Abstract
The potential of bone marrow cells to differentiate into myelin-forming cells and to repair the demyelinated rat spinal cord in vivo was studied using cell transplantation techniques. The dorsal funiculus of the spinal cord was demyelinated by x-irradiation treatment, followed by microinjection of ethidium bromide. Suspensions of a bone marrow cell fraction acutely isolated from femoral bones in LacZ transgenic mice were prepared by centrifugation on a density gradient (Ficoll-Paque) to remove erythrocytes, platelets, and debris. The isolated cell fraction contained hematopoietic and nonhematopoietic stem and precursor cells and lymphocytes. The cells were transplanted into the demyelinated dorsal column lesions of immunosuppressed rats. An intense blue β-galactosidase reaction was observed in the transplantation zone. The genetically labeled bone marrow cells remyelinated the spinal cord with predominately a peripheral pattern of myelination reminiscent of Schwann cell myelination. Transplantation of CD34+ hematopoietic stem cells survived in the lesion, but did not form myelin. These results indicate that bone marrow cells can differentiate in vivo into myelin-forming cells and repair demyelinated CNS.
Keywords: axon, bone marrow, demyelination, glia, myelin, multiple sclerosis, Schwann cell
INTRODUCTION
Transplantation of oligodendrocytes (Archer et al., 1994; Blakemore and Crang, 1988) or peripheral myelin-forming cells such as Schwann cells (Blakemore, 1977; Blakemore and Crang, 1985; Honmou et al., 1996) or olfactory ensheathing cells (Franklin et al., 1996; Imaizumi et al., 1998; Kato et al., 2000) can elicit remyelination in animal models and recovery of electrophysiological function (Honmou et al., 1996; Imaizumi et al., 1998; Utzschneider et al., 1994). While possible, preparation of such cells from a patient for autologous cell therapy approaches is problematic because biopsies must be obtained from either brain or nerve. Neural precursor or stem cells derived from brain have the capacity to self-renew and to generate progeny capable of differentiating into multiple distinct cell lineages of neurons and glia (Gage et al., 1995; Lois and Alvarez-Buylla, 1993; Morshead et al., 1994; Reynolds and Weiss, 1992).
Transplantation of human neural stem cells obtained from embryonic tissue can lead to neuronal and astrocytic differentiation (Chalmers-Redman et al., 1997; Moyer et al., 1997; Svendsen et al., 1997) and remyelination in the newborn mouse brain (Flax et al., 1998). Neural precursor cells derived from adult human brain have been reported to achieve remyelination and recovery of impulse conduction upon transplantation into demyelinated rodent spinal cords (Akiyama et al., 2001). These studies have engendered intense interest because they suggest the possibility of using such cells for repair strategies in neurological diseases (Akiyama et al., 2001; Chalmers-Redman et al., 1997; Moyer et al., 1997; Svendsen et al., 1997; Yandava et al., 1999). However, issues remain with regard to the relative inaccessibility of these cells for autologous cell therapies and the need for cell expansion using trophic factors.
Recent work demonstrates that neural stem cells can generate hematopoietic cells in vivo indicating that neural precursor cells are not restricted to a neural lineage (Bjornson et al., 1999). Moreover, bone marrow stromal cells can differentiate into astrocytes when injected into the lateral ventricles of neonatal mice (Kopen et al., 1999), or exclusively into neurons in vitro under appropriate cell culture conditions (Woodbury et al., 2000). Recent work indicates that after intravenous injection of bone marrow cells of x-irradiated rats that neuronal cells can differentiate in brain from the injected bone marrow cells (Brazelton et al., 2000; Mezey et al., 2000). We report that transplantation of an acutely isolated bone marrow cell fraction (devoid of erythrocytes, platelets, and debris) into the demyelinated rat spinal cord results in relatively extensive remyelination. The cells were separated on density gradient, but not expanded with trophic factors. These results indicate that an acutely prepared bone marrow cell fraction can develop a myelinating phenotype in vivo when placed into an appropriate CNS lesion, and the potential of developing an easily accessible and renewable source of autologous donor cells for cell transplantation studies in demyelinating disease.
MATERIALS AND METHODS
Preparation of Bone Marrow Cells, Schwann Cells, and CD34+ Hematopoietic Cells
Bone marrow (10 μl) was obtained from femoral bones in adult LacZ transgenic mice (ROSA26; Jackson Laboratory). The samples were diluted in L-15 medium (2 ml) containing 3 ml Ficoll. The cells were collected from the lymphocytic layer after centrifugation (2,000 rpm, 15 min), and resuspended in 2 ml serum-free medium neural progenitor cell maintenance medium (NPMM). After centrifugation (2,000 rpm, 15 min), the cells were collected in NPMM. Primary Schwann cell cultures were established from the sciatic nerve of neonatal mouse (P1-3) according to the method of Honmou et al. (1996). Briefly, the cell suspension resulting from enzymatically and mechanically dissociating sciatic nerves was plated onto 100-mm2 poly (L-lysine)-coated tissue culture plates at 8 × 105 cells per plate and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (vol/vol) fetal calf serum (FCS). CD34+ hematopoietic stem cells were prepared from the lymphocytic fraction of mouse bone marrow prepared by density gradient as above, and isolated by magnetic cell sorting using a MiniMACS system (Miltenyi Biotec, Auburn, CA).
