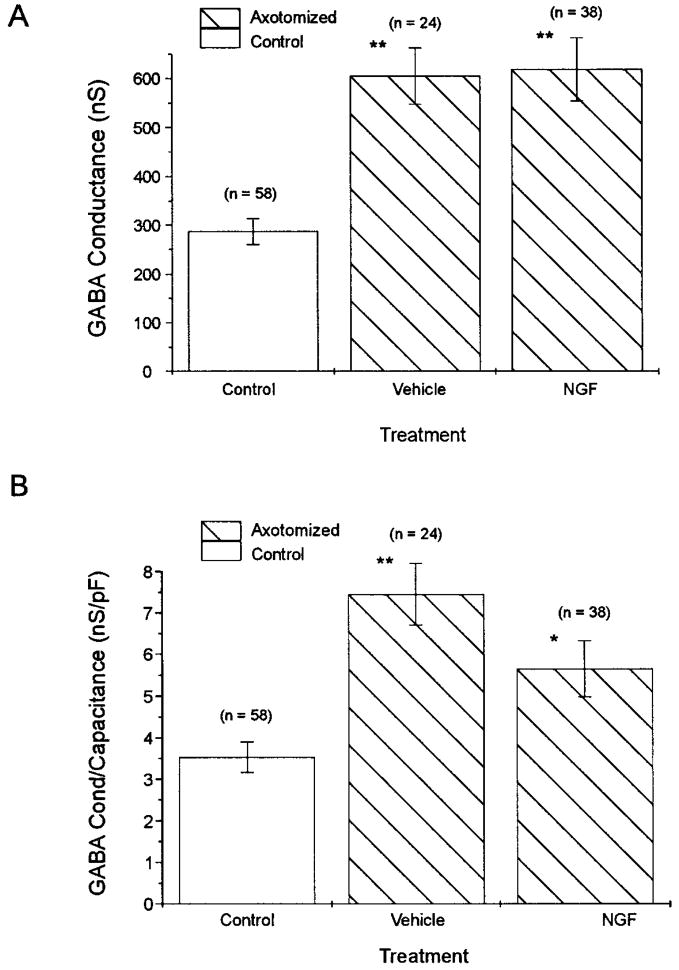
GABA-induced whole cell current and conductance were compared in uninjured control cutaneous afferent neurons and axotomized neurons from animals in which the transected sciatic nerve stump was continuously perfused with NGF or vehicle solution. GABA (100 μM) pressure microejected onto cutaneous afferent neurons elicited an inward current (Fig. 4 A), whereas control solution did not. The whole cell GABA current response to the voltage-clamp pulse protocol (Fig. 4 B2) is shown before GABA application (Fig. 4 B1, open arrow) and at the peak response to GABA (Fig. 4 B1, filled arrow). The GABA-induced current is observed to be a rapidly desensitizing inward current accompanied by an increase in conductance readily seen at slow time base (Fig. 4 A). To measure the peak conductance increase before the onset of desensitization, the hyperpolarizing voltage-clamp pulses were repeated at 2.5 Hz (Oyelese et al. 1995a). The peak whole cell current in the uninjured control neuron in Fig. 4, A and B, was 32 nA. Whole cell currents recorded in axotomized DRG neurons supplied with NGF or vehicle solution were indistinguishable from each other, but were larger than those observed in uninjured control neurons (Fig. 4, C and D). Peak currents measured in the NGF- and vehicle-treated neurons in Fig. 4, C and D, were 73 and 77 nA, respectively. Slope conductance was determined from plots of GABA-induced whole cell current at −60, −80, and −100 mV (Fig. 4 E) for the control (333 nS), vehicle-treated (806 nS), and NGF-treated neurons (824 nS) shown in Fig. 4, B–D, respectively. GABA-induced slope conductance of axotomized cutaneous afferent neurons supplied with NGF was similar to that observed in neurons supplied with vehicle solution [61 8 ± 64 nS (n = 38) vs. 605 ± 57 nS (n = 24) ; P > 0.05 (Fig. 5 A) ]. This GABA-induced conductance in NGF and vehicle-treated axotomized neurons represented a twofold increase over levels in uninjured control cells (288 ± 27 nS; n = 58, P < 0.01). An increase in the size of NGF-treated axotomized neurons studied versus vehicle-treated and uninjured control neurons was observed (Table 2). Because measured GABA conductance may vary with neuronal size (Oyelese et al. 1995a), GABA conductance was normalized for neuronal size to correct for observed increases in conductance secondary to differences in neuronal size. Normalized conductance (conductance/capacitance) was significantly increased in both vehicle- and NGF-treated neurons (Fig. 5 B), and although the increase tended to be greater in vehicle- versus NGF-treated neurons, this difference did not reach the level of significance (P > 0.05, Table 2).
FIG. 4.
γ-Aminobutyric acid (GABA)-induced whole cell current in control neurons and in axotomized neurons after vehicle and NGF treatment. Comparison of GABA-induced whole cell currents in control (A and B), vehicle-treated (C), and NGF-treated (D) neurons revealed a larger response to GABA after axotomy. Responses in vehicle-treated (C) and NGF-treated (D) axotomized neurons were similar. A: whole cell current at slow time scale showing desensitizing inward current and conductance increase in response to a 3-s application of GABA (100 μM) in control cutaneous neuron in B. Neurons were voltage clamped at −60 mV and subjected to voltage-clamp protocol in B2 that was repeated at a rate of 2.5 Hz. Response to individual pulse step at fast time base (B1) showing whole cell current before GABA application (open arrows) and at peak of response to GABA (filled arrows). GABA-induced conductance was greater in axotomized neurons treated with vehicle solution or NGF compared with control (E).
FIG. 5.
