Abstract
The systemic injection of human mesenchymal stem cells (hMSCs) prepared from adult bone marrow has therapeutic benefits after cerebral artery occlusion in rats, and may have multiple therapeutic effects at various sites and times within the lesion as the cells respond to a particular pathological microenvironment. However, the comparative therapeutic benefits of multiple injections of hMSCs at different time points after cerebral artery occlusion in rats remain unclear. In this study, we induced middle cerebral artery occlusion (MCAO) in rats using intra-luminal vascular occlusion, and infused hMSCs intravenously at a single 6 h time point (low and high cell doses) and various multiple time points after MCAO. From MRI analyses lesion volume was reduced in all hMSC cell injection groups as compared to serum alone injections. However, the greatest therapeutic benefit was achieved following a single high cell dose injection at 6 h post-MCAO, rather than multiple lower cell infusions over multiple time points. Three-dimensional analysis of capillary vessels in the lesion indicatedthat the capillary volume was equally increased in all of the cell-injected groups. Thus, differences in functional outcome in the hMSC transplantation subgroups are not likely the result of differences in angiogenesis, but rather from differences in neuroprotective effects.
Keywords: Regeneration, Stem cell, Stroke, Transplantation, Meschenchymal stem cell
1. Introduction
Intravenous administration of human mesenchymal stem cells (hMSCs) prepared from adult bone marrow has been reported to ameliorate functional deficits after middle cerebral artery occlusion (MCAO) in rats (Chen et al., 2001; Li et al., 2002; Iihoshi et al., 2004; Nomura et al., 2005; Honma et al., 2006; Horita et al., 2006; Liu et al., 2006). Several hypotheses to account for these therapeutic benefits have been suggested, including neuroprotective effects from release or stimulation of growth factors and cytokines, the induction of neovascularization, and the replacement of damaged cells (Hirouchi and Ukai, 2002; Kurozumi et al., 2004; Radtke et al., 2007).
A cell based-therapy may have the advantage of exerting multiple therapeutic effects at various sites and times within the lesion as the cells respond to a particular pathological micro-environment (Chopp et al., 2000; Pluchino et al., 2003; Iihoshi et al., 2004; Nomura et al., 2005; Pluchino et al., 2005; Zhang et al., 2005; Honma et al., 2006; Horita et al., 2006; Liu et al., 2006; Ukai et al., 2007; Onda et al., 2008). Although a single injection of hMSCs several hours after ischemia onset can reduce infarction size and improve functional outcome in rodent cerebral ischemia models (Chen et al., 2001; Li et al., 2002; Iihoshi et al., 2004; Nomura et al., 2005; Honma et al., 2006; Horita et al., 2006; Liu et al., 2006, Ukai et al., 2007; Onda et al., 2008), the potential therapeutic benefits of multiple injections of hMSCs remain unclear.
In the present study, hMSCs were intravenously delivered in a cerebral ischemia model to investigate whether multiple injections may improve the therapeutic outcome. hMSCs were intravenously infused into the rats after MCAO in four different protocols. MRI studies, histological examinations, and behavioral studies were performed to evaluate the therapeutic benefits of treatment.
2. Results
2.1. Magnetic resonance image analysis
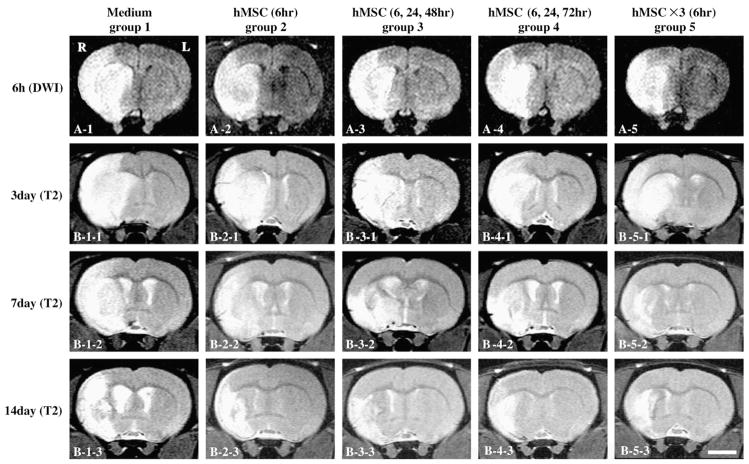
The ischemic lesion size was estimated using in vivo MRI (see Experimental procedures). Medium alone (sham control: group 1) or hMSCs (low or high dose) in medium were delivered intravenously immediately after the initial 6 h MRI scan (groups 2 and 5), and at multiple time point injections (groups 3 and 4; 24 h and 48 h, and 24 h and 1 week, respectively) in addition to the initial injection 6 h after MCAO. A single infusion of 1×106 cells or 3×106 was injected into group 2 and group 5, respectively, at the 6 h time point. Groups 3 and 4 with the multiple time point injections received 1×106 cells for each of the 3 time points for a total 3×106 cells. DWI is more useful for lesion volume detection in the acute infarction phase, although T2WI is more suitable for later time points. Therefore, DWI was used at only the 6 h time point, and T2WIs were obtained for group 1 (n=20), group 2 (n=13), group 3 (n=14), group 4 (n=14), and group 5 (n=13) at 6 h and 1, 3, 7, 14, and 28 days. Coronal forebrain sections were obtained at the level of caudato-putamen complex. DWI and T2WI are shown in Figs. 1A and B, respectively. Note the reduction in density in lesions on the right side of the images with increasing time after MCAO induction.