Animal Preparation and Transplantation
Experiments were performed on 12-week-old Wistar rats. A focal demyelinated lesion was created in the dorsal column of the spinal cord using x-irradiation and ethidium bromide injection (EB-X; Honmou et al., 1996). Briefly, rats were anesthetized with ketamine (75 mg/kg) and xylazine (10 mg/kg) i.p., and a 40-Gray surface dose of x-irradiation was delivered through a 2 × 4 cm opening in a lead shield (4 mm thick) to the spinal cord caudal to the tenth thoracic level (T10) using a Softex M-150 WZ radiotherapy machine (100 kV, 1.15 mA, SSD 20 cm, dose rate 200 cGy/min). Three days after irradiation, rats were anesthetized as above and, using sterile technique, a laminectomy was performed at T11. The demyelinating lesion was induced by the direct injection of ethidium bromide (EB) into the dorsal column via a drawn glass micropipette. Injections of 0.5 μl of 0.3 mg/ml EB in saline were made at depths of 0.7 and 0.4 mm. A suspension of cells (1 × 104 cells/μl) in 1 μl medium was injected into the middle of the EB-X-induced lesion 3 days after the EB injection. Transplant-receiving rats were immunosuppressed with Cyclosporin A (10 mg/kg/day).
Histological Examination
The rats were deeply anesthetized with sodium pentobarbital (60 mg/kg, i.p.) and perfused through the heart first with phosphate-buffered solution (PBS) and then with a fixative solution containing 2% glutaraldehyde and 2% paraformaldehyde in 0.14 M Sorensen’s phosphate buffer, at pH 7.4. After in situ fixation for 10 min, the spinal cord was carefully excised, cut into 1-mm segments, and placed into fresh fixative. The tissue was washed several times in Sorensen’s buffer, postfixed with 1% OsO4 for 2 h at 25°C, dehydrated in graded ethanol solutions, passed through propylene oxide, and embedded in EPON. Semithin sections (1 μm) were cut, counterstained with 0.5% methylene blue, 0.5% azure II in 0.5% borax, and examined with a light microscope (Zeiss: Axioskop FS). Thin sections were counterstained with uranyl and lead salts and examined with a JEOL JEM1200EX electron microscope operating at 60 kV.
A 50 × 50 μm standardized region in the central core of the dorsal funiculus near the site of cell injection was used for the morphometric analysis. Remyelinated axons and the number of cell bodies associated with the axons were counted in this region; density was scaled to square millimeters (mm2). The diameters of the axons and cell bodies, the number of cells with multilobular nuclei and cells showing myelination of more than one axon were counted in the same standardized region. Measurements and counts were obtained from five sections per rat and five separate rats (n = 5) were analyzed for each experimental condition. All variances represent standard error (± SEM).
Detection of β-Galactosidase Reaction Products In Vivo
Rats were perfused through the heart with fixative (0.5% glutaraldehyde in phosphate buffer). After removal of the spinal cords they were left in fixative for 1 h. Sections were cut either with a vibratome (500 μm) or a cryostat (50 μm) and β-galactosidase expressing myelin-forming cells were detected by incubating the sections at 37°C overnight with X-Gal in a final concentration of 1 mg/ml in X-Gal developer (35 mM K3Fe(CN)6/35 mM K4Fe(CN)6 · 3H2O/2 mM MgCl2 in PBS) to form a blue color within the cell. The slices were then fixed for an additional 3 h in 3.6% (vol/vol) glutaraldehyde in phosphate buffer (0.14 M) and were examined by light microscopy for the presence of a blue reaction product (β-galactosidase reaction product). Sections were counterstained with hematoxylin to observe nuclei. Some sections were also processed for GFAP visualization using immunohistochemistry as described below.
Immunohistochemistry
Three weeks after transplantation, the antigenic phenotypes were analyzed in vivo. The rats were deeply anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and perfused through the heart with PBS, followed by fixative containing 4% paraformaldehyde in 0.14 M Sorensen’s phosphate buffer (pH 7.4). Spinal cords were removed, kept in 4% paraformaldehyde in phosphate buffer overnight, dehydrated, and embedded in paraffin. Transverse sections (3 μm) were cut. Paraffin-embedded sections were mounted on slides, dewaxed in xylene, and dehydrated in ethanol. After rehydration in PBS the sections were treated with 1% hydrogen peroxide. Anti-NSE polyclonal antibody (1: 1,000) and anti-GFAP polyclonal antibody (1:200) were applied separately overnight. Secondary antibody was biotinylated goat anti-rabbit IgG antibody. A Histofine kit (Nichirei, Toyko) was used for signal visualization.