GABA-induced whole cell conductance in control and axotomized neurons normalized for cell size. A difference in neuronal size was observed after treatment of axotomized neurons with NGF. To ensure that observed changes in GABA conductance were not strictly a result of these cell size changes, conductance was normalized for cell capacitance. GABA conductance was significantly increased after axotomy and treatment with vehicle solution, and this increase was not altered by treatment with NGF (A). Normalized conductance was similarly increased in vehicle- and NGF-treated axotomized neurons (B). (Values are means ± SE; n = number of neurons; *P < 0.05, **P < 0.01 for comparisons with uninjured cutaneous neurons.)
Effect of BDNF on AP waveform and GABAA-receptor-mediated conductance
The effect of BDNF on the injury-induced changes in AP waveform was minimal. Approximately 25% of axotomized neurons in BDNF-treated animals had APs with inflections on the downslope (i.e., 75% lacked an inflection, Fig. 3 D), versus 18% of neurons in axotomized vehicle-treated animals and 47% of neurons in uninjured control animals. However, AP duration in neurons from BDNF-treated animals did not differ from values in vehicle-treated or uninjured control animals [0.97 ± 0.14 ms (n = 20) vs. 0.76 ± 0.75 ms in vehicle-treated and 1.09 ± 0.11 ms in uninjured controls; P > 0.05 for both].
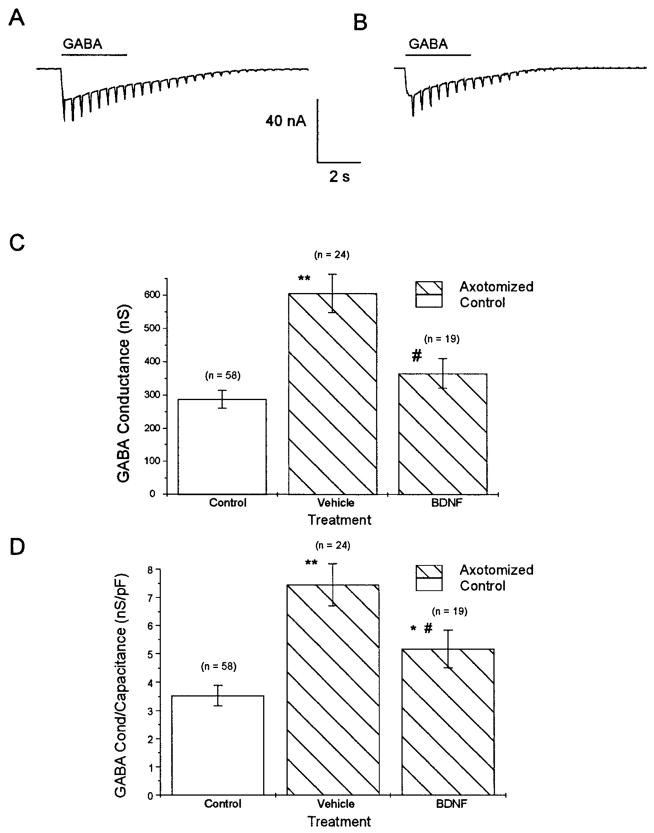
Cutaneous afferent neurons from axotomized animals whose transected nerve stumps were perfused with BDNF displayed GABA-induced whole cell currents that were similar to those of uninjured controls in magnitude (Fig. 6, A and B). GABAA-receptor-mediated conductance in BDNF-treated axotomized animals was not significantly different from uninjured controls [365 ± 44 nS (n = 19) vs. 288 ± 27 nS; P > 0.05]; an attenuation of the injury-induced increase in GABAA-receptor-mediated conductance was observed versus axotomized neurons implanted with vehicle- filled pumps (Fig. 6C; P < 0.05). Normalized GABA conductance was compared in these groups of axotomized neurons (because BDNF-treated neurons had a smaller diameter than vehicle-treated neurons) and was significantly lower in BDNF-treated neurons than vehicle-treated neurons (Fig. 6D), but was also significantly larger than in control neurons. Lower doses of BDNF (0.25 μg/μl at 0.5 μl/h) had no detectable effect on GABAA-receptor-mediated conductance or AP waveform (data not shown).
FIG. 6.
Comparison of GABA-induced whole cell currents and conductance in control and BDNF-treated cutaneous afferent neurons. Whole cell GABA currents were comparable in uninjured control and BDNF-treated neurons. In representative neurons GABA-induced whole cell current was 32 nA for control (A) and 31 nA for BDNF-treated (B) neurons. GABA conductance was significantly attenuated compared with vehicle-treated axotomized neurons (C). GABA conductance normalized for capacitance was compared to correct for size differences in groups studied. Normalized conductance in BDNF-treated neurons was attenuated compared with vehicle-treated axotomized neurons; however, a significant increase over uninjured controls was also observed (D). Thus BDNF did not appear to completely reverse axotomy-induced increase in GABA conductance even though a significant attenuation was observed. (Values are means ± SE; *P < 0.05, **P < 0.01 vs. uninjured controls; #P < 0.05 for comparisons with vehicle-treated axotomized neurons.)
