Abstract
Outward K+ currents were recorded using a whole cell patch-clamp configuration, from acutely dissociated adult rat cutaneous afferent dorsal root ganglion (DRG) neurons (L4 and L5) identified by retrograde labeling with Fluoro-gold. Recordings were obtained 16–24 h after dissociation from cells between 39 and 49 mm in diameter with minimal processes. These cells represent medium-sized DRG neurons relative to the entire population, but are large cutaneous afferent neurons giving rise to myelinated axons. Voltage-activated K+ currents were recorded routinely during 300-ms depolarizing test pulses increasing in 10-mV steps from −40 to +50 mV; the currents were preceded by a 500-ms conditioning prepulse of either −120 or −40 mV. Coexpression of at least three components of K+ current was revealed. Separation of these components was achieved on the basis of sensitivities to the K+ channel blockers, 4-aminopyridine (4-AP) and dendrotoxin (DTx), and by the current responses to variation in conditioning voltage. Changing extracellular K+ concentration from 3 to 40 mM resulted in a shift to the right of the I–V curve commensurate with K+ being the principal charge carrier. Presentation of 100 mM 4-AP revealed a rapidly activating K+ current sensitive to low concentrations of 4-AP. High concentrations of 4-AP (6 mM) extinguished all inactivating current, leaving almost pure sustained current (IK). On the basis of the relative distribution of K+ currents neurons could be separated into three distinct categories: fast inactivating current (IA), slow inactivating current (ID), and sustained current (IK); only IA and IK; and slow inactivating current and IK. However, IK was always the dominant outward current component. These results indicate that considerable variation in K+ currents is present not only in the entire population of DRG neurons, as previously reported, but even within a restricted size and functional group (large cutaneous afferent neurons).
INTRODUCTION
Dorsal root ganglion (DRG) neurons are the primary neurons for somatic and visceral afferentation. There has been much interest in the electrophysiological and pharmacological properties of these neurons both because they better our understanding of normal sensory physiology and because after nerve injury these neurons often display aberrant firing properties that result in abnormal sensations (Burchiel 1984; Desantis and Duckworth 1982; Devor and Wall 1990; Kajander et al. 1992; Wall and Devor 1983). DRG neurons are heterogeneous in size and in electrophysiological properties (Harper and Lawson 1985a,b). Although cell size is an approximation of functional class of DRG neurons, size alone is not an absolute predictor of functional type of neuron. This presents a difficulty in biophysical studies where the neurons are acutely dissociated for in vitro patch-clamp study because they are disconnected from their targets and cannot be assessed by functional tests. Moreover, there is considerable overlap in the size of DRG neurons between different functional classes. For example, medium-sized DRG neurons, which give rise to myelinated axons, can innervate either skin (cutaneous afferents) or muscle structures such as Golgi tendon organs or muscle spindles. Work in our laboratory has used in vivo retrograde marking techniques to identify muscle and cutaneous afferents after cell dissociation. These studies indicate striking differences in action potential waveform, Na+ channel organization and γ-aminobutyric acid-A (GABAA) receptor properties between relatively large cutaneous and muscle afferent neurons (Honmou et al. 1994; Oyelese and Kocsis 1996; Oyelese et al. 1995, 1997).
The importance of different K+ current components in neuronal function is widely recognized (Albert and Nerbonne 1995; Ficker and Heinemann 1992; Foehring and Surmeier 1993; Storm 1988, 1990; McFarlane and Cooper 1991; Wu and Barish 1992). For example, fast transient K+ current, commonly called A current, has been shown to be instrumental in transducing graded stimulating currents into graded firing rates and was first recognized and analyzed in large molluscan ganglion cells (Connor and Stevens 1971a,b; Hagiwara et al. 1961; Neher 1971). Since these initial studies, transient currents have been described in numerous other types of neurons and excitable cells (see Pallotta and Wagoner 1992; Rogawski 1985; Rudy 1988; Storm 1988). Recently, Gold et al. (1996) characterized a number of currents previously unidentified in adult rat DRG neurons. The characterization of these currents was generally from small, nociceptive type neurons, a different class than the larger mechanoreceptor type examined in this study. These large cutaneous neurons express three distinct classes of current: dominant sustained (K current; IK), fast inactivating (A current; IA), and slow inactivating (D current; ID). These classifications are more similar to McFarlane and Cooper’s (1991) in neonatal rat sensory neurons. D currents have been reported to differ from A currents in so far as they have slower inactivation rates, steady-state properties at different voltages, and enhanced sensitivity to 4-aminopyridine (4-AP), dendrotoxin (DTx), and mast cell degranulating peptide (For reviews, see Castle et al. 1989; Dolly 1988; Moczydlowski et al. 1988; Strong 1990). It is not clear however, whether D current is, in effect, a residue of a number of currents other than sustained current (Foehring and Surmeier 1993).
Previous work suggests a predominance of sustained current with a paucity of outward transient currents in adult rat DRG neurons (Robertson and Taylor 1986). Gold et al. (1996) indicated that within the population of DRG neurons they examined there are possibly six kinetically and pharmacologically distinct K+ channel types in dissociated adult DRG neurons. However, a detailed characterization of potassium currents in identified subpopulations of adult DRG neurons has yet to be made. In the present study, we recorded from a subclass of identified cutaneous afferent DRG neurons that give rise to myelinated axons, which likely include Aβ fibers, which are functionally involved in tactile sensation of the skin. We demonstrate that these neurons have at least two types of inactivating current and a dominant sustained current, which is manifest in all cells examined. The neurons could be categorized into three groups based on the relative distribution of their currents. Thus these results indicate a diversity of K+ currents in a restricted class of neurons innervating skin that are likely to be functionally related to tactile sensation.
METHODS
Cell identification and culture techniques
Cutaneous afferent DRG neurons were identified using retrograde Fluoro-gold labeling. Fluoro-gold solution (2–4%), in distilled water, was injected under the skin of the rat’s foot (Honmou et al. 1994; Schmued and Fallon 1986). An attempt was made to limit labeling to cutaneous afferents [injections into the lateral plantar region have been shown to expose cutaneous afferents, i.e., small- and medium-sized cells, with distinctly different kinetics to those of muscle afferents (Oyelese and Kocsis 1996; Oyelese et al. 1995)]. Three weeks postinjection the adult Wistar rats (180–240 g) were exsanguinated under pentobarbital anesthesia (60 mg/kg ip), and the lumbar DRG (L4, L5) were excised and prepared for dissociation and culture (see Caffrey et al. 1992; Honmou et al. 1994). Results reported in this study are from 103 identified adult cutaneous afferent DRG neurons. Our analysis was limited to relatively large (39–49 mm diam) cutaneous afferents, which correspond to medium-sized neurons of the entire DRG neuronal population. These neurons give rise to myelinated axons and correspond to skin mechanoreceptors, but we could not distinguish between high- and low-threshold mechanoreceptors. We did not study smaller diameter nociceptive neurons in this study.
Electrophysiological techniques and analysis
The neurons were studied 16–22 h after plating in an attempt to avoid variations in expressed types and amounts of current as a function of time and to circumvent space clamp problems; neurite outgrowth was minimal at time of testing. Coverslips plated with the DRG neurons were rinsed with normal bath solution (see Table 1, E2), placed in a recording chamber on the stage of an inverted phase-contrast microscope (Nikon Diaphot), and perfused with solution. Recording electrodes were fabricated from thin-walled, single-filamented, borosilicate glass tubing (Warner Instrument) with a micropipette puller (Model P-97, Sutter Instruments) and fire-polished with the use of a Narashige MI 83 to a resistance of 1–2 MΩ seal resistances were ≥1 GΩ. All recordings were made at room temperature (21 ± 10°C).
TABLE 1.