RESULTS
The dorsal funiculus of the spinal cord consists largely of myelinated axons (Fig. 1A,B). To induce a focal region of demyelination, the lumbar spinal cord was first x-irradiated to inhibit mitosis of endogenous glial cells. Subsequently, the central region of the dorsal funiculus was injected with a nucleic acid chelator, ethidium bromide, to kill glia cells including oligodendrocytes (Blakemore and Crang, 1985; Honmou et al, 1996). The lesion induced by this procedure is characterized by virtually complete loss of endogenous glial elements (astrocytes and oligodendrocytes) with preservation of axons (Fig. 1C). The higher power light micrograph of the lesion shows compact fields of demyelinated axons in close apposition separated by areas of myelin debris and macrophages (Fig. 1D). The lesion occupies nearly the entire dorsoventral extent of the dorsal columns for 5–7 mm longitudinally. Virtually no endogenous invasion of Schwann cells or astrocytes occurs before 6–8 weeks, at which time these glial elements begin to invade the lesion from its peripheral borders. Thus, a demyelinated and glial-free environment in vivo is present for at least 6 weeks.
Fig. 1.
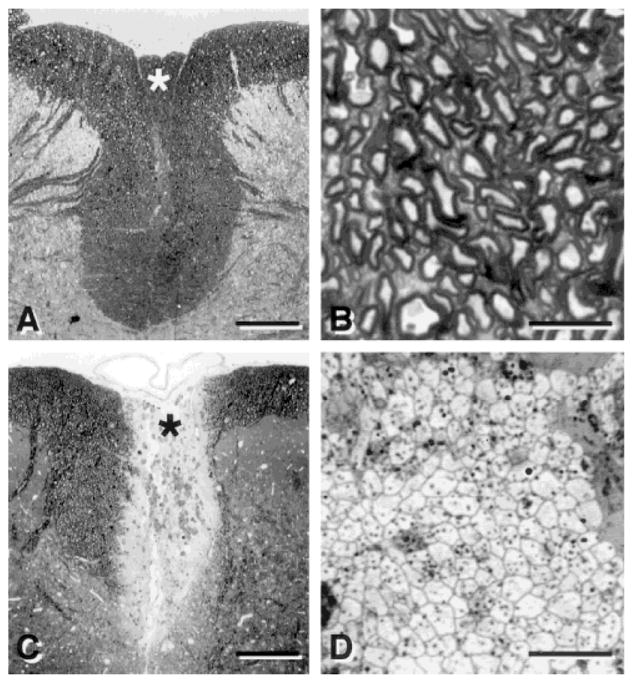
Light micrographs of transverse sections of the dorsal spinal cord showing the dorsal funiculus in normal (A) and demyelinated (C) rats. Examination at higher magnification shows normal (B) and demyelinated (D) axons in the dorsal columns. B and D were prepared from the area around the asterisk in A and C, respectively. Scale bar = 250 μm in A,C; 10 μm in B,D.
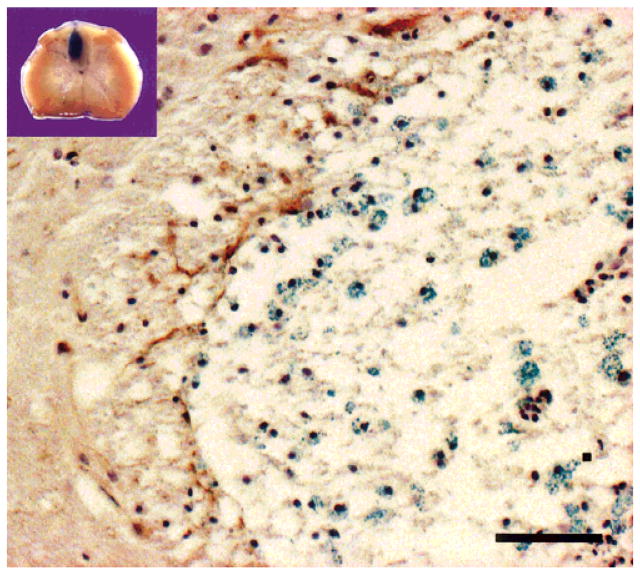
Three weeks after transplantation of LacZ transgenic mouse bone marrow (BM) cells into the central region of the lesion in immunosuppressed rats (see Methods), intense blue β-galactosidase reaction product was observed in the transplantation sites of the dorsal funiculus in vibratome sections (Fig. 2, inset). Light microscopic examination of the tissue revealed numerous β-galactosidase-positive cells within the BM transplantation zone of the dorsal funiculus (Fig. 2, blue profiles). There was little GFAP staining within the lesion zone and few astrocyte-like cells were observed with electron microscopy within the lesion zone, suggesting that astrocytes did not significantly form within the lesion. Some GFAP-positive cells were present at the peripheral margin of the lesion (Fig. 2, left of center). These results indicate that few astrocytes are present in the transplant region, and that there is a preponderance of myelin-forming cells derived from donor cells in the lesion zone.
Fig. 2.
Inset shows intense blue β-galactosidase reaction product after transplantation of LacZ transgenic mouse bone marrow cells into the central region of a demyelinated lesion in the rat spinal cord. Numerous cells with β-galactosidase blue reaction are observed in the demyelinated dorsal funiculus 3 weeks after transplantation of LacZ transgenic mouse bone marrow cells. The image is from the upper left region of the dorsal funiculus at the border of the lesion with lateral white matter. The section was processed for β-galactosidase and GFAP and counterstained with hematoxylin to observe nuclei. Scale bar = 25 μm.