Fig. 1.
Evaluation of the ischemic lesion volume with Diffusion Weighted Images (DWI) and T2Weighted Images (T2WI). hMSCs were intravenously injected at various times and doses after the initial MRI scan (6 h after MCAO). DWI obtained at 6 h after MCAO in group 1 (A1), group 2 (A2), group 3 (A3), group 4 (A4), and group 5 (A5). T2WI obtained at 3, 7 and 14 days after MCAO in group 1 (B1-1 to 3), group 2 (B2-1 to 3), group 3 (B3-1 to 3), group 4 (B4-1 to 3), and group 5 (B5-1 to 3). Scale bar=5 mm.
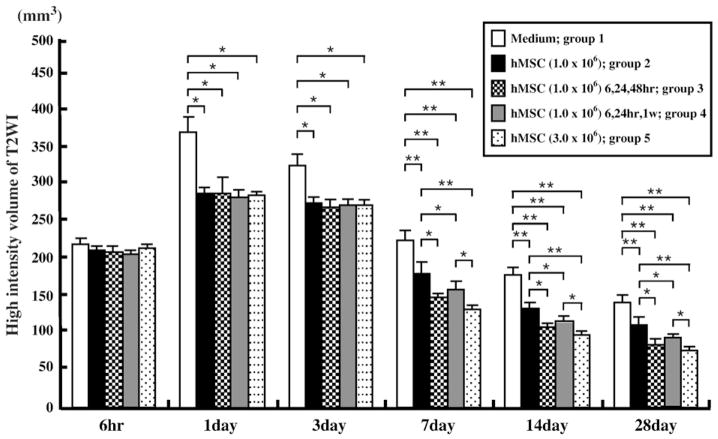
Lesion volume (mm3) was determined by analysis of high intensity areas on serial images collected through the cerebrum (see Experimental procedures). The lesion volumes estimated at 6 h after MCAOwere the same among the five groups (Figs. 1A1–5). DWI lesion volumes at 6 h after MCAO were 220.0±3.4 in group 1, 223.0±3.0 in group 2, 217.5±3.4 in group 3, 217.3±4.3 in group 4, and 219.0±4.5 in group 5. The lesion volume was reduced for the hMSC-injected groups compared to that of the sham control (group 1) at one day after MCAO and at later times (Fig. 2). The reduction in lesion volume showed no significant difference between the four hMSC-injected groups at 1 and 3 days post-MCAO. However, the multi-time point hMSC injection groups (groups 3 and 4) showed greater lesion volume reduction at 7, 14, and 28 days post-MCAO than in the group with a single cell injection (group 2) at 6 h. Rats given a single high dose of hMSCs (3.0×106) injected at a single time point of 6 h after MCAO (group 5) showed a greater reduction in gross lesion volume as compared to rats in groups 2 and 4. These results are summarized in Fig. 2.
Fig. 2.
A summary of lesion volumes evaluated by MRI (T2WI) were obtained at 6 h and 1, 3, 7, 14, and 28 days after MCAO in sham control (group 1) and hMSC-treated groups (groups 2–5). *P<0.05, **P<0.01.
