Abstract
To examine the mechanisms responsible for the more rapid nerve regeneration observed after a previous (conditioning) nerve injury, adult rats were subjected to a midthigh sciatic nerve transection by using one of three protocols designed to facilitate or restrict nerve regeneration: 1) ligation, in which transected axons were prevented from regenerating; 2) cut, in which transected axons were permitted to extend into peripheral target tissue but were separated from the denervated peripheral nerve stump; and 3) crush, in which axons could regenerate normally through the denervated distal nerve tract. The affected dorsal root ganglia (DRG) were subsequently removed, dissociated, and cultured for up to 3 days, and the timing of neurite initiation, rate of outgrowth, and arborization pattern of previously injured neurons were compared with control DRG. Our results indicate that conditioning lesions have at least four distinct and differentially regulated effects on neuronal morphogenesis: 1) conditioning lesions promote earlier neurite initiation, 2) prior nerve injury decreases the ability of neurons to extend long neurites following a second axotomy, 3) exposure to the environment of a denervated peripheral nerve stimulates greater initial rates of neurite outgrowth, and 4) conditioning lesions reduces initial neuritic branching frequency, resulting in straighter neurites whose growth cones extend further distances from their cell bodies. The primary effect of all conditioning lesions on cultured DRG neurons appeared to be to advance the timing of morphogenesis, resulting in conditioning-lesioned neurons that exhibited characteristics consistent with control neurons that had been cultured for an additional day or more. A secondary effect of conditioning lesions on neurite outgrowth rates was dependent on the local environment of the axons prior to culturing.
Keywords: neurite outgrowth, neurite initiation, nerve regeneration, conditioning lesion, neurite arborization
It has been well known for many years, that a prior, or conditioning, nerve lesion can, in many cases, lead to more rapid nerve regeneration following a second nerve injury (Carlson, 1983; Edwards et al., 1981; Forman et al., 1980; Jacob and McQuarrie, 1993; McQuarrie, 1978; Sjöberg and Kanje, 1990a,b; Tanaka et al., 1992; Thomas, 1970), but the mechanisms responsible for this “conditioning lesion effect” are not well understood. After more than two decades of study, it remains unclear which influences are responsible for conditioning-lesion-induced enhancement of regeneration and which aspects of neuronal morphogenesis are altered by prior lesioning.
Peripheral nerve injury induces a complex series of changes in both the axotomized neurons and in associated cells. Effects on axotomized neurons include depolarization and calcium influx (Mattson et al., 1990), changes in gene expression (Gold et al., 1994; Goldstein et al., 1988; Herdegen et al., 1992; Lankford et al., 1996; Leah et al., 1991; Miller et al., 1989; Moskowitz and Oblinger, 1995; Watson, 1968; Wells and Vaidya, 1994; Woolf et al., 1990), electrophysiological properties (Kocsis et al., 1982; Oyelese et al., 1995; Oyelese and Kocsis, 1996; Rizzo et al., 1995; Waxman et al., 1994), and axonal transport (McQuarrie and Graffstein, 1982; McQuarrie and Jacobs, 1991; Moskowitz and Oblinger, 1995; Wujek, 1983) in axotomized neurons, as well as loss of retrogradely transported trophic factors from peripheral target tissues (Kristenson and Olson, 1975). Axotomy also induces local inflammatory responses that promote macrophage invasion into injured nerves (Beuche and Freide, 1984; Perry et al., 1987). Satellite cells in the dorsal root ganglia (DRG; Friede and Johnstone, 1967; Leech, 1967; Pannese, 1964) and Schwann cells in the denervated nerve tract (Abercrombie and Johnson, 1946; Pelligerino and Spencer, 1985; Salzer and Bunge, 1980; Sjöstrand, 1965) proliferate, and denervated Schwann cells increase their production of trophic factors (Heuman et al., 1987; Lindholm, 1987; Meyer et al., 1992; Sebert and Shooter, 1993; Windebank and Poduslo, 1986) and laminin (Bignami et al., 1994; Cornbrooks et al., 1983). Many of these axotomy-induced changes in neurons (Jenkins and Hunt, 1991; Jenkins et al., 1993; Van der Zee et al., 1989) and Schwann cells (Heumann et al., 1987; Meyer et al., 1992; Thomas, 1970) persist at least several weeks, or until reinnervation is established and may therefore contribute to conditioning lesion-induced enhancement of regeneration.
Although the more rapid regrowth of peripheral axons following a second nerve injury is most often explained in terms of changes in the growth environment of the previously denervated nerve tract (Kanje et al., 1991; Lu and Richardson, 1991; Ouedega et al., 1994; Politis et al., 1982; Sjöberg and Kanje, 1990a; Sjöberg et al., 1988), other data support the idea that prior nerve injury alters the growth properties of the neurons themselves (Hu-Tsai et al., 1994; Richardson and Verge, 1984, 1987). Different in vivo conditioning lesion studies have not only yielded different conclusions about the effects of conditioning lesions on the rate of outgrowth vs. the timing of neurite initiation but also have reported significantly different growth rates and delay times before initiation of outgrowth for both single-and double-lesioned axons in the same model systems (Bisby and Pollock, 1983; Forman et al., 1980; McQuarrie et al., 1977, 1978; Sjöberg and Kanje, 1990a; see Grafstein and McQuarrie, 1978 for review). Unfortunately, most in vivo studies have had to make a series of assumptions to calculate axonal growth rates and extrapolate the timing of neurite initiation, including 1) that there is no axonal retraction prior to initiation of outgrowth, 2) that there is no axonal branching or collateral sprouting from uninjured axons, 3) that rates of axonal outgrowth are uniform throughout the period investigated, and 4) that rates of growth are similar for all types of neurons within the nerve. Each of these assumptions have been at least partially invalidated (Diamond et al., 1987; Duce et al., 1976; Jenq et al., 1988; McQuarrie, 1985; Ramón y Cajal, 1928; Sjöberg and Kanje, 1990a,b), implying that rates of outgrowth and times of axonal initiation, calculated by using these assumptions, are unreliable.
The relative roles of changes in neuronal growth properties and changes in local growth environment in conditioning lesion-induced enhancement of nerve regeneration are also unclear. For example, although one set of experiments showed an increase in initial axonal growth rates following conditioning lesions distant from the site of the test lesion, supporting the idea that conditioning lesion-induced enhancement of regeneration is due to changes in neuronal growth properties rather than a more favorable growth environment of the previously denervated nerve tract, other experiments in the same study showed that freeze-killing Schwann cells immediately distal to the test lesion blocked the conditioning lesion effect, implying that the local growth environment does play a critical role in the conditioning lesion effect (Sjöberg and Kanje, 1990a). Furthermore, although one laboratory reported that prior peripheral axotomy promoted greater extension of DRG axons into the central nervous system after a dorsal root crush (Richardson and Verge, 1984, 1987), another study found no effect of peripheral lesions on regrowth of central DRG axons (Oblinger and Lasek, 1984).
The strongest evidence that axotomy-induced changes in intrinsic neuronal growth characteristics play a significant role in the conditioning lesion-induced enhancement of nerve regeneration comes from studies of cultured neurons showing that a prior conditioning nerve lesion in vivo can promote earlier neurite outgrowth from dissociated rat DRG neurons (Hu-Tsai et al., 1994) or frog retina explants (Agranoff et al., 1976; Landreth and Agranoff, 1976) in vitro. These results argue that prior axotomy alters the growth properties of neurons themselves, independent of effects on the local growth environment. However, indirect effects of the lesions on trophic factor production by support cells, which were also present in the cultures, could not be ruled out.
To test the hypothesis that conditioning lesions alter the growth properties of neurons, independent of changes in the local growth environment, and to assess whether conditioning lesion-induced changes in neuronal morphogenesis involve primarily changes in timing, rate, or pattern of neurite growth, we subjected adult rats to sciatic nerve injury and examined the attachment and survival, axon initiation, total neurite outgrowth, and branching frequency of cultured dissociated neurons from the affected dorsal root ganglia. Nerve injury protocols that were either permissive or nonpermissive for nerve regeneration were used to discriminate between changes in growth properties related to axotomy and/or regeneration alone and those that were due to prior exposure to growth-promoting substances in the denervated nerve tract. Although the pattern of morphogenesis varied greatly between individual neurons within each condition, our results indicated that neurite initiation, rate of outgrowth, and branching frequency were affected by prior conditioning lesions and that the rate of outgrowth was differentially affected by regeneration-permissive and -nonpermissive lesions.
MATERIALS AND METHODS
Surgery and cell culture
Adult female Wistar rats (140–180 g) were anethesized with an intraperitoneal injection of ketamine (40 mg/kg) and xylazine (2.5 mg/kg) and subjected to a unilateral midthigh sciatic nerve transection using one of three protocols designed to differentially impact nerve regeneration. In the ligation protocol, the nerve was tied off with 4-0 suture silk, cut distal to the suture, and the proximal cut end was then pulled into a silicon-plugged cuff to prevent regeneration. In the crush protocol, the nerve was clamped for 5 seconds at 90° angles with a pair of number 5 forceps, severing axons but leaving the nerve tract intact to facilitate functional regeneration. In the cut protocol, a 1-cm section of nerve was removed, allowing axons to regenerate into peripheral tissue, but depriving them of their normal peripheral nerve growth environment and inhibiting function repair. To more directly compare the effects of different lesioning protocols, for some experiments, two different conditioning lesions were performed on opposite legs of a single animal. In some experiments the proximal stump of the ligated nerve was injected with 0.05 ml of 4% Fluoro-Gold (Fluorochrome, Inc., Englewood, CO) to label cell bodies of neurons whose axons were transected. One day to 5 weeks after surgery, rats were deeply anesthetized with sodium pentobarbital (I.P., 60 mg/kg) and DRG L4 and L5 were removed, trimmed of connective tissue and nerve segments, and dissociated with collagenase and papain as described elsewhere (Oyelese et al., 1995). Dissociated neurons were plated on polyornithine- and laminin-coated coverslips in media containing 45% DMEM, 45% Ham’s F12, and 10% fetal calf serum at a density of half ganglion per 35-mm coverslip.
Immunohistochemistry
After several hours or days in culture, cell-bearing coverslips were fixed with 4% ice-cold paraformaldehyde, permeablized with Triton X-100, and blocked with bovine serum albumin and normal goat serum. Cultures were then stained with the monoclonal antibody SMI-32, directed against nonphosphorylated rat neurofilament proteins, and secondary and tertiary antibodies 501 and 405 (Sternberger Monoclonals Incorporated, Baltimore, MD), and visualized by using standard PAP labeling procedure described by Sternberger et al. (1970). Cells were visually inspected during the diaminobenzidine (DAB) reaction using a Nikon TMS inverted microscope to ensure dense staining of all processes for experiments involving measurement of neurites. DAB reaction time was reduced in experiments involving Fluoro-Gold labeling to prevent obscuration of the fluorescence signal. Stained coverslips were mounted on glass slides by using Aqua Mount or Aqua polymount (Lerner Laboratories, Pittsburgh, PA).
Morphometric analysis
Fluoro-Gold-labeled and neurofilament-stained cultures were examined by using a Leitz Aristoplan fluorescence microscope with a UV excitation filter. Neurofilament-positive cells were scored for Fluoro-Gold fluorescence and for the presence or absence of at least one neurite whose length was more than twice the cell body diameter. Percentages of Fluoro-Gold-labeled and unlabeled neurons with neurites were calculated separately.
Neurofilament-stained coverslips without Fluoro-Gold labeling were examined by using a 40X objective on a Nikon Microphot attached to a MTI CCD72 video camera (total screen magnification 1,320X). Measurements of cell body sizes and neurite lengths of DAB-labeled cells were made by using Image 1 software (Georgia Instruments, Atlanta, GA).
To distinguish between processes that represented new neurite initiation and those that could represent residual axonal stumps or abortive filopodia sprouting, neurons were scored as representing one of three growth categories; neurons were scored as “no neurites” if they exhibited no visible cell processes, “short” if they exhibited only processes that were less than twice the cell diameter in length, and “long” if they exhibited at least one process that was twice as long as the cell diameter. Neurons in the “long” category were considered to have initiated new neurite outgrowth. All neurons in each condition were scored for neurite initiation. Additional detailed data were collected on the first 200 or 500 neurons on each coverslip. For this group of neurons, cell body diameter (measured along the longest and shortest axes), total neurite length, numbers of main processes extending from the cell body, and number of branch points also were recorded. Average interbranch distances were calculated for each neuron by using the formula: Average Interbranch Distance = Total Neurite Length ÷ (Number of Main Processes + [2 × Number of Branch Points]). For some early time points, the numbers of neurons with morphologically identifiable growth cones also were counted and recorded. Data for each cell were recorded separately and entered into a spreadsheet to facilitate later comparisons between subpopulations of neurons. Cells that could not be measured or scored were recorded as empty data sets. At least 97% of neurons could be scored for neurite outgrowth in each condition. Overall, total neurite outgrowth could be measured in roughly two thirds of all neurons with neurites at day 1 in culture, and numbers of branch points could also be counted in half of neurite-bearing neurons, but the percentages of neurons from which these data could be obtained decreased with time in culture as more processes began to overlap. The percentages of neurons cultured 1 week after surgery, for which each type of information could be obtained, are shown in Table 1. Similar numbers were obtained for neurons cultured 1 day, 2 weeks, 3 weeks, and 5 weeks after surgery.
TABLE 1.