NGF diminished the loss of kinetically slow sodium currents in axotomized cutaneous afferent neurons
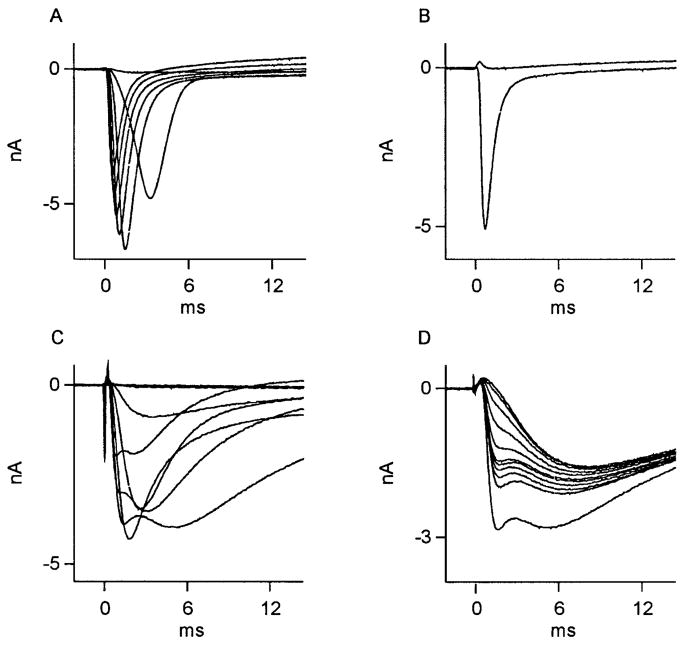
Axotomy results in a decrease in the occurrence of kinetically slow, TTX-resistant Na+ current and an increase in the expression of kinetically fast, TTX-sensitive Na+ current in cutaneous afferent DRG neurons (Rizzo et al. 1995). Given the above data suggestive of NGF regulation of the TTX-resistant, Na+-dependent mechanisms underlying the AP inflection in axotomized neurons, we carried out experiments that were designed to determine whether NGF treatment could influence the expression of Na+ currents in the somatic membrane of axotomized cutaneous afferent neurons. Figure 7, A and C, show superimposed Na+ currents, recorded at the indicated test pulses following a fixed pre-pulse from axotomized, identified cutaneous afferents perfused with vehicle only. This figure reveals how, qualitatively, two possible Na + current forms were encountered: either a relatively “pure,” kinetically fast, TTX-sensitive current (Fig. 7, A and B) or a mixture of kinetically fast and slow currents (Fig. 7C). In Fig. 7D, successive test pulses applied to another (axotomized, vehicle-treated) neuron during TTX perfusion reveals TTX resistance of the slow component. Of 16 axotomized, identified cutaneous afferent DRG neurons whose axon stumps were perfused with vehicle only, 10 revealed a singular, kinetically fast variety of Na + current of the form shown in Fig. 7A, and 6 revealed a combination of kinetically fast and slow Na+ currents of the form shown in Fig. 7C. No axotomized neurons from animals perfused with vehicle solution were observed to express singular kinetically slow, TTX-resistant sodium currents.
FIG. 7.
Effect of vehicle treatment on Na+ currents in axotomized cutaneous neurons. Na+ currents recorded from 2 DRG neurons whose ligated axons were perfused with vehicle (Ringer) solution. Of 16 neurons studied, Na+ currents in 10 had appearance shown in A. Here, superimposed traces were recorded during test potentials from −40 to +20 mV in steps of 10 mV, after a fixed holding potential of −60 mV. These currents were from a kinetically fast population of Na+ channels completely sensitive to 1 μM TTX (maximal inward current observed at −20 mV: subsequent stimulations have decreasing inward current as reversal potential for Na+ is approached). B: current during test pulses to 0 mV, before, then during, perfusion with TTX. Of 16 vehicle-perfused neurons, 6 had Na+ currents whose complex appearance suggested presence of ≥2 kinetically distinct currents, such as those in C and D. In C, superimposed traces were recorded during test potentials from −50 to +10 mV in steps of 10 mV, after a fixed holding potential of −60 mV. Subsequent, stronger depolarizations revealed rapidly activating and inactivating currents followed by a 2nd, more slowly activating and inactivating component. The 2 components displayed a differential sensitivity to external 1 μM TTX (D). Here, successive depolarizations to +10 mV at 1-s intervals during TTX perfusion show early component of Na+ current to be selectively sensitive (maximum inward current at −20 mV).
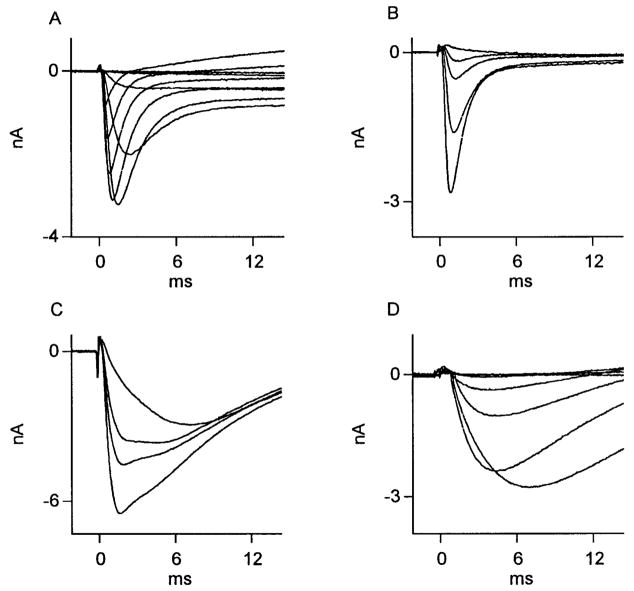
Axotomized neurons treated with NGF showed a higher prevalence of TTX-resistant Na+ current than axotomized neurons treated with vehicle. Although kinetically fast sodium currents of the type recorded in neurons from axotomized, vehicle-treated animals were also recorded in neurons from axotomized NGF-treated animals (Fig. 8, A and B), a significantly larger proportion of neurons from axotomized NGF-treated animals expressed kinetically slow sodium currents (see Table 3). Moreover, relatively pure kinetically slow sodium currents, not observed in vehicle-treated neurons, were encountered in NGF-treated neurons (Fig. 8D). More than 50% of neurons expressed a combination of kinetic forms such as that depicted in Fig. 8C. Of 19 axotomized, identified cutaneous afferent DRG neurons whose axon stumps were perfused with NGF, 6 revealed kinetically fast currents of the form shown in Fig. 8A, 10 revealed a combination of fast and slow current of the form shown in Fig. 8C, and 3 revealed slow currents of the form shown in Fig. 8D. The results are summarized in Table 3. It appears that by depriving DRG neurons of target derived NGF, axotomy leads to the selective loss of slow Na+ current.