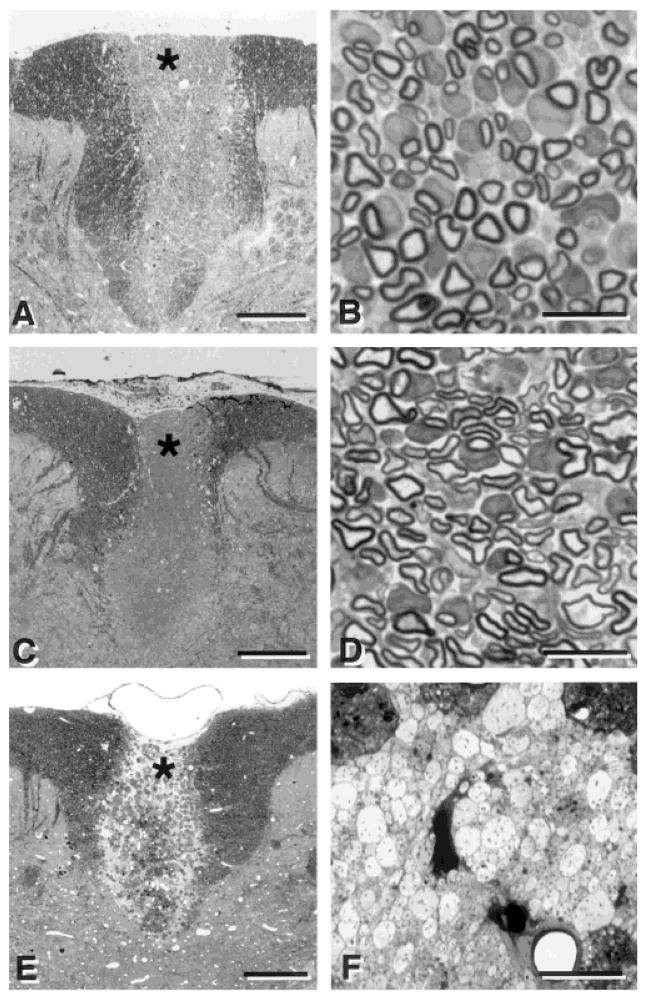
Histological examination of the transplant region in plastic sections (Fig. 3A,B) indicated that the transplanted dorsal funiculus was extensively myelinated. Many of the cells have large cytoplasmic and nuclear domains surrounding axons. Without immunosuppression (n = 3), no myelination or cell survival was observed after cell transplantation of the mouse BM cells. Remyelination was observed across the entire coronal dimension of the dorsal columns and throughout the anteroposterior extent of the lesion. Figure 3C,D shows the pattern of remyelination observed after transplantation of allogeneic Schwann cells (SC) in the EB-X lesion model. Note that with both BM and SC transplantation, several cells have large nuclear and cytoplasmic domains surrounding the remyelinated axons, which is characteristic of peripheral myelin (Berthold, 1995). SC transplantation led to a similar pattern of remyelination (Fig. 3C,D), but as discussed below there were subtle differences. Control experiments were carried out using a cell that should not myelinate the axons (CD34+ hematopoietic bone marrow cells). These cells did not produce myelin, but some survived within the lesion (Fig. 3E,F). This finding, together with the β-galactosidase identification described in Figure 2, strongly suggests that the injected BM cells were responsible for the remyelination and not recruitment of endogenous cells.
Fig. 3.
Low- (left) and high- (right) power micrographs of the dorsal funiculus after transplantation of bone marrow cells (A,B), Schwann cells (C,D) and CD34+ hematopoietic cells (E,F). Note the relatively extensive myelination after transplantation of bone marrow cells (B) and Schwann cells (D). Transplantation of CD34+ hematopoietic cells derived from mouse bone marrow into the demyelinated rat spinal cord did not result in myelination, although the transplanted cells were present and survived in the demyelinated lesion. B, D, and F are prepared from the area around the asterisk in A, C and E, respectively. Scale bar = 250 μm in A,C,E; 10 μm in B,D,F.