Summary of Neuronal Population Sizes Analyzed for Each Growth Parameter at Successive Time Points in Culture
| Treatment and culture age |
No. of experiments |
Neurons examined |
Scored for outgrowth (%) |
Neurites counted (%) |
Neurites measured (%) |
Branching frequency (%) |
|---|---|---|---|---|---|---|
| C1 | ||||||
| Control | 23 | 6,734 | 98 | 69 | 86 | 62 |
| Ligation | 25 | 7,706 | 97 | 64 | 66 | 49 |
| Cut | 14 | 4,088 | 98 | 59 | 76 | 54 |
| Crush | 12 | 6,312 | 96 | 59 | 52 | 41 |
| C2 | ||||||
| Control | 9 | 3,197 | 96 | 62 | 49 | 38 |
| Ligation | 11 | 3,412 | 94 | 46 | 33 | 22 |
| Cut | 9 | 1,802 | 95 | 50 | 56 | 41 |
| Crush | 8 | 2,425 | 95 | 55 | 33 | 26 |
| C3 | ||||||
| Control | 11 | 3,995 | 88 | 64 | 26 | 22 |
| Ligation | 11 | 3,690 | 93 | 47 | 24 | 18 |
| Cut | 9 | 1,801 | 93 | 49 | 43 | 34 |
| Crush | 9 | 2,979 | 95 | 53 | 29 | 23 |
Table indicates the total numbers of neurons examined in control and conditioning lesioned dorsal root ganglion (DRG) cultures plated one week after a conditioning lesion, as well as the percent of neurons which could be scored for the presence of neurites at least twice their cell diameter in length, and the percentages of process-bearing neurons for which numbers of neurites, total neurite length and branching frequency could be determined. Note that the neurite initiation frequency data should be considered the most reliable since neurite initiation could be scored for >90% of all neurons for virtually every condition and age examined. Branching frequency data at days 2 and 3 should be considered less reliable since only a third to a quarter of process-bearing neurons could have their process fully measured and branchpoints counted at these time points. However, the total numbers of neurons from which branching frequency data was obtained was still very high, with average interbranch distances calculated for 483–1,875 neurons for 1, 2, and 3 day cultures.
Statistical treatment of data
We used several different approaches to analyze the effects of conditioning lesion on neuronal survival and morphogenesis and to distinguish between the effects of conditioning lesions on all types of DRG neurons, the effects of lesions that were specific to subcategories of DRG neurons, and the indirect effects of conditioning lesions on one growth parameter through effects on differential cell survival or on other growth parameters.
To identify conditioning lesion-induced changes in neuronal morphology that were consistent across many experiments, we computed the percentage of neurite-bearing neurons, average numbers of neurites per neurite-bearing neuron, average cell body size, average total neurite length, and average interbranch distance for neurons in each treatment condition for each experiment and compared the results of each treatment condition by using the unpaired Student’s t-test. Trends related to culture age also were examined by using the unpaired Student’s t-test. Effects of treatments on overall neuronal viability were assessed by comparing total cell counts in control and conditioning-lesioned cultures using both paired and unpaired Student’s t-tests.
To distinguish between morphological changes affecting only subpopulations of neurons and those affecting the population as a whole, data from all cells exposed to a particular experimental condition were first pooled. The effects of treatment conditions and time in culture on the size distribution of neurons were assessed visually, by plotting histograms of the size distribution of neurons and statistically, by performing a one-way analysis of variance on cell size measurements from different treatment conditions. The percentage of neurite-bearing neurons, average numbers of neurites per neurite-bearing neuron, and average total neurite outgrowth were then computed for neurons in each 10-μm size range, and average interbranch distances were calculated for neurons in discrete total outgrowth categories and compared across conditions. A χ2 test was used to compare the proportion of neurons in each neurite outgrowth category (“no neurites,” “short,” or “long”) under different treatment conditions, by using the percentage of neurons in each outgrowth category in control cultures to calculate the expected value for experimental conditions.
To determine whether conditioning lesions induced changes in numbers of neurites, total neurite outgrowth, or neurite branching, independent of effects on other morphological parameters, we first computed correlation coefficients between each pair of the morphometric variables. We then compared treatment conditions by using a multiple analysis of covariance (MANCOVA) program in which potentially confounding variables with a 5% or greater correlation with the parameter being tested were identified as covariants. Thus, the effects of conditioning lesions on numbers of neurites per process-bearing neuron, total neurite outgrowth, and average interbranch frequency were assessed by using a MANCOVA in which cell body size, cell body size + numbers of main neurites, and cell body size + numbers of main neurites + total neurite outgrowth were identified, respectively, as covariants.
MANCOVA statistical tests were performed by using Systat for Windows 5.0. Unpaired Student’s t-tests and ANOVAs were performed by using the analysis toolpack in Microsoft Excel 5.0 for Windows. χ2 values were calculated by using a self-written Microsoft Excel subroutine with P values obtained from standard statistical tables.
Unless otherwise specified in the text, differences in growth parameters that were statistically significant by using one analytical approach were also statistically significant by using all approaches. Readers should note, however, that the absolute magnitude of the effects on any growth parameter varies somewhat, depending on the method used to calculate the effect. Graphs showing effects of treatments on subcategories of neurons based on pooled data cannot be directly compared with graphs representing average effects of treatments based on an experiment by experiment analysis.
All procedures involving live animals have been approved by Yale Animal Care and Use Committee.
Unless otherwise specified, all chemicals were obtained from Sigma Chemical Co. (St. Louis, MO). Values in text and figures are shown as means ± standard error. Values for n refer to the number of experiments analyzed unless otherwise specified as number of cells examined. Each experiment represented surgery on a single animal and analysis of a single culture dish for each time point in culture. With the exception of experiments involving conditioning intervals of 2–5 weeks, each data point shown represents at least three experiments on three different animals. For experiments involving conditioning intervals of 2–5 weeks duration, each data point represents one to three experiments.
RESULTS
General appearance of DRG cultures
The overall appearance of adult DRG cell cultures has been described in detail elsewhere (Birch et al., 1992; Lankford et al., 1995). Briefly, both dissociated cell cultures of control adult rat DRGs and cultures of DRGs from animals subjected to a prior sciatic nerve ligation, nerve crush, or nerve cut protocols, consisted primarily of neurofilament-positive cells with 10- to 80-μm cell bodies and a variable complement of putative Schwann cells, satellite cells, and fibroblasts. Immediately after plating, neuronal cell bodies typically appeared rounded, with a variable proportion of neurons retaining short axonal stumps, less than two cell body diameters in length, and a very small percentage of neurons (typically less than 5%) retaining longer processes. Numbers of nonneuronal cells increased with time in culture but were highly variable and did not differ significantly between different treatment conditions.
Both control cultures and cultures derived from conditioning-lesioned DRGs showed extensive neurite outgrowth after several days in vitro, and no differences in the overall appearance of control and conditioning-lesioned neurons were detected in cells cultured for 3 days or more. At 24 hours after plating, however, a greater density of neurites was apparent in cultures of conditioning-lesioned neurons than in control cultures (Fig. 1). To assess the nature of this apparent increase in early neuritic arborization, we examined neuronal attachment and survival rates, timing of neurite initiation, rate of neurite outgrowth, and branching patterns in control cultures and cultures of DRG subjected to a sciatic nerve ligation, nerve crush, or nerve cut protocol, and compared the effects of control conditions and conditioning lesions of different durations on each aspect of neuronal morphogenesis.
Fig. 1.
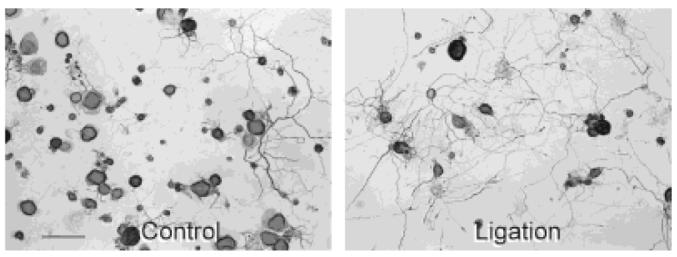
Effects of conditioning lesions on gross appearance of dorsal root ganglion (DRG) cultures. Representative light micrographs of neurofilament stained control (A) and ligation (B) protocol cultures of adult rat DRG neurons after 1 day in vitro. L4 and L5 DRG from the control and conditioning-lesioned side of the animal were dissociated and plated 1 week after subjecting rats to a unilateral midthigh sciatic nerve ligation with the proximal cut end of the nerve pulled through a silicon-plugged cuff to prevent regeneration through the denervated nerve segment. Note the enhanced neurite outgrowth in the culture exposed to a previous axotomy in vivo. Scale bar = 100 μm.
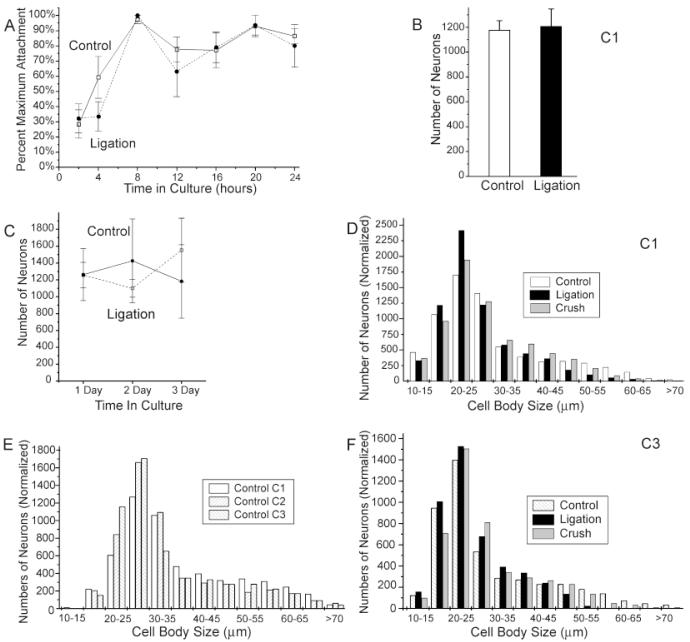
Neuronal attachment, survival, and size distribution
DRG neurons from both the control and 1-week ligation condition attached rapidly to the polyornithine-laminin substrate, with 28 ± 9% (n = 8 experiments) of control neurons and 32 ± 9% (n = 7 experiments) of ligated neurons becoming attached to the coverslip within 2 hours after plating, and essentially all viable neurons attaching within 8 hours (Fig. 2A). Numbers of attached neurons declined slightly between 8 and 24 hours after plating, presumably due to the death of damaged neurons, but no decrease in cell numbers was observed between 1 and 3 days in culture, and trypan blue exclusion tests detected few or no nonviable neurons attached to coverslips at any age. After 24 hours in vitro, control and ligation protocol cultures, plated at a density of half ganglion per coverslip, contained an average of 1,177 ± 77 (n = 13 experiments) and 1,207 ± 140 (n = 140 experiments) neurons per coverslip, respectively, for all experiments that were completely counted (Fig. 2B). No statistically significant differences were detected between cell counts at 1, 2, and 3 days in vitro for any treatment condition, or between treatment conditions at any time point (Fig. 2C).
Fig. 2.
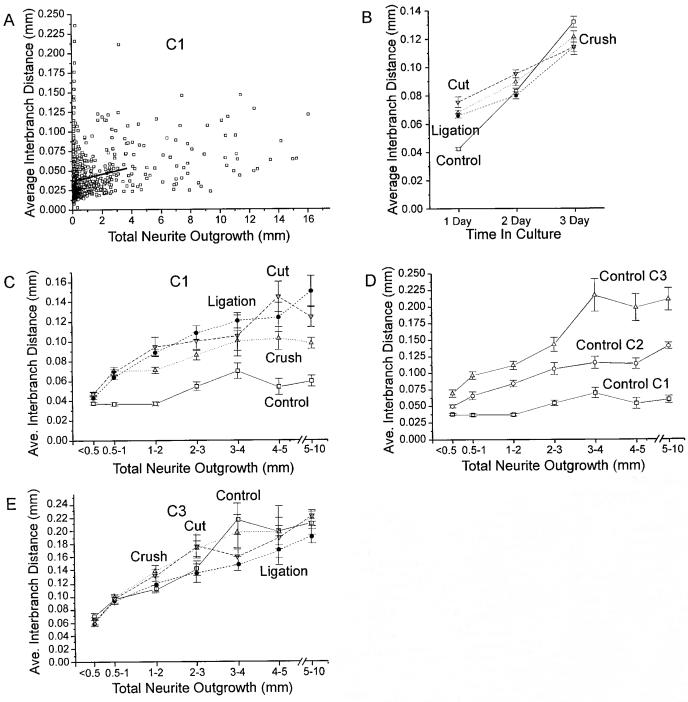
Effects of 1-week conditioning lesions on neuronal attachment, survival, and cell size distributions. A: Time course of cell attachment for control and ligation protocol neurons. Each value represents the average of at least three experiments in which cultures were fixed at successive time points after plating. To correct for differences between the numbers of viable neurons obtained for each dissociation procedure, total cell counts for each time point were divided by the maximum number of cells counted for that treatment condition in that experiment. B,C: Effects of conditioning lesions on cell survival. B: Bar graph comparing the total numbers of neurons in 13 control and 10 ligation cultures after 24 hours in vitro. C: Line graph illustrating the numbers of neurons counted for four experiments after 1, 2, and 3 days in culture. Note that there is no statistically significant difference between conditions at any time point and no statistically significant difference between time points for either treatment condition. D—F: Histograms illustrating the distribution of cell sizes for individual treatment conditions at different time points in culture. Actual cell counts were normalized to the same total numbers of cells in each condition. Note the increased numbers of neurons in the 20- to 25-μm cell size category and decrease in neurons in larger cell size categories in the conditioning-lesioned conditions. Distribution of cell sizes in all conditioning-lesioned conditions at day 1 in vitro differ significantly from controls at P < 0.000001 with an ANOVA. Error bars for this and all subsequent figures represent standard errors. Open squares and solid lines indicate control condition. Filled circles and dashed lines indicate ligation protocol. Cultures ages of 1, 2, and 3 days are indicated as C1, C2, and C3, respectively, in this and all subsequent figures.
