Abstract
Intravenous infusion of bone marrow cells has demonstrated therapeutic efficacy in animal models of cerebral ischemia and spinal cord injury. We intravenously delivered human mesenchymal stem cells (SH2+, SH3+, CD34−, and CD45−) immortalized with a human-telomerase gene (hTERT-MSCs) and transfected with eGFP or LacZ into rats 12 h after induction of transient middle cerebral artery occlusion (MCAO), to study their potential therapeutic benefit. hTERT-MSCs were delivered at 12 h after lesion induction. Lesion size was assessed using MR imaging and spectroscopy, and histological methods. Functional outcome was assessed using the Morris water maze and a treadmill test. Intravenous delivery of hTERT-MSCs reduced lesion volume and the magnitude of the reduction and functional improvement was positively correlated with the number of cells injected. The reduction of lesion size could be assessed in vivo with MRI and MRS and was correlated with subsequent histological examination of the brain. This work demonstrates that highly purified hTERT-MSCs reduce cerebral infarction volume and improve functional outcome.
Keywords: Bone marrow, Neuroprotection, Regeneration, Stroke, Transplantation
Introduction
Transplantation of bone marrow cells into cerebral ischemia models (Chen et al., 2001; Iihoshi et al., 2004; Kurozumi et al., 2004; Li et al., 2002) has demonstrated reduced lesion size and improved functional outcome. Moreover, bone marrow cell transplantation into demyelinated (Akiyama et al., 2002; Inoue et al., 2003; Sasaki et al., 2001) and spinal cord injury models (Hofstetter et al., 2002) has demonstrated remyelination and improved functional recovery, respectively. The precise cell population in bone marrow responsible for these putative therapeutic effects is uncertain. However, bone marrow-derived mesenchymal stem cells (MSCs) have been suggested to differentiate into bone (Kobune et al., 2003; Prockop, 1997), cartilage (Kobune et al., 2003), cardiac myocytes (Toma et al., 2002), and neurons and glia (Brazelton et al., 2000; Iihoshi et al., 2004; Sanchez-Ramos et al., 2000; Sasaki et al., 2001; Woodbury et al., 2000) both in vitro and in vivo. MSCs are thought to represent a very small proportion of cells in the mononuclear population of bone marrow (Iihoshi et al., 2004; Kobune et al., 2003; Prockop et al., 2001), and a method to isolate and expand them in culture would provide a valuable tool to study their potential therapeutic efficacy in vivo.
Bone marrow cells can be enriched in MSCs by selecting for plastic-adherent cells. As originally described by Friedenstein (1976), these cells will grow to confluency in appropriate culture conditions as flattened fibroblast-like cells. MSCs may be present in different proportions in the cell fraction of various species (Woodbury et al., 2000). MSCs have a distinct cell surface antigen pattern including SH2+, SH3+, and CD34− (Majumdar et al., 1998). Methodologies have been established to culture human MSCs in very high purity (Kobune et al., 2003; Majumdar et al., 1998; Sekiya et al., 2002). However, these cells had reduced mitogenic activity after about 5 cell doublings over the course of about 6 weeks (Kobune et al., 2003). In order to generate larger numbers of human MSCs, we transfected these cells with the human telomerase gene (Kobune et al., 2003). The hTERT-MSCs remained mitogenically active for over a year (Kobune et al., 2003). In the present study, we intravenously delivered immortalized human MSCs into immunosuppressed rats 12 h after induction of unilateral cerebral ischemia.
Our results indicate that systemic delivery of hTERT-MSCs 12 h after cerebral ischemic lesion induction results in reduced lesion size as determined by MRI and histological analysis and improved behavioral outcome. This suggests that the mesenchymal stem cell component of bone marrow is an important cellular element of bone marrow that confers neuroprotection in this model system, and that immortalized and expanded human MSCs retain their neuroprotection properties.