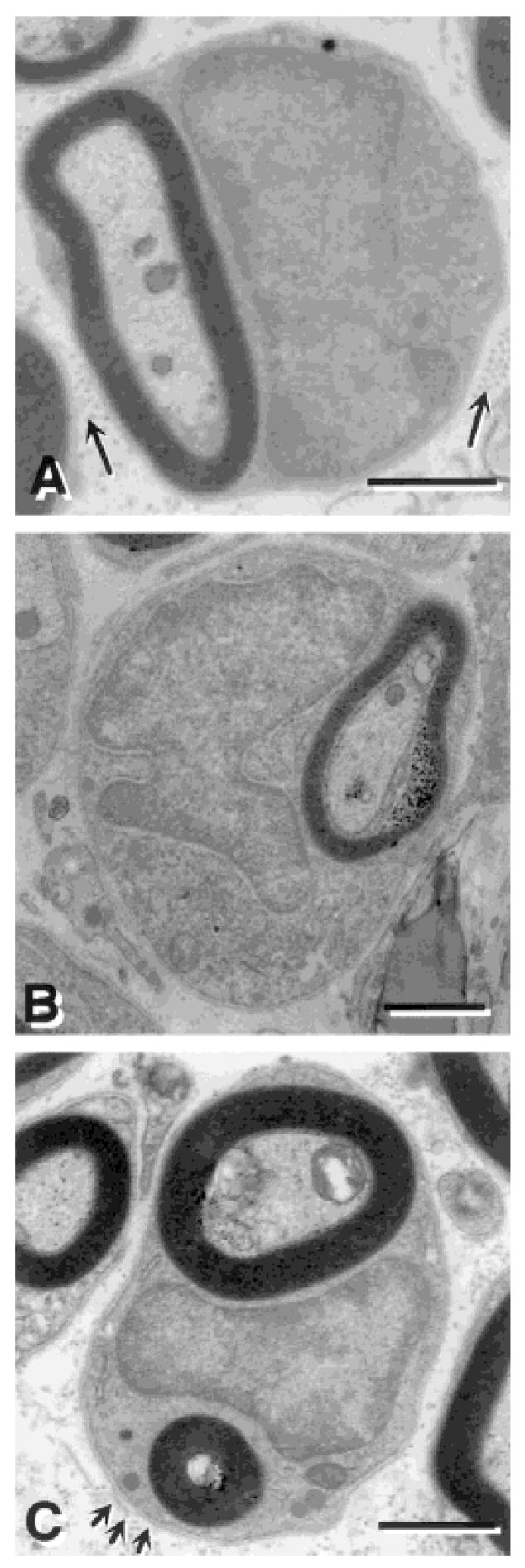
Electron microscopic analysis revealed several features suggesting the similarity of many of the myelin-forming cells to peripheral myelin-forming cells (Fig. 4). The cells had large nuclear and cytoplasmic regions and a basement membrane (Fig. 4C; arrows), and collagen was deposited in the extracellular space surrounding the cells. (Fig. 4A, arrows). It is important to note that some of the myelinated axons were not associated with these peripheral myelin-forming cell characteristics and may be myelinated by oligodendrocytes. Although we have not carried out a quantitative analysis of central versus peripheral myelination in the transplanted rats, the morphological observations suggest that a substantial number of the myelinated axons are from peripheral-like myelin forming cells. We emphasize that some oligodendrocyte-like myelination was present and future studies should address the relative amount of central versus peripheral myelin.
Fig. 4.
Electron micrographs of remyelinated axons in the dorsal funiculus of the spinal cord after bone marrow transplantation. A: Many myelin-forming cells were similar to peripheral myelin-forming cells, characterized by large nuclear and cytoplasmic regions, and collagen (arrows) in the extracellular space. B: Several of the myelin-forming cells have multilobular nuclei in the BM transplantation group. C: Some of the myelin-forming cells in the BM transplantation group engaged more than one axon, while no Schwann cells were observed to engage more than one axon. Arrows in C point to a basement membrane surrounding the myelin-forming cell. Scale bar = 1 μm in A,B; 2 μm in C.
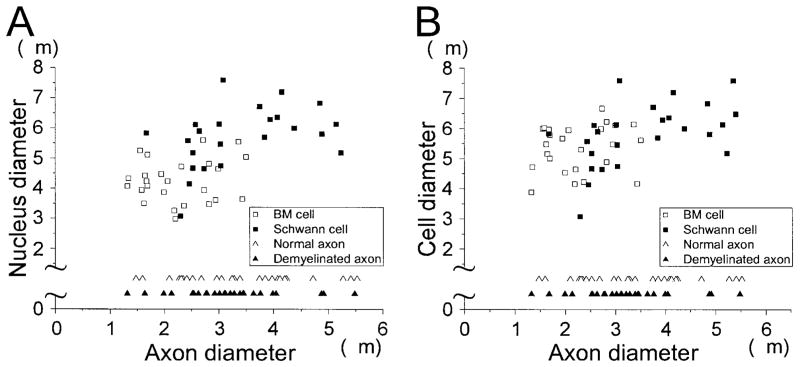
As noted above, there are several similarities between BM- and SC-induced remyelination; however, several differences were also observed. First, although the density of cell bodies associated with myelinated axons is similar after BM and SC transplantation (BM: 7,300 ± 494.3 cells/mm2, n = 5; SC: 7,360 ± 1156.5 cells/mm2, n = 5), the density of myelinated axons is less for BM transplantation (BM: 23,950 ± 2,196.7 axons/mm2, n = 5; SC: 58,400 ± 2616.9 axons/mm2, n = 5). This compares with 50,960 ± 1330.3 (n = 5) axons/mm2 for normal dorsal columns. Second, the number of myelin-forming cells that have multilobular nuclei is larger in the BM transplantation group (Fig. 4B); 13% of myelin-forming cells (950 ± 150.0 cells/mm2, n = 5) showed multilobular nuclei in the BM transplantation group, as compared with 3.3% (240 ± 98.0 cells/mm2, n = 5) in the SC transplantation group. Third, cells associated with two or three myelin segments (Fig. 4C) were present in the BM transplantation group (2.7%: 200 ± 75.6 cells/mm2, n = 5), but none were observed in the SC transplantation group. Fourth, the distribution of cell body and nucleus sizes is similar in both groups, but axonal diameter is smaller in the BM transplantation group (Fig. 5A,B). Finally, there is some neuronal and glial differentiation in the EB-X lesions after BM transplantation, but not after SC transplantation: 5% of cells in the EB-X lesions showed NSE immunoreactivity, and 3.9% showed GFAP immunoreactivity, indicating that at least some of the bone marrow cells differentiated into neuronal-like and glial-like cells, respectively, in vivo (data not shown).