Neuronal cell sizes in both control and conditioning lesion protocol cultures ranged from approximately 10 μm to greater than 70 μm in diameter, with the majority of neurons falling between 15 and 35 μm in size (Fig. 2D-F). However, the distribution of cell sizes appeared to be shifted slightly downward in the conditioning-lesioned cultures (Fig. 2D). The proportions of neurons with at least 50-μm diameter cell bodies were reduced in 1-day cultures in the ligation, cut, and crush protocol conditions, whereas the proportions of neurons in the 20- to 25-μm range were significantly increased, resulting in a decrease in average neuronal cell size from 29.5 ± 0.2 μm for control neurons to 26.6 ± 0.1, 27.4 ± 0.2, and 28.6 ± 0.1 μm for the ligation, cut, and crush protocol, respectively. (All treatment conditions differ significantly at P < 0.00005 by using ANOVA.) A similar, but smaller, reduction in the proportion of neurons with larger cell bodies also was observed in control cultures after 2 or 3 days in culture (Fig. 2E), but differences between control and conditioning-lesioned cultures could still be detected at 3 days in vitro (Fig. 2F). (Conditioning lesions differ significantly from controls at P < 0.0005 by using ANOVA.)
Because the numbers of surviving neurons did not differ between treatment conditions or decrease with time in culture, the reduction in the proportion of neurons with large cell bodies in cultures of conditioning-lesioned neurons and in control cultures with time in vitro implied a post-axotomy shrinkage of neuronal cell bodies rather than selective death of larger neurons. Differential survival of neuronal subpopulations could not be ruled out, however, and it was necessary to evaluate the relationships between cell size and other morphological characteristics and to compare the effects of conditioning lesions on morphogenesis of different size categories of neruons, as well as the effects on the cell population as a whole.
Conditioning lesion-induced promotion of neurite initiation
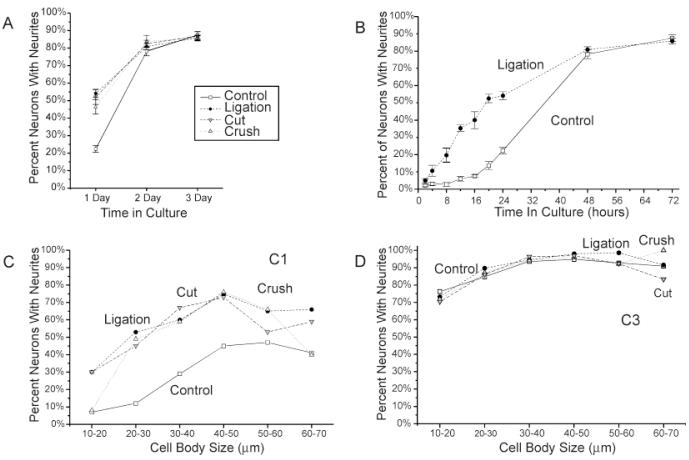
As reported previously (Kocsis et al., 1994; Lankford et al., 1995, 1996), the majority of control adult rat DRG neurons initiate neurites after a delay of more than 24 hours in vitro. Although this study, which used a different lot of fetal calf serum and of laminin, found a slightly higher proportion of neurons initiating processes during the first 24 hours in vitro than our previous reports, only 22 ± 2% (n = 23 experiments) of control neurons in this study exhibited at least one process more than twice as long as their cell diameter 24 hours after plating. In contrast, neurons from DRG subjected to a conditioning lesion 1 week prior to culturing were roughly twice as likely to initiate processes during the first 24 hours in culture; 54 ± 2%, 52 ± 4%, and 46 ± 4% (n = 25, 14, and 12 experiments, respectively) of neurofilament-positive cells in the ligation, cut, and crush protocol conditions, respectively, generated at least one process of criterion length by 24 hours in vitro (Fig. 3A). Percentages of neurite-bearing neurons in each conditioning lesion condition differ significantly from controls at P < 0.000001 by using the unpaired Student’s t-test. χ2 tests on pooled data for outgrowth status were significant at P < 0.0005 for each lesioning condition. There was no significant difference between lesion protocols.
Fig. 3.
Effects of 1-week conditioning lesions on neurite initiation. A: Comparison between the numbers of process-bearing neurons in control and three types of conditioning-lesioned cultures at 1, 2, and 3 days in vitro. Note that all conditioning-lesion conditions show an increase in neurite initiation at day 1 in culture, but no difference from control after 3 days in vitro. The percentage of neurite-bearing neurons in all conditioning-lesioned conditions differ significantly from controls at the P < 0.000001 level or lower (unpaired Student’s t-test). B: Time course of neurite initiation. Graph showing the percentage of attached neurofilament-positive cells exhibiting at least one process more than twice the cell diameter in cultures fixed at successive time points after plating. Neurite initiation frequencies in the ligation condition differ significantly from controls at the P < 0.002 level or better for time points between 12 and 24 hours in culture by using the unpaired Student’s t-test and at P < 0.02 or less for all time points after 2 hours by using the χ2 test. Each data point represents at least three to five experiments, except 2-hour data points, which represent seven experiments. C,D: Comparison of neurite initiation frequencies for neurons in different size classes in control and conditioning-lesioned cultures at 1 and 3 days in vitro. Note that neurons in all cell size categories in conditioning-lesioned cultures are more likely to initiate neurite after 24 hours in vitro than control neurons of similar sizes, but no difference is observed in 3-day cultures. Note also that because the numbers of neurons in each size category differ between treatment conditions, panels C and D cannot be directly compared with panel A, in which data for all cells in each experiment were pooled. In this and all subsequent figures: open squares and solid lines, control condition; filled circles and short dashed lines indicate ligation protocol; downward triangles and long dashed lines, cut condition; up triangles and dotted lines, crush condition.
Although conditioning lesions increased the percentages of process-bearing neurons at 24 hours in vitro, prior nerve injury did not appear to affect the ability of neruons to ultimately generate neurites. Numbers of precesses-bearing neurons did not differ significantly between treatment conditions after 2 or 3 days in vitro (Fig. 3A), with 87 ± 2%, 86 ± 2%, 87 ± 3%, and 86 ± 1 of control, ligation, cut, and crush protocol neurons, respectively, initiation process by day 3 (n = 10, 13, 8, and 8 experiments, respectively).
Time course studies of neurite initiation indicated that conditioning lesions advanced the onset of neurite initiation in vitro by approximately 12 hours. Neurons in ligation protocol cultures began initiating processes almost immediately after plating, and as early as 2 hours in vitro, 5 ± 1% (n = 7 experiments) of ligation protocol neurons exhibited at least one process more than twice their cell diameter, compared with only 2.1 ± 0.8% (n = 7 experiments) of control neurons (P < 0.01, χ2 test). The percentage of process-bearing neurons in ligation protocol cultures increased significantly to 11 ± 3% (n = 3 experiments) and 20 ± 4% (n = 3 experiments) of attached neurons at 4 and 8 hours in vitro, respectively (Fig. 3B), whereas the percentages of neurite-bearing neurons in control cultures did not begin to increase until after 12 hours in vitro and remained substantially below those for ligation protocol cultures until after the first day in vitro. The appearance of morphologically identifiable growth cones in both control and ligation protocol cultures followed similar time courses as those for the presence of criterion length neurites, and a partial time course study of neurite initiation in crush protocol cultures showed a pattern of neurite initiation similar to the ligation protocol (data not shown).
The increased percentages of neurite-bearing neurons in conditioning-lesioned cultures at 24 hours in vitro could not be accounted for by changes in size distribution of the neuronal population in conditioning-lesioned cultures. Although proportionally fewer large neurons were present in conditioning-lesioned cultures, neurons with larger cell bodies were actually more likely to initiate processes during the first 24 hours in vitro than smaller cells (Fig. 3C). Furthermore, conditioning-lesioned neurons in essentially all cell size categories showed increased probabilities of neurite initiation at 24 hours in vitro (Fig. 3C).
Although the timing of neurite initiation varied with cell size and prior lesioning status, neurons in all cell size categories and all treatment conditions also showed similar capacities for ultimately generating neurites, and by day 3 in vitro, the majority of neurons in all cell size categories and all treatment conditions had initiated at least one neurite (Fig. 3D).
Roles of axotomy vs. local environment in promotion of neurite initiation
Although all size classes of neurons in conditioning-lesioned protocol cultures exhibited an increased probability of early neurite initiation, retrograde labeling of cut axons with Fluoro-Gold revealed that only neurons whose axons were transected were more likely to initiate axons during the first 24 hours in culture (see Fig. 4A,B for appearance of Fluoro-Gold-labeled cells). Neurite initiation rates for neurons whose axons exited the main nerve proximal to the site of axotomy, and were therefore uncut and unlabeled, were not statistically different from control cultures at 24 hours in vitro: 23 ± 2% (n = 5 experiments) of Fluoro-Gold-negative ligation protocol neurons initiated neurites after 24 hours in vitro, compared with 22 ± 2% (n = 23 experiments) of control neurons. Fluoro-Gold-labeled neruons, in contrast, showed even higher rates of neurite initiation after 24 hours than ligation protocol cultures as a whole; 77 ± 6% (n = 5 experiments) of Fluoro-Gold-positive neurons initiated processes, compared with 54 ± 2% (n = 23 experiments) for all ligation protocol neurons P < 0.0004, Student’s t-test; Fig. 4C). Fluoro-Gold injections themselves had no detectable effect on overall neurite initiation frequencies. Possible differences in total neurite outgrowth and branching frequency between Fluoro-Gold-labeled and unlabeled neurons in ligation protocol cultures could not be assessed due to fluorescence bleaching. Conditioning lesion-induced increases in early neurite initiation could not be mimicked by exposing neurons to conditioned media from ligation protocol cultures or Schwann cell cultures (Fig. 4D).
Fig. 4.
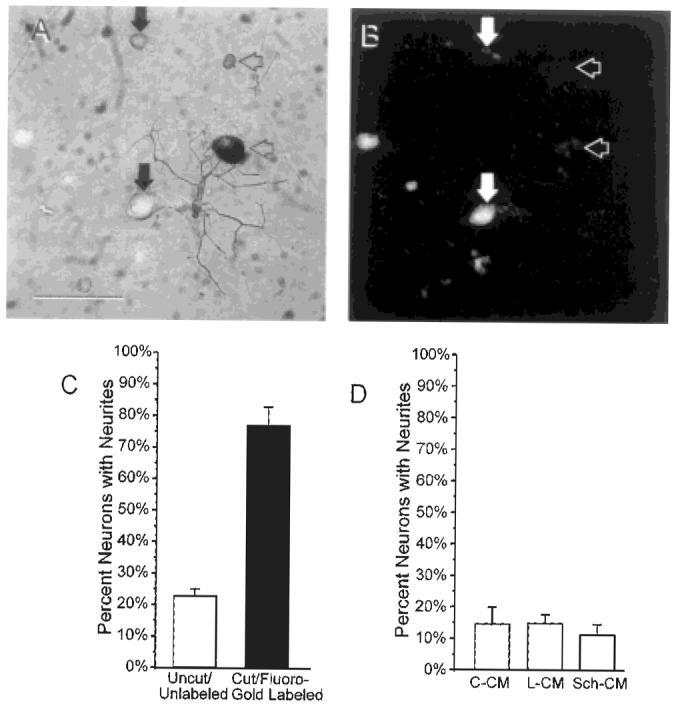
Effects of axotomy vs. local environment on neurite initiation. Transmitted light (A) and fluorescence micrograph (B) of a ligation protocol culture in which Fluoro-Gold was injected into the silicon-plugged cuff to retrogradely label cell bodies of neurons with ligated axons. Vertical filled arrowheads indicated Fluoro-Gold-labeled neurons. Horizontal open arrowheads indicate neurofilament-positive cells that are not labeled with Fluoro-Gold. C: Graph showing the percentage of Fluoro-Gold-positive and Fluoro-Gold-negative neurons with at least one process of criterion length. Neurite initiation frequencies for Fluoro-Gold-labeled neurons differed significantly from unlabeled neurons at P < 0.0004 (unpaired Student’s t-test). D: Graph showing the percentage of neurite-bearing neurons in 24-hour cultures exposed continuously to conditioned media from control DRG cultures (C-CM), conditioned media from ligation protocol DRG cultures (L-CM) or conditioned media from Schwann cell cultures (Sch-CM). Note that neurite initiation frequencies in cultures exposed to conditioned media from ligated DRG or Schwann cells did not differ significantly from cultures exposed to control DRG conditioned media or from no addition controls. Scale bar = 100 μm.