Materials and methods
Preparation of human mesenchymal stem cells and immortalization
Human bone marrow from healthy adult volunteers was obtained by aspiration from the posterior iliac crest after informed consent was obtained; this study was approved by the Institutional Review Board at our university. Bone marrow mononuclear cells were isolated, and were plated in 150-cm2 plastic tissue culture flasks and incubated overnight. After washing away the free cells, the adherent cells were cultured in Mesenchymal Stem Cell Basal Medium (MSCBM, Cambrex) containing Mesenchymal Cell Growth Supplement (MCGS, Cambrex), 4 mM L-glutamine, in a humidified atmosphere of 5% CO2 at 37°C. After reaching confluence, they were harvested and cryopreserved as primary MSCs or used for gene transduction.
The primary MSCs were immortalized with a retroviral vector, BABE-hygro-hTERT (Kawano et al., 2003). MSCs within 40 population doublings (PD) were used in this study. The morphological features of the MSC were the same as those previously described by Kobune et al. (2003). Adult normal human dermal fibroblasts were obtained from Takara Bio Inc. in Japan, and were cultured in DMEM containing 10% FBS in a humidified atmosphere of 5% CO2 at 37°C.
Phenotypic characterization of the primary MSCs and hTERT-MSCs
Flow cytometric analysis of MSCs was performed as previously described (Kobune et al., 2003). Briefly, cell suspensions were washed twice with PBS containing 0.1% bovine serum albumin (BSA). For direct assays, one million cells were incubated with FITC-conjugated CD34, CD45, CD9 (Immunotech, Marseille, France), CD166 (ALCAM) (Antigenix America, Huntington, NY, USA), CD105 (SH-2), CD106 (Ancell, Bayport, MN, USA), or CD157 (MBL, Nagoya, Japan), and PE-conjugated CD117 (Immunotech), or CD133 (Miltenyi Biotech, Auburn, CA) at 4°C for 30 min, and then washed twice with PBS containing 0.1% BSA. For indirect assays, cells were immunolabeled with anti-human CD73 (SH-3) (Alexis Biochemicals, San Diego, CA, USA). As the secondary antibody, goat anti-mouse IgG (H + L)-FITC (Immunotech) was used. The cells were analyzed by cytometric analysis using an EPICS XL flow cytometer (Becton-Dickinson, San Diego, CA, USA) with EXPO32 software.
Cerebral ischemic model
The rat middle cerebral artery occlusion model was used as a stroke model. We induced transient middle cerebral artery occlusion (MCAO) for 45 min by using a previously described method of intraluminal vascular occlusion (Longa et al., 1989). Adult male Sprague–Dawley rats (n = 60) weighing 250 to 300 g were initially anesthetized with 5% isoflurane and maintained under anesthesia with 1.5% isoflurane in a mixture of 70% N2O and 30% O2 with mechanical ventilation. Rectal temperature was maintained at 37°C with an infrared heat lamp. The left femoral artery was cannulated for measuring blood pH, pO2, and pCO2 throughout the surgery. A length of 20.0–22.0 mm 3-0 surgical dermalon suture with the tip rounded by heating near a flame was advanced from the external carotid artery into the lumen of the internal carotid artery until it blocked the origin of the MCA. Forty-five minutes after MCAO, reperfusion was performed by withdrawal of the suture until the tip cleared the internal carotid artery.
Transplantation procedures
Adex1CAlacZ adenovirus and Adex1CAeGFP adenovirus were used to transduce either the LacZ gene or GFP gene into the MSCs. Details of the construction procedures are described elsewhere (Nakagawa et al., 1998; Nakamura et al., 1994; Takiguchi et al., 2000). This adenoviral vector carries an adenovirus serotype-5 genome lacking the E1A, E1B, and E3 regions to prevent virus replication, and contains the Escherichia coli β-galactosidase gene, lacZ gene, between the CAG promoter (Niwa et al., 1991), composed of the cytomegalovirus enhancer plus the chicken β-actin promoter, and the rabbit β-globin polyadenylation signal in the place of the E1A and E1B regions. The recombinant adenovirus was propagated and isolated in 293 cells. Viral solutions were stored at −80°C until use. For in vitro adenoviral infection, 1.0 ×107 MSCs were placed with Adex1CAlacZ at 50 MOI for 1 h and incubated at 37°C in DMEM containing 10% fetal calf serum.