FIG. 8.
Effect of NGF treatment on Na+ currents in axotomized cutaneous neurons. Na+ currents recorded from 3 DRG neurons whose transected axons were perfused with NGF. Of 19 neurons studied, 6 expressed kinetically fast Na+ currents (A). Here, superimposed traces were recorded during test potentials from −50 to +30 mV in steps of 10 mV, after a fixed holding potential of −55 mV. Successive traces from same neuron during 1 μM TTX perfusion (B) revealed these currents to be entirely TTX sensitive. C and D: 13 of 19 neurons revealed presence of a kinetically slow TTX-resistant current. In C, successive Na+ current recordings at 0 mV from a conditioning potential of −60 mV during perfusion with TTX eliminate early, but not late portion of current. Fast, early component of Na+ current appeared to be lacking in 3 of 19 NGF-treated neurons, as depicted in D, which shows Na + current traces recorded from another neuron during test potentials from −50 to 0 mV in steps of 10 mV after a fixed holding potential of −60 mV (peak inward current was recorded at −10 mV). This neuron was not exposed to TTX.
TABLE 3.
Effect of NGF and vehicle treatment in vivo on expression of fast and slow Na+ current in axotomized cutaneous afferent DRG neurons
| Axotomized + | Slow Only | Fast/Slow Combination | Fast Only | Total |
|---|---|---|---|---|
| Vehicle perfusion | 0 | 6 | 10 | 16 |
| NGF perfusion | 3* | 10* | 6 | 19 |
Values are numbers of neurons. For abbreviations, see Table 2.
NGF (0.25 μg/μl) was deliverd directly to the transected sciatic nerve stump in vivo at 0.5 μl/h for 14 days. Delivery of vehicle solution served as a control.
P < 0.001 with respect to the occurrence of the slow Na+ current in 6 of 16 neurons after vehicle treatment as determined by χ2 formulation with 2 degrees of freedom.
DISCUSSION
The aim of the present study was to determine the role of the neurotrophins NGF and BDNF in the maintenance of certain electrophysiological properties in adult cutaneous afferent DRG neurons. The ability of either NGF or BDNF to mitigate the changes in GABAA-receptor-mediated conductance, AP waveform, and sodium current expression in cutaneous afferent DRG neurons after axotomy was examined. Our findings suggest a differential effect of NGF and BDNF in the modulation of these electrophysiological properties within cutaneous afferent neurons in the adult rat.
Effect of NGF and BDNF on axotomy-induced changes in AP waveform
Although delivery of vehicle solution to the axotomized sciatic nerve stump did not prevent the reduction in the expression of inflected APs in cutaneous afferent neurons after axotomy, NGF treatment produced a marked increase in the relative proportion of inflected APs, but the effect of BDNF was minimal. These findings are in agreement with previous studies showing that NGF regulates the expression of TTX-resistant, inflected APs in cutaneous Aδ-high-threshold mechanoreceptors in vivo (Lewin et al. 1992; Ritter and Mendell 1992) and in DRG neurons in vitro (Aguayo and White 1992). Interestingly, the proportion of neurons with inflected APs did not decrease 3 wk after crush injury (Oyelese and Kocsis 1996) in which the transected axons were permitted to regenerate into the distal nerve stump where there is an increased synthesis of NGF and BDNF (Funakoshi et al. 1993; Heumann et al. 1987a; Meyer et al. 1992). The observed preservation of AP properties in the earlier study was not likely due to BDNF, because no significant alteration in AP expression of axotomized neurons was observed after BDNF treatment in the present study.
The ionic currents underlying inflections in APs of DRG neurons have been attributed to sodium (Honmou et al. 1994) and calcium fluxes (Ransom and Holtz 1977; Yoshida et al. 1978) because of the sensitivity of the inflection to calcium channel blockers and the dependence of the primary spike on sodium. Studies have demonstrated the sensitivity of TTX-resistant sodium-selective currents to cadmium (Frelin et al. 1986; Kostyuk et al. 1981; Roy and Narahashi 1992). Our pharmacological testing on inflected APs in cutaneous afferent neurons showing TTX resistance, cadmium sensitivity, and the persistence of AP inflections in calcium- free solutions are consistent with the presence of a kinetically slow sodium current. Thus the ionic currents underlying the AP inflections in our population of cutaneous neurons appear to be sodium dependent. A diminished voltage-dependent potassium conductance could have a similar AP-broadening effect and thus various cation-specific mechanisms could contribute to the repolarization phase of APs in cutaneous neurons.
NGF increases the expression of TTX-resistant sodium channels (Rudy et al. 1987) and calcium currents (Garber et al. 1989; Plummer et al. 1989) in PC12 cells. Although BDNF can also increase sodium current density in PC12 cells that express the high-affinity BDNF receptor TrkB (Fanger et al. 1995), its effect on the expression of TTX-resistant channels is unknown. NGF, but not BDNF, increases calcium currents in basal forebrain neurons in vitro (Levine et al. 1995). Although we show that NGF increased the expression of TTX-resistant, kinetically slow sodium currents, further voltage-clamp studies will be necessary to determine the specific ionic current or combination of currents, modulated by NGF, that contributes to the AP inflection.