In an attempt to determine whether the conditioning lesion-induced advance in the timing of neurite initiation was caused by an increase in trophic factor production by Schwann cells or satellite cells in response to nerve injury, we exposed control neurons to conditioned media from ligation protocol cultures or Schwann cell cultures. The effects of conditioning lesions on neurite initiation could not be reproduced by exposure to conditioned media. No statistically significant differences were found between the numbers of process-bearing neurons in cultures of control neurons exposed to 50% conditioned media from other control cultures, and numbers of process-bearing neurons in cultures exposed to conditioned media from ligation protocol cultures or cultured Schwann cells (Fig. 4D; n = 5 experiments).
Conditioning lesion-induced increases in neurite numbers
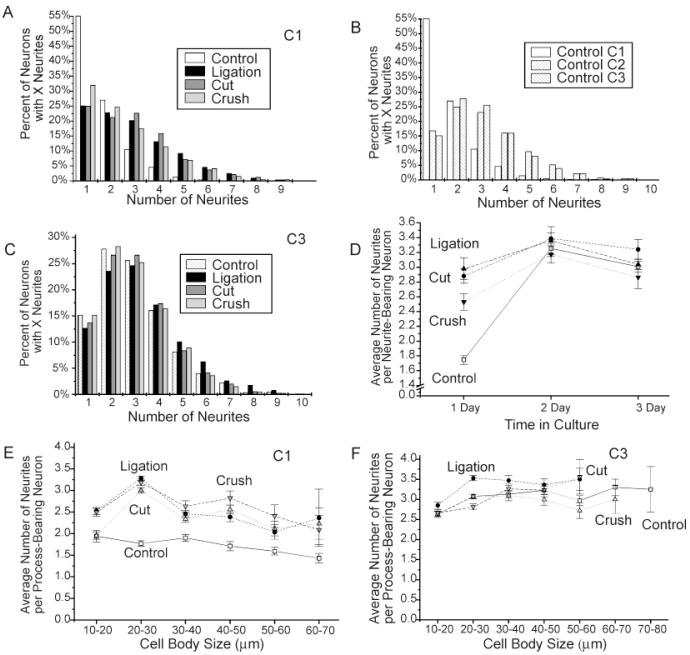
In addition to increases in the numbers of neurons that initiated processes during the first 24 hours in vitro, individual neurons in the conditioning-lesion conditions were also more likely than control neurons to initiate multiple processes during the first 24 hours in culture. Comparison of numbers of neurites initiated by individual neurons in control, ligation, cut, and crush protocol cultures after 1 day in vitro reveals a dramatic shift in the distribution of neurite numbers in each of the conditioning-lesioned conditions toward increasing neurite numbers (Fig. 5A). Neurite-bearing neurons in conditioning-lesioned cultures were more likely than neurons in control cultures to initiate more than one process and more likely to initiate greater numbers of processes. In control cultures, less than half of all neurite-bearing neurons initiated more than one process after 1 day in vitro, and only 7% of neurite-bearing neurons in control cultures exhibited four or more processes. In conditioning lesion cultures, by comparison, roughly three fourths of all neurite-bearing neurons initiated more than one process during the first 24 hours in culture and 32%, 31%, and 26% of neurite-bearing neurons in the ligation, cut, and crush conditions, respectively, initiated four or more processes (n = 888, 2,533, 1,141, and 2,603 neurons, respectively, for control, ligation, cut, and crush protocol conditions).
Fig. 5.
Effects of axotomy on the numbers of neurites per process-bearing neuron. A—C: Frequency histograms showing the percentages of neurons in each treatment condition after 1 (A) and 3 days (C) in vitro and for control neurons at 1, 2, and 3 days in culture (B). Note the increase in the numbers of neurons with multiple processes in each of the conditioning-lesioned conditions at day 1 and for control neurons with time in culture. D: Plot showing the effects of conditioning lesions on neurite numbers with time in vitro. Note that the differences between control and conditioning-lesioned neurons decrease with time in vitro, and only neurons in the ligation protocol condition initiate more processes than control by day 3 in vitro. All conditioning-lesion conditions differ significantly from controls at P < 0.00002 or lower (unpaired Student’s t-test). Numbers of neurites in the crush condition differ significantly from the ligation and cut condition at P < 0.03 (unpaired Student’s t-test). E,F: Effects of conditioning lesions on neurite initiation for different size classes of neurons. Note that conditioning lesions increase the average numbers of processes initiated from process-bearing neurons in essentially all cell size classes after 1 day in vitro, but differences disappear for day 3 except for ligation protocol neurons in the smaller cell size categories. Average numbers of neurites per process-bearing neuron were significantly increased at P < 0.00005 for all conditioning-lesion conditions at day 1 in culture when cell body size was controlled by using MANCOVA.
As with neurite initiation frequency, numbers of neurons with multiple processes increased with time in culture, and differences in the numbers of neurites initiated by control and conditioning-lesioned neurons were either eliminated or greatly attenuated by day 3 in vitro. Although only 45% of process-bearing neurons in control cultures exhibited more than one process at day 1 in vitro, 85% of neurite-bearing neurons in control exhibited multiple neurites by day 3 (Fig. 5B). After 3 days in vitro, the distribution of neurite numbers in control and conditioning-lesioned cultures were essentially indistinguishable (Fig. 5C), with 31%, 39%, 33%, and 31%, of control, ligation, cut, and crush protocol neurons, respectively, initiating more than four processes (n = 2,119, 1,403, 1,695, and 2,979 neurons, respectively). The average numbers of neurites per process-bearing neuron in control cultures increased dramatically from 1.73 ± 0.06 (n = 18 experiments) neurites per neuron at day 1 to 3.00 ± 0.03 (n = 11 experiments) neurites per neuron on day 3, whereas the average numbers of neurites per process-bearing neuron were much higher in conditioning-lesioned conditions at day 1 in vitro (2.9 ± 0.1, 3.0 ± 0.1, and 2.5 ± 0.1 neurites per process-bearing neuron, respectively for ligation, cut, and crush protocol conditions n = 22, 11, and 13 experiments) but showed little additional increase during the next 2 days in culture. All conditioning-lesion conditions differ significantly from control at P < 0.0001 on day 1 by using the unpaired Student’s t-test.) By day 3, neurite-bearing neurons in control, ligation, cut, and crush protocol cultures were not significantly different (3.01 ± 0.08, 3.2 ± 0.1, 3.04 ± 0.07, 2.8 ± 0.2 neurites per process-bearing neurons, respectively (n = 11, 11, 9, and 9 experiments, respectively; Fig. 5D).
Neurons with larger cell bodies were slightly less likely than smaller neurons to initiate multiple processes during the first 24 hours in vitro (correlation coefficients: −0.179, −0.157, −0.122, and −0.0337 for control, ligation, cut, and crush protocol conditions, respectively), but conditioning-lesioned neurons in essentially all cell size categories exhibited more processes on average than controls (Fig. 5E), and differences in neurite numbers remained statistically significant when differences in cell body size distributions were controlled (neurite numbers in all conditioning-lesioned conditions differ significantly from controls at P < 0.0005 by using MANCOVA; crush condition differs significantly from ligation and cut conditions at P < 0.0005). With time in culture, neurons in all cell size classes increased the numbers of neurites they produced; however, the increase in neurite numbers was greater for larger neurons and for control neurons and differences in numbers of neurites in different cell size categories and treatment conditions were largely eliminated by 3 days in vitro (Fig. 5F). After 3 days in vitro, only neurons in the ligated condition showed a statistically significant increase in the numbers of neurites per neuron after controlling for differences in cell sizes (P < 0.0005, MANCOVA).
Complex effects of conditioning lesions on neurite extension
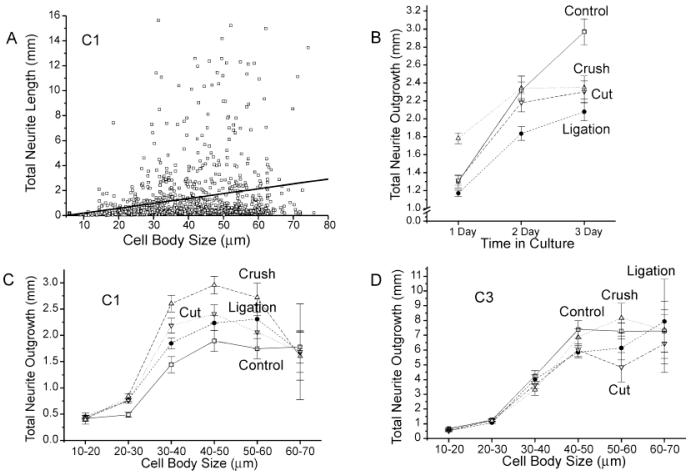
In this study, neurite outgrowth was quite rapid under all treatment conditions, with process-bearing neurons in all treatment conditions averaging more than 1 mm in total process length during the first 24 hours in culture and some neurons in each condition extending processes of more than 10 mm in total length during this period. Total neurite outgrowth from individual neurons was highly variable but outgrowth correlated moderately with cell body size under all treatment condition (Fig. 6A; correlation coefficients: 0.234, 0.372, 0.365, and 0.369 for neurons in the control, ligation, cut, and crush conditions, respectively, after 24 hours in vitro).
Fig. 6.
Effects of conditioning lesions on total neurite length. A: Plot illustrating the distribution of total neurite lengths for individual neurons in the control condition by cell body size after 1 day in vitro. A best-fit line also is shown. Note that there is considerable variability in total outgrowth for neurons of any size category, but that neurons with larger cell bodies are more likely to extend processes with greater total lengths. B: Effects of conditioning lesions on neurite outgrowth for the entire neuronal population over time in culture. Note that only neurons in the crush protocol condition show an increase in average total neurite outgrowth compared with controls at 1 day in vitro (P < 0.000005, unpaired Student’s t-test), and that, by day 3 in vitro, average total neurite outgrowth in the control condition is significantly greater than all conditioning-lesioned condition (P < 0.002 or lower, unpaired Student’s t-test). C,D: Effects on conditioning lesions on total neurite lengths for neurons in different size classes after 1 (C) and 3 days (D) in vitro. Note that although the average total neurite outgrowth for the entire neuronal population after 1 day in vitro is only increased in the crush protocol condition, average total neurite lengths for neurons in most cell size categories are increased in all conditioning-lesioned conditions at day 1. Note also that because the numbers of neurons in each size category differ between treatment conditions, panels C and D cannot be directly compared with panel B, in which neurite lengths for all neurons in an individual experiment were pooled. Total neurite length in ligation, crush, and cut protocol conditions differ significantly from controls at P < 0.003, P < 0.0005, and P < 0.002, respectively, by using MANCOVA.
The ability of neurons to extend long neurites appeared to be reduced in all treatment conditions in which neurons were exposed to conditioning lesion 1 week prior to culturing, but the effects of conditioning lesions on initial outgrowth rates were distinctly different, depending on the nature of the lesion. Despite the earlier onset of neurite initiation, and consequently greater period of outgrowth in the conditioning-lesioned cultures, average total neurite outgrowth after 3 days vitro was reduced by nearly one third in conditioning-lesioned cultures from 3.0 ± 0.1 mm for control neurons to 2.0 ± 0.1, 2.3 ± 0.1, and 2.2 ± 0.1 mm in ligation, cut, and crush conditions, respectively (P < 0.00001, P < 0.0004, and P < 0.002 by using unpaired Student’s t-test). In contrast to the uniform effects of all conditioning lesions on neurite initiation and total outgrowth after 3 days, the extent of neurite outgrowth in conditioning-lesioned cultures after 1 day appeared to be dependent on the nature of the lesion. Neurons from the crush condition, which permits nerve regeneration through the distal nerve segment, showed a significant increase in initial neurite outgrowth from an average of 1.30 ± 0.07 mm in control cultures to 1.78 ± 0.06 mm in crush cultures after 1 day in vitro (P < 0.000001, Student’s t-test). In contrast, neurons from the ligation protocol condition, which prevents regeneration through the distal nerve segment, showed a slight, but not statistically significant, decrease in neurite outgrowth to 1.17 ± 0.03 mm. Neurons in the cut condition, which permits axonal outgrowth into skin and muscle but eliminates the direct connection to the denervated peripheral nerve, showed no effect of conditioning lesions on initial neurite outgrowth (1.31 ± 0.05 mm).
The reduction in neurite lengths in the ligation condition could be largely, but not completely, accounted for by the reduction in average cell size. When neurite lengths for neurons in discrete cell size ranges were compared, neurons in all but the smallest and largest cell size categories showed small increases in average neurite length in each of the conditioning-lesioned conditions after 24 hours in vitro (Fig. 6C). Only neurons in the 10- to 20-μm and 60- to 70-μm cell size categories showed no effect of lesions on neurite length in 1-day cultures. A MANCOVA showed that total neurite lengths in all conditioning lesions conditions differed significantly from controls when differences in cell sizes and numbers of neurites were both taken into account (P < 0.0005).