Approximately 1 ×104 to 1 ×107 hTERT-MSCs in 1 ml total fluid volume (MSCBM) were injected into a left femoral vein 12 h after MCAO (just after the initial MRI measurement). Experiments consisted of six groups. In group 1 (control), rats were given medium alone (without donor cell administration) injected intravenously at 12 h after MCAO. In groups 2 to 5, rats were given hTERT-MSCs (1.0 ×104, 1.0 ×105, 1.0 ×106, 1.0 ×107) injected intravenously at 12 h after MCAO, respectively. In groups 6 and 7, rats were given primary MSCs (1.0 ×106) or fibroblasts (1.0 ×106) injected intravenously at 12 h after MCAO, respectively.
MRI and MRS
Rats were anesthetized with ketamine (75 mg/kg) and xylazine (10 mg/kg) i.p. Each rat was placed in an animal holder/MRI probe apparatus and positioned inside the magnet. The animal’s head was held in place inside the imaging coil. All MRI measurements were performed using a 7-T, 18-cm-bore superconducting magnet (Oxford Magnet Technologies) interfaced to a UNITYINOVA console (Oxford Instruments, UK and Varian, Inc., Palo Alto, CA). T2-weighted images were obtained from a 1.0-mm- thick coronal section using a 3-cm field of view, TR = 3000 ms, TE = 30 ms, and reconstructed using a 128 ×128 image matrix. Magnetic Resonance Spectroscopy was carried out using TR = 1500 ms, TE = 20 ms, Average = 1024, the voxel size of 2.5 ×2.5 ×2.5 mm3. Accurate positioning of the brain was performed to center the image slice 5 mm posterior to the rhinal fissure with the head of the rat held in a flat skull position. MRI measurements were obtained 12 h and 1 week after MCAO.
The ischemic lesion area was calculated from T2-weighted images using imaging software (Scion Image, Version Beta 4.0.2, Scion Corporation), based on the previously-described method (Kurozumi et al., 2004, 2005; Neumann-Haefelin et al., 2000). For each slice, the higher intensity lesions in T2-weighted images where the signal intensity were 1.25 times higher than the counterpart in the contra-lateral brain lesion were marked as the ischemic lesion area, and infarct volume was calculated taking slice thickness (1 mm/slice) into account.
Histological analysis
TTC-staining, HE-staining, and quantitative analysis of infarct volume
One week after transplantation, the rats were deeply anesthetized with sodium pentobarbital (50 mg/kg, i.p.). The brains were removed carefully and dissected into coronal 1-mm sections using a vibratome. The fresh brain slices were immersed in a 2% solution of 2,3,5-triphenyltetrazolium chloride (TTC) in normal saline at 37°C for 30 min.
For hematoxylin–eosin staining, the rats were deeply anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and perfused through the heart, first with PBS, and then with a fixative solution containing 10% paraformaldehyde in 0.14 M Sorensen’s phosphate buffer, pH 7.4. Brains were removed and placed in 10% paraformaldehyde in phosphate-buffer overnight, dehydrated, and embedded in paraffin. Transverse sections (5 μm) were cut, and were counterstained with hematoxylin and eosin.
The cross-sectional area of infarction in each brain slice was examined with a dissection microscope and was measured using an image analysis software, NIH image. The total infarct volume for each brain was calculated by summation of the infarcted area of all brain slices.