2.2. Histological determination of infarction volume
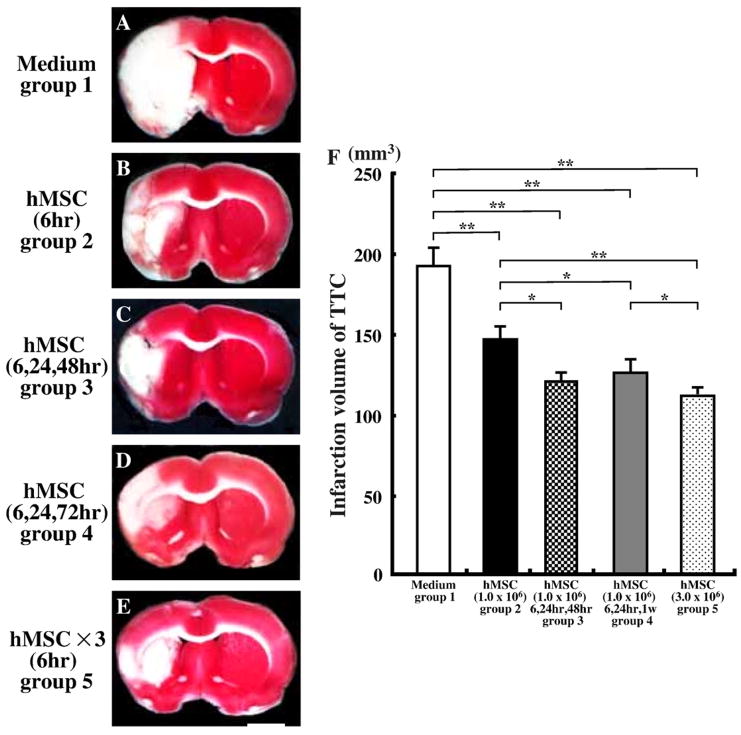
The animals were perfused and stained with TTC to obtain a second independent measure of infarction volume. Normal brain (grey matter) tissue typically stains with TTC, but infarcted lesions show no or reduced staining (Bederson et al., 1986). TT -staining that was obtained at 2 weeks after MCAO for the 5 experimental groups (Fig. 3). Note the reduced staining on the lesion side for all of the hMSC transplantation groups (Figs. 3B–E) as compared to sham control (Fig. 3A). Lesion volume was calculated by measuring the area of reduced TTC staining in the forebrain (see Experimental procedures). There was about 20–40% reduction in infarction size in all hMSCs-treated groups compared to sham control animals. The reduction in lesion volume between the transplant groups was less for group 2 than for the other groups. Rats in group 5 (single high dose injection at 6 h) showed the greatest reduction in lesion volume. There were no significant differences between rats in group 3 (Fig. 3C) and group 4 (Fig. 3D). These results are summarized in Fig. 3F (n=5 for each group).
Fig. 3.
Brain slices stained with 2, 3, 5-triphenyl tetrazolium chloride (TTC) to visualize the ischemic lesions at 14 days after MCAO. TTC-stained brain slices from group 1 (A), group 2 (B), group 3 (C), group 4 (D), and group 5 (E). (F) A summary of lesion volumes evaluated with TTC-stained brain slices at14 days after MCAO. Scale bar=3 mm. *P<0.05, **P<0.01.
2.3. Identification of donor cells in vivo
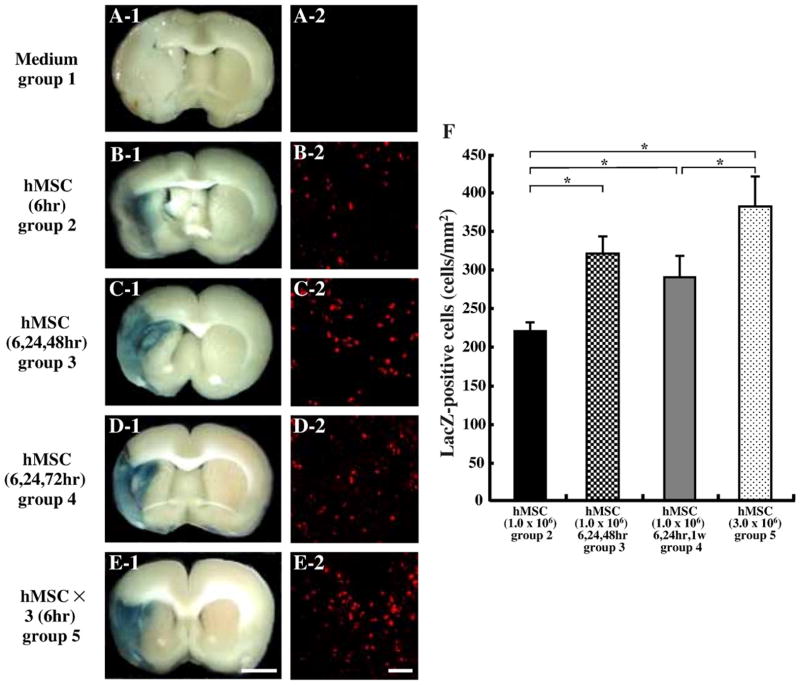
LacZ-transfected hMSCs that had been administered intravenously after MCAO were identified by X-gal staining in vivo (n=5 for each group). The LacZ-expressing hMSCs were observed primarily in the lesion. Note the abundance of beta-gal-reaction blue product in and around the lesion, indicating that, with systemic delivery, the hMSCs reached the lesion site (Figs. 4B-1–E-1). Blue staining was virtually absent in the sham control group (Fig. 4A-1).
Fig. 4.
Intravenously administrated hMSCs accumulated in the ischemic hemisphere. hMSCs were transfected with the reporter gene LacZ. Transplanted LacZ-positive hMSCs (blue cells) were present in the ischemic lesion in group 2 (B-1), group 3 (C-1), group 4 (D-1), and group 5 (E-1). Brains from sham control (medium alone) injected animals with comparable X-gal staining is shown in group 1 (A-1). Confocal images of group 2 (B-2), group 3 (C-2), group 4 (D-2), and group 5 (E-2) demonstrated a large number of LacZ-positive cells in the lesion. The numbers of LacZ-positive cells were obtained from one section per rat and five separate rats were analyzed, resulting in the demonstration of large numbers of LacZ-positive cells (F). Scale bar=3 mm (1), and 50 μm (2). *P<0.05.