Although conditioning lesion-induced increases in neurite length after 1 day in vitro were statistically significant, the effects were small, and it is unclear whether the increased neurite lengths are due to faster rates of outgrowth or simply longer growth periods. The increase in average total neuritic outgrowth for process-bearing neurons in 1-day cultures in the crush condition, for example, would be equally consistent with either a 37% increase in the rate of neurite outgrowth or with an approximately 9-hour earlier onset of outgrowth. Because time course data indicated that conditioning lesions advance the onset of neurite initiation by an estimated 8–12 hours, these results do not provide strong support for conditioning lesions-induced increases in the rate of neurite extension in a neutral environment. Rates of neurite outgrowth in the crush protocol, or regeneration permissive condition, do, however, appear to be increased, compared with lesions that prevent effective nerve regeneration. Although neurons in the ligation, cut, and crush conditions all showed similar reductions in cell body sizes and similar advances in the onset of neurite initiation, average neurite length in crush condition was significantly increased, compared with either the ligation or cut protocol, indicating that prior exposure to something in the denervated nerve segment may temporarily stimulate neurons to extend processes more rapidly. (Total neurite outgrowth from neurons in the crush condition differ significantly from the ligation and cut condition at P < 0.0005 by using a MANCOVA). Comparisons of average neurite lengths for the longest 5% of neurite-bearing neurons in each condition, which presumably represent the earliest initiated fastest growing neurites in the population, also support an increase in neurite outgrowth rate for crush condition neurons relative to other conditioning lesions. The longest 5% of neurons in the crush condition extended 10.0 ± 0.2 mm, compared with 6.9 ± 0.3 mm and 7.9 ± 0.4 mm for ligation and cut conditions, respectively, and 10.1 ± 0.4 mm for control cells, implying that the maximum rate of neurite outgrowth in previously injured neurons is increased in neurons that have been previously exposed to the environment of a denervated nerve tract.
The apparent positive effects of prior nerve regeneration on subsequent rates of neurite extension were only transitory, however. After 2–3 days in vitro, the differences between average neurite lengths in the crush protocol and other conditioning-lesioned conditions were greatly reduced (Fig. 6B), and by day 3 in vitro, average neurite lengths for neurons of similar sizes in different treatment conditions were essentially indistinguishable (Fig. 6D). There was no statistically significant effect of any conditioning lesion on total neurite outgrowth after 3 days in vitro when differences in cell body size and neurite numbers were controlled for.
Reduction in neurite branching frequency in conditioning-lesioned cultures
Neurites of adult rat DRG neurons branch extensively on polyornithine- and laminin-coated coverslips. Neuritic branching frequency varies considerably for neurites of any given length, but average distances between neuritic branch points tend to increase with increasing total neuritic outgrowth (correlation coefficients 0.325, 0.450, 0.304, and 0.290, respectively, for control, ligation, cut, and crush conditions at 1 day in vitro). Interbranch distances in 1-day cultures also correlate weakly with cell body size in the control condition (correlation coefficient 0.082) and weakly inversely with numbers of neurites in all treatment conditions (correlation coefficients: −0.100, −0.049, −0.074, and −0.052 for control, ligation, cut, and crush conditions, respectively).
Average interbranch distances increase with time in culture for neurons in all treatment conditions, but increases in interbranch distances were greater for control neurons than conditioning-lesioned cells (Fig. 7B). Average interbranch distances for all control neurons more than tripled from day 1 to day 3 in vitro, increasing from 0.041 ± 0.001 mm at 1 day in vitro to 0.083 ± 0.002 mm at 2 days and 0.132 ± 0.004 mm at 3 days (average interbranch distances in control cultures at day 1, 2, and 3 all differ significantly at P < 0.00001 by using the unpaired Student’s t-test). By comparison, interbranch distances in conditioning-lesioned cultures doubled over the same period, increasing from 0.066 ± 0.002, 0.075 ± 0.004, and 0.068 ± 0.002 mm in ligation, cut, and crush protocol cultures, respectively, at day 1 in vitro, to 0.114 ± 0.005, 0.115 ± 004, and 0.121 ± 0.004 mm at day 3 in vitro (average interbranch distances for all conditioning-lesioned conditions at day 3 differ significantly from controls at P < 0.001 and from day 1 values at P < 0.00001 by using the unpaired Student’s t-test).
Fig. 7.
Effects of conditioning lesions on neurite branching frequency. A: Plot illustrating the distribution of branching frequencies for individual neurons in the control condition after 1 day in vitro plotted as a function of total neurite length with best-fit line. B: Effects of conditioning lesions on branching frequency with time in culture. Note that average interbranch distances for neurons in all conditioning-lesioned conditions are greater than in control cultures at day 1 in vitro (P < 0.000001, Student’s t-test), but not at day 3 in vitro. C—E: Effects of conditioning lesions on average interbranch distances for neurons with different total neurite lengths at day 1 (C) and day 3 (E) in vitro, and for control neurons on days 1, 2, and 3 in vitro. Note that average interbranch distances increased for conditioning-lesioned neurons in all cell size categories at day 1, but not day 3 in vitro. Average interbranch distances were significantly increased for all conditioning lesion protocols at day 1 in vitro (P < 0.0005, MANCOVA).
Although branching frequency increased with increasing neurite length, increases in interbranch distance in conditioning-lesioned cultures or with time in vitro could not be attributable to differences in neurite length alone. Increases in interbranch distances were observed for neurons of all neurite lengths in all conditioning-lesioned conditions at 1 day in vitro (Fig. 7C), and average interbranch distances also increased with time in culture for control neurons with similar total neurite lengths (Fig. 7D). When differences in total neurite outgrowth, as well cell body size and neurite numbers were controlled for, average interbranch distances in all conditioning-lesion conditions still differed significantly from controls after 1 day in culture (P < 0.0005, MANCOVA), and branching frequency in 1-day control cultures differed significantly from that in 3-day cultures (P < 0.0005, MANCOVA). Similar to the effects of conditioning lesions on other morphological parameters, differences between neuritic branching frequencies in control and conditioning-lesioned neurons appeared early in culture, and statistically significant differences in neuritic branching frequency between control and ligation protocol cultures could be detected as early as 4 hours after plating (P < 0.0005, MANCOVA). Differences between branching frequencies in control and conditioning-lesioned conditions disappeared with time in culture, however, and no statistically significant difference could be detected after 3 days in vitro when differences in cell body size, neurite numbers, and total neurite outgrowth were controlled.
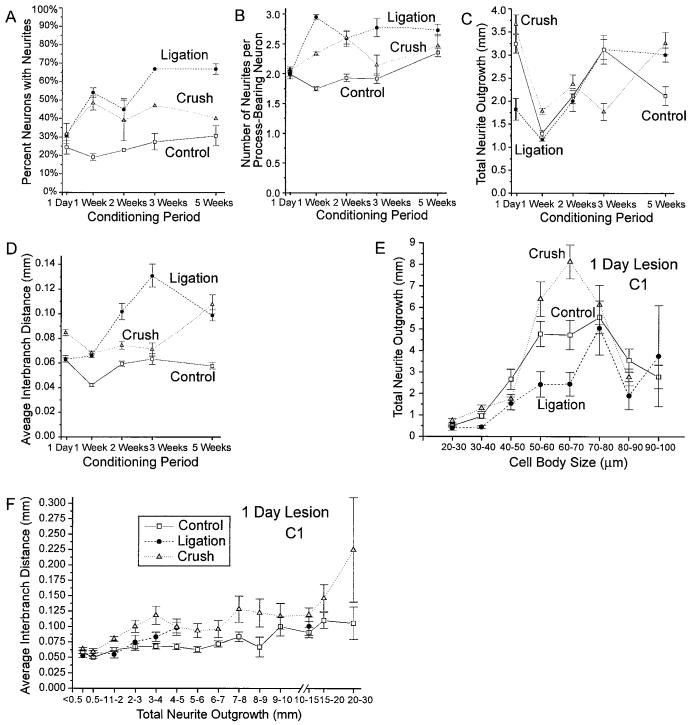
Effects of conditioning period
Consistent with studies of conditioning lesion enhancement of nerve regeneration in vivo, nerve crush or ligation protocols performed 1–3 weeks before removing the DRG resulted in significant changes in neuritic arborization of cultured neurons, whereas conditioning intervals of 24 hours or 5 weeks had less predictable effects and revealed significant differences between the crush and ligation protocols (Fig. 8A-D; n = 6, 3, and 4 experiments, respectively for 1-day control, ligation, and crush conditioning lesions; 24, 25, and 13 experiments for 1-week lesions; and 1–3 experiments each for longer conditioning intervals). Following a 24-hour conditioning period, neurons of similar sizes or total neurite lengths in the crush protocol condition showed significant increases in total neurite outgrowth (Fig. 8E; P < 0.003, MANCOVA), and average interbranch distance (Fig. 8F; P < 0.0005, MANCOVA) after 1 day in vitro, but neurons in the ligation condition showed only a decrease in neurite outgrowth (P < 0.04, MANCOVA) with no effect on neuritic branching when differences in cell body size and total neurite length were controlled (Fig. 8E,F; n = 813, 195, and 789 neurons with measured processes and 725, 179, and 660 neurons whose branching frequency could be determined in 24-hour cultures of control, ligation, and crush protocol neurons, respectively, following a 1-day conditioning interval). Following a conditioning interval of 5 weeks, when substantial recovery would be expected in the intact animal, neurons exposed to the crush protocol showed no significant change in morphogenesis, compared with control neurons, whereas neurons in the ligation condition continued to exhibit increases in early neurite initiation (P < 0.005, χ2 test), neurite numbers ( < 0.03, MANCOVA), as well as a reduction in total neurite outgrowth (P < 0.0005, MANCOVA), and a reduction in branching frequency (P < 0.0005, MANCOVA) after 1 day in vitro when other parameters were controlled for (n = 442, 447, and 147 neurons with measured processes and 361, 277, and 86 neurons whose branching frequency could be determined in 24-hour cultures of control, ligation, and crush protocol neurons, respectively, following a 5-week conditioning interval).
Fig. 8.
Effects of conditioning period on conditioning lesion-induced changes in neuronal morphogenesis after day in vitro. A—D: Effects of conditioning lesions of different durations on neurite initiation, numbers of neurites per neuron, total neurite outgrowth, and average interbranch distances at day 1 in vitro. (n = 6, 3, and 4 experiments, respectively for 1-day control, ligation, and crush conditioning lesions; 24, 25, and 13 experiments, respectively, for 1-week lesions and one to three experiments each for longer conditioning intervals). E: Effects of a 1-day conditioning lesion on total neurite outgrowth for neurons of different cell sizes on day 1 in vitro. Total outgrowth for neurons in the ligation and crush condition differ significantly from controls at P < 0.05 and P < 0.003, respectively, and from each other at P < 0.0005 when controlling for difference in cell body size and neurite numbers (MANCOVA; n = 813, 195, and 789 control ligation and crush neurons, respectively, measured). F: Effects of a 1-day conditioning lesion on average interbranch distance for neurons with different total neurite lengths after 1 day in vitro. Average interbranch distances for neurons in the crush condition differ significantly from control at P < 0.0005 when other morphological parameters are controlled (MANCOVA; n = 725, 179, and 660 control ligation and crush protocol neurons, respectively, for which interbranch distances could be determined).
DISCUSSION
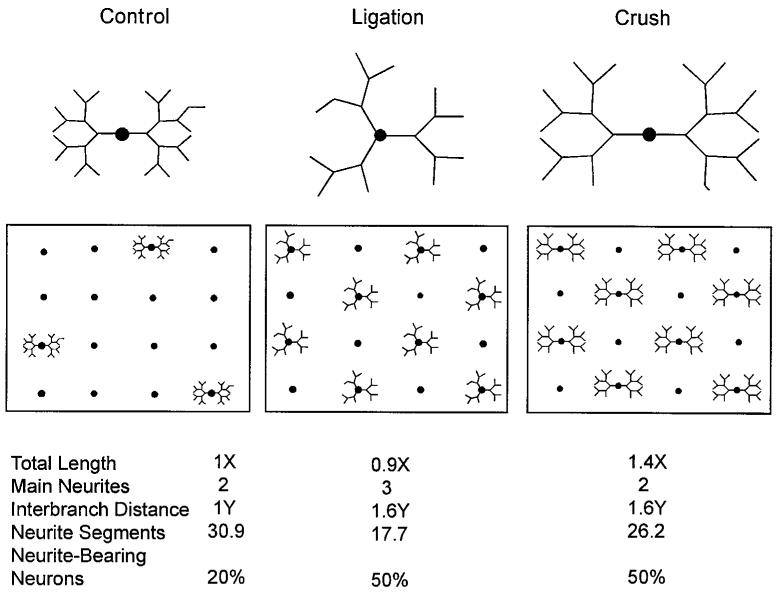
The results of this study indicate that prior or conditioning nerve lesions have at least four distinct effects on neuronal morphogenesis, which may contribute to, or mitigate against, the enhanced nerve regeneration observed following a second nerve lesion in vivo: 1) promotion of earlier neurite initiation, 2) reduction in the ability of neurons to extend long neurites following a second axotomy, 3) stimulation of increased initial rates of neurite outgrowth, and 4) reduction in initial neuritic branching frequency, resulting in more rectilinear neurites whose growth cones extend further distances from their cell bodies. Three of these effects were observed in all nerve injury protocols, but the increase in initial neurite outgrowth rates was dependent on the ability of the conditioning-lesioned axons to regenerate through the distal nerve segment (see Fig. 9 for a schematic diagram of neurons with the average morphological characteristic of neurons in the control, ligation, and crush protocol conditions after 1 day in vitro). Taken together, our results suggest that the primary effect of conditioning lesions on cultured DRG neurons is to advance the timing of morphogenesis, resulting in conditioning-lesioned neurons that exhibited characteristics consistent with control neurons that had been cultured for an additional day or more. A secondary effect of conditioning lesions on neurite outgrowth rates was dependent on the local environment of the axons prior to culturing.
Fig. 9.