Detection of donor MSC cells and phenotypic analysis in vivo
One week after transplantation, eGFP-expressing cells were detected in vivo. Brains of the deeply-anesthetized rats were removed, fixed in 4% paraformaldehyde in phosphate-buffer, dehydrated with 30% sucrose in 0.1 M PBS for overnight, and frozen in powdered dry ice. Coronal cryostat sections (10 μm) were processed. To excite the eGFP fluorescence, a 488-nm laser line generated by an argon laser was used. Confocal images were obtained using a Zeiss laser scanning confocal microscope with the use of Zeiss software.
Phenotypic analysis of the transplanted cells in vivo was carried out using laser scanning confocal microscopy. Coronal cryostat sections (10 μm) were processed for immunohistochemistry. To identify the cell type derived from the donor bone marrow, double-labeling studies were performed with the use of antibodies to beta-galactosidase (rhodamine-labeled polyclonal rabbit anti-beta-galactosidase antibody, DAKO), neurons (FITC-labeled monoclonal mouse NeuN, DAKO), and astrocytes (FITC-labeled monoclonal mouse anti-glial fibrillary acidic protein [GFAP], SIGMA).
To excite the FITC fluorochrome (green), a 488-nm laser line generated by an argon laser was used, and for the Rhodamine fluorochrome (red), a 543-nm laser line from a HeNe laser was used. Confocal images were obtained using a Zeiss laser scanning confocal microscope with the use of Zeiss software.
Behavior study
Morris water maze test
Acquisition of spatial learning was tested using procedures adapted from Morris (n = 10) (Morris, 1981). The apparatus consisted of a 1.3-m-diameter white steel tank filled to a depth of 30 cm of water that was made opaque with white tempera paint and maintained at 24°C. The walls of the room contained visual cues that remained constant across the experiments. A ceramic 8-cm-diameter circular platform was submerged under 2.5 cm of water in one quadrant of the tank during all training trials. On the first training day, a single habituation trial was performed by placing each animal on the hidden platform for 60 s. If the animal fell or jumped from the platform, it was removed from the water and placed back on the platform. Quadrant search, and swim speed were monitored by a video camera mounted from the ceiling and connected to a computerized tracking image analyzer system.
Treadmill stress test
Rats were trained 20 min per day for 2 days a week to run on a motor driven treadmill at a speed of 20 m/min with a slope of 0°. Rats were placed on a moving belt facing away from the electrified grid and induced to run in the direction opposite of the movement of the belt. Thus, to avoid foot-shocks (with intensity in 1.0 mA), the rats had to move forward. Only the rats that had leaned to avoid the mild electrical shock were included in this study (n = 10). The maximum speed at which the rats could run on a motor-driven treadmill was recorded.
Statistical analysis
The lesion volume and the behavior scores (Morris water maze test and treadmill stress test) recorded were statistically analyzed. Data are presented as mean values ±SD. Differences among groups were assessed by ANOVA with Scheffes post hoc test to identify individual group differences. Differences were deemed statistically significant at P < 0.05.
Results
Morphological and surface antigen characteristics of primary and immortalized human MSCs
Primary human MSCs cultured as plastic adherent cells could be maintained in culture for about 6 weeks which corresponded to about 10 population doublings (PD). The cells grow to confluency in about 1 week, and are expanded by replating just prior to confluency. Fig. 1A shows the morphological features of these cells at about 10 PD. Characteristic flattened and spindle-shaped cells can be recognized. A characteristic feature of MSCs is a CD34−, CD45−, SH2+(CD105), SH3+(CD73) cell surface phenotype (Kobune et al., 2003). Flow cytometric analysis of the primary MSCs indicated this cell surface phenotype (Fig. 1C). Further indication of MSC phenotype was collaborated by CD117−, CD133−, CD166+, CD9+, CD157+, CD166+ (ALCAM) cell surface expression (data not shown). Primary MSCs become senescent by about 6 weeks in culture (about 10 PD). However, after immortalization by a retroviral vector, BABE-hygro-hTERT, the cells could be maintained for at least 1 year utilizing the same culture conditions as for the primary MSCs. Fig. 1B is a photomicrograph of hTERT-MSCs maintained for 5 months (PD = 30), a time beyond the survival period of primary MSCs. Note the similar flattened and spindle-shaped morphology of immortalized cells. Flow cytometric analysis of the hTERT-MSCs was essentially identical to primary MSCs (Fig. 2D). These data are in agreement with previous work (Deans and Moseley, 2000) suggesting that the transduction of hTERT cDNA prolonged the life span of human MSCs without transformation.