Immunohistochemical studies were performed in order to identify LacZ-positive cells in and around the lesion zone in animals transplanted with LacZ-transfected hMSCs (n=5 for each group). The micrographs of group 2 (Fig. 4B-2), group 3 (Fig. 4C-2), group 4 (Fig. 4D-2), and group 5 (Fig. 4E-2) demonstrated a large number of LacZ-positive cells in the lesion. There were no LacZ-positive cells in the sham control group (Fig. 4A-2). The number of LacZ-positive cells was obtained from one section per rat and five separate rats of each group were analyzed, demonstrating a large number of LacZ-positive cells in the lesions in group 5, which were larger than those in groups 2 and 4. We described the phenotypical features of these cells in a previous study (Honma et al., 2006; Liu et al., 2006). These results are summarized in Fig. 4F.
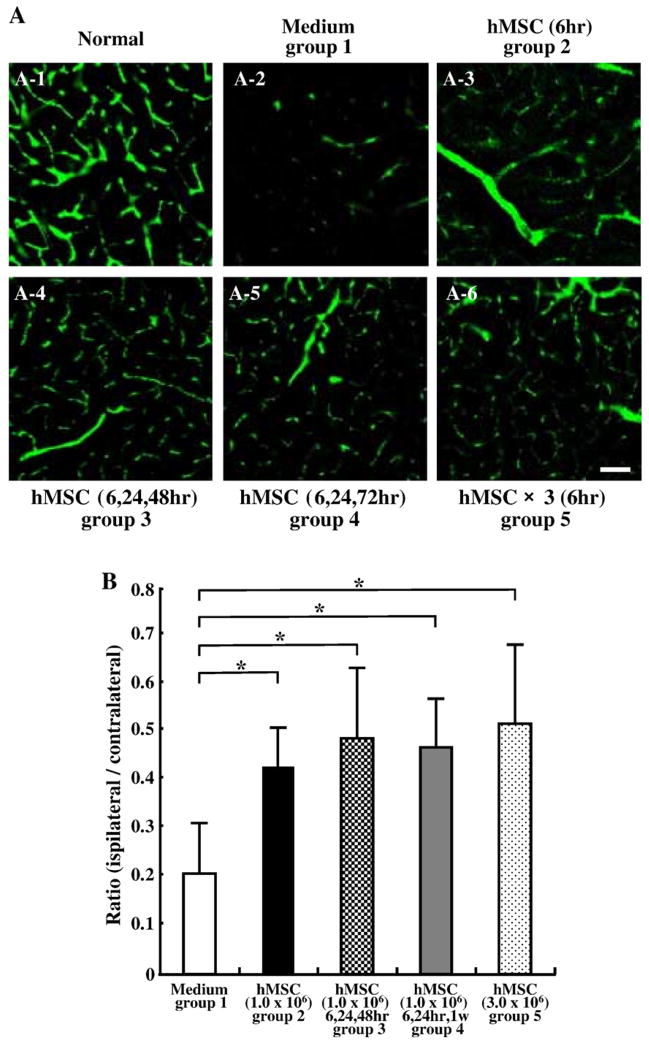
2.4. Analysis of capillary vessel volume
In order to examine whether the administration of hMSCs induced angiogenesis, three-dimensional analyses of capillary vessels in the lesions were performed using Zeiss LSM5 PASCAL software (n=4 for each groups). Fig. 5A-1 shows a three-dimensional capillary image in the normal rat brain (n=4). The capillary vascular volume was expressed as a ratio of ischemic hemisphere over that of the contralateral control hemisphere. At 14 days after MCAO, the ratio (ipsilateral/contralateral) was increased in all hMSC-treated groups (Figs. 5A-3 to 6) compared to the sham control group (Fig. 5A-2). These results were summarized in Fig. 5B.
Fig. 5.
Fourteen days after MCAO, angiogenesis in boundary zone was analyzed using a three-dimensional analysis system. Panel A-1 shows the three-dimensional capillary image with systemically perfused FITC-dextran in the normal rat brain. The capillary vascular volume was expressed as a ratio by dividing that obtained from the ischemic hemisphere by that of the contralateral control hemisphere. At 14 days after MCAO, the ratio (ipsilateral/contralateral) was increased in group 2 (A-3), group 3 (A-4), group 4 (A-5), and group 5 (A-6) compared to group 1 (A-2). These results were summarized in panel B. Scale bar=150 μm. *P<0.05.
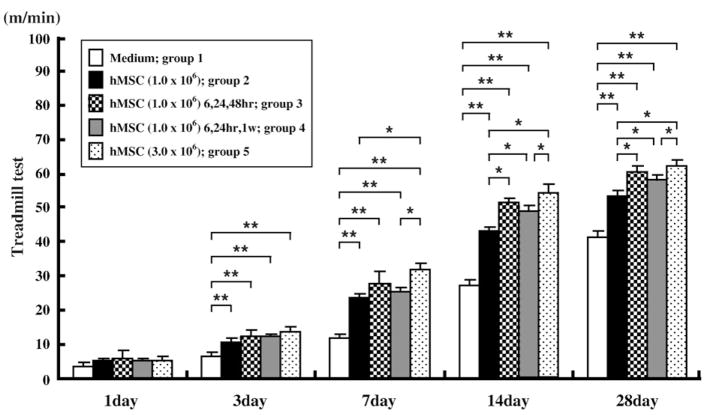
Fig. 6.