Solutions
| Extracellular
|
|||
|---|---|---|---|
| Intracellular (I) | E1 | E2 | |
| TMA Cl, mMa | 100 | 100 | 140 |
| KCl, mMb | 40 | 40 | 3 |
| CaCl2, mMb | 0 | 1 | 1 |
| MgCl2, mMb | 1 | 1 | 1 |
| CdCl2, μMb | 0 | 100 | 100 |
| TTX, μMc | 0 | 1 | 1 |
| HEPES, mMd | 0 | 10 | 10 |
| EGTA, mMb | 10 | 0 | 0 |
| pH | 7.2 | 7.4 | 7.4 |
Ethylene glycol-bis (β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) and N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) buffers were prepared with the hydroxide of the main cation; cation concentrations given in the table include contributions from the added hydroxide. For some experiments, 10 and 100 μM and 1, 3, and 6 mM 4-aminopyridineb; 10 and 100 μM, and 1, 10 and 20 mM tetraehtylammonium chlorideb; and 1 or 2 μM DTxe, were added to the extracellular solution, E2.
TMA, tetramethylammonium Aldrich Chemical, Milwaukee, WI.
Sigma Chemical, St. Louis, MO.
Calbiochem, La Jolla, CA.
American Bioanalytical, Natick, MA.
RBI Research Biochemicals, Natick, MA.
Voltage-clamp recordings were made in the whole cell patch-clamp configuration (Hamill et al. 1981) using an EPC 9 amplifier driven by the Pulse program (Heka-Electronik). The neurons were voltage-clamped at −80 mV for all experimental manipulations. Capacity and leakage subtraction were performed using a P/± 6 subtraction protocol. Series resistance compensation of ≥45% was employed routinely to minimize any voltage error. Cells in which series resistance compensation was possible but <45% were discounted from the study. The current was sampled at 50 kHz, low-pass filtered (four pole Bessel) at 10 kHz unless otherwise stated and initially digitized and stored on computer (Macintosh, Quadra 700).
Data were analyzed using Heka analysis programs (Adams and List) and IgorPro (WaveMetrics). All analysis was performed on recordings from neurons stable for ≥10 min to facilitate diffusion of the electrode solution into the neuron, thus, ensuring more stable and uniform responses. Where necessary, currents were zero-subtracted (DC leak current based on the data points corresponding to the first stored segment are subtracted from 0). Inactivation time constants were estimated from exponential fits via the Heka program for Macintosh, which uses the root mean square (RMS) deviation between fit and data. The RMS value is used as residual for the simplex fit algorithm (see: Heka Patch Clamp EPC9, Pulse Fit Manual 8.0, 1997).
Solutions
Normal bath solution contained (in mM) 140 tetramethylammonium (TMA), 3 KCl, 10 N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 1 CaCl, and 1 MgCl (see Table 1). Tetrodotoxin (TTX, 1 μM) and CdCl (100 μM) were added to block Na+ and Ca2+ currents, respectively. Recordings could be obtained for ≤2 h in this solution. Osmolarity of the perfusion solution was adjusted to 305–310 mosmol with glucose, and pH was adjusted to 7.4 using TMA OH. In an attempt to reduce errors caused by series resistance artifacts, TMA was used as the primary nonpermeant monovalent cation, thus reducing current amplitude. Electrodes were filled with (in mM) 100 TMA, 40 KCl, 10 ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, and 1 MgCl; osmolarity was adjusted to 300–305 mosmol with glucose, and the pH was adjusted to 7.4 using TMA OH. Preliminary studies showed no detectable effect on outward currents of either 100 μM (n = 6), or 200 μM (n = 6), CdCl.
The recording chamber (volume 1.0 ml) was perfused continuously at 0.75–1.25 ml/min. Application of the drugs was by changing the chamber perfusate via a four-way delivery tube attached to a single vent in the chamber. Preliminary experiments perfusing tetraethylammonium (TEA; Sigma) at 40, 20, and 10 mM were performed, but results were nonspecific, i.e., these concentrations reduced all K+ components. Lower concentrations of TEA, 10 and 100 μM, also were examined but were of limited success in making clear the different components of outward current. However, in a few examples, 100 μM TEA did expose fast and sustained portions of K current (n = 5; not shown). An effective concentration was established for 4-AP (applied in the perfusion solution) by sequentially presenting 1, 10, 50, 100, and 200 μM and 1 mM. DTx, from the African green mamba Dendroaspis angusticeps (Research Biochemicals International) was presented at 1 and 2 μM. Initial pulse protocols gave some indication of the compliment of currents that may have been manifest in the cell being examined. This determined what type of pharmacological protocol followed when attempting to dissect out the various types of current. Presentation of the relevant blockers generally could determine if a particular current, or currents, were absent in any one cell.
RESULTS
Depolarization-activated K+ currents
K+ current was recorded selectively by replacing Na+ ions with a nonpermeant ion and by using blockers of Na+ and Ca2+ current, TTX and CdCl, respectively. In initial studies using different concentrations of K+ in the external solution, the large amplitude of this current made identification of the other components difficult. As the concentration of K+ was replaced systematically by an impermeant cation, peak currents were reduced to workable levels and the other components of the potassium current could be identified ore readily. However, IK always remained the largest portion of current and was present in all recordings. From a holding potential of −80 mV, isolated outward currents were elicited by stepping to a conditioning voltage of either −40 or −120 mV and then depolarizing the membrane to −40 mV and on up to +50 mV, in 10-mV increments (see Fig. 2). Activation of these currents was rapid and decay only partial during a 300-ms depolarization pulse. Absolute current amplitudes and rates of rise of the currents increased with increasing depolarization; +50 mV produced the largest and most rapidly rising current in each recording. With symmetrical K+ concentrations (40 mM inside and outside), the I–V curve shifted to the right, and the K+ equilibrium potential was close to the predicted reversal potential of 0 mV. This is commensurate with K+ being the principal charge carrier (Fig. 1). Fast transient (IA) components began to be activated from stimulations of less than −40 mV. IA activated two or three times faster than the slow component in all cells tested. Inactivation of IA was fast, proceeding with a time constant of 36 ± 5 ms (mean ± SD) at 0 mV test potential, decreasing to 5 ± 5 ms at +50 mV (n = 9); the rates of inactivation of the slow component were 250 ± 10 ms at 0 mV and 45 ± 8 ms at +50 mV (n = 9). A single exponential fit indicated a voltage dependence of inactivation of the A-type current; however, it was clear that a double exponential fit was closer to the rate at which inactivation was taking place. This was almost certainly because of the two or more elements of current that could be present in any particular recording in this series of experiments (see Fig. 2). It also was observed that cells having the fast transient, A-type current, also had large tail currents and that those tail currents could be enlarged by increasing external K+ concentration (Fig. 1). The fact that the time course of inactivation is voltage dependent may give some indication of the class of K+ current involved here. The time course of inactivation of A current carried by various Kv 4 channels is voltage independent; whereas, in Kv 1.4 channels, it tends to be highly voltage dependent.
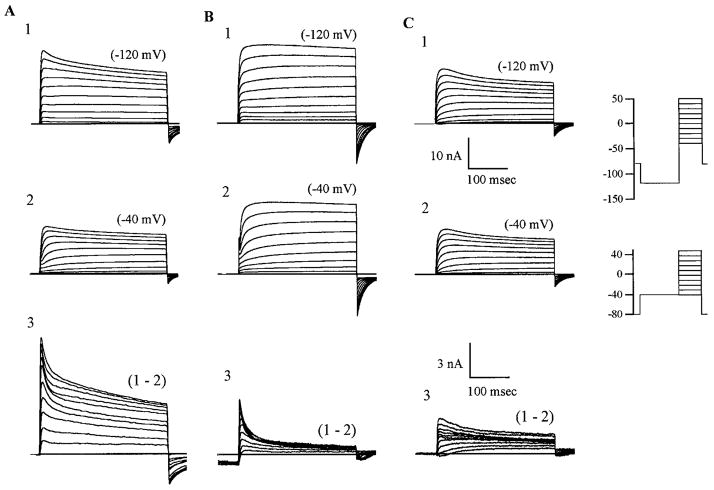
FIG. 2.
Voltage-dependent current isolation protocol reveals distinct classes of identified cutaneous DRG neurons based on the relative contribution of transient and sustained potassium currents. Recordings from 3 categories can be seen in A1, B1, and C1 recorded after the −120-mV prepulse protocol; A2, B2, and C2 were initiated via the −40-mV prepulse protocol. A: typical type of traces (of this shape and amplitude) that were recorded in the majority (~58%) of experiments. This cell type manifested ≥3 distinct components of current. B1–B2: this cell type (occurring in ~28% of recordings) has a large amount of fast inactivating component in its complement of currents. C1–C2: cell type that lacks the fast inactivating component but has a slower inactivating voltage-activated component sensitive to the conditioning potential (occurring in ~15% of recordings). Lower scale bars relate to subtractionss only (subtractions are enlarged for clarity of comparison). These are typical traces taken from the mean of each group.