Effect of NGF and BDNF on axotomy-induced changes in GABAA-receptor-mediated conductance
Axotomy increases GABA-induced conductance in cutaneous afferent neurons (Oyelese and Kocsis 1996). Neither NGF nor vehicle solution treatment had an effect on these injury-induced changes; however, BDNF treatment attenuated the injury-induced increase in GABA response. The absence of a modulatory effect of NGF on GABAA-receptor-mediated currents is consistent with previous studies in vitro (Aguayo and White 1992), but contrary to recent reports that GABA-induced currents in vitro are augmented in the presence of NGF (Bevan and Winter 1995). The finding in this study that NGF regulated the AP inflection, but not GABA-induced conductance, is consistent with the suggestion based on studies in crush-lesioned animals that these electrophysiological properties may be regulated by distinct mechanisms in axotomized cutaneous afferent neurons (Oyelese and Kocsis 1996). As with crush lesion neurons, an attenuation in injury-induced increase in GABA conductance was observed after BDNF perfusion. Because a decrease was not observed with NGF, and BDNF is available to regenerating axons after a crush lesion (Meyer et al. 1992), it is possible that the decrease in GABA response observed in that study was mediated in part by BDNF.
Possible mechanisms for the injury-induced increase in GABA conductance include increased receptor density and increased single-channel conductance. Macrophage-derived interleukin-1, which is increased in response to nerve injury (Heumann et al. 1987b; Lindholm et al. 1987; Murphy et al. 1995), has been observed to have a long-lasting potentiating effect on GABAA-receptor-mediated conductance (Miller et al. 1990). The precise mechanisms by which BDNF attenuated the injury-induced increase in GABAA-receptor-mediated conductance of medium-sized cutaneous neurons in this study remain unclear. Studies have shown that only about one-sixth of cutaneous afferent neurons express trkB (McMahon et al. 1994), although this population of trkB-expressing neurons overlaps with that of both small trkA-and large trkC-expressing neurons.
Effect of NGF on axotomy-induced changes in sodium current expression
Uninjured cutaneous afferent neurons express TTX-resistant, slow sodium currents either singularly or in combination with a TTX-sensitive, fast sodium current (Honmou et al. 1994; Rizzo et al. 1995). After axotomy, there is selective loss of the slow current while the fast, TTX-sensitive current predominates (Rizzo et al. 1995). Additionally, Jakeman et al. (1995) have demonstrated increased [3H] saxitoxin binding in L5 and L6 DRG after axotomy, suggesting an increased density of TTX-sensitive Na+ channels.
In the present study, NGF significantly enhanced the expression of slow TTX-resistant current that is substantially reduced in axotomized neurons. However, NGF did not appear to prevent the increase in amplitude of kinetically fast sodium current observed after axotomy. Singular kinetically fast sodium currents are not observed in uninjured cutaneous afferent neurons (Rizzo et al. 1995). NGF has been observed to increase the density of sodium currents in PC12 cells (D’Arcangelo et al. 1993; Fanger et al. 1995; Kalman et al. 1990; Mandel et al. 1987; Toledo-Arai et al. 1995). Moreover, NGF upregulates the expression of sodium channel β1 subunit as well as (multiple) α-subunit mRNA in cultured embryonic DRG neurons (Zur et al. 1995). Increases in the expression of TTX-resistant sodium channels in PC12 cells (Rudy et al. 1987) and TTX-resistant sodium currents in DRG neurons (Aguayo and White 1992) in response to NGF have been reported. Evidently, the increased expression of fast sodium current after axotomy in cutaneous afferent neurons is not responsive to NGF, and is regulated by mechanisms that are distinct from those controlling the expression of slow sodium channels. Additionally, NGF did not regulate the TTX-resistant slow sodium current in all cutaneous afferent DRG neurons, suggesting that perhaps other neurotrophins may regulate this current in some cutaneous neurons.
Role of neurotrophins in the regulation of injury-induced changes in cutaneous afferent neurons
The survival and phenotypic differentiation of sensory neurons is mediated by target-derived trophic influences during development (reviewed in Johnson et al. 1986). Although most adult sensory neurons do not require trophic influences for survival (Lindsay 1988), continued trophic support is required for the maintenance of the differentiated phenotype of some adult sensory neurons, as demonstrated by the ability of NGF to maintain neuropeptide expression in vitro and to reverse changes in neuropeptide expression and some electrophysiological properties after axotomy (Fitzgerald et al. 1985; Lindsay and Harmar 1989; Lindsay et al. 1989; Verge et al. 1995). The role of neurotrophins in phenotypic regulation in the intact adult animal is less clearly defined, because studies examining responses to NGF and NGF antisera treatment have produced conflicting results (Mayer et al. 1982; Ritter et al. 1993). It has been suggested that axotomized adult neurons undergo a “dedifferentiation” (Kuno et al. 1974; Titmus and Faber 1990); thus in this state they might once again be susceptible to the modulatory influences of neurotrophins. Indeed sodium channel mRNA expression reverts to an embryonic pattern after axotomy, with a reemergence of type III sodium channel mRNA that is expressed at high levels embryonically but undetectable in normal adult neurons (Dib-Hajj et al. 1996; Waxman et al. 1994). In vivo deprivation of NGF in developing rats results in the withdrawal of cutaneous Aδ-afferents normally innervating high-threshold mechanoreceptors within the epidermis to the dermis where they innervate D hair receptors instead (Lewin et al. 1992). Although this phenotypic shift can occur only within a specific time window (Lewin et al. 1992), it is possible that one of the effects of axotomy is to reinduce a state of plasticity in which the phenotypic expression of sensory neurons is amenable to modulation by trophic influences. Although the DRG neuronal size population we examined has not been shown to largely express TrkA, the NGF high-affinity receptor, we see a strong regulatory effect of NGF on our observed injury-induced changes. This effect of NGF on myelinated cutaneous afferents is not without precedent, because NGF has been shown to regulate AP duration in vivo in Aβ-afferents of developing rats (Ritter and Mendell 1992). Whether this indicates a regulatory ability of NGF through other receptors (e.g., p75 low-affinity NGF receptor) or other signal transduction mechanisms is unknown and deserves further study.