Fig. 5.
Comparison of axon diameter between bone marrow (BM) transplant group and Schwann cell (SC) transplant group. Nucleus (A) and cell body (B) sizes are plotted versus axon diameters.
DISCUSSION
Bone marrow stromal cells were recently reported to differentiate into neurons and astrocytes in cell culture (Woodbury et al., 2000) and into astrocytes when transplanted into normal (Azizi et al., 1998) and ischemic (Eglitis et al., 1999) brain. Intravenous injection of bone marrow cells into mice has also been shown to lead to neuronal differentiation in brain (Brazelton et al., 2000; Mezey et al., 2000). These investigators have suggested that BM stromal cells could, in principle, be harvested from a patient and be used for potential cell therapy approaches in neurological disease. The emphasis in these studies has been on neuronal and astrocyte differentiation. However, it is well documented that in addition to committed myelin-forming cells such as oligodendrocytes (Utzschneider et al., 1994) and Schwann cells (Blakemore and Crang, 1985; Honmou et al., 1996), less committed embryonic stem cells (Brustle et al., 1999) and adult neural precursor cells (Akiyama et al., 2001) can differentiate into myelin-forming cells when transplanted into the demyelinated CNS. In the present study, we found that transplantation of an acutely isolated fraction of BM cells into the demyelinated rat spinal cord results in relatively extensive remyelination, demonstrating that BM cells can differentiate into a myelinating phenotype in vivo and repair demyelinated CNS.
Several lines of evidence indicate that the remyelination was from the transplanted BM cells, and not from endogenous host cells. The lesion model includes focal x-irradiation to block mitosis and ethidium bromide injection to kill all cells in the lesion zone. Indeed, no endogenous remyelination has been reported in this model system for 6–8 weeks (Blakemore and Crang, 1985; Honmou et al., 1996). However, the possible recruitment of cells outside of the lesion zone from the injection procedure (vehicle injection) cannot be overlooked. Sham injections of CD34+ hematopoietic stem cells did not result in remyelination, although some of the cells survived near the injection site. Moreover, when we did not immunosuppress with Cyclosporine A, no myelination was observed in the BM transplant cohort. Perhaps more importantly, an intense blue β-galactosidase reaction product was observed in the lesion after transplantation of the transgenic LacZ BM cells.
Recent studies have shown that neural precursor cells prepared from the CNS can remyelinate CNS axons upon grafting with an anatomical pattern of remyelination similar to Schwann cells, i.e., large cytoplasmic and nuclear regions surrounding the remyelinated axons and a basement membrane (Akiyama et al., 2001; Keirstead et al., 1999). Keirstead et al. (1999) report both oligodendrocyte-like and Schwann cell-like remyelination after transplantation of neural precursor cells derived from neonatal rodent brain. Many of the myelinated axons induced by transplantation of bone marrow cells in the present study are characteristic of peripheral myelin. We have not as yet fully examined the tissue for oligodendrocyte-like myelin, but some myelin profiles are suggestive of oligodendrocyte myelination.
Although the peripheral-like myelinating cells have many similarities to Schwann cell myelination there are a number of noted differences. In particular, the BM-induced remyelinating cells show larger and multilobular nuclei and larger cytoplasmic regions. In addition, while no Schwann cells were observed to engage more than one axon, some of the BM cells (2.7%) were associated with 2–3 adjacent axons. It is unclear whether this multiple engagement of axons is with functional axons or whether some of the members of the axonal set are actually degenerating or otherwise abnormal. However, this multiple axon pattern is different for the SC- and BM-transplanted groups. It is interesting that neural precursor cells and olfactory ensheathing cells (Akiyama et al., 2001; Kato et al., 2000) transplanted into the EB-X lesion model show peripheral-like myelin and some of the features such as multilobular nuclei and multiple axonal myelination described in the present report for BM cells. Another difference was that the BM transplants had a lower density of myelinated axons than in the SC transplantation group, but similar number of cell bodies associated with myelin were observed in both groups. One possibility is that internodes are shorter in the BM cohort and that the large nuclei in the myelin-forming BM cells increases axonal spacing.