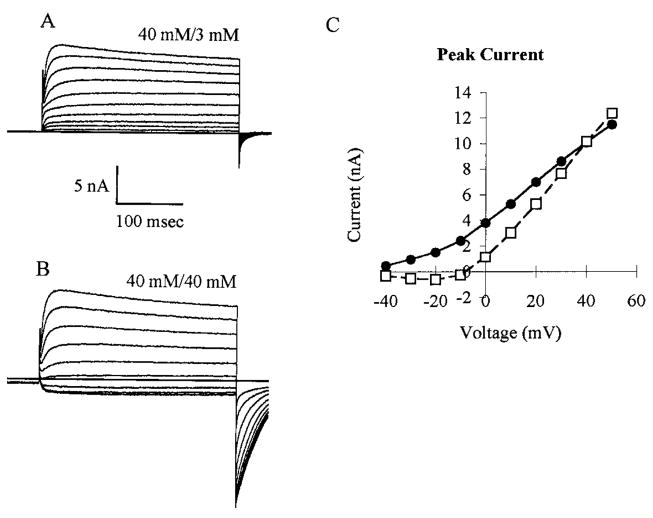
FIG. 1.
Whole cell potassium currents recorded from dorsal root ganglion (DRG) neurons showing external KCl concentration change. KCl concentration was changed from 3 mM (A and •) to 40 mM (B and □) to confirm K+ is the principal charge carrier of the current. Currents were recorded after a 300-ms depolarization pulse increasing in 10-mV steps from −40 to +50 mV; these were preceded by 500-ms prepulses of −120 mV. C: peak current-voltage curves from the records in A and B. Commensurate with K+ being the principal charge carrier, the I–V curve shifts to the right. With depolarizing steps an initial inward current is observed followed by an outward current.
Current identification
FAST, SLOW AND SUSTAINED-CURRENTS
Fast transient outward currents (A currents) traditionally have been identified in both mammalian and nonmammalian neurons using a variety of pharmacological and kinetic techniques (Thompson 1977). Criteria for their identification include rapid activation and inactivation, dependence on the holding potential, and sensitivity to both 4-AP and DTx (Wu and Barish 1992). Although in the majority of cells, K+ current components could not be completely isolated by conditioning voltage alone [similarly Albert and Nerbonne (1995) also reported a lack of complete isolation of component currents by conditioning voltage alone]. In some cases, A current could be dissected from the sustained current and K-type elements using two different prepulse voltages (Vc) with identical stimulation pulse protocols (Vp; Fig. 2), but this was dependent upon the types of component current expressed in the individual neuron.
Figure 2 shows an example of the matrix of current families recorded at voltages between −40 and +50 mV after a 500-ms conditioning prepulse to −120 mV (Vc = −120 mV) or to −40 mV (Vc = −40 mV) in the standard perfusion solution. Figure 2A1 demonstrates the predominant composition of currents occurring in ~58% of cells examined (n = 52). In this configuration of currents, subtraction of responses (1–2) demonstrated fast and slow activating and inactivating components that were sensitive to the Vc. It was expected that the sustained portion of the K+ current would be unchanged by Vc, rendering currents that were initiated by a variation in Vc evident. However, in this configuration of currents (Fig. 2 A), it appeared that a small portion of the sustained current was sensitive to Vc and therefore contaminated the subtraction. Figure 2 B shows that in some of the neurons the K+ current components, IA and IK, were clearly defined after subtraction. Subtraction C3 of figure 2 shows a clear dissection of the slower inactivating component. Cells that had IA and IK only, occurred much less often, being recorded in ~24% (n = 22) of cells examined in this study. Exposure of IA was by varying pharmacology and conditioning voltage; 24% relates to all cells exhibiting a decay time constant of or around 5 ms at +50 mV. Cells lacking IA, but showing the slower inactivating component along with IK, were seen in ~12% of the cells examined (n = 11), 12% relates to those cells having a slower inactivating current with a decay time constant on or around 45 ms at +50 mV. Figure 3 demonstrates the voltage-dependence of activation. It shows a double component of current (on a much faster activating and inactivating current) initiated by depolarizations positive to −30 mV. At depolarizations negative to −30 mV only a single exponential was needed, indicating a single slower inactivating component of current.
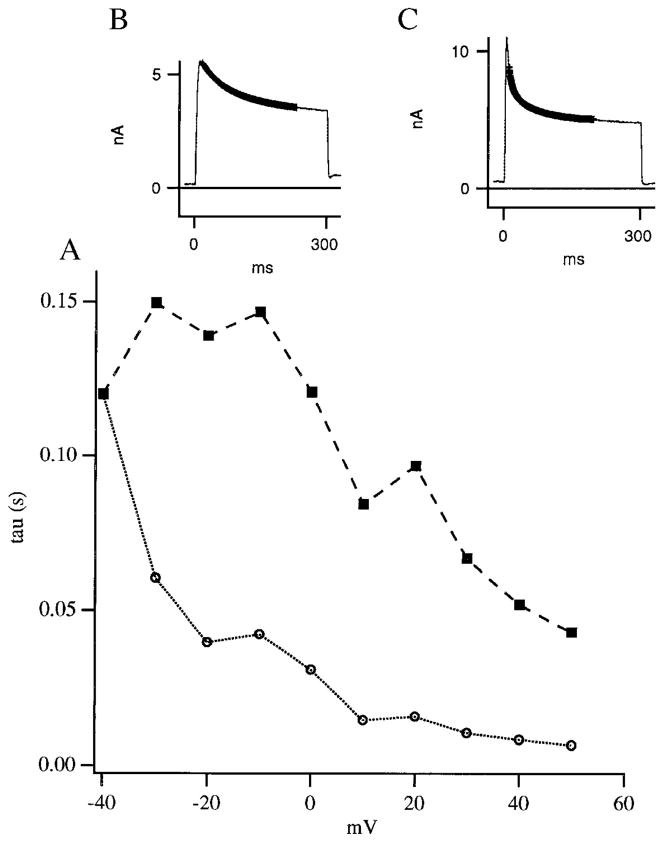
FIG. 3.
Voltage-dependence of inactivation: inactivation against test voltage using double exponential fit (A). This example is typical of cells where a substantial A current could be dissected. For depolarizations positive to −30 mV, ≥2 exponentials were required for an adequate fit of the fast inactivating current (indicating the presence of >1 element of current). ■ represents the slower component of current; ○, the faster component, in this typical recording. B and C: thicker lines (B, −20-mV depolarization; C, 40-mV depolarization) demonstrate the goodness of fit, and corresponds to the theoretical exponential fits. Scales of the y axes in B and C have been adjusted to make a clearer comparison of the difference in rate of inactivation.
Currents varied between cells and, in some cases, were easily visible but in others close to undetectable. Figure 4 shows the means at the maximum stimulation (+50 mV) for the individual A, D, and K currents in the three different types of cells (n = 5, in each group) quantifying their relative contributions in the population of cells examined, thus providing the objective basis for grouping the cells into three distinct categories.
FIG. 4.