In the present study, the phenotype of cutaneous afferent neurons expressing inflected APs, low GABA conductance, and slow sodium current was altered after axotomy. Our data indicate that there is selective rescue of some of these properties following treatment with NGF or BDNF. Thus we conclude that a consequence of the loss of peripherally derived trophic influences after axotomy is an alteration in the membrane ion channel and receptor composition in sensory neurons and that expression of somatic sodium channels and GABA receptors is selectively regulated by distinct neurotrophins.
Acknowledgments
We thank H.-F. Mi for preparing the DRG neuronal cultures and B. Toftness for computer support. BDNF was a generous gift from Regeneron, Tarrytown, NY.
This work was supported in part by the Medical Research Service of the Department of Veterans Affairs and National Institutes of Health Grants NS-10174, NS-01606, and NMSS RG-1912.
References
- Aguayo LG, Weight FF, White G. TTX-sensitive action potentials and excitability of adult rat sensory neurons in serum- and exogenous nerve growth factor-free medium. Neurosci Lett. 1991;121:88–92. doi: 10.1016/0304-3940(91)90656-e. [DOI] [PubMed] [Google Scholar]
- Aguayo LG, White G. Effects of nerve growth factor on TTX-and capsaicin-sensitivity in adult rat sensory neurons. Brain Res. 1992;570:61–67. doi: 10.1016/0006-8993(92)90564-p. [DOI] [PubMed] [Google Scholar]
- Bevan S, Winter J. Nerve growth factor differentially regulates the chemosensitivity of adult rat cultured sensory neurons. J Neurosci. 1995;15:4918–4926. doi: 10.1523/JNEUROSCI.15-07-04918.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F, Armstrong CM. Inactivation of the sodium channel. I Sodium current experiments. J Gen Physiol. 1977;70:549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhisitkul RB, Kocsis JD, Gordon TR, Waxman SG. Trophic influence of the distal nerve segment on GABAA receptor expression in axotomized adult sensory neurons. Exp Neurol. 1990;109:273–278. doi: 10.1016/s0014-4886(05)80017-2. [DOI] [PubMed] [Google Scholar]
- Carroll SL, Silos-Santiago I, Frese SE, Ruit KG, Milbrandt J, Snider WD. Dorsal root ganglion neurons expressing trk are selectively sensitive to NGF deprivation in utero. Neuron. 1992;9:779–788. doi: 10.1016/0896-6273(92)90040-k. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Paradiso K, Shepherd D, Brehm P, Halegoua S, Mandel G. Neuronal growth factor regulation of two different sodium channel types through distinct signal transduction pathways. J Cell Biol. 1993;122:915–921. doi: 10.1083/jcb.122.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib-hajj S, Black JA, Felts P, Waxman SG. Down-regulation of subunits for Na channel α-SNS in spinal sensory neurons following axotomy. Proc Natl Acad Sci USA. 1996;93:14950–14954. doi: 10.1073/pnas.93.25.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiStefano PS, Friedman B, Radziejewski C, Alexander C, Boland P, Schick CM, Lindsay RM, Wiegand SJ. The neurotrophins BDNF, NT-3 and NGF display distinct patterns of retrograde axonal transport in peripheral and central neurons. Neuron. 1992;8:983–993. doi: 10.1016/0896-6273(92)90213-w. [DOI] [PubMed] [Google Scholar]
- Fanger GR, Jones JR, Maue RA. Differential regulation of neuronal sodium channel expression by endogenous and exogenous tyrosine kinase receptors expressed in rat pheochromocytoma cells. J Neurosci. 1995;15:202–213. doi: 10.1523/JNEUROSCI.15-01-00202.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M, Wall PD, Goedert M, Emson PC. Nerve growth factor counteracts the neurophysiological and neurochemical effects of chronic sciatic nerve section. Brain Res. 1985;332:131–141. doi: 10.1016/0006-8993(85)90396-8. [DOI] [PubMed] [Google Scholar]
- Frelin C, Cognard C, Vigne P, Lazdunski M. Tetrodotoxin-sensitive and tetrodotoxin-resistant Na+ channels differ in their sensitivity to Cd2+ and Zn2+ Eur J Pharmacol. 1986;122:245–250. doi: 10.1016/0014-2999(86)90109-3. [DOI] [PubMed] [Google Scholar]
- Funakoshi H, Frisén J, Barbany G, Timmusk T, Zachrisson O, Verge VMK, Persson H. Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J Cell Biol. 1993;123:455–465. doi: 10.1083/jcb.123.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber SS, Hoshi T, Aldrich RW. Regulation of ionic currents in pheochromocytoma cells by nerve growth factor and dexamethasone. J Neurosci. 1989;9:3976–3989. doi: 10.1523/JNEUROSCI.09-11-03976.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin PD, Johnson EM., Jr Effects of long-term nerve growth factor deprivation on the nervous system of the adult rat: an experimental autoimmune approach. Brain Res. 1980;198:27–42. doi: 10.1016/0006-8993(80)90341-8. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher ME, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pfluegers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J Physiol Lond. 1985;359:31–46. doi: 10.1113/jphysiol.1985.sp015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumann R, Korsching S, Bandtlow C, Thoenen H. Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection. J Cell Biol. 1987a;104:1623–1631. doi: 10.1083/jcb.104.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumann R, Lindholm D, Bandtlow C, Meyer M, Radeke MJ, Misko TP, Shooter E, Thoenen H. Differential regulation of mRNA encoding nerve growth factor and its receptor in rat sciatic nerve during development, degeneration and regeneration: role of macrophages. Proc Natl Acad Sci USA. 1987b;84:8735–8739. doi: 10.1073/pnas.84.23.8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himes BT, Tessler A. Death of some dorsal root ganglion neurons and plasticity of others following sciatic nerve section in adult and neonatal rats. J Comp Neurol. 1989;284:215–230. doi: 10.1002/cne.902840206. [DOI] [PubMed] [Google Scholar]