The BM cells that we injected into the spinal cord were prepared acutely and were not maintained in culture. The cells were isolated by centrifugation through a density gradient (Ficoll-Paque: “lymphocytic” layer) to remove erythrocytes, platelets and debris. The recovered “lymphocytic” layer contained stromal cells, hematopoietic and nonhematopoietic stem and precursor cells, and lymphocytes (Azizi et al., 1998). We found that grafting this crude cellular BM fraction was sufficient to elicit relatively extensive remyelination. Although we suspect that the stromal cells are important, as is the case for neuronal differentiation from BM, we are uncertain as to which of the numerous cell types within the BM cell fraction is responsible for the in vivo differentiation of myelin-forming cells. However, transplantation of CD34+ cells did not result in myelination, suggesting that hematopoietic stem cells are not a candidate cell. The vast majority of cell bodies and nuclei present within the lesion zone were associated with myelin-forming cells, suggesting that this was the predominant cell type to differentiate in our lesion. Although we are uncertain as to the precise cell type from BM that develops a myelinating phenotype, it is clear that this simple crude BM cell fraction is sufficient to achieve myelin repair in our lesion model.
Another unresolved question is why such a large proportion of cells differentiated in vivo into myelin-forming cells in our study, while others report largely neuronal and astrocytic differentiation with BM stromal cell transplantation in normal or ischemic brain (Eglitis et al., 1999). It is well established that neural precursor cells can differentiate in vivo into different cell types depending on the local host environment (Akiyama et al., 2001; Brustle et al., 1999; Hammang et al., 1997; Lundberg and Bjorklund, 1996; Snyder et al., 1997). Much work suggests that the extracellular environment into which neural precursor cells are transplanted has an important influence on their fate in vivo (Akiyama et al., 2001; Brustle et al., 1999; Hammang et al., 1997; Lundberg and Bjorklund, 1996; Snyder et al., 1997). The lesion into which the cells were transplanted in the present study was enriched in axons because virtually all glia, including astrocytes and oligodendrocytes, were killed by the lesion protocol (Blakemore and Crang, 1985; Honmou et al., 1996). This abundance of axon membrane may be an important local environmental signal for myelin-forming cell differentiation. Additionally, the lesion has areas of partitioned myelin debris and macrophages not observed in normal CNS. The possibility of interactions between macrophages, or released cytokines, and the transplanted cells should be considered. Also, very immature pluripotent stem cells may be present in the crude fraction of bone marrow that could give rise to myelin-forming cells. Future studies using more specific cell separation and identification techniques of the BM cell fraction will be helpful in addressing this issue.
Although a number of groups have demonstrated the potential of various cell components of bone marrow to differentiate into neurons and glia (Azizi et al., 1998; Brazelton et al., 2000; Eglitis et al., 1999; Mezey et al., 2000; Prockop, 1997; Woodbury et al., 2000), we have shown in the present study that BM cells devoid of debris, erythrocytes and platelets can repair demyelinated lesion when transplanted in vivo. The cells are relatively easy to isolate from small aspirates of bone marrow that can be obtained under local anesthesia, and the isolation of the BM cell fraction to be used for transplantation can be achieved within minutes after BM collection. Thus, these cells have the potential to provide an efficient and renewable source of cells for autologous transplantation studies for demyelinating diseases.
Acknowledgments
Grant sponsor: Japanese Science and Technology Agency; Grant number: 12207; Grant sponsor: Japanese Ministry of Education, Science, Sports and Culture; Grant number: 09671434; Grant number: 11770765; Grant number: 12470295; Grant sponsor: Japanese Ministry of Health and Welfare; Grant number: 11-3; Grant sponsor: Japan Heart Foundation Research; Grant sponsor: Kanae Foundation; Grant sponsor: National Multiple Sclerosis Society (USA); Grant number: RG2135; Grant sponsor: National Institutes of Health; Grant number: NS10174; Grant sponsor: Medical and Rehabilitation and Development Research Services of the Department of Veterans Affairs.
References
- Akiyama Y, Honmou O, Kato T, Uede T, Hashi K, Kocsis JD. Transplantation of clonal neural precursor cells derived from adult human brain establishes functional peripheral myelin in the rat spinal cord. Exp Neurol. 2001;167:27–39. doi: 10.1006/exnr.2000.7539. [DOI] [PubMed] [Google Scholar]
- Archer DR, Leven S, Duncan ID. Myelination by cryopreserved xenografts and allografts in the myelin-deficient rat. Exp Neurol. 1994;125:268–277. doi: 10.1006/exnr.1994.1029. [DOI] [PubMed] [Google Scholar]
- Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats—similarities to astrocyte grafts. Proc Natl Acad Sci USA. 1998;95:3908–3913. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthold C, Rydmark M. Morphology of normal peripheral axons. In: Waxman SG, Kocsis JD, Stys PK, editors. The axon: structure, function and pathophysiology. New York: Oxford University Press; 1995. pp. 13–50. [Google Scholar]
- Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999;283:534–537. doi: 10.1126/science.283.5401.534. [DOI] [PubMed] [Google Scholar]