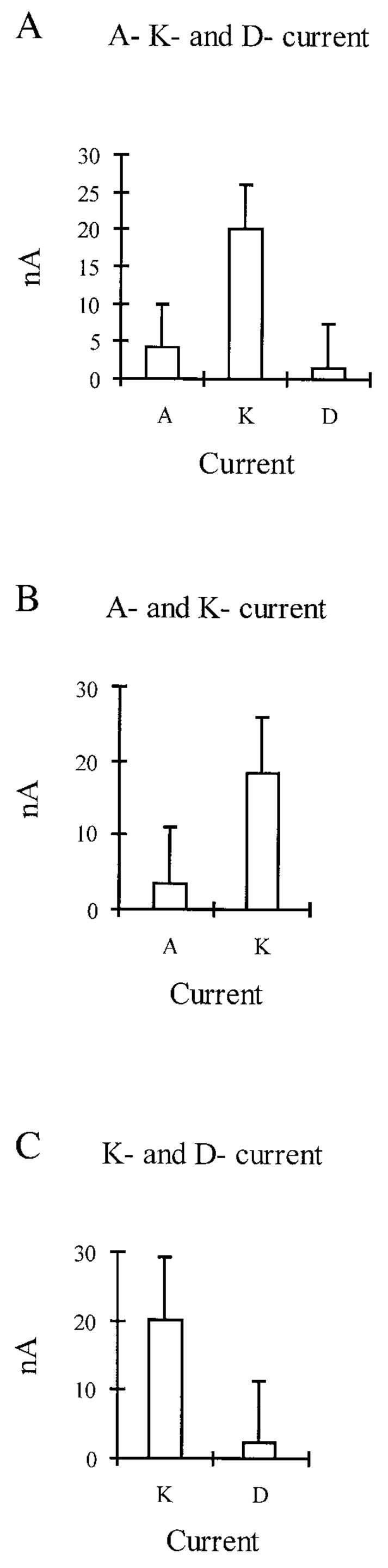
Relative contributions of the 3 different currents (at +50-mV stimulation) in the population of cells examined. Currents were expressed in different quantities in morphologically identical cells. A: typical compliment of currents in the majority of cells, where all 3 currents are manifest. B: cells expressing only A and K current. C: cells that appeared to manifest only K and D current. Values represent means of current (n = 5, most representative in each group) ± SE. Because various pharmacological protocols were used to separate the currents in differerent experiments, it was not possible to lump all cells from all experiments into the 3 groups for this type of figure.
Effects of 4-AP in high and low concentrations
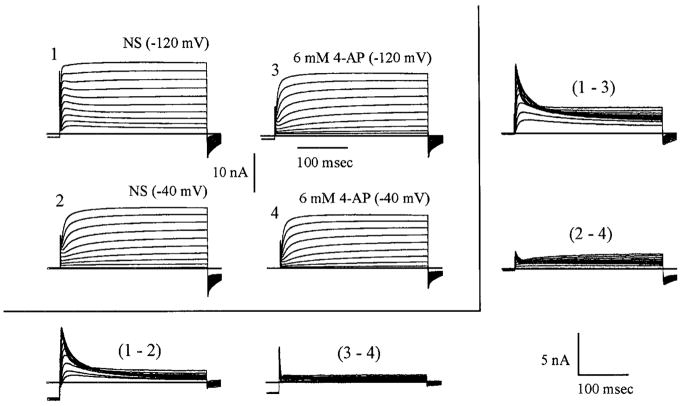
Concentrations of 4-AP as low as 100 μM have been reported to block virtually all slowly inactivating current while sparing the initial fast transient A current in mouse hippocampal cells (Wu and Barish 1992). Preliminary studies with concentrations as low as 10 μM 4-AP showed effects in some cells; 100 μM 4-AP was selected as a more effective concentration, one by which the results could be compared with studies on other cell types. In this study, 100 μM 4-AP reduced the slow inactivating component (see Fig. 5) in some cells (3 of 10), whereas in others had little or no effect. This is as would be expected if all neurons do not express the full compliment of fast, slow, and sustained currents. Fast and slowly inactivating transient currents, along with a small amount of sustained current, can be seen in Fig. 5 (1–2). After presentation of 100 μM 4-AP (3–4), which has Vc as the variable, little if any difference (peak currents remaining almost identical) was observed. It should be noted, however, that the 100 μM 4-AP appeared to be having a biphasic effect, being a more effective block after a −40-mV conditioning prepulse than with a Vc of −120 mV. Figure 5, [(1–3) and (2–4)] also reveals that inhibition of the slowly inactivating component does occur in some cells.
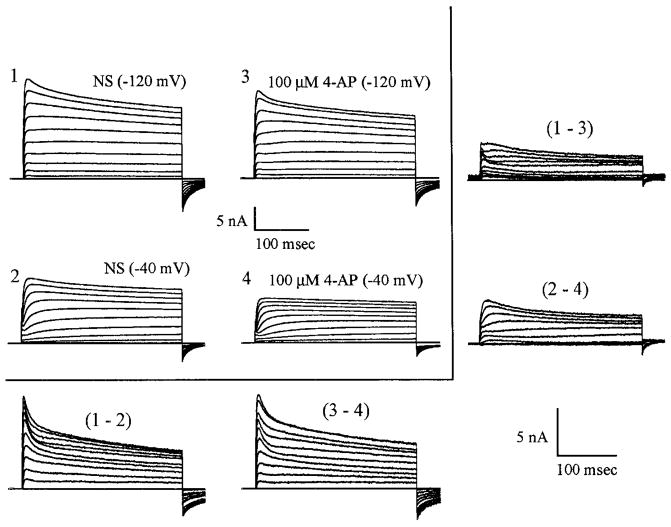
FIG. 5.
Separation of current types based on voltage activation and sensitivity to low concentrations of 4-aminopyridine (4-AP). Transient current has 2 components, a fast transient current that is voltage dependent and insensitive to 100 mM 4-AP and a slowly inactivating current that is not blocked by depolarized voltages but is blocked by 100 mM 4-AP. 1–4: matrix of total outward currents recorded during the 2 standard pulse protocols before (1 and 2) and after (3 and 4) 100 μM 4-AP. Comparison of subtractions of 1 – 2 and 3 – 4 indicates that 100 μM 4-AP leaves the fast inactivating component only partially blocked in this cell. Subtractions 1 – 3 and 2 – 4, however, do confirm that a slower inactivating component of K+ current is extinguished by the 4-AP at this concentration. Subtractions are enlarged for clarity (see scale bars).
In neurons having only fast inactivating and sustained current (as in Fig. 6), low concentrations of 4-AP had no effect because there was no slowly inactivating component for them to affect. Figure 6 shows the effect of high concentrations of 4-AP (6 mM) on the fast inactivating current in DRG neurons that expressed only IA and IK. This dose eliminated the fast inactivating current (n = 5), leaving only the sustained current in cells with this compliment of currents. This series of experiments demonstrated a relatively pure sustained current. The effects of low concentrations (100 μM) of 4-AP could be reversed almost completely in 15–20 min (n = 10), whereas, higher concentrations (6 mM) required up to 45 min (n = 3) or more.
FIG. 6.
Presentation of high concentrations of 4-AP. From this example, having only fast inactivating and sustained current, a clear picture of the pure sustained current is visible. Presentation of 6 mM 4-AP can be seen to extinguish all fast inactivating current (compare subtraction 1 – 2 with 3 – 4) isolating total IK because IK is reported as being unaffected by 4-AP. In this cell, a prepulse of −120 mV initiates voltage-activated A-type current that is shown when the −40-mV prepulse is subtracted from it (1 – 2). Subtractions 1 – 3 and 2 – 4 show which currents are removed by the high concentration of 4-AP. Small amount of inactivating current picked-up on subtraction 2 – 4 probably is caused by a slight drop in membrane potential during the 20 min taken to acquire the data. Subtracts are enlarged for clarity.
Effects of DTx
A preliminary set of experiments was performed to determine an effective concentration of DTx in these neurons. Although 500 nM was observed to affect the currents, the outcome was unpredictable; 1 μM was more consistent and 2 μM had no further discernible effect. Therefore 1 μM DTx was selected as appropriate. Foehring and Surmeier (1993) reported that DTx blocked virtually all slowly inactivating current while sparing the initial fast transient A current in rat cortical neurons.