- Honmou O, Utzschneider DA, Rizzo MA, Bowe CM, Waxman SG, Kocsis JD. Delayed depolarization and slow sodium currents in cutaneous afferents. J Neurophysiol. 1994;71:1627–1637. doi: 10.1152/jn.1994.71.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakeman LB, Kwan J, Bonhaus DW, Hunter JC. Sodium channel density is increased in rat dorsal root ganglia following chronic constriction injury (CCI) Soc Neurosci Abstr. 1995;21:1815. [Google Scholar]
- Johnson EM, Jr, Rich KM, Yip HK. The role of NGF in sensory neurons in vivo. Trends Neurosci. 1986;9:33–37. [Google Scholar]
- Kalman D, Wong B, Horvai AE, Cline MJ, O’Lague PH. Nerve growth factor acts through cAMP-dependent protein kinase to increase the number of sodium channels in PC12 cells. Neuron. 1990;2:355–366. doi: 10.1016/0896-6273(90)90048-k. [DOI] [PubMed] [Google Scholar]
- Kashiba H, Senba E, Kawai Y, Ueda Y, Tohyama M. Axonal blockade induces the expression of vasoactive intestinal polypeptide and galanin in dorsal root ganglion neurons. Brain Res. 1992;577:19–28. doi: 10.1016/0006-8993(92)90532-e. [DOI] [PubMed] [Google Scholar]
- Kingery WS, Fields RD, Kocsis JD. Diminished dorsal root GABA sensitivity following chronic peripheral nerve injury. Exp Neurol. 1988;100:478–490. doi: 10.1016/0014-4886(88)90033-7. [DOI] [PubMed] [Google Scholar]
- Kostyuk PG, Veselovsky NS, Tsyndrenko AY. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons. I Sodium currents. Neuroscience. 1981;6:2423–2430. doi: 10.1016/0306-4522(81)90088-9. [DOI] [PubMed] [Google Scholar]
- Kuno M, Miyata Y, Munoz-Martinez EJ. Differential reaction of fast and slow-motoneurones to axotomy. J Physiol Lond. 1974;240:725–739. doi: 10.1113/jphysiol.1974.sp010631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ES, Dreyfuss CF, Black IB, Plummer MR. Differential effects of NGF and BDNF on voltage-gated calcium currents in embryonic basal forebrain neurons. J Neurosci. 1995;15:3084–3091. doi: 10.1523/JNEUROSCI.15-04-03084.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin GR, Ritter AM, Mendell LM. On the role of nerve growth factor in the development of myelinated nociceptors. J Neurosci. 1992;12:1896–1905. doi: 10.1523/JNEUROSCI.12-05-01896.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm D, Heumann R, Meyer M, Thoenen H. Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature Lond. 1987;330:658–659. doi: 10.1038/330658a0. [DOI] [PubMed] [Google Scholar]
- Lindsay RM. Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurons. J Neurosci. 1988;8:2394–2405. doi: 10.1523/JNEUROSCI.08-07-02394.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay RM, Harmar AJ. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature Lond. 1989;337:362–364. doi: 10.1038/337362a0. [DOI] [PubMed] [Google Scholar]
- Lindsay RM, Lockett C, Sternberg J, Winter J. Neuropeptide expression in cultures of adult sensory neurons: modulation of substance P and calcitonin gene-related peptide levels by nerve growth factor. Neuroscience. 1989;33:53–65. doi: 10.1016/0306-4522(89)90310-2. [DOI] [PubMed] [Google Scholar]
- Mandel G, Cooperman SS, Maue RA, Goodman RH, Brehm P. Selective induction of brain type II Na+ channels by nerve growth factor. Proc Natl Acad Sci USA. 1987;85:924–928. doi: 10.1073/pnas.85.3.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer N, Lembeck F, Goedert M, Otten U. Effects of antibodies against nerve growth factor on the postnatal development of substance P-containing sensory neurons. Neurosci Lett. 1982;29:47–52. doi: 10.1016/0304-3940(82)90362-7. [DOI] [PubMed] [Google Scholar]
- Mc Mahon SB, Armanini MP, Ling LH, Phillips HS. Expression and coexpression of Trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron. 1994;12:1161–1171. doi: 10.1016/0896-6273(94)90323-9. [DOI] [PubMed] [Google Scholar]
- Meyer M, Matsuoka I, Wetmore C, Olson L, Thoenen H. Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J Cell Biol. 1992;119:45–54. doi: 10.1083/jcb.119.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LG, Galpern WR, Dunlap K, Dinarello CA, Turner TJ. Interleukin-1 augments γ-aminobutyric acid receptor function in brain. Mol Pharmacol. 1990;39:105–108. [PubMed] [Google Scholar]
- Murphy PG, Grondin J, Altares M, Richardson PM. Induction of interleukin-6 in axotomized sensory neurons. J Neurosci. 1995;15:5130–5138. doi: 10.1523/JNEUROSCI.15-07-05130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyelese AA, Eng DL, Richerson GB, Kocsis JD. Enhancement of GABAA receptor-mediated conductances induced by nerve injury in a subclass of sensory neurons. J Neurophysiol. 1995a;74:673–683. doi: 10.1152/jn.1995.74.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyelese AA, Kocsis JD. GABAA receptor-mediated conductance and action potential waveform in cutaneous and muscle afferent neurons of the adult rat: differential expression and response to nerve injury. J Neurophysiol. 1996;76:2383–2392. doi: 10.1152/jn.1996.76.4.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyelese AA, Rizzo MA, Waxman SG, Kocsis JD. Differential effect of neurotrophins on injury-induced plasticity of GABAA receptors and sodium currents in cutaneous afferent DRG neurons. Soc Neurosci Abstr. 1995b;21:1055. [Google Scholar]