The maximum speed at which the rats could run on a motor driven treadmill was recorded. Twenty four hours after MCAO, the maximum velocity on the treadmill test was at its maximum deficit, and there was no significant difference observed between the groups. All of the groups of hMSC-treated rats showed a greater functional recovery than did sham control rats at up to 3 days. Rats in group 5 showed a greater functional recovery than did rats in group 2 and group 4 at up to 28 days. *P<0.05, **P<0.01.
2.5. Functional analysis
Behavioral testing began at 2 days before lesion induction in all rats. All rats performed this task before and after lesion induction. The maximum speed at which the rats could run on a motor driven treadmill was recorded, and all rats reached a maximum treadmill velocity of about 70 m/min2 before MCAO. Twenty four hours after MCAO, the maximum velocity on the treadmill test was at its maximum deficit, and there was no significant difference among the groups.
All groups of hMSC-treated rats showed greater improved functional recovery in the treadmill stress test as compared to the sham control rats (group 1) (n=11) at up to 3 days. Rats in group 5 (n=11) showed a greater functional recovery than did rats in group 2 (n=12) and group 4 (n=11). There was no significant difference between rats in group 3 and 4 (n=12). Group 5 was significantly different from all groups except group 3. These results are summarized in Fig. 6.
3. Discussion
The results of the present study demonstrate that the intravenous infusion of hMSCs in MCAO rats resulted in an improved outcome, which is consistent with previous studies showing the therapeutic benefits of the injection of hMSCs in experimental cerebral ischemic models (Chen et al., 2001; Nomura et al., 2005; Honma et al., 2006; Horita et al., 2006; Liu et al., 2006; Ukai et al., 2007; Onda et al., 2008). Although proposed mechanisms involved include neuroprotective effects due to the release or stimulation of growth factors and cytokines, the induction of neovascularization, and the replacement of damaged cells (Hirouchi and Ukai, 2002; Kurozumi et al., 2004; Radtke et al., 2007), a cell based-therapy may have multiple therapeutic effects at various sites and times within the lesion as the cells respond to a particular pathological microenvironment. Thus, the ideal therapeutic protocol in terms of number of cells and at what times to be delivered remains uncertain because of the complicated therapeutic mechanisms.
Rats in group 5 (single high hMSC dose) showed about a 10% greater reduction in gross lesion volume and improved functional recovery than did rats in group 4 which were given the same number of cells as in group 5, but infused in 1/3 dose at three temporal time points. Moreover, rats in group 5 showed about 20% reduction in lesion volume and improved functional recovery than did rats in group 2 (single 6 h lower MSC dose injection). This suggests that a relatively large dose of hMSCs administered at an early time point is more effective than temporally dispersed (fractionated) dosing. Interestingly, multiple injections of 1×106 cells at 6, 24 and 48 h showed nearly identical efficacy as a single 3×106 cell dose at 6 h. This suggests that relatively high dosing of hMSCs within the first 2 days is the most effective protocol. Three-dimensional analysis of capillary vessels in the lesion indicated that the capillary volume was equally increased in all of the cell-injected groups. Thus, the differences in functional outcome between groups 2 and 5 are not likely the result of differences in angiogenesis, but rather may be from differences in neuroprotective effects.
Although the mechanism of cellular homing following stroke remains uncertain, hMSC migration may involve various chemotaxic molecules such as stromal-derived factor-1 or monocyte chemoattractant protein-1 (Wang et al., 2002; Hill et al., 2004; Wang et al., 2008). In the present study, the density of LacZ-positive cells in the lesion of group 5 estimated from the immunohistochemical analysis was about 400 cells/mm3. Transduction efficiency of LacZ gene was about 90%. Infarcted volume was about 120 mm3. The number of intravenously injected cells was 3×106 cells. Thus, an estimate of the percentage of intravenously injected cells accumulating in the lesion was about 1.8%, and a very limited number of LacZ-positive cells were seen in other apparently non-injured regions, which is consistent with previous studies (Liu et al., 2006; Shen et al., 2007; Ukai et al., 2007). Further studies to dissect homing mechanisms will be important in the context of developing strategies to enhance the therapeutic benefits of hMSC following systemic administration.
A systemically delivered cell based-therapy may have the advantage of exerting multiple therapeutic effects at various sites and times within the lesion, and these approaches are being used in clinical studies for a number of neurological diseases including Krabbe’s disease (Escolar et al., 2005), Hurler’s syndrome (Staba et al., 2004), metachromatic leukodystrophy (Koc et al., 2002), and stroke (Bang et al., 2005), suggesting the prospect of the future use of hMSCs for clinical studies in a large number of CNS diseases. However, the therapeutic benefits may differ between diseases or the phase of pathological conditions. Thus, optimal therapeutic protocols using hMSCs should be extensively investigated in each pathophysiological environment.
