Abstract
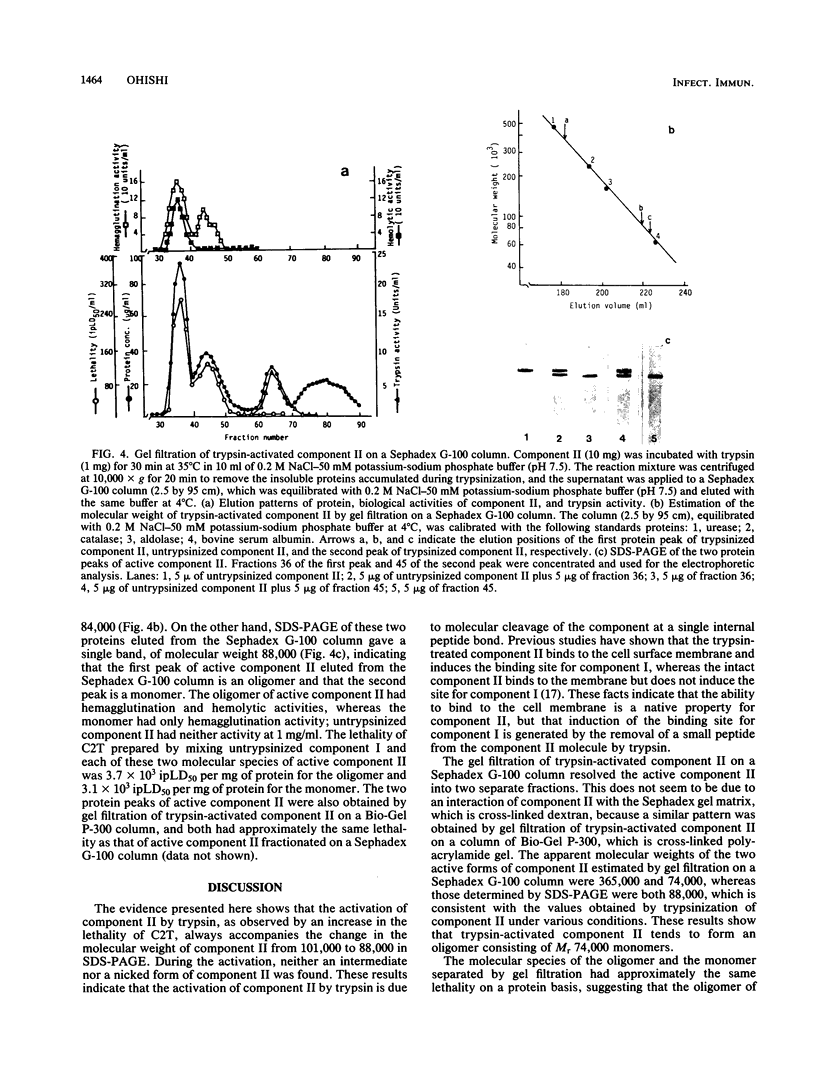
C2 toxin (C2T) elaborated by Clostridium botulinum types C and D is composed of two dissimilar protein components, designated components I and II. The biological activity of C2T is enhanced by treating the toxin with trypsin. This activation of C2T is observed as a result of mixing untrypsinized component I and trypsinized component II but not as a result of mixing trypsinized component I and untrypsinized component II. The data presented here show that the maximum lethality of C2T, determined by mixing untrypsinized component I and trypsinized component II, was attained by treating component II with trypsin at a ratio of 10:1 on a protein basis for 30 min at 35 degrees C at pH 7.5. The activation of component II was always accompanied by a change in the molecular weight of the component from 101,000 to 88,000 as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). However, the gel filtration of trypsinized component II resulted in the separation of two active components, with apparent molecular weights, estimated from the elution volume by gel filtration, of 365,000 and 74,000. The high-molecular-weight component II had hemagglutination and hemolytic activities, whereas the low-molecular-weight component II has only hemagglutination activity. These two molecular species of active component II had approximately the same lethality, when mixed with component I, and gave a single band in SDS-PAGE, with a molecular weight of 88,000, the same as that of trypsin-activated component II under different reaction conditions. The results indicate that the activation of C2T by trypsin is due to the molecular conversion of component II from molecular weight 101,000 to 88,000 as determined by SDS-PAGE and that the trypsin-activated component II tends to form an oligomer of the active component II.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aktories K., Bärmann M., Ohishi I., Tsuyama S., Jakobs K. H., Habermann E. Botulinum C2 toxin ADP-ribosylates actin. Nature. 1986 Jul 24;322(6077):390–392. doi: 10.1038/322390a0. [DOI] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baenziger J. U., Fiete D. Structural determinants of Ricinus communis agglutinin and toxin specificity for oligosaccharides. J Biol Chem. 1979 Oct 10;254(19):9795–9799. [PubMed] [Google Scholar]
- Draper R. K., Chin D., Simon M. I. Diphtheria toxin has the properties of a lectin. Proc Natl Acad Sci U S A. 1978 Jan;75(1):261–265. doi: 10.1073/pnas.75.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERLANGER B. F., KOKOWSKY N., COHEN W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys. 1961 Nov;95:271–278. doi: 10.1016/0003-9861(61)90145-x. [DOI] [PubMed] [Google Scholar]