Idealized schematic illustrating the average appearance of neurons in a control, ligation, and crush protocol cultures after 1 day in vitro and the percentage of process-bearing neurons in each condition. Total length of the neurites in the control condition is actually longer than that in the ligation condition, but the length of the ligation protocol neurons appears to be greater because of the reduced neurite branching. Numbers below diagram describe normalized values used for the construction of control, ligation, and crush protocol neuronal schematics using experimental values for average neurite lengths, numbers of main process, branching frequencies, and neurite initiation frequencies.
We found no convincing evidence in this study for conditioning lesion effects on either neuronal attachment to a laminin substrate or cell survival. Both control and ligation protocol neurons rapidly attached to the substrate within 8 hours after plating, and total numbers of surviving neurons did not differ between control and ligation protocol cultures at 1, 2, or 3 days in vitro. Although the proportion of neurons in the larger cell size classes was slightly reduced in conditioning-lesioned cultures, the lack of reduction in total neuronal numbers suggests that this difference was most likely due to a postaxotomy shrinkage, as described by other investigators (Himes and Tessler, 1989; Wells and Vaidya, 1989), rather than to increased death of larger neurons. Because we could not rule out the possibility of differential survival of subclasses of neurons in various treatment conditions, however, and because some neurons in each culture could not be fully analyzed (see Table 1), it was necessary to compare the effects of conditioning lesions on neurite initiation, total neurite outgrowth, and branching frequency for subpopulations of neurons of similar sizes and/or total neuritic lengths, as well as on the whole neuronal population, to rule out the possibility that apparent differences in morphogenesis were actually due to differences in the cell population examined.
Neurite initiation
Comparisons between neurite initiation frequencies and neurite numbers for control and conditioning-lesioned neurons at successive time points in culture indicate that prior axotomy advances the onset of neurite initiation without affecting the ability of neurons to initiate processes. Neurons from ganglia subjected to a ligation, crush, or cut protocol 1–5 weeks before dissociation were significantly more likely than control cells to initiate neurites within the first 24 hours in culture and were more likely to generate multiple processes during this period. After 2–3 days in vitro, however, the percentage of process-bearing neurons did not differ significantly between treatment conditions and only neurons in the ligation protocol showed a small increase in average numbers of neurites per process-bearing neurons.
The increase in early neurite initiation by adult rat DRG neurons in this study agrees with a previous study (Hu-Tsai et al., 1994) showing that roughly twice as many neurons from rat DRG subjected to a 2-week-old sciatic nerve crush initiate process during the first 24 hours in vitro, compared with control cells. The results of our study also extend previous observations to provide strong evidence for a specific effect of conditioning lesions on neurite initiation and for direct effects of prior lesioning on growth properties of the neurons themselves. The lack of reduction in neuronal cell numbers in conditioning-lesioned cultures in this study shows that the increased proportion of neurite-bearing neurons after 24 hours in vitro cannot be explained by increased cell death of cell types less likely to initiate process at early time points. Neurons in this study were plated at a density related to the number of ganglia dissociated, rather than the numbers of viable neurons, but no statistically significant differences were detected between numbers of neurons in cultures of control and conditioning-lesioned neurons at 1, 2, or 3 days in vitro. Vital dye staining also showed that essentially all attached neurons in each condition were viable, and nearly 90% of neurons in each condition initiated processes by day 3 in vitro. Furthermore, although the reduction in average neuronal cell body size in conditioning-lesioned cultures suggest a possible loss of larger neurons, neurons with larger cell bodies were actually more likely to initiate neurons during the first 24 hours in culture, and conditioning-lesioned neurons in all cell size classes showed increased probability of neurite initiation at 24 hours in vitro. These results indicate that the reduction in neurite initiation frequency of conditioning-lesioned neurons at 1 day in culture could not be explained by differences in the cell size populations between control and conditioning-lesioned cultures.
Comparisons between total neurite lengths and neurite initiation frequencies illustrate that the increased number of neurons with criterion length processes was not an artifact of increased growth rates. In this study, average total neurite outgrowth for process-bearing neurons was actually slightly reduced in ligation and cut protocol cultures for each time point examined, although the numbers of neurons with process more than twice their diameter were increased in all conditioning-lesioned protocols at 1 day in vitro. Time course data showing growth cone formation and neurite initiation within 2–4 hours after plating in ligation protocol cultures, compared with an 8- to 12-hour delay in control cultures, provides additional strong support for a specific effect of conditioning lesions on neurite initiation.
The increase in the numbers of neurite-bearing neurons at the earliest time point examinable in ligation protocol cultures suggests that at least some previously axotomized neurons are primed to begin initiating processes very soon after a second axotomy. Such a priming effect could be a function of the increased mRNA (Watson, 1968; Wells and Vaidya, 1994) or protein synthesis (Chong et al., 1994; McQuarrie and Graffstein, 1982; Moskowitz and Oblinger, 1995; Moskowitz et al., 1993; Van der Zee et al., 1989; Weise et al., 1992; Woolf et al., 1990) or elevated expression of immediate early genes (Gold et al., 1994; Herdegen et al., 1992; Jenkins and Hunt, 1991; Lankford et al., 1996; Robinson, 1994) associated with prior axotomy, but the nature of the signal is not clear (see Lankford et al., 1996 for a review of mechanisms involved in regulating neurite initiation). Previous studies in this laboratory indicated that neurite initiation in DRG cells is preceded by a large intracellular calcium signal approximately 1 day before observable neurite initiation and is required for neurite initiation (Kocsis et al., 1994; Lankford et al., 1995). However, in this study, conditioning lesions performed 1 day before culturing did not increase in the numbers of process-bearing DRG neurons at 1 day in culture, suggesting that axotomy-induced elevation of intracellular calcium was unlikely to be the sole cause of conditioning lesion-induced advance in neurite initiation.
Neurite outgrowth
Although several studies have reported more rapid growth of conditioning-lesioned sensory axons in vivo (Sjöberg and Kanje, 1990b; McQuarrie and Grafstein, 1982; see also Grafstein and McQuarrie, 1978), in this study, conditioning lesions did not uniformly increase neurite outgrowth in vitro, but instead had two distinct, and partially opposing, effects on neurite outgrowth. Prior axotomy alone appeared to reduce the ability of neurons to subsequently extend long neurites, whereas prior regeneration in vivo resulted in more rapid rates of initial neurite outgrowth in vitro. The effects of conditioning lesions on neurite length, therefore, varied with both the nature of the lesion and the duration of the culture period.
The reduction in average total neurite outgrowth from neurons in the conditioning-lesioned cultures, particularly at day 3, suggests that prior axotomy has a negative effect on neurite extension. Average total neurite outgrowth for all neurons in each of the conditioning-lesioned conditions were significantly reduced, compared with controls at day 3 in vitro, and total neurite lengths in the ligated protocol cultures were significantly reduced at all time points examined. At least part of the reduction in average total neurite extension after 1 day in culture may be attributable to a reduction in average cell body size in the conditioning-lesioned conditions, because neurite lengths for neurons in similar size ranges increased slightly for conditioning-lesioned neurons in most cell size classes at day 1 in vitro. However, reductions in cell body sizes could not explain the apparent reduction in rate of neurite outgrowth for conditioning-lesioned, but not control, neurons with time in culture, because average cell body sizes were not significantly reduced with time in culture. Furthermore, average total neurite outgrowth for conditioning-lesioned neurons in the same size classes were either reduced or unchanged, compared with controls after 3 days in vitro, although neurons in the conditioning lesion condition initiate processes much earlier than controls.
In contrast to the apparent inhibitory effects of prior axotomy on neurite outgrowth, regeneration of axons through a denervated nerve tract appears to increase the initial rate of extension of DRG neurites. Average total neurite outgrowth for all neurons in the crush condition was significantly elevated, compared with the control, ligation, or cut protocol condition at 1 day in vitro. Average total neurite lengths for neurons in most cell size classes also were increased in the crush condition, compared with both the control condition and other lesion protocols at day 1 in culture, and differences in neurite length remained statistically significant when differences in cell body sizes and numbers of neurites were controlled. Although the increases in average neurite outgrowth in the crush protocol, compared with control neurons, was small enough to be explained by the earlier onset of neurite initiation in the conditioning-lesioned conditions, the increase in outgrowth of crush protocol neurons, relative to ligation or cut protocol cultures, can only be explained by more rapid neurite extension, because neurons in all conditioning-lesion conditions initiated processes with similar time courses. Total neurite lengths in the crush condition were not increased relative to other conditions at day 3, however, implying that prior exposure to factors in the denervated nerve track may stimulate more rapid initial neurite elongation but cannot increase total outgrowth in the absence continuous exposure to the stimulating agents.
Neuritic branching
The most unexpected result in this study was the apparently strong effect of prior axotomy on neuritic branching, which could not have been reasonably detected in in vivo studies and was not mentioned in other in vitro studies. Average interbranch distances were significantly increased in 1-day cultures of 1-week conditioning-lesioned neurons from all three conditioning lesion protocols, both for the cell population as a whole, and for neurons in each total outgrowth category examined. Increases in interbranch distances also were observed in 1-day cultures of neurons exposed to a ligation protocol 2, 3, or 5 weeks before culturing or a crush protocol 2 or 3 weeks before culturing. Interbranch distances increased with time in culture, however, in all treatment conditions, and no statistically significant increases in interbranch distances could be detected by day 3 in vitro.
The causes of the reduced branching frequency in 1-day cultures of conditioning-lesioned neurons are difficult to ascertain, but at least two possible mechanisms may be considered. The general reduction in branching frequency with both increased time in culture and increased total outgrowth for both control and conditioning-lesioned neurons implies that DRG neurites initially form many side branches as they extend, but that most branches are retracted over time as the main branch extends and incorporates structural materials from unneeded side branches. The reduced branching frequency of conditioning-lesioned neurons at 1, but not 3 days in culture, compared with controls, suggests that conditioning-lesioned neurons may more quickly retract side branches than control neurons. Possibly, the same mechanism that permits more rapid neurite initiation also facilitates more rapid disassembly of neuritic structures and retraction of unneeded side branches. Alternatively, because at least one trophic factor, nerve growth factor (NGF), has been implicated in side branch sprouting from some sensory neurons (Diamond et al., 1987), severing neuronal contact with peripheral trophic factor sources could reduce the tendency of conditioning-lesioned neurons to form side branches. A role for trophic factor loss in reduction of neuritic branching frequency also could explain the increased branching frequency in crush protocol cultures compared with other types of conditioning lesions, because denervated Schwann cells are sources of several trophic factors (Heumann et al. 1987; Lindholm, 1987; Meyer et al., 1992; Sebert and Shooter, 1993; Windebank and Poduslo. 1986). A possible role for NGF in conditioning lesion-induced differences in neurite branching is uncertain, however, because effects of anti-NGF antibodies on collateral sprouting were observed for uninjured neurons in vivo (Diamond et al., 1987).
Time course of conditioning lesion effects
Morphometric analysis of neurons subjected to conditioning lesions 1–5 weeks before culturing indicate that, similar to studies of nerve regeneration in vivo (Forman et al., 1980; Sjöberg and Kanje, 1990a,b), conditioning lesion-induced effects on neuronal morphogenesis in vitro persist at least several weeks after the initial axotomy. Examining neurons plated 1 day after conditioning lesions, however, indicates that different morphological responses have different onset times. For neurons subjected to a conditioning lesion 1 day prior to dissociation, average total neurite outgrowth after 24 hours in vitro was significantly reduced in the ligation condition, and average interbranch distances were reduced in the crush condition, but neurite initiation frequencies and numbers of neurites per neuron were unchanged in cultures subjected to any of the conditioning lesion protocols only 1 day before culturing. These observations suggest that neurite initiation, outgrowth, and branching may be regulated by different mechanisms, with changes leading to reduced neuritic outgrowth and reduced branching being triggered earlier than changes leading to more rapid neurite initiation.
Effects of in vivo growth environment on in vitro growth
Although all conditioning lesion protocols resulted in earlier neurite initiation and reduced neuritic branching at early time points in culture, notable differences were detected between lesion protocols that permitted normal nerve regeneration and those that restricted or inhibited regeneration. Neurons in the ligation and cut protocol cultures, both of which prevent normal regeneration in vivo, showed highly similar patterns of morphogenesis, whereas neurons in the regeneration-permissive crush protocol condition showed several distinct differences in growth patterns compared with other conditioning lesions. Compared with the ligation or cut conditions, the crush protocol had smaller effects on both neurite initiation and branching frequency. Neurite initiation frequencies, numbers of neurites per neuron, and average interbranch distances at 1 day in vitro in the crush condition were intermediate between values for neurons in the control condition and conditioning lesion protocols that did not permit regeneration. Unlike neurons in the cut or ligation protocol, neurons subjected to a nerve crush 1 week before culturing also showed an increase in average total neurite outgrowth after 24 hours in vitro. These observations suggest that regeneration through a previously denervated nerve segment may promote more rapid neurite extension after a subsequent nerve injury, but that prior regeneration also may have a slight inhibitory effect on neurite initiation and on the suppression or elimination of side branches.