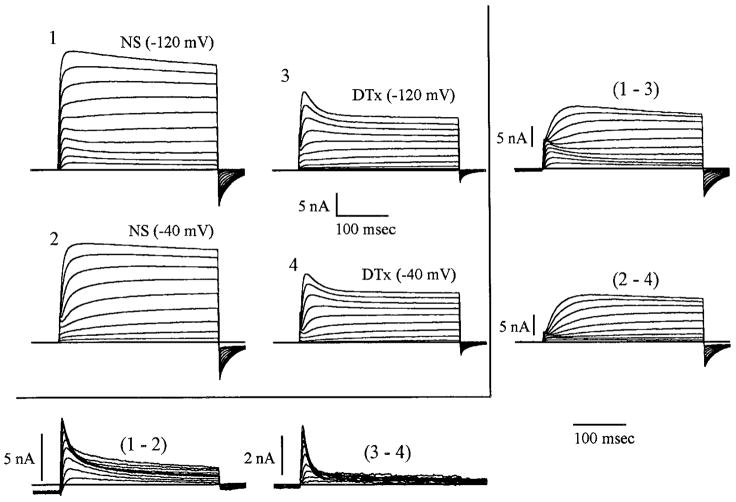
Figure 7 shows an example of the effects of 1 μM DTx. In this cell, fast and slow inactivating currents were observed before presentation of the drug; the fast inactivating component being evident, while the subtraction (1 – 2) was contaminated with the slow inactivating component. The families of currents observed in Fig. 7 (3 and 4) are typical current configurations obtained within 2 min of presentation of this concentration of DTx (n = 17). Subtraction (3 – 4) shows raw fast inactivating current without the slow inactivating component, which was eliminated by the DTx, [subtraction (1 – 3)]. In this cell, the slow inactivating component can be seen to be Vc sensitive because in the −120-mV conditioning prepulse, >6 nA of slow inactivating current is made visible, whereas the −40-mV conditioning prepulse (2– 4) shows <2 nA. In Fig. 7, subtractions (1 – 3) and (2 – 4) indicate that DTx (1 μM) also has a blocking effect on the sustained current. DTx, at this concentration, did not wash out after 20 min; beyond this time the input resistance decreased, causing recordings to become more unstable as a function of time.
FIG. 7.
Slow inactivating current block by dendrotoxin (DTx). In this example, 1 μM DTx blocked a portion of K+ current. Fast inactivating component of the current was left largely untouched. Approximately 3 nA of this current was seen after presentation of the DTx (3 – 4), 3 or 4 nA before (1 – 2). In subtraction (1 – 3), it can be seen that the DTx is affecting both slow and sustained current. Slow inactivating current was initiated by the −40- to −10-mV depolarizing pulses and the sustained current by the 0- to +50-mV depolarizations. It would appear that the slow inactivating current is also voltage sensitive because more of this type is evident in the −120-mV prepulse protocol (subtraction 1 – 3) than in the −40-mV prepulse protocol (subtraction 2 – 4).
Effects of TEA
Generally, TEA was too nonspecific to be useful in dissection of currents in these neurons. High concentrations (10, 20, and 40 mM) presented extracellularly (not shown) reduced all K+ components. Lower concentrations of 10 mM (n = 5) and 100 mM (n = 5) also could be seen to reduce IK, IA, and the slowly inactivating components of current (not shown), reduction being in proportion to concentration. The relative difference of amounts of IK made evident on subtraction of currents initiated via the −40-mV prepulse protocol from the −120-mV prepulse protocol increased, and the relative difference of IA was reduced after presentation of the drug. However, total peak IK was reduced for both prepulse protocols by TEA. Total peak IA also was reduced by TEA in both the −120-mV prepulse and in the −40-mV prepulse conditions. This difference is probably the amount of A-type current that is voltage activated but not the total because the difference is a relative one.
DISCUSSION
The DRG neurons examined in this study represent a subclass of cutaneous afferent neurons that give rise to myelinated axons inclusive of the Aβ class and are involved functionally in tactile sensation of the skin. They have distinct electrophysiological properties compared with DRG neurons of similar size that have peripheral projections to muscle. In particular, these large cutaneous afferent neurons have both kinetically fast and slow Na+ currents, broad inflected action potentials, and relatively small GABAA receptor-mediated conductance: in contrast, muscle afferents display only fast currents, narrow noninflected action potentials, and relatively large GABAA receptor-mediated conductance (Oyelese and Kocsis 1996). In response to nerve ligation, cutaneous afferent neurons lose their kinetically slow Na+ currents and increase their fast Na+ currents, the action potentials become narrow, and the GABAA receptor responses are increased (Oyelese et al. 1997; Rizzo et al. 1994). Moreover, the Aβ axons that arise from the larger cutaneous afferent fibers sprout in the dorsal horns and occupy vacated synapses in lamina II that previously were occupied by nociceptive C-fiber terminals (Woolf et al. 1995). It has been suggested that this synaptic reorganization in the dorsal horn, after peripheral nerve injury, may contribute to allodynia, whereby, normally nonnoxious afferent inputs such as stroking of the skin are sensed as painful stimuli. Although the importance of K+ currents in regulating action potential firing patterns is well recognized, little is known of the detailed distribution of K+ channels on cutaneous afferent neurons innervating the skin that facilitates tactile sensation. In this study, we characterized various K+ currents on identified cutaneous afferent neurons innervating the skin whose cell size suggests that they correspond to skin mechanoreception. Reduction in K+ currents as reported here could contribute to the abnormal firing properties of these afferent neurons.
The main findings of the present study are that on identified cutaneous afferent DRG neurons of the adult rat: 1) IK is the predominant current recorded in all cells; 2) the K+ current components from these cells can be dissected into at least three types: two inactivating (1 slow, the other fast) and one sustained; 3) the K+ current components were manifest in different ratios with some of the components being expressed, or not, in cells morphologically indistinct from one another; 4) generally, three types of cells based on the relative distribution of their K+ currents were categorized; and 5) effects of DTx and 4-AP varied in identified cutaneous afferent DRG neurons from the adult rat.
Although three neuronal categories were identified, based on K+ current distribution, it should be noted that they are not “hard” and “fast” groups; the expression of current components also could be described as a spectrum, ranging from cells having all the component elements to those evincing the sustained component with little or none of the other currents present. This suggests that there is a spectrum of underlying K+ currents varieties that are expressed in varying proportions among these neurons. Whether these differences reflect different functional subclasses of skin mechanoreceptive neurons is uncertain.
Sustained or K current
Although, Robertson and Taylor (1986) reported that in rat DRG neurons they had observed no fast transient current, outward current being totally composed of the sustained type, this possibly is explained by the fact that current components such as the fast inactivating or slow inactivating elements were masked by the much larger, sustained K current. More recently, Gold et al. (1996) postulated that this sustained, or noninactivating, current can be dissected further. They reported that in functionally unidentified rat DRG neurons, IK can be divided into three distinct classes; this dissection generally appears to be in cells of a smaller diameter than cells examined here. Large cutaneous afferent neurons may not express the same elements or quantities of currents as those seen in cells of a smaller diameter. This sustained K+ current was always the predominant element recorded in all neurons tested. In our experiments, the sustained component in these cells was sensitive to micromolar concentrations of DTx and TEA; it was insensitive to millimolar concentrations of 4-AP.
Fast inactivating or A current
Medium-sized cutaneous DRG neurons (39–49 μm) expressing A-type currents in the adult rat appear similar to those described by McFarlane (McFarlane and Cooper 1991) for rat nodose neurons and to those reported by Safronov and Vogel (1995) in the somata of rat motoneurons. More recently, Safronov et al., (1996) reported the only difference between A current in small neonatal DRG neurons and in that from newborn rats was that the kinetics of the channels in motoneurons were slowed after formation of cell-attached or inside-out patch configurations. In acutely dissociated adult DRG neurons, the fast inactivating component is distinctive and could be separated by a combination of kinetic and pharmacological criteria.
In studies examining hippocampal cells (Ficker and Heinemann 1992), it has been suggested that A current, reported to be resistant to DTx, can be divided into subclasses with and without a binding site for the toxin (Halliwell et al. 1986). As in other neurons, these fast transient potassium currents in adult DRG neurons are thought to modulate the timing of repetitive action potential generation, the repolarization of single action potentials, and the time required to reach the threshold to fire an action potential (Budde et al. 1992; Hammond and Crepal 1992; Numann et al. 1987; Storm 1988, 1990; Wu and Barish 1992). In this study, the A component of whole cell potassium currents was relatively large and was expressed in ~80% of cells examined, indicating the important role of fast inactivating current in this type of cell. In preliminary kinetic analysis, the gating mechanism of the fast transient current was both voltage activated and inactivated [see Fig. 6, subtractions (1 – 2) and (1 – 3)]. The voltage dependence of inactivation is unusual for this channel; this may be an A current with gating properties different from those commonly described. It also was observed that cells having A current also had large tail currents, and that those tail currents could be enlarged by increasing external K+ concentration. A current, in this study, was sensitive to TEA in micromolar concentrations, to 4-AP in millimolar concentrations, and insensitive to micromolar concentrations of DTx.