- Gill D. M., King C. A. The mechanism of action of cholera toxin in pigeon erythrocyte lysates. J Biol Chem. 1975 Aug 25;250(16):6424–6432. [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Svennerholm L. Tissue receptor for cholera exotoxin: postulated structure from studies with GM1 ganglioside and related glycolipids. Infect Immun. 1973 Aug;8(2):208–214. doi: 10.1128/iai.8.2.208-214.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J. The interaction of Ricinus communis agglutinin with normal and tumor cell surfaces. Biochim Biophys Acta. 1972 May 9;266(2):543–547. doi: 10.1016/0005-2736(72)90109-5. [DOI] [PubMed] [Google Scholar]
- Ohishi I., Iwasaki M., Sakaguchi G. Purification and characterization of two components of botulinum C2 toxin. Infect Immun. 1980 Dec;30(3):668–673. doi: 10.1128/iai.30.3.668-673.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi I., Iwasaki M., Sakaguchi G. Vascular permeability activity of botulinum C2 toxin elicited by cooperation of two dissimilar protein components. Infect Immun. 1981 Mar;31(3):890–895. doi: 10.1128/iai.31.3.890-895.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi I. Lethal and vascular permeability activities of botulinum C2 toxin induced by separate injections of the two toxin components. Infect Immun. 1983 Apr;40(1):336–339. doi: 10.1128/iai.40.1.336-339.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi I., Miyake M. Binding of the two components of C2 toxin to epithelial cells and brush borders of mouse intestine. Infect Immun. 1985 Jun;48(3):769–775. doi: 10.1128/iai.48.3.769-775.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi I. NAD-glycohydrolase activity of botulinum C2 toxin: a possible role of component I in the mode of action of the toxin. J Biochem. 1986 Aug;100(2):407–413. doi: 10.1093/oxfordjournals.jbchem.a121728. [DOI] [PubMed] [Google Scholar]
- Ohishi I. Response of mouse intestinal loop to botulinum C2 toxin: enterotoxic activity induced by cooperation of nonlinked protein components. Infect Immun. 1983 May;40(2):691–695. doi: 10.1128/iai.40.2.691-695.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi I., Tsuyama S. ADP-ribosylation of nonmuscle actin with component I of C2 toxin. Biochem Biophys Res Commun. 1986 Apr 29;136(2):802–806. doi: 10.1016/0006-291x(86)90511-5. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Pihl A. Isolation and properties of abrin: a toxic protein inhibiting protein synthesis. Evidence for different biological functions of its two constituent-peptide chains. Eur J Biochem. 1973 May;35(1):179–185. doi: 10.1111/j.1432-1033.1973.tb02823.x. [DOI] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr, Gill D. M. Diphtheria. Science. 1973 Oct 26;182(4110):353–358. doi: 10.1126/science.182.4110.353. [DOI] [PubMed] [Google Scholar]
- Richards R. L., Moss J., Alving C. R., Fishman P. H., Brady R. O. Choleragen (cholera toxin): a bacterial lectin. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1673–1676. doi: 10.1073/pnas.76.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M., Nogimori K., Yajima M., Ase K., Ui M. A role of the B-oligomer moiety of islet-activating protein, pertussis toxin, in development of the biological effects on intact cells. J Biol Chem. 1983 Jun 10;258(11):6756–6761. [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]