Because all three conditioning lesions resulted in axotomy, differences between morphogenesis of neurons in the crush vs. the cut or ligation protocol condition are most likely due to differences in the local environments of the cut axons. Previous studies have found that axotomy induces an increase in neurotrophin production by Schwann cells in the denervated nerve tract (Heumann et al., 1987; Lindholm, 1987; Meyer et al., 1992; Sebert and Shooter, 1993; Windebank and Poduslo, 1986). However, other reports also have indicated that Schwann cells may produce substances that inhibit axon initiation (Tapia et al., 1985). This apparent production by Schwann cells of both growth-promoting and neurite initiation inhibiting factors could account for the relatively slower neurite initiation, but greater average rates of outgrowth from neurons in the crush protocol conditions, compared with other conditioning-lesion conditions. Because NGF also has been associated with axonal branching in at least some classes of sensory neurons (Diamond et al., 1987), exposure to NGF produced by Schwann cells in the denervated nerve also may account for the relatively greater branching frequency in crush protocol cultures, compared with other conditioning lesions.
An alternative hypothesis for explaining differences in growth properties of neurons in different conditioning-lesion conditions, that different types of lesion may produce differing degrees of local trauma, cannot be ruled out, but is somewhat less satisfactory. The major trauma in all of the protocols would seem to be the surgery itself, cutting through the skin and separating muscle to expose the sciatic nerve for ligation, cut, or crush injuries. If anything, the cut protocol procedure took less time and would seem to involve no more cell damage than the crush protocol, unless some of the axons were spared in crush condition. Total neurite outgrowth and branching frequency in the cut condition, however, differed significantly from the crush, but not the presumably more traumatic ligation protocol condition. Furthermore, preliminary studies in which sciatic nerves were subjected to a series of six cuts moving progressively more proximal over a period of 3–5 minutes showed no differences in growth patterns between neurons subjected to this more severe cut procedure and those subjected only to a simple cut protocol (data not shown).
Sources of conditioning lesion effects
Although the precise mechanisms responsible for conditioning lesion-induced changes in neuronal morphogenesis cannot be determined from this study, our results indicate that the effects of conditioning lesions on timing of neurite initiation and neuritic branching are unlikely to be due to any positive stimulus from the denervated nerve, such as retrograde transport of trophic factors from denervated regions of the peripheral nerve to the cell body. In this study, neurons in all conditioning-lesion conditions showed similar patterns of increased early neurite initiation and decreased branching at early times in culture, regardless of the ability of the neurons to extend axons into the denervated region. Furthermore, neurite initiation frequencies, numbers of neurites per neuron, and average interbranch distances at day 1 in vitro were actually slightly reduced in the crush condition, which permitted direct axonal contact with the denervated nerve segment, compared with other conditioning lesions that prevented or reduced such exposure.
Changes in trophic factor production by satellite cells are also unlikely to contribute directly to the more rapid neurite initiation in conditioning-lesioned cultures. Retrograde labeling of neurons with cut axons revealed that only axotomized neurons in ligation protocol cultures had an increased probability of neurite initiation after 24 hours in vitro, and conditioned media from ligation protocol cultures had no effect on neurite initiation. These results indicate that prior axotomy alters the ability of neurons to rapidly initiate neurites rather than altering the ability of associated cells to stimulate axon initiation. These effects on the neurite initiation capacity of axotomized neurons could be a result of direct effects of axotomy on neurons, such as membrane depolarization and calcium influx, or indirect effects, such as loss of signals from target tissue.
Possible contributions of nonneuronal cells to changes in neurite branching frequency in conditioning-lesioned cultures are more difficult to assess, because fluorescence fading makes it impractical to measure neurite outgrowth and count branch points from significant numbers of Fluoro-Gold-labeled and unlabeled neurons. Preliminary results, however, have not found any effects of conditioned media from ligation protocol cultures on neuritic branching of control neurons (unpublished result), indicating that soluble factors produced by nonneurons in culture are unlikely to be responsible for the reduced branching frequency in conditioning-lesioned cultures. It remains possible, however, that satellite cells or other nonneuronal cells alter their production of extracellular matrix molecules or cell adhesion proteins after a conditioning lesion and indirectly affect neurite branching by altering substrate adhesivity.
Taken together, these observations suggest that the effects of conditioning lesions on neurite initiation and branching are due to direct effects of the axotomy, such as injury-induced depolarization and calcium influx, or to loss of contact with factors from peripheral target tissues, and not to indirect effects of lesions on nonneuronal cells. The increased rates of initial outgrowth in crush protocol cultures, compared with the ligation or cut condition, however, imply that factors in the denervated nerve segment also may contribute to increased rates of neurite outgrowth.
The results of these experiments indicate that “the conditioning lesion effect” is a composite of a number of different effects on neurite initiation, extension, and branching. The evidence that some of the effects of conditioning lesions can have opposite effects on outgrowth, with prior axotomy both promoting more rapid neurite initiation and reducing the ability of neurons to extend long process in the absence of specific trophic support, may provide an explanation for the often contradictory conclusions reported by different in vivo studies about both the nature and source of conditioning lesion-induced enhancement of nerve regeneration. Because different components of the conditioning lesion effect appear to be produced by different consequences of a particular lesioning protocol, slight differences in nerve crush protocols or time points examined may be expected to produce different effects on regeneration and therefore cause individual investigators to reach conflicting conclusions about the nature of the conditioning lesion effect or the importance of factors from the denervated nerve segment.
Acknowledgments
Grant sponsor: NIH; Grant number: NS10174; Grant sponsor: Medical Research Service of the Department of Veterans Affairs.
LITERATURE CITED
- Abercrombie M, Johnson ML. Quantitative histology of Wallerian degeneration. I. Nuclear population in rabbit sciatic nerve. J. Anat. 1946;80:37–50. [PubMed] [Google Scholar]
- Agranoff BW, Fields P, Gaze RM. Neurite outgrowth from explanted Xenopus retina: An effect of prior optic section. Brain Res. 1976;113:225–234. doi: 10.1016/0006-8993(76)90938-0. [DOI] [PubMed] [Google Scholar]
- Beuche W, Freide RL. The role of non-resident cells in Wallerian degeneration. J. Neurocytol. 1984;13:767–796. doi: 10.1007/BF01148493. [DOI] [PubMed] [Google Scholar]
- Bignami A, Chi NH, Dahl D. Laminin in rat sciatic nerve undergoing Wallerian degeneration. Immunofluorescence study with laminin and neurofilament antisera. J. Neuropathol. Exp. Neurol. 1984;48:94–103. doi: 10.1097/00005072-198401000-00008. [DOI] [PubMed] [Google Scholar]
- Birch BD, Eng DL, Kocsis JD. Intracellular Ca2+ transients during neurite regeneration of an adult mammalian neuron. Proc. Natl. Acad. Sci. USA. 1992;89:7878–7982. doi: 10.1073/pnas.89.17.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisby MA, Pollock B. Increased regeneration rate of peripheral axons following double lesions: Enhancement of the conditioning lesion phenomenon. J. Neurobiol. 1983;14:467–472. doi: 10.1002/neu.480140607. [DOI] [PubMed] [Google Scholar]
- Carlson RC. Delayed induction of the cell body response and enhancement of regeneration following a conditioning lesion of frog peripheral nerve at 15°C. Brain Res. 1983;279:9–18. doi: 10.1016/0006-8993(83)90158-0. [DOI] [PubMed] [Google Scholar]
- Chong MS, Reynolds ML, Irwin N, Coggeshall RE, Emson PC, Benowitz l.I., Woolf CJ. GAP-43 expression in primary sensory neurons following central axotomy. J. Neurosci. 1994;14:4375–4384. doi: 10.1523/JNEUROSCI.14-07-04375.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornbrooks CJ, Corey DJ, MacDonald JA, Timple R, Bunge RP. In vivo and in vitro observations on laminin production by Schwann cells. Proc. Natl. Acad. Sci. USA. 1983;80:3850–3854. doi: 10.1073/pnas.80.12.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J, Coughlin M, Macintyre L, Holms M, Visheau B. Evidence that endogenous β nerve growth factor is responsible for the collateral sprouting, but not the regeneration of nociceptive axons in adult rats. Proc. Natl. Acad. Sci. USA. 1987;84:6596–6600. doi: 10.1073/pnas.84.18.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duce IR, Reeves JF, Keen P. A scanning electron microscope study of the development of axonal sprouts at the cut ends of spinal nerve roots in the rat. Cell Tissue Res. 1976;170:507–513. doi: 10.1007/BF00361708. [DOI] [PubMed] [Google Scholar]
- Edwards DL, Alpert RM, Grafstein B. Recovery of vision in regeneration of goldfish optic axons: Enhancement of axonal outgrowth by a conditioning lesion. Exp. Neurol. 1981;72:672–686. doi: 10.1016/0014-4886(81)90016-9. [DOI] [PubMed] [Google Scholar]
- Forman DS, McQuarri IG, Labore FW, Wood DK, Stone LS, Braddock CH, Fuchs DA. Time course of conditioning lesion effect on axon regeneration. Brain Res. 1980;182:180–185. doi: 10.1016/0006-8993(80)90842-2. [DOI] [PubMed] [Google Scholar]
- Freide RL, Johnstone MA. Response of thymidine labeling of nuclei in grey matter and nerve following sciatic nerve transection. Acta Neuropathol. 1967;7:218–231. doi: 10.1007/BF00686373. [DOI] [PubMed] [Google Scholar]
- Gold BC, Austin DR, Storm-Dickerson T. Multiple signals underlie the axotomy-induced up-regulation of c-Jun in adult sensory neurons. Neurosci. Lett. 1994;176:123–127. doi: 10.1016/0304-3940(94)90886-9. [DOI] [PubMed] [Google Scholar]
- Goldstein ME, Weiss SR, Lazzarini RA, Schneidman PS, Lees JF, Schlaepfer WW. mRNA levels of all three neurofilament proteins decline following nerve transection. Mol. Brain Res. 1988;3:287–292. doi: 10.1016/0169-328x(88)90051-4. [DOI] [PubMed] [Google Scholar]
- Grafstein B, McQuarrie IG. Role of the nerve cell body in axonal regeneration. In: Cotman Carl W., editor. Neuronal Plasticity. Raven Press; New York: 1978. pp. 155–195. [Google Scholar]
- Herdegen T, Fiallos-Estrada CE, Schmidt W, Bravo R, Zimmerman M. Transcription factors c-JUN, JUN D and CREB, but not FOS and KROX-24 are differentially regulated in neurons following axotomy of rat sciatic nerve. Mol. Brain Res. 1992;14:155–165. doi: 10.1016/0169-328x(92)90170-g. [DOI] [PubMed] [Google Scholar]
- Heumann R, Korsching S, Bandlow C, Thoenen H. Changes in nerve growth factor synthesis in non-neuronal cells in response to sciatic nerve transection. J. Cell Biol. 1987;104:1623–1631. doi: 10.1083/jcb.104.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himes BT, Tessler A. Death of some dorsal root ganglion neurons and plasticity of others following sciatic nerve section in adult and neonatal rats. J. Comp. Neurol. 1989;284:215–230. doi: 10.1002/cne.902840206. [DOI] [PubMed] [Google Scholar]
- Hu-Tsai M, Winter J, Emson PC, Woolf CJ. Neurite outgrowth and GAP-43 mRNA expression in cultured adult rat dorsal root ganglion neurons: Effects of BDNF or prior peripheral axotomy. J. Neurosci. Res. 1994;39:634–645. doi: 10.1002/jnr.490390603. [DOI] [PubMed] [Google Scholar]
- Jacob JM, McQuarrie IC. Accelerated axon outgrowth in rat sciatic nerve at one week after axotomy. J. Neurobiol. 1993;24:356–367. doi: 10.1002/neu.480240308. [DOI] [PubMed] [Google Scholar]
- Jenkins SR, Hunt SP. Long-term increase in the levels of c-jun mRNA and Jun protein-like immunoreactivity in motor and sensory nerve following axon damage. Neurosci. Lett. 1991;129:107–110. doi: 10.1016/0304-3940(91)90731-8. [DOI] [PubMed] [Google Scholar]
- Jenkins SR, McMahon SB, Bond AB, Hunt SP. Expression of c-Jun as a response to dorsal root and peripheral nerve section in damaged and adjacent intact sensory neurons in the rat. Eur. J. Neurosci. 1993;5:751–759. doi: 10.1111/j.1460-9568.1993.tb00539.x. [DOI] [PubMed] [Google Scholar]
