Abstract
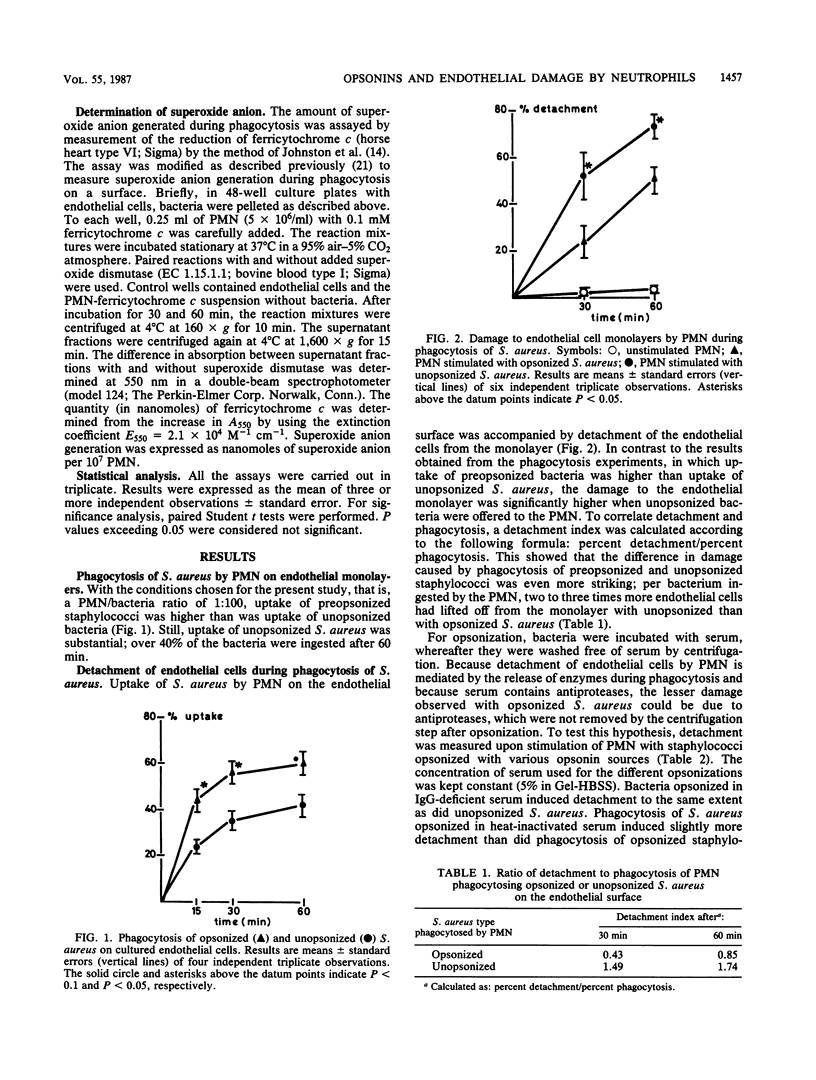
When phagocytosis of Staphylococcus aureus by human polymorphonuclear leukocytes (PMN) takes place on the surface of cultured human endothelial cells, the endothelial monolayers are damaged by lysosomal enzymes that are released by the PMN. Because PMN can phagocytose opsonized as well as unopsonized staphylococci on an endothelial surface, we studied the role of bacterial opsonization in the damage caused to the endothelium. Phagocytosis of unopsonized S. aureus was accompanied by greater damage (expressed as the percentage of the endothelial cells detached from the culture plates) of the monolayers than was phagocytosis of opsonized S. aureus: 52 +/- 10% and 24 +/- 7%, respectively, after 30 min of phagocytosis and 73 +/- 5% and 50 +/- 6%, respectively, after 60 min of phagocytosis. When correlated to the amount of phagocytosis, this difference was even greater (uptake was 35 +/- 4% for unopsonized S. aureus and 56 +/- 5% for opsonized S. aureus after 30 min and 42 +/- 3% and 60 +/- 5%, respectively, after 60 min). Total release of lysozyme and myeloperoxidase and generation of superoxide anion were the same during phagocytosis of opsonized or unopsonized staphylococci. Adherence of PMN to the endothelial cells was greater during phagocytosis of unopsonized S. aureus: 42 +/- 4% verus 27 +/- 3% during phagocytosis of opsonized staphylococci. Possibly, increased adherence of the PMN resulted in a locally higher concentration of enzymes which induced more damage. We conclude that opsonization of bacteria not only improves bacterial uptake, but also protects bystander cells from damage by the phagocytosing PMN.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson J. S., Mills E. L., Sawyer M. K., Regelmann W. R., Nelson J. D., Quie P. G. Recurrent infections and delayed separation of the umbilical cord in an infant with abnormal phagocytic cell locomotion and oxidative response during particle phagocytosis. J Pediatr. 1981 Dec;99(6):887–894. doi: 10.1016/s0022-3476(81)80011-x. [DOI] [PubMed] [Google Scholar]
- Bar-Shavit Z., Ofek I., Goldman R., Mirelman D., Sharon N. Mannose residues on phagocytes as receptors for the attachment of Escherichia coli and Salmonella typhi. Biochem Biophys Res Commun. 1977 Sep 9;78(1):455–460. doi: 10.1016/0006-291x(77)91276-1. [DOI] [PubMed] [Google Scholar]
- Boogaerts M. A., Yamada O., Jacob H. S., Moldow C. F. Enhancement of granulocyte-endothelial cell adherence and granulocyte-induced cytotoxicity by platelet release products. Proc Natl Acad Sci U S A. 1982 Nov;79(22):7019–7023. doi: 10.1073/pnas.79.22.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freimer N. B., Ogmundsdóttir H. M., Blackwell C. C., Sutherland I. W., Graham L., Weir D. M. The role of cell wall carbohydrates in binding of microorganisms to mouse peritoneal exudate macrophages. Acta Pathol Microbiol Scand B. 1978 Apr;86(2):53–57. doi: 10.1111/j.1699-0463.1978.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Griffin J. A., Leider J. E., Silverstein S. C. Studies on the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J Exp Med. 1975 Nov 1;142(5):1263–1282. doi: 10.1084/jem.142.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Killen P. D., Harker L. A., Striker G. E., Wright D. G. Neutrophil-mediated endothelial injury in vitro mechanisms of cell detachment. J Clin Invest. 1981 Dec;68(6):1394–1403. doi: 10.1172/JCI110390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Schwartz B. R., Reidy M. A., Schwartz S. M., Ochs H. D., Harker L. A. Activated neutrophils disrupt endothelial monolayer integrity by an oxygen radical-independent mechanism. Lab Invest. 1985 Feb;52(2):141–150. [PubMed] [Google Scholar]