- Plummer MR, Logothetis DE, Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989;2:1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- Raivich G, Hellweg R, Kreutzberg GW. NGF receptor-mediated reduction in axonal uptake and retrograde transport following sciatic nerve injury and during regeneration. Neuron. 1991;7:151–164. doi: 10.1016/0896-6273(91)90083-c. [DOI] [PubMed] [Google Scholar]
- Ransom RB, Holz RW. Ionic determinants of excitability in cultured mouse dorsal root ganglion and spinal cord cells. Brain Res. 1977;136:445–453. doi: 10.1016/0006-8993(77)90069-5. [DOI] [PubMed] [Google Scholar]
- Ritter AM, Lewin GR, Kremer NE, Mendell LM. Requirement for nerve growth factor in the development of myelinated nociceptors in vivo. Nature Lond. 1991;350:500–502. doi: 10.1038/350500a0. [DOI] [PubMed] [Google Scholar]
- Ritter AM, Lewin GR, Mendell LM. Regulation of myelinated nociceptor function by nerve growth factor in neonatal and adult rats. Brain Res Bull. 1993;30:245–249. doi: 10.1016/0361-9230(93)90251-6. [DOI] [PubMed] [Google Scholar]
- Ritter AM, Mendell LM. Soma membrane properties of physiologically identified sensory neurons in the rat: effects of nerve growth factor. J Neurophysiol. 1992;68:2033–2041. doi: 10.1152/jn.1992.68.6.2033. [DOI] [PubMed] [Google Scholar]
- Rizzo MA, Kocsis JD, Waxman SG. Slow sodium conductances of dorsal root ganglion neurons: intraneuronal homogeneity and interneuronal heterogeneity. J Neurophysiol. 1994;72:2796–2815. doi: 10.1152/jn.1994.72.6.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo MA, Kocsis JD, Waxman SG. Selective loss of slow and enhancement of fast Na+ currents in cutaneous afferent DRG neurons following axotomy. Neurobiol Dis. 1995;2:87–96. doi: 10.1006/nbdi.1995.0009. [DOI] [PubMed] [Google Scholar]
- Roy ML, Narahashi T. Differential properties of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels in rat dorsal root ganglion neurons. J Neurosci. 1992;12:2104–2111. doi: 10.1523/JNEUROSCI.12-06-02104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, Kirschenbaum B, Rukenstein A, Greene LA. Nerve growth factor increases the number of functional Na channels and induces TTX-resistant Na channels in PC12 pheochromocytoma cells. J Neurosci. 1987;7:1613–1625. doi: 10.1523/JNEUROSCI.07-06-01613.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmued LC, Fallon JH. Fluoro-gold: a new fluorescent retrograde axonal tracer with numerous unique properties. Brain Res. 1986;377:147–154. doi: 10.1016/0006-8993(86)91199-6. [DOI] [PubMed] [Google Scholar]
- Titmus MJ, Faber DS. Axotomy-induced alterations in the electrophysiological characteristics of neurons. Prog Neurobiol. 1990;35:1–51. doi: 10.1016/0301-0082(90)90039-j. [DOI] [PubMed] [Google Scholar]
- Toledo-Arai JJ, Brehm P, Halegoua S, Mandel G. A single pulse of nerve growth factor triggers long-term neuronal excitability through sodium channel gene induction. Neuron. 1995;14:607–611. doi: 10.1016/0896-6273(95)90317-8. [DOI] [PubMed] [Google Scholar]
- Verge VMK, Richardson PM, Weisenfeld-Hallin Z, Hökfelt T. Differential effect of nerve growth factor on neuropeptide expression in vivo: a novel role in peptide suppression in adult sensory neurons. J Neurosci. 1995;15:2081–2096. doi: 10.1523/JNEUROSCI.15-03-02081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall PD, Devor M. The effect of peripheral nerve injury on dorsal root potentials and on transmission of afferent signals into the spinal cord. Brain Res. 1981;209:95–111. doi: 10.1016/0006-8993(81)91174-4. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Kocsis JD, Black JA. Type III sodium channel mRNA is expressed in embryonic but not adult spinal sensory neurons, and is reexpressed following axotomy. J Neurophysiol. 1994;72:466–470. doi: 10.1152/jn.1994.72.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells MR, Vaidya U. Morphological alterations in dorsal root ganglion neurons after peripheral axon injury: association with changes in metabolism. Exp Neurol. 1983;104:32–38. doi: 10.1016/0014-4886(89)90006-x. [DOI] [PubMed] [Google Scholar]
- Wells MR, Vaidya U. Morphological alterations in dorsal root ganglion neurons after peripheral axon injury: association with changes in metabolism. Exp Neurol. 1989;104:32–38. doi: 10.1016/0014-4886(89)90006-x. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Matsuda Y, Samejima A. Tetrodotoxin-resistant sodium and calcium components of action potentials in dorsal root ganglion cells of the adult mouse. J Neurophysiol. 1978;41:1096–1106. doi: 10.1152/jn.1978.41.5.1096. [DOI] [PubMed] [Google Scholar]
- Zur KB, Oh Y, Waxman SG, Black JA. Differential up-regulation of sodium channel α- and β-subunit mRNAs in cultured embryonic DRG neurons following exposure to NGF. Mol Brain Res. 1995;30:97–103. doi: 10.1016/0169-328x(94)00283-k. [DOI] [PubMed] [Google Scholar]