- Jenq C-B, Jenq LL, Bear HM, Coggeshall RE. Conditioning lesions of peripheral nerves change regenerated axon numbers. Brain Res. 1988;457:63–69. doi: 10.1016/0006-8993(88)90057-1. [DOI] [PubMed] [Google Scholar]
- Kanje M, Skottner A, Lundborg G, Sjöberg J. Does insulin-like growth factor (IGF-1) trigger the cell body reaction in the rat sciatic nerve? Brain Res. 1991;563:285–287. doi: 10.1016/0006-8993(91)91547-e. [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Waxman SG, Hildbrand C, Ruiz JA. Regenerating mammalian nerve fibers: Changes in the action potential waveform and firing characteristics following blockage of potassium conductance. Proc. R. Soc. Lond. [Biol.] 1982;217:77–87. doi: 10.1098/rspb.1982.0095. [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Rand MN, Lankford KL, Waxman SG. Intracellular calcium mobilization and neurite outgrowth in mammalian neurons. J. Neurobiol. 1994;25:252–264. doi: 10.1002/neu.480250306. [DOI] [PubMed] [Google Scholar]
- Kristensson K, Olson Y. Retrograde transport of horseradish peroxidase in transacted axons. II. Relations between rate of transfer from the site of injury to the perikaryon and the onset of chromatolysis. J. Neurocytol. 1975;4:653–661. doi: 10.1007/BF01181628. [DOI] [PubMed] [Google Scholar]
- Landreth GE, Agranoff BW. Explant culture of adult goldfish retina: Effect of prior optic nerve crush. Brain Res. 1976;118:299–303. doi: 10.1016/0006-8993(76)90714-9. [DOI] [PubMed] [Google Scholar]
- Lankford KL, Rand MN, Waxman SG, Kocsis JD. Blocking Ca2+ mobilization with thapsigargin reduces neurite initiation in cultured adult rat DRG neurons. Dev. Brain Res. 1995;84:151–163. doi: 10.1016/0165-3806(94)00159-w. [DOI] [PubMed] [Google Scholar]
- Lankford KL, Kenney A, Kocsis JD. Mechanisms controlling neurite initiation. Prog. Brain Res. 1996;108:55–81. doi: 10.1016/s0079-6123(08)62532-7. [DOI] [PubMed] [Google Scholar]
- Leah JD, Herdegen T, Bravo R. Selective expression of Jun proteins following axotomy and axonal transport block in peripheral nerves in the rat: Evidence for a role in the regenerative process. Brain Res. 1991;566:198–207. doi: 10.1016/0006-8993(91)91699-2. [DOI] [PubMed] [Google Scholar]
- Leech RW. Changes in satellite cells of rat dorsal root ganglia during central chromatolysis. Neurology. 1967;17:349–358. doi: 10.1212/wnl.17.4.349. [DOI] [PubMed] [Google Scholar]
- Lindholm D, Heuman R, Meyer M, Thoenen H. Changes in nerve growth factor synthesis in non-neuronal cells of the rat sciatic nerve. Nature. 1987;330:658–659. doi: 10.1038/330658a0. [DOI] [PubMed] [Google Scholar]
- Lu X, Richardson PM. Inflammation near the nerve cell body enhances axonal regeneration. J. Neurosci. 1991;11:972–978. doi: 10.1523/JNEUROSCI.11-04-00972.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Murrian M, Guthrie PB. Localized calcium influx orients axon formation in embryonic hippocampal pyramidal neurons. Dev. Brain Res. 1990;52:201–209. doi: 10.1016/0165-3806(90)90236-r. [DOI] [PubMed] [Google Scholar]
- McQuarrie IG. The effect of conditioning lesions on regeneration of motor axons. Brain Res. 1978;152:597–602. doi: 10.1016/0006-8993(78)91116-2. [DOI] [PubMed] [Google Scholar]
- McQuarrie IG. Effects of conditioning lesions on sprout formation at nodes of Ranvier. J. Comp. Neurol. 1985;231:239–249. doi: 10.1002/cne.902310211. [DOI] [PubMed] [Google Scholar]
- McQuarrie IG, Grafstein B. Protein synthesis and axonal transport in goldfish retinal ganglion cells during regeneration accelerated by a conditioning lesion. Brain Res. 1982;251:25–37. doi: 10.1016/0006-8993(82)91270-7. [DOI] [PubMed] [Google Scholar]
- McQuarrie IG, Jacobs JM. Conditioning nerve crush accelerates cytoskeletal protein transport in sprouts that form after a subsequent nerve crush. J. Comp. Neurol. 1991;305:139–147. doi: 10.1002/cne.903050113. [DOI] [PubMed] [Google Scholar]
- McQuarrie IG, Grafstein B, Gershen MD. Axonal regeneration in the rat sciatic nerve: Effect of conditioning lesion and of dbcAMP. Brain Res. 1977;132:443–453. doi: 10.1016/0006-8993(77)90193-7. [DOI] [PubMed] [Google Scholar]
- McQuarrie IG, Grafstein B, Dryfus CF, Gershen MD. Regeneration of adrenergic axons in rat sciatic nerve: Effect of a conditioning lesion. Brain Res. 1978;41:21–34. doi: 10.1016/0006-8993(78)90614-5. [DOI] [PubMed] [Google Scholar]
- Meyer M, Matauka I, Westmore C, Olson L, Thoenen H. Enhanced synthesis of brain derived neurotrophic factors in the lesioned peripheral nerve: Different mechanism are responsible for the regulation of BDNF and NGF mRNA. J. Cell Biol. 1992;119:45–54. doi: 10.1083/jcb.119.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller FD, Telzlaff W, Bisby MA, Fawcett JW, Milner RJ. Rapid induction of the major αtubulin mRNA Ta1, during nerve regeneration in the adult rat. J. Neurosci. 1989;9:1452–1463. doi: 10.1523/JNEUROSCI.09-04-01452.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz PF, Oblinger MC. Sensory neurons selectively upregulate synthesis and transport of the βIII-tubulin protein during axonal regeneration. J. Neurosci. 1995;15:1545–1555. doi: 10.1523/JNEUROSCI.15-02-01545.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz PF, Smith R, Picket J, Frankfurter A, Oblinger MM. Expression of the class III β-tubulin gene during axonal regeneration of rat dorsal root ganglion neurons. J. Neurosci. Res. 1993;34:129–134. doi: 10.1002/jnr.490340113. [DOI] [PubMed] [Google Scholar]
- Oblinger MM, Lasek RJ. A conditioning lesion of the peripheral axons of the dorsal root ganglion cells accelerates regeneration of only their peripheral axons. J. Neurosci. 1984;4:1736–1774. doi: 10.1523/JNEUROSCI.04-07-01736.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oblinger MM, Lasek RJ. Axotomy induced alterations in the synthesis and transport of neurofilaments and microtubules in dorsal root ganglion cells. J. Neurosci. 1988;8:1747–1758. doi: 10.1523/JNEUROSCI.08-05-01747.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudega M, Varon S, Hagg T. Regeneration of adult rat sensory axons into intraspinal nerve grafts: Promoting effects of conditioning lesion and graft pre-degeneration. Exp. Neurol. 1994;129:194–206. doi: 10.1006/exnr.1994.1161. [DOI] [PubMed] [Google Scholar]
- Oyelese AA, Kocsis JD. GABAA receptor-mediated conductance and action potential waveform in cutaneous muscle afferent neurons in the adult rat: Differential expression and response to nerve injury. J. Neurophysiol. 1996;76:2383–2392. doi: 10.1152/jn.1996.76.4.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyelese AA, Eng DL, Richardson GB, Kocsis JD. Enhancement of GABAA receptor-mediated conductances induced by nerve injury in a subclass of sensory neurons. J. Neurophysiol. 1995;74:673–683. doi: 10.1152/jn.1995.74.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannese E. Number and structure of perisomatic satellite cells of spinal ganglia under normal conditions or during axon regeneration and neuronal hypertrophy. Z. Zellforsh. 1964;63:568–592. doi: 10.1007/BF00339491. [DOI] [PubMed] [Google Scholar]
- Pellegrino R, Spencer PS. Schwann cell mitosis in responses to regenerating peripheral axons in vivo. Brain Res. 1985;341:16–25. doi: 10.1016/0006-8993(85)91467-2. [DOI] [PubMed] [Google Scholar]
- Perry VH, Brown MC, Corgan S. The macrophage response to central and peripheral nerve injury. J. Exp. Med. 1987;165:1218–1223. doi: 10.1084/jem.165.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politis MJ, Ederle K, Spencer PS. Tropism in nerve regeneration in vivo. Attraction of regenerating axons by diffusible factors derived from cells in the distal nerve stump of transected peripheral nerves. Brain Res. 1982;253:1–12. doi: 10.1016/0006-8993(82)90667-9. [DOI] [PubMed] [Google Scholar]
- y Cajal S. Ramón.Degeneration and regeneration of the nervous system 1928I Translated by R.M. May. Oxford University Press; Cambridge [Google Scholar]
- Richardson BM, Verge VMK. Peripheral injury enhances central regeneration of primary sensory neurones. Nature. 1984;309:791–793. doi: 10.1038/309791a0. [DOI] [PubMed] [Google Scholar]
- Richardson BM, Verge VMK. Axonal regeneration in dorsal spinal roots is accelerated by peripheral axonal transection. Brain Res. 1987;411:406–408. doi: 10.1016/0006-8993(87)91096-1. [DOI] [PubMed] [Google Scholar]
- Rizzo MA, Kocsis JD, Waxman SG. Selective loss of slow and enhancement of fast Na+ currents in cutaneous afferent dorsal root ganglion neurons following axotomy. Neurobiol. Dis. 1995;2:87–96. doi: 10.1006/nbdi.1995.0009. [DOI] [PubMed] [Google Scholar]
- Robinson GA. Immediate early gene expression in axotomized and regenerating retinal ganglion cells of the adult rat. Mol. Brain Res. 1994;24:43–54. doi: 10.1016/0169-328x(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Salzer JL, Bunge RP. Studies of Schwann cell proliferation. I. An analysis in tissue culture of proliferation during development, Wallerian degeneration, and direct injury. J. Cell Biol. 1980;84:739–752. doi: 10.1083/jcb.84.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebert ME, Shooter EM. Expression of mRNA for neurotrophic factors and their receptors in the rat dorsal root ganglion and sciatic nerve following injury. J. Neurosci. Res. 1993;36:357–367. doi: 10.1002/jnr.490360402. [DOI] [PubMed] [Google Scholar]
- Sjöberg J, Kanje M. The initial period of peripheral nerve regeneration and the importance of local environment for conditioning lesion effect. Brain Res. 1990a;529:79–84. doi: 10.1016/0006-8993(90)90812-p. [DOI] [PubMed] [Google Scholar]
- Sjöberg J, Kanje M. Effects of repetitive conditioning crush lesion on regeneration of the rat sciatic nerve. Brain Res. 1990b;530:167–169. doi: 10.1016/0006-8993(90)90676-3. [DOI] [PubMed] [Google Scholar]
- Sjöberg J, Kanje M, Edström A. Influence of non-neuronal cells on regeneration of the rat sciatic nerve. Brain Res. 1988;453:221–226. doi: 10.1016/0006-8993(88)90161-8. [DOI] [PubMed] [Google Scholar]
- Sjöstrand J. Proliferative changes in glial cells during nerve regeneration. Z. Zellforsh. 1965;68:481–493. doi: 10.1007/BF00347712. [DOI] [PubMed] [Google Scholar]
- Sternberger LA, Hardy PH, Cucil JJ, Meyer HG. The unlabeled antibody enzyme method of immunohistochemistry: Preparation and properties of soluble antigen-antibody complex (horseradish peroxidase antiperoxidase) and its use in identification of spirochetes. J. Histochem. Cytochem. 1970;18:315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Zhang Q-L, Webster H.deF. Myelinated fiber regeneration after sciatic nerve crush: Morphometric observations in young adult and aging mice and the effects of macrophage suppression and conditioning lesions. Exp. Neurol. 1992;118:53–61. doi: 10.1016/0014-4886(92)90022-i. [DOI] [PubMed] [Google Scholar]
- Tapia M, Inestrosa NC, Alvarez J. Early axonal regeneration: Repression by Schwann cells and a protease? Exp. Neurol. 1985;131:124–132. doi: 10.1016/0014-4886(95)90014-4. [DOI] [PubMed] [Google Scholar]
- Thomas PK. The cellular response to nerve injury. 3. The effects of repeated crush injuries. J. Anat. 1970;106:463–470. [PMC free article] [PubMed] [Google Scholar]
- Van der Zee CEEM, Nielander HB, Vos JP, Lopes de Silva S, Verhaagen J, Oestreicher B, Schrama LH, Schotman P, Gispen H. Expression of growth-associated protein B-50 (GAP43) in dorsal ganglia and sciatic nerve during regenerative sprouting. J. Neurosci. 1989;9:3505–3512. doi: 10.1523/JNEUROSCI.09-10-03505.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson WE. Observations on the nucleolar and total cell body nucleic acid of injured nerve cells. J. Physiol. (Lond.) 1968;196:655–676. doi: 10.1113/jphysiol.1968.sp008528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells MR, Vaidya U. Morphological alterations in dorsal root ganglion neurons after peripheral axon injury: Association with changes in metabolism. Exp. Neurol. 1989;104:32–38. doi: 10.1016/0014-4886(89)90006-x. [DOI] [PubMed] [Google Scholar]
- Wells MR, Vaidya U. RNA transcription in axotomized dorsal root ganglion neurons. Mol. Brain Res. 1994;27:163–166. doi: 10.1016/0169-328x(94)90198-8. [DOI] [PubMed] [Google Scholar]
- Weise UH, Ruth JL, Emson PC. Differential expression of growth associated protein (GAP-43) mRNA in rat primary sensory neurons after peripheral nerve lesion: A non-radioactive in situ hybridization study. Brain Res. 1992;592:141–156. doi: 10.1016/0006-8993(92)91669-6. [DOI] [PubMed] [Google Scholar]
- Windebank AJ, Poduslo JF. Neuronal growth factors produced by adult peripheral nerves after injury. Brain Res. 1986;385:197–200. doi: 10.1016/0006-8993(86)91567-2. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Reynolds ML, Molander C, O’Brien C, Lindsay RM, Benowitz LI. The growth-associated protein GAP-43 appears in dorsal root ganglion cells and in the dorsal horn of the rat spinal cord following injury. Neuroscience. 1990;34:465–478. doi: 10.1016/0306-4522(90)90155-w. [DOI] [PubMed] [Google Scholar]
- Wujek JR. Correlation of axonal regeneration and slow component B in two branches of a single axon. J. Neurosci. 1983;3:243–251. doi: 10.1523/JNEUROSCI.03-02-00243.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]