Slow-inactivating current
Slowly inactivating current is distinct from A current showing differences in activation and inactivation at different voltages (inactivating more slowly during depolarizing voltage steps) and showing an enhanced sensitivity to 4-AP and DTx (Castle et al. 1989; Dolly 1988; Moczydlowski et al. 1988). This would seem to indicate that the slow inactivating current is similar to Foehring and Sumeier’s (1993) proposed K3 channel population, which showed similar sensitivities, in rat neocortical neurons. Slow-inactivating current has been identified or observed in a number of different neurons: rat neostriatal neurons (Surmeier et al. 1989, 1991), nodose neurons (Stansfeld et al. 1986, 1987), adult rat dorsal root ganglion neurons (Stansfeld and Feltz 1988), rat amygdala neurons (Gean and Schinnick-Gallager 1989), rat hippocampal neurons (Ficker and Heinemann 1992; Halliwell et al. 1986; Storm 1988), mouse hippocampal neurons (Wu and Barish 1992), rat cortical neurons (Foehring and Surmeier 1993), and rat visual cortical neurons (Albert and Nerbonne 1995). Other studies have reported only one class of transient potassium current in different types of neurons: neocortical neurons (Ahmed 1988; Zona et al. 1988), neonatal rat motor neurons (Takahashi 1990), cerebella granuale cells (Cull-Candy et al. 1989), and hippocampal neurons (Numann et al. 1987; Segal and Barker 1984). Some of these reports are seemingly in contradiction (e.g., Segal and Barker 1984; Wu and Barish 1992). One possible explanation for these apparent contradictions is that the investigators may have been studying cells, which, although morphologically indistinct, are composed of subpopulations of cells expressing the different components of K+ current.
The precise function of the slow inactivating current is, as yet, to be clarified. Because many of the reports of this type of current are in fast conducting axons, or neurons that have these axons, it has been postulated that this could reflect their requirement of rapid firing and secure conduction (Brew and Forsythe 1995). In the present study, the slow inactivating component was seen to be sensitive to micromolar concentrations of TEA and DTx, and to millimolar concentrations of 4-AP.
Pharmacology
In the present study, a slow inactivating current sensitive to 100 μM 4-AP could only be detected in 3 cutaneous afferent neurons of 10. This is not necessarily an accurate reflection of the extent of the distribution of this slow inactivating current in these cells because it was also often not possible to clearly dissect the outward potassium currents by varying prepulse potentials when there were more than two components expressed in any given neuron (as in Fig. 2A). The activation and inactivation of the fast and slow components overlapped causing difficulty in distinguishing one component from the other.
Others have reported that a 100 μM concentration of 4-AP produced similar blocking effects to 1 μM DTx (Wu and Barish 1992) on the slow inactivating current. This is most clear on examination of the shapes of the family of currents after presentation of the DTx compared with those after 100 μM 4-AP (cf. Figs. 4 and 6). However, DTx is seen to affect the sustained portion of the current as well as the slow inactivating portion [Fig. 7, subtractions (1 – 3) and (2 – 4)]; whereas, 100 μM 4-AP was seen to reduce only the slow inactivating portion of current in the three affected cells (see Fig. 5). Thus DTx (1 μM) appears to be having more of a nonspecific effect than the 100 μM 4-AP.
A variety of effects of the convulsant peptide DTx have been reported, and the type of neuron being examined appears to be of paramount importance when comparing results. This compound has been described as blocking a number of different types of K+ current. Stansfeld and Feltz (1988) reported a series of experiments where DTx inhibited fast activating current in DRG neurons of the rat. Other workers report that fast inactivating A current is insensitive to DTx in rat cortical neurons (Foehring and Surmeier 1993). In earlier studies, Stansfeld et al. (1986) also had shown that DTx blocks slowly inactivating outward current in rat visceral sensory neurons; other workers showed that it is a selective blocker of sustained potassium current in the DRG neurons of the guinea pig (Penner et al. 1986). In the light of these reports, the lack of specificity of DTx is not particularly surprising.
Ficker and Heinemann (1992) report that in rat hippocampal cells concentrations as high as 10 mM 4-AP could not completely abolish fast transient current identified in those cells. In contrast to this finding, in our studies, concentrations of 5 or 6 mM 4-AP could be seen to effectively extinguish all A-type current in the adult rat DRG neurons of those cells comprising current configurations, allowing us to examine this effect. However, it should be noted that in cells that had current component configurations with more slow inactivating current but still had fast inactivating current, these components were difficult to define because of the difficulty of kinetic separation.
Relationship of K+ channels to cutaneous afferent axonal function
It generally is assumed that action potential activity in the DRG cell body is not a participant in sensory processing; DRG neurons are pseudobipolar, and impulse activity from the periphery to central targets does not require spike activity in the DRG somata. However, the complement of ion channels on the DRG cell body is thought to be representative of channels that are functionally important on the axon. For example, cutaneous afferent myelinated axons and their cell bodies possess kinetically distinct Na+ channels (Honmou et al. 1994; Rizzo et al. 1994; Oyelese et al. 1997), and both muscle and cutaneous afferent neurons have GABAA receptors (Oyelese and Kocsis 1996; Oyelese et al. 1995) as do their axons (Bhisitkul et al. 1990). It has been speculated that the slow Na+ current on cutaneous afferents could be functionally important for transduction of mechanical stimulation to afferent spike activity (Honmou et al. 1994; Kocsis et al. 1983). In addition, GABAA receptors are involved in presynaptic inhibition on the intraspinous terminal fields of primary afferents (Eccles et al. 1963; Levy 1977). The physiological relevance of the diversity of K+ channels, reported here, on cutaneous afferent neurons may be in the functional role they assume in their distribution on the axon for the regulation of frequency-response properties.
Several studies have indicated that 4-AP–sensitive K+ channels are important in repolarization of primary afferent axonal action potentials and the maintenance of spike waveform, whereas TEA-sensitive K+ channels are important in spike frequency adaptation (Baker et al. 1993; Barrett et al. 1988; Bowe et al. 1987; Eng et al. 1988; Gordon et al. 1988; Kocsis et al. 1987). Moreover, blockade of the 4-AP–sensitive K+ channel on cutaneous afferent axons unmasks a slow Na+ current and gives rise to burst firing (Honmou et al. 1994; Kocsis et al. 1983). The identification of the K+ current components on the cell bodies of these axons, as reported here, suggests their presence on the axons. Indeed, loose patch-clamp recordings from myelinated axons indicate a diversity of K+ channels, including sustained and inactivating channels (Roper and Schwarz 1989). It will be important to determine the role of these K+ currents in normal axonal function and processing of afferent input. DRG neuronal cell bodies can become a source of ectopic impulse generation and contribute to the pathophysiology subsequent to nerve injury (Burchiel 1984; Desantis and Duckworth 1982; Kajander et al. 1992). The relative distribution of Na+ channel subtypes on cutaneous afferent neurons changes after axotomy with a kinetically slow Na+ current being reduced and a kinetically fast Na+ current increasing (Honmou et al. 1994; Oyelese et al. 1997; Rizzo et al. 1995). Preliminary work in our laboratory (Everill and Kocsis 1997; Everill et al. 1996) indicates that, overall, K+ currents are reduced on cutaneous afferent neurons after axotomy. Given the well-established role of K+ currents in regulating firing properties of neurons, it will be important to determine if specific changes in of K+ current components on DRG neurons contribute to DRG hyperexcitability after nerve injury.
Acknowledgments
We thank H.-F. Mi for the preparation of neuronal cultures.
This work was supported in part by the Medical Research Service of the Department of Veterans Affairs and by the National Institutes of Health.