- Hayward A. R., Harvey B. A., Leonard J., Greenwood M. C., Wood C. B., Soothill J. F. Delayed separation of the umbilical cord, widespread infections, and defective neutrophil mobility. Lancet. 1979 May 26;1(8126):1099–1101. doi: 10.1016/s0140-6736(79)91786-0. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973 Nov;52(11):2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEBANOFF S. J. INACTIVATION OF ESTROGEN BY RAT UTERINE PREPARATIONS. Endocrinology. 1965 Feb;76:301–311. doi: 10.1210/endo-76-2-301. [DOI] [PubMed] [Google Scholar]
- Lee D. A., Hoidal J. R., Clawson C. C., Quie P. G., Peterson P. K. Phagocytosis by polymorphonuclear leukocytes of Staphylococcus aureus and Pseudomonas aeruginosa adherent to plastic, agar, or glass. J Immunol Methods. 1983 Sep 30;63(1):103–114. doi: 10.1016/0022-1759(83)90213-2. [DOI] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Van Kessel K. P., Van Kats-Renaud H. J., Van Strijp J. A., Visser M. R., Verhoef J. Measurement of antibody-mediated binding of human polymorphonuclear leukocytes to HSV-1 infected anchorage fibroblasts. J Immunol Methods. 1986 Apr 3;88(1):101–107. doi: 10.1016/0022-1759(86)90057-8. [DOI] [PubMed] [Google Scholar]
- Van Oss C. J., Gillman C. F. Phagocytosis as a surface phenomenon. Contact angles and phagocytosis of non-opsonized bacteria. J Reticuloendothel Soc. 1972 Sep;12(3):283–292. [PubMed] [Google Scholar]
- Vandenbroucke-Grauls C. M., Thijssen H. M., Verhoef J. Interaction between human polymorphonuclear leucocytes and Staphylococcus aureus in the presence and absence of opsonins. Immunology. 1984 Jul;52(3):427–435. [PMC free article] [PubMed] [Google Scholar]
- Vandenbroucke-Grauls C. M., Thijssen H. M., Verhoef J. Phagocytosis of staphylococci by human polymorphonuclear leukocytes is enhanced in the presence of endothelial cells. Infect Immun. 1985 Oct;50(1):250–254. doi: 10.1128/iai.50.1.250-254.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbroucke-Grauls C. M., Thijssen H. M., van Kessel K. P., van Asbeck B. S., Verhoef J. Injury to endothelial cells by phagocytosing polymorphonuclear leukocytes and modulatory role of lipoxygenase products. Infect Immun. 1987 Jun;55(6):1447–1454. doi: 10.1128/iai.55.6.1447-1454.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugh H. A., Peters R., Peterson P. K., Verhoef J. Phagocytosis and killing of staphylococci by human polymorphonuclear and mononuclear leucocytes. J Clin Pathol. 1978 Jun;31(6):539–545. doi: 10.1136/jcp.31.6.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoef J., Peterson P. K., Quie P. G. Kinetics of staphylococcal opsonization, attachment, ingestion and killing by human polymorphonuclear leukocytes: a quantitative assay using [3H]thymidine labeled bacteria. J Immunol Methods. 1977;14(3-4):303–311. doi: 10.1016/0022-1759(77)90141-7. [DOI] [PubMed] [Google Scholar]
- WOOD W. B., Jr, SMITH M. R., PERRY W. D., BERRY J. W. Studies on the cellular immunology of acute bacteremia. I. Intravascular leucocytic reaction and surface phagocytosis. J Exp Med. 1951 Dec 1;94(6):521–534. doi: 10.1084/jem.94.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weening R. S., Voetman A. A., Hamers M. N., Meerhof L. J., Bot A. A., Roos D. Oxidative damage to lysosomal enzymes in human phagocytosing neutrophils. Adv Exp Med Biol. 1982;141:247–257. doi: 10.1007/978-1-4684-8088-7_26. [DOI] [PubMed] [Google Scholar]
- ZUCKER-FRANKLIN D. ELECTRON MICROSCOPE STUDY OF THE DEGRANULATION OF POLYMORPHONUCLEAR LEUKOCYTES FOLLOWING TREATMENT WITH STREPTOLYSIN. Am J Pathol. 1965 Sep;47:419–433. [PMC free article] [PubMed] [Google Scholar]