- Blakemore WF. Remyelination of CNS axons by Schwann cells transplanted from the sciatic nerve. Nature. 1977;266:68–69. doi: 10.1038/266068a0. [DOI] [PubMed] [Google Scholar]
- Blakemore WF, Crang AJ. The use of cultured autologous Schwann cells to remyelinate areas of persistent demyelination in the central nervous system. J Neurol Sci. 1985;70:207–223. doi: 10.1016/0022-510x(85)90088-7. [DOI] [PubMed] [Google Scholar]
- Blakemore WF, Crang AJ. Extensive oligodendrocyte remyelination following injection of cultured central nervous system cells into demyelinating lesions in adult central nervous system. Dev Neurosci. 1988;10:1–11. doi: 10.1159/000111949. [DOI] [PubMed] [Google Scholar]
- Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- Brustle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD, Duncan ID, McKay RD. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999;285:754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- Chalmers-Redman RM, Priestley T, Kemp JA, Fine A. In vitro propagation and inducible differentiation of multipotential progenitor cells from human fetal brain. Neuroscience. 1997;76:1121–1128. doi: 10.1016/s0306-4522(96)00386-7. [DOI] [PubMed] [Google Scholar]
- Eglitis MA, Dawson D, Park KW, Mouradian MM. Targeting of marrow-derived astrocytes to the ischemic brain. NeuroReport. 1999;10:1289–1292. doi: 10.1097/00001756-199904260-00025. [DOI] [PubMed] [Google Scholar]
- Flax JD, Aurora S, Yang C, Simonin C, Wills AM, Billinghurst LL, Jendoubi M, Sidman RL, Wolfe JH, Kim SU, Snyder EY. Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nature Biotechnol. 1998;16:1033–1039. doi: 10.1038/3473. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Gilson JM, Franceschini IA, Barnett SC. Schwann cell-like myelination following transplantation of an olfactory bulb-ensheathing cell line into areas of demyelination in the adult CNS. Glia. 1996;17:217–224. doi: 10.1002/(SICI)1098-1136(199607)17:3<217::AID-GLIA4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Gage FH, Coates PW, Palmer TD, Kuhn HG, Fisher LJ, Suhonen JO, Peterson DA, Suhr ST, Ray J. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci USA. 1995;92:11879–11883. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammang JP, Archer DR, Duncan ID. Myelination following transplantation of EGF-responsive neural stem cells into a myelin-deficient environment. Exp Neurol. 1997;147:84–95. doi: 10.1006/exnr.1997.6592. [DOI] [PubMed] [Google Scholar]
- Honmou O, Felts PA, Waxman SG, Kocsis JD. Restoration of normal conduction properties in demyelinated spinal cord axons in the adult rat by transplantation of exogenous Schwann cells. J Neurosci. 1996;16:3199–3208. doi: 10.1523/JNEUROSCI.16-10-03199.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T, Lankford KL, Waxman SG, Greer CA, Kocsis JD. Transplanted olfactory ensheathing cells remyelinate and enhance axonal conduction in the demyelinated dorsal columns of the rat spinal cord. J Neurosci. 1998;18:6176–6185. doi: 10.1523/JNEUROSCI.18-16-06176.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Honmou O, Uede T, Hashi K, Kocsis JD. Transplantation of human olfactory ensheathing cells elicits remyelination of demyelinated rat spinal cord. Glia. 2000;30:209–218. doi: 10.1002/(sici)1098-1136(200005)30:3<209::aid-glia1>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Ben-Hur T, Rogister B, O’Leary MT, Dubois-Dalcq M, Blakemore WF. Polysialylated neural cell adhesion molecule-positive CNS precursors generate both oligodendrocytes and Schwann cells to remyelinate the CNS after transplantation. J Neurosci. 1999;19:7529–7536. doi: 10.1523/JNEUROSCI.19-17-07529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci USA. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg C, Bjorklund A. Host regulation of glial markers in intrastriatal grafts of conditionally immortalized neural stem cell lines. NeuroReport. 1996;7:847–852. doi: 10.1097/00001756-199603220-00002. [DOI] [PubMed] [Google Scholar]
- Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Moyer MP, Johnson RA, Zompa EA, Cain L, Morshed T, Hulsebosch CE. Culture, expansion, and transplantation of human fetal neural progenitor cells. Transplant Proc. 1997;29:2040–2041. doi: 10.1016/s0041-1345(97)00221-2. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Snyder EY, Yoon CH, Flax JD, Macklis JD. Multipotent neural progenitors can differentiate toward replacement of neurons undergoing targeted apoptotic degeneration in adult mouse neocortex. Proc Natl Acad Sci USA. 1997;94:11663–11668. doi: 10.1073/pnas.94.21.11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen CN, Caldwell MA, Shen J, ter Borg MG, Rosser AE, Tyers P, Karmiol S, Dunnett SB. Long-term survival of human central nervous system progenitor cells transplanted into a rat model of Parkinson’s disease. Exp Neurol. 1997;148:135–146. doi: 10.1006/exnr.1997.6634. [DOI] [PubMed] [Google Scholar]
- Utzschneider DA, Archer DR, Kocsis JD, Waxman SG, Duncan ID. Transplantation of glial cells enhances action potential conduction of amyelinated spinal cord axons in the myelin-deficient rat. Proc Natl Acad Sci USA. 1994;91:53–57. doi: 10.1073/pnas.91.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Yandava BD, Billinghurst LL, Snyder EY. “Global” cell replacement is feasible via neural stem cell transplantation: evidence from the dysmyelinated shiverer mouse brain. Proc Natl Acad Sci USA. 1999;96:7029–7034. doi: 10.1073/pnas.96.12.7029. [DOI] [PMC free article] [PubMed] [Google Scholar]