References
- Ahmed Z. Expression of membrane currents in rat neocortical neurons in serum-free culture. II Outward currents. Dev Brain Res. 1988;40:297–305. doi: 10.1016/0165-3806(88)90142-3. [DOI] [PubMed] [Google Scholar]
- Albert JL, Nerbonne JM. Calcium-independent depolarization-activated potassium currents in superior collicus-projecting rat visual cortical neurons. J Neurophysiol. 1995;73:2163–2178. doi: 10.1152/jn.1995.73.6.2163. [DOI] [PubMed] [Google Scholar]
- Baker M, Howe JR, Richie JM. Two types of 4-AP-sensitive potassium current in rabbit schwann cells. J Physiol (Lond) 1993;464:321–342. doi: 10.1113/jphysiol.1993.sp019637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett EF, Morita K, Scappaticci KA. Effects of tetraethylammonium on the depolarizing after-potential and passive properties of lizard mylinated axons. J Physiol (Lond) 1988;402:65–78. doi: 10.1113/jphysiol.1988.sp017194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhisitkul RB, Kocsis JD, Gordon TR, Waxman SG. Trophic influence of distal nerve segment on GABAA receptor expression in axotomized adult sensory neurons. Exp Neurol. 1990;109:273–278. doi: 10.1016/s0014-4886(05)80017-2. [DOI] [PubMed] [Google Scholar]
- Bowe CM, Kocsis JD, Targ EF, Waxman SG. Physiological effects of 4-aminopyridine on demyelinated mammalian motor and sensory fibers. Ann Neurol. 1987;22:264–268. doi: 10.1002/ana.410220212. [DOI] [PubMed] [Google Scholar]
- Brew HM, Forsythe ID. Two voltage-dependent K+ conductances with complementary functions in postsynaptic integration at a central auditory synapse. J Neurosci. 1995;15:8011–8022. doi: 10.1523/JNEUROSCI.15-12-08011.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde T, Mager R, Pape HC. Different types of potassium outward current in relay neurons acutely isolated from the rat geniculate nucleus. Eur J Neurosci. 1992;4:708–722. doi: 10.1111/j.1460-9568.1992.tb00180.x. [DOI] [PubMed] [Google Scholar]
- Burchiel KJ. Spontaneous impulse generation in normal and denervated dorsal root ganglia: sensitivity to the alpha-adrenergic stimulation and hypoxia. Exp Neurol. 1984;85:257–272. doi: 10.1016/0014-4886(84)90139-0. [DOI] [PubMed] [Google Scholar]
- Caffrey JM, Eng DL, Black JA, Waxman SG, Kocsis JD. Three types of sodium channels in adult rat dorsal root ganglion neurons. Brain Res. 1992;592:283–297. doi: 10.1016/0006-8993(92)91687-a. [DOI] [PubMed] [Google Scholar]
- Castle NA, Haylett DG, Jenkinson DH. Toxins in the charaterization of potassium channels. Trends Neurosci. 1989;12:59–65. doi: 10.1016/0166-2236(89)90137-9. [DOI] [PubMed] [Google Scholar]
- Connor JA, Stevens CF. Prediction of repetitive firing behaviour from voltage clamp study of isolated neuron somata. J Physiol (Lond) 1971a;213:31–53. doi: 10.1113/jphysiol.1971.sp009366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JA, Stevens CF. Voltage clamp studies of a transeient outward membrane current in gastropod neuron somata. J Physiol (Lond) 1971b;213:21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy SG, Marshall CG, Ogden D. Voltage-activated membrane currents in rat cerebellar granuale neurons. J Physiol (Lond) 1989;414:179–199. doi: 10.1113/jphysiol.1989.sp017683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desantis M, Duckworth JW. Properties of primary afferent neurons from muscle which are spontaneously active after a lesion of there peripheral. Exp Neurol. 1982;75:261–274. doi: 10.1016/0014-4886(82)90159-5. [DOI] [PubMed] [Google Scholar]
- Devor M, Wall PD. Cross-excitation in dorsal root ganglia of nerve-injured and intact rats. J Neurophysiol. 1990;64:1733–1746. doi: 10.1152/jn.1990.64.6.1733. [DOI] [PubMed] [Google Scholar]
- Dolly JO. Potassium channels—what can the protein chemistry contribute? Trends Neurosci. 1988;11:186–188. doi: 10.1016/0166-2236(88)90118-x. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Schmidt R, Wallis WD. Pharmacological studies on presynaptic inhibition. J Physiol (Lond) 1963;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng DL, Gordon TR, Kocsis JD, Waxman SG. Development of 4-AP and TEA sensitivities in mammalian myelinated nerve fibers. J Neurophysiol. 1988;60:2168–2179. doi: 10.1152/jn.1988.60.6.2168. [DOI] [PubMed] [Google Scholar]
- Everill B, Kocsis JD. Nerve injury reduces total potassium conductance of two of three identified currents in adult cutaneous afferent dorsal root ganglion neurons. Neurosci Soc Abstr. 1997;23:1745. [Google Scholar]
- Everill B, Rizzo MA, Kocsis JD. Characterization of fast and slow potassium channels of cells from the cell bodies of dorsal root ganglion neurons. Neurosci Soc Abstr. 1996;22:349. [Google Scholar]
- Ficker E, Heinemann U. Slow and fast transient potassium currents in cultured rat hippocampal cells. J Physiol (Lond) 1992;445:431–455. doi: 10.1113/jphysiol.1992.sp018932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foehring RC, Surmeier DJ. Voltage-gated potassium currents in acutely dissociated rat cortical neurons. J Neurophysiol. 1993;70:51–63. doi: 10.1152/jn.1993.70.1.51. [DOI] [PubMed] [Google Scholar]
- Gean PW, Schinnick-Gallager P. The transient potassium current, the A current, is involved in spike frequency adaptation in rat amygdala neurons. Brain Res. 1989;480:160–169. doi: 10.1016/0006-8993(89)91578-3. [DOI] [PubMed] [Google Scholar]
- Gold MS, Shuster MJ, Levine JD. Characterization of six voltage-gated K+ currents in adult rat sensory neurons. J Neurophysiol. 1996;75:2629–2646. doi: 10.1152/jn.1996.75.6.2629. [DOI] [PubMed] [Google Scholar]
- Gordon TR, Kocsis JD, Waxman SG. Evidence for the presence of two types of potassium channels in the rat optic nerve. Brain Res. 1988;447:1–9. doi: 10.1016/0006-8993(88)90959-6. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Kusano K, Saito N. Membrane changes in ochidium nerve cells in potassium rich media. J Physiol (Lond) 1961;155:470–489. doi: 10.1113/jphysiol.1961.sp006640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell JV, Othman IB, Plechen-Mathews A, Dolly JO. Central action of dendrotoxin: selection conduction of a transient K conductance in hippocampus and binding to localized acceptors. Proc Natl Acad Sci USA. 1986;83:493–497. doi: 10.1073/pnas.83.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hammond C, Crepal F. Evidence for a slowly activating K+ current in prefrontal cortical cells. Eur J Neurosci. 1992;4:1087–1092. doi: 10.1111/j.1460-9568.1992.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurons. J Physiol (Lond) 1985a;359:31–46. doi: 10.1113/jphysiol.1985.sp015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper AA, Lawson SN. Electrical properties of dorsal root ganglion neurons with different peripheral nerve conduction velocities. J Physiol (Lond) 1985b;359:47–63. doi: 10.1113/jphysiol.1985.sp015574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honmou O, Utzschneider DA, Rizzo MA, Bowe CM, Waxman SG, Kocsis JD. Delayed depolarization and slow sodium currents in cutaneous afferents. J Neurophysiol. 1994;71:1627–1637. doi: 10.1152/jn.1994.71.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajander KC, Wakisaka S, Bennett GJ. Spontaneous discharge originates in the dorsal root ganglion at the onset of a painful peripheral neuropathy. Neurosci Lett. 1992;138:225–228. doi: 10.1016/0304-3940(92)90920-3. [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Eng DL, Gordon TR, Waxman SG. Functional differences between 4-aminopyridine and tetraethylammonium-sensitive potassium channels in myelinated axons. Neurosci Lett. 1987;75:193–198. doi: 10.1016/0304-3940(87)90296-5. [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Ruiz JA, Waxman SG. Maturation of mammalian myelinated fibers: changes in action potential characteristics following 4-aminopyridine application. J Neurophysiol. 1983;50:449–463. doi: 10.1152/jn.1983.50.2.449. [DOI] [PubMed] [Google Scholar]