4. Experimental procedures
4.1. Preparation of hMSCs
Human bone marrow from healthy adult volunteers was obtained by aspiration from the posterior iliac crest after informed consent was obtained. All procedures in this study were approved by the institutional review board at our university (Kobune et al., 2003). Bone marrow mononuclear cells were isolated and plated in 150-cm2 plastic tissue culture flasks at the density of 3000 cells/cm2 in mesenchymal stem cell basal medium (MSCBM, Cambrex) containing mesenchymal cell growth supplement (MCGS, Cambrex) and 4 mM L-glutamine, and were maintained in a humidified atmosphere of 5% CO2 at 37°C overnight. After washing away the free cells, the adherent cells were cultured by replacing the medium. When cultures reached subconfluence (5000 cells/cm2), the adherent cells were detached with trypsin-EDTA solution (SIGMA) and subcultured at 2000 cells/cm2. The cells were propagated without any differentiation process, and cryopreserved as primary hMSCs before reaching passage 5, which characteristic feature was previously reported as a CD34−, CD45−, CD73+, CD105+cell surface phenotype (Kobune et al., 2003; Honma et al., 2006).
4.2. Cerebral ischemic model
The rat middle cerebral artery was permanently occluded as a stroke model. We induced MCAO using a previously described method of intra-luminal vascular occlusion (Longa et al., 1989; Nomura et al., 2005). Adult female Sprague–Dawley rats weighing 250–300 g were anesthetized with ketamine (50 mg/kg) and xylazine (10 mg/kg) i.p. A length of 20.0–22.0 mm 4-0 surgical suture (Dermalon, Sherwood Davis and Geck, UK) with the tip rounded by heating near a flame was advanced from the external carotid artery into the lumen of the internal carotid artery until it blocked the origin of the MCA.
4.3. Transplantation procedures
Transplantation protocols consisted of five groups. In group 1 (sham control), rats were injected intravenously with medium alone through the femoral vein (without donor cell administration) at 6 h after MCAO (just after the initial MRI measurement). In group 2, rats were injected intravenously with hMSCs (1.0×106) in 1 ml total fluid volume (DMEM) at 6 h after MCAO. In group 3, rats were injected intravenously with hMSCs (1.0×106 each) at 6, 24, and 48 h after MCAO. In group 4, rats were injected intravenously with hMSCs (1.0×106 each) at 6, 24, and 168 h after MCAO. In group 5, rats were injected intravenously with a single high dose of hMSCs (3.0×106) at 6 h after MCAO. All rats (including sham control rats) were injected daily with cyclosporine (10 mg/kg) i.p.
AxCALacZ-F/RGD adenovirus was used to transduce the LacZ gene into the hMSCs, in order to trace the transplanted hMSC in vivo. For in vitro adenoviral infection, 1.0×106 hMSCs were co-cultured with AxCALacZ-F/RGD at a multiplicity of infection (MOI) of 3.0×103 pu/cell for 1 h, and incubated at 37°C in medium containing 10% fetal calf serum.
4.4. MR imaging
Each rat was placed in an animal holder/MRI probe apparatus and positioned inside the magnet, after anesthetization with ketamine (75 mg/kg) and xylazine (10 mg/kg) i.p. The animal’s head was held in place inside the imaging coil. All MRI measurements were performed using a 7-T, 18-cm-bore superconducting magnet (Oxford Magnet Technologies) interfaced to a UNITYINOVA console (Oxford Instruments, UK and Varian, Inc., Palo Alto, CA, USA).
T2 weighted images (T2WI) were obtained from a 1.0-mm-thick coronal section with a 0.5 mm gap using a 30 mm×30 mm field of view, TR=3000 ms, TE=37 ms, and reconstructed using a 256×128 image matrix. Diffusion weighted images (DWI) were obtained under the same conditions as T2 WI, except for the b value (b value=966) and image matrix (128×128). Accurate positioning of the brain was performed to center the image slice 5 mm posterior to the rhinal fissure with the head of the rat held in a flat skull position. MRI measurements were obtained at 6 h and 1, 3, 7, 14, and 28 days after MCAO.
The ischemic lesion area was calculated from both T2WI and DWI using imaging software (Scion Image, Version Beta 4.0.2, Scion Corporation), based on the previously described methods (Neumann-Haefelin et al., 2000; Nomura et al., 2005). For each slice, the higher intensity lesions in both T2WI and DWI, where the signal intensity was 1.25 times higher than the comparable region in the contralateral side of the brain, were marked as the ischemic lesion areas, and the infarct volume was calculated while taking slice thickness (1 mm/slice) into account. We used only rats which showed 200–250 mm3 of high intensity area in DWI at 6 h after MCAO, which pre-selected rats were randomly used for each group.
4.5. Histological analysis
4.5.1. TTC staining and quantitative analysis of infarct volume
The rats were anesthetized with ketamine (50 mg/kg) and xylazine (10 mg/kg) i.p. at two weeks after transplantation, and the brains were carefully removed and dissected into 1 mm coronal sections using a vibratome. The fresh brain slices were immersed in a 2% solution of 2, 3, 5-triphenyl tetrazolium chloride (TTC) in normal saline at 37°C for 30 min (Bederson et al., 1986). The cross-sectional area of infarction in each brain slice was examined with a dissection microscope and was measured using image analysis software (Adobe Photoshop). The total infarct volume for each brain was calculated by summation of the infracted area from all brain slices.
4.5.2. Detection of donor hMSCs and phenotypic analysis in vivo
4.5.2.1. X-gal staining
At two weeks after transplantation, brains from deeply anesthetized rats were removed and fixed in 0.5% glutaraldehyde in phosphate buffer for 1 h. Brain slices (1000 μm) were cut with a vibratome, and β-galactosidase expressing cells were detected by incubating the sections at 37°C overnight in X-gal at a final concentration of 1 mg/ml in X-Gal developer (35 mM K3Fe(CN)6/35 mM K4Fe(CN)63H2O/2 mM MgCl2 in phosphate-buffered saline) to form blue reaction product within the transplanted cells.
4.5.2.2. Immunohistochemistry
At two weeks after transplantation, analysis of the transplanted cells in vivo was performed using laser scanning confocal microscopy. Brains of the deeply anesthetized rats were removed, fixed in 4% paraformaldehyde in phosphate buffer, dehydrated with 30% sucrose in 0.1 M PBS for overnight, and frozen in powdered dry ice. The coronal forebrain section was obtained at the level of Bregma, and cryostat sections (10 μm) were processed for immunohistochemistry. In order to identify the transplanted hMSCs, immuno-labeling studies were performed with the use of antibodies to beta-galactosidase (rhodamine-labeled polyclonal rabbit anti-beta-galactosidase antibody, DAKO). A 543-nm laser line from a HeNe laser was used to excite the rhodamine fluorochrome (red). Confocal images were obtained using a Zeiss laser scanning confocal microscope and Zeiss software.
4.5.3. Capillary vessels in ischemic brain
In order to examine capillary vessels in the ischemic brain, fluorescein isothiocyanate (FITC) dextran (2×106 molecular weight, Sigma; 0.1 mL of 50 mg/mL) was administered intravenously to the ischemic rats after two weeks of MCAO. Brains were removed and brain slices (100 μm) were cut with a vibratome. A 488-nm laserline generated by an argon laser was used to excite the FITC (green). Confocal images were obtained using a Zeiss laser scanning confocal microscope and Zeiss software, and vessel volumes were measured in three dimensions using Zeiss LSM software.
4.6. Treadmill stress test
A treadmill stress test was performed to evaluate the functional outcome of the experimental rats. Rats were trained 20 min per day for 2 days a week to run on a motor driven treadmill at a speed of 20 m/min. Rats were placed on a moving belt and induced to run in the direction opposite of the movement of the belt. The maximum speed at which the rats could run on the motor driven treadmill was recorded.
4.7. Statistical analysis
The lesion volume, the capillary vascular volume, and the behavior scores (treadmill stress test) were statistically analyzed. Data are presented as mean values±S.D. Differences among groups were assessed by ANOVA (StatView) for Figs. 2–4, 6, and Kruskal–Wallis test for Fig. 5 to identify individual group differences. Differences were deemed statistically significant at P<0.05.
Acknowledgments
This work was supported in part by grants from the Japanese Ministry of Education, Science, Sports and Culture (16390414, 20390388), the National Multiple Sclerosis Society (U.S.A) (RG2135; CA1009A10), the National Institutes of Health (NS43432), and the Medical and Rehabilitation and Development Research Services of the Department of Veterans Affairs.