- Levy RA. The role of GABA in primary afferent depolarization. Neurobiology. 1977;9:211–267. doi: 10.1016/0301-0082(77)90002-8. [DOI] [PubMed] [Google Scholar]
- Mc Farlane S, Cooper E. Kinetics and voltage dependence of Atype currents on neonal rat sensory neurons. J Neurophysiol. 1991;66:1380–1391. doi: 10.1152/jn.1991.66.4.1380. [DOI] [PubMed] [Google Scholar]
- Moczydlowski E, Lucchesi K, Ravindran A. An emerging pharmacology of peptide toxins targeted against potassium channels. J Membr Biol. 1988;105:95–111. doi: 10.1007/BF02009164. [DOI] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage-clamp on snail neurons. J Physiol (Lond) 1971;58:36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numann RE, Wadman WJ, Wong RKS. Outward currents of single hippocampal cells obtained from the adult guinea pig. J Physiol (Lond) 1987;393:331–353. doi: 10.1113/jphysiol.1987.sp016826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyelese AA, Eng DL, Richerson GB, Kocsis JD. Enhancement of GABAA receptor-mediated conductances induced by nerve injury in a subclass of sensory neurons. J Neurophysiol. 1995;74:673–683. doi: 10.1152/jn.1995.74.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyelese AA, Kocsis JD. GABAA receptor-mediated conductance and action potential waveform in cutaneous and muscle afferent neurons of the adult rat: differential expression and response to nerve injury. J Neurophysiol. 1996;76:2383–2392. doi: 10.1152/jn.1996.76.4.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyelese AA, Rizzo MA, Waxman SG, Kocsis JD. Differential effects of NGF and BDNF on axotomy-induced changes in GABAA-receptor-mediated conductance and sodium currents in cutaneous afferent neurons. J Neurophysiol. 1997;77:31–42. doi: 10.1152/jn.1997.78.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallotta B, Wagoner PK. Voltage-dependent potassium channels since Hodgkin and Huxley. Physiol Rev. 1992;72(Suppl):S49–S67. doi: 10.1152/physrev.1992.72.suppl_4.S49. [DOI] [PubMed] [Google Scholar]
- Penner R, Petersen M, Pierau F, Dreyer F. Dendrotoxin: a selective blocker of non-inactivating potassium current in guinea-pig dorsal root ganglion neurons. Pflügers Arch. 1986;407:365–369. doi: 10.1007/BF00652619. [DOI] [PubMed] [Google Scholar]
- Rizzo MA, Kocsis JD, Waxman SG. Slow sodium conductances of dorsal root ganglion neurons: intraneuronal homogeneity and interneuronal heterogeneity. J Neurophysiol. 1994;72:2796–2815. doi: 10.1152/jn.1994.72.6.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo MA, Kocsis JD, Waxman SG. Selective loss of slow enhancement of fast Na+ currents in cutaneous afferent DRG neurons following axotomy. Neurobiol Dis. 1995;2:87–96. doi: 10.1006/nbdi.1995.0009. [DOI] [PubMed] [Google Scholar]
- Robertson B, Taylor WR. Effects of γ-aminobutyric acid and (−)-baclofen on calcium and potassium currents in cat dorsal root ganglion neurons in vitro. Br J Pharmacol. 1986;89:661–672. doi: 10.1111/j.1476-5381.1986.tb11170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski MA. The A-current how ubiquitous a feature of excitable cells is it? Trends Neurosci. 1985;8:214–219. [Google Scholar]
- Roper J, Schwarz Z. Heterogeneous distribution of fast and slow potassium channels in myelinated rat nerve fibers. J Physiol (Lond) 1989;416:93–110. doi: 10.1113/jphysiol.1989.sp017751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988;25:729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Safronov BV, Bischoff U, Vogel W. Single voltage-gated K+ channels and their functions in small dorsal root ganglion neurons of rat. J Physiol (Lond) 1996;493:393–408. doi: 10.1113/jphysiol.1996.sp021391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safronov BV, Vogel W. Single voltage-activated Na+ and K+ channels in the somata of rat motoneurons. J Physiol (Lond) 1995;460:675–691. doi: 10.1113/jphysiol.1995.sp020863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmued LC, Fallon JH. Fluoro-gold: a new fluorescent retrograde axonal tracer with numerous unique properties. Brain Res. 1986;377:147–154. doi: 10.1016/0006-8993(86)91199-6. [DOI] [PubMed] [Google Scholar]
- Segal M, Barker JL. Rat hippocampal neurons in culture: potassium conductances. J Neurophysiol. 1984;51:1409–1433. doi: 10.1152/jn.1984.51.6.1409. [DOI] [PubMed] [Google Scholar]
- Stansfeld CE, Feltz A. Dendrotoxin-sensitive K+ channels in dorsal root ganglion cells. Neurosci Lett. 1988;93:49–55. doi: 10.1016/0304-3940(88)90011-0. [DOI] [PubMed] [Google Scholar]
- Stansfeld CE, Marsh SJ, Halliwell JV, Brown DA. 4- Aminopyridine and dendrotoxin induce repetitive firing in rat visceral sensory neurons by blocking a slowly inactivating outward current. Neurosci Lett. 1986;64:299–304. doi: 10.1016/0304-3940(86)90345-9. [DOI] [PubMed] [Google Scholar]
- Stansfeld CE, Marsh SJ, Parcej DN, Dolly JO, Brown DA. Mast cell degranulating peptide and dendrotoxin selectively inhibit a fast-activating potassium current and bind to common neuronal proteins. Neuroscience. 1987;23:893–902. doi: 10.1016/0306-4522(87)90166-7. [DOI] [PubMed] [Google Scholar]
- Storm JF. Temporal integration by a slowly inactivating K+ current in hippocampal neurons. Nature. 1988;336:379–381. doi: 10.1038/336379a0. [DOI] [PubMed] [Google Scholar]
- Storm JF. Potassium currents in hippocampal pyramidal cells. Prog Brain Res. 1990;83:161–187. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- Strong PN. Potassium channel toxins. Pharmacol Ther. 1990;46:137–162. doi: 10.1016/0163-7258(90)90040-9. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Bargas J, Kitai ST. Two types of A- current differing in voltage-dependence are expressed by neurons of the rat neostriatum. Neurosci Lett. 1989;103:331–337. doi: 10.1016/0304-3940(89)90122-5. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Stefani A, Foehering RC, Kitai ST. Developmental regulation of a slowly-inactivating potassium conductance in rat neostriatal neurons. Neurosci Lett. 1991;122:41–46. doi: 10.1016/0304-3940(91)90188-y. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Membrane currents in visually identified motorneurons of neonatal rat spinal cord. J Physiol (Lond) 1990;423:27–46. doi: 10.1113/jphysiol.1990.sp018009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SH. Three pharmacologically distinct potassium channels in molluscan neurons. J Physiol (Lond) 1977;265:465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall PD, Devor M. Sensory afferent impulses originate from dorsal root ganglia as well as from the periphery in normal and nerve injured rats. Pain. 1983;17:321–339. doi: 10.1016/0304-3959(83)90164-1. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Shortland P, Reynolds M, Ridings J, Doubell T, Coggeshall RE. Reorganization of central terminals of myelinated primary afferents in the rat dorsal horn following peripheral axotomy. J Comp Neurol. 1995;360:121–134. doi: 10.1002/cne.903600109. [DOI] [PubMed] [Google Scholar]
- Wu RL, Barish ME. Two pharmacologically and kinetically distinct transient potassium currents in cultured embryonic mouse hippocampal neurons. J Neurosci. 1992;12:2235–2246. doi: 10.1523/JNEUROSCI.12-06-02235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zona C, Pirrone G, Avoli M, Dichter M. Delayed and fast transient potassium currents in rat neocortical neurons in cell culture. Neurosci Lett. 1988;94:285–290. doi: 10.1016/0304-3940(88)90032-8. [DOI] [PubMed] [Google Scholar]