References
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–1308. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001;189:49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- Chopp M, Zhang XH, Li Y, Wang L, Chen J, Lu D, Lu M, Rosenblum M. Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. Neuroreport. 2000;11:3001–3005. doi: 10.1097/00001756-200009110-00035. [DOI] [PubMed] [Google Scholar]
- Escolar ML, Poe MD, Provenzale JM, Richards KC, Allison J, Wood S, Wenger DA, Pietryga D, Wall D, Champagne M, Morse R, Krivit W, Kurtzberg J. Transplantation of umbilical-cord blood in babies with infantile Krabbe’s disease. N Engl J Med. 2005;352:2069–2081. doi: 10.1056/NEJMoa042604. [DOI] [PubMed] [Google Scholar]
- Hill WD, Hess DC, Martin-Studdard A, Carothers JJ, Zheng J, Hale D, Maeda M, Fagan SC, Carroll JE, Conway SJ. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J Neuropathol Exp Neurol. 2004;63:84–96. doi: 10.1093/jnen/63.1.84. [DOI] [PubMed] [Google Scholar]
- Hirouchi M, Ukai Y. Current state on development of neuroprotective agents for cerebral ischemia. Nippon Yakurigaku Zasshi. 2002;120:107–113. doi: 10.1254/fpj.120.107. [DOI] [PubMed] [Google Scholar]
- Honma T, Honmou O, Iihoshi S, Harada K, Houkin K, Hamada H, Kocsis JD. Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Exp Neurol. 2006;199:56–66. doi: 10.1016/j.expneurol.2005.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horita Y, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Intravenous administration of glial cell line-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in the adult rat. J Neurosci Res. 2006;84:1495–1504. doi: 10.1002/jnr.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iihoshi S, Honmou O, Houkin K, Hashi K, Kocsis JD. A therapeutic window for intravenous administration of autologous bone marrow after cerebral ischemia in adult rats. Brain Res. 2004;1007:1–9. doi: 10.1016/j.brainres.2003.09.084. [DOI] [PubMed] [Google Scholar]
- Kobune M, Kawano Y, Ito Y, Chiba H, Nakamura K, Tsuda H, Sasaki K, Dehari H, Uchida H, Honmou O, Takahashi S, Bizen A, Takimoto R, Matsunaga T, Kato J, Kato K, Houkin K, Niitsu Y, Hamada H. Telomerized human multipotent mesenchymal cells can differentiate into hematopoietic and cobblestone area-supporting cells. Exp Hematol. 2003;31:715–722. doi: 10.1016/s0301-472x(03)00177-2. [DOI] [PubMed] [Google Scholar]
- Koc ON, Day J, Nieder M, Gerson SL, Lazarus HM, Krivit W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH) Bone Marrow Transplant. 2002;30:215–222. doi: 10.1038/sj.bmt.1703650. [DOI] [PubMed] [Google Scholar]
- Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, Honmou O, Houkin K, Date I, Hamada H. BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol Ther. 2004;9:189–197. doi: 10.1016/j.ymthe.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- Liu H, Honmou O, Harada K, Nakamura K, Houkin K, Hamada H, Kocsis JD. Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain. 2006;129:2734–2745. doi: 10.1093/brain/awl207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin T, Kastrup A, de Crespigny A, Yenari MA, Ringer T, Sun GH, Moseley ME. Serial MRI after transient focal cerebral ischemia in rats: dynamics of tissue injury, blood–brain barrier damage, and edema formation. Stroke. 2000;31:1965–1973. doi: 10.1161/01.str.31.8.1965. [DOI] [PubMed] [Google Scholar]
- Nomura T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. I.V. infusion of brain-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Neuroscience. 2005;136:161–169. doi: 10.1016/j.neuroscience.2005.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onda T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Therapeutic benefits by human mesenchymal stem cells (hMSCs) and Ang-1 gene-modified hMSCs after cerebral ischemia. J Cereb Blood Flow Metab. 2008;28:329–340. doi: 10.1038/sj.jcbfm.9600527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalini A, Bergami A, Furlan R, Comi G, Constantin G, Martino G. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436:266–271. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- Radtke C, Spies M, Sasaki M, Vogt PM, Kocsis JD. Demyelinating diseases and potential repair strategies. Int J Dev Neurosci. 2007;25:149–153. doi: 10.1016/j.ijdevneu.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staba SL, Escolar ML, Poe M, Kim Y, Martin PL, Szabolcs P, Allison-Thacker J, Wood S, Wenger DA, Rubinstein P, Hopwood JJ, Krivit W, Kurtzberg J. Cord-blood transplants from unrelated donors in patients with Hurler’s syndrome. N Engl J Med. 2004;350:1960–1969. doi: 10.1056/NEJMoa032613. [DOI] [PubMed] [Google Scholar]
- Shen LH, Li Y, Chen J, Cui Y, Zhang C, Kapke A, Lu M, Savant-Bhonsale S, Chopp M. One-year follow-up after bone marrow stromal cell treatment in middle-aged female rats with stroke. Stroke. 2007;38:2150–2156. doi: 10.1161/STROKEAHA.106.481218. [DOI] [PubMed] [Google Scholar]
- Ukai R, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Mesenchymal stem cells derived from peripheral blood protects against ischemia. J Neurotrauma. 2007;24:508–520. doi: 10.1089/neu.2006.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Li Y, Chen J, Gautam SG, Zhang Z, Lu M, Chopp M. Ischemic cerebral tissue and MCP-1 enhance rat bone marrow stromal cell migration in interface culture. Exp Hematol. 2002;30:831–836. doi: 10.1016/s0301-472x(02)00829-9. [DOI] [PubMed] [Google Scholar]
- Wang Y, Deng Y, Zhou GQ. SDF-1alpha/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Res. 2008;1195:104–112. doi: 10.1016/j.brainres.2007.11.068. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li Y, Chen J, Cui Y, Lu M, Elias SB, Mitchell JB, Hammill L, Vanguri P, Chopp M. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp Neurol. 2005;195:16–26. doi: 10.1016/j.expneurol.2005.03.018. [DOI] [PubMed] [Google Scholar]