Fig. 1.
May–Giemsa staining of primary MSCs (A) and hTERT-MSCs (B) (scale bar = 20 μm). Flow cytometric analysis of the expression of surface antigens on primary MSCs (C) and hTERT-MSCs (D). The cells were immunolabeled with the FITC-conjugated monoclonal antibody specific for the indicated surface antigen. Dead cells were eliminated by forward and side scatter.
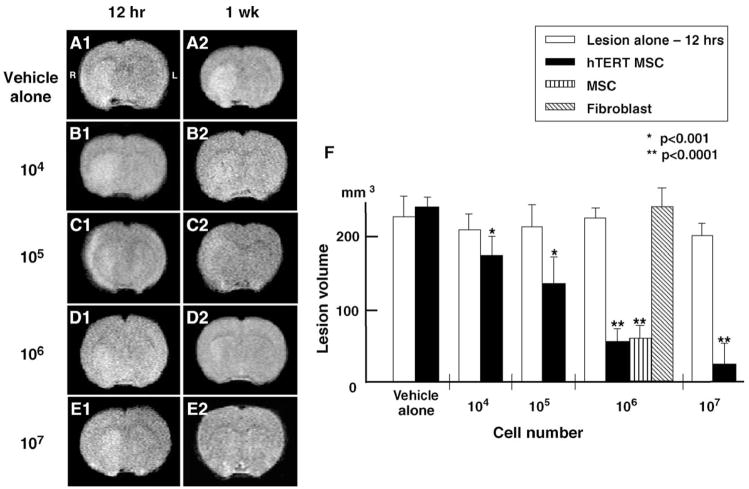
Fig. 2.
Evaluation of the ischemic lesion volume with MRI. T2-weighted images were obtained from experimental animals 12 h after MCAO and 1 week after intravenous delivery of varying concentrations of hTERT-MSCs. The left-hand column (panels A1–E1) shows single brain images obtained 12 h post injury. The right hand column (panels A2–E2) show images 1 week after treatment. Panel F indicates summary of the lesion volumes in each group.
Characterization of ischemic lesion size by magnetic resonance image analysis
An initial estimate of lesion size was obtained using in vivo MRI (see Materials and methods). Brain images (T2- weighted) were collected from all experimental animals 12 h after MCAO and 1 week after intravenous delivery of varying concentrations of hTERT-MSCs (Figs. 2A–E). The cells were intravenously delivered immediately after the 12 h MRI. The left-hand column in Fig. 2 (A1–E1) shows single brain images obtained 12 h post injury for five rats. These coronal forebrain sections were obtained at the level of caudato-putamen complex. Note the reduction in density in lesions on the right side of the brains that were subjected to ischemic injury. Lesion volume (mm3) was determined by analysis of high-intensity areas on serial images collected through the cerebrum (see Materials and methods). The infarction volume at 12 h post lesion induction was 214 ±23 mm3, n = 25.
In order to determine the efficacy of hTERT-MSCs transplantation in modulating the ischemic lesion volume, varying concentrations of cells (104, 105, 106, 107) were delivered intravenously at 12 h after lesion induction, and animals were re-imaged 1 week later. Five animals were used for each cell concentration. In sham control (vehicle alone) (Fig. 2F), there was no change in MRI-estimated lesion volume between 12 h and 1 week post MCAO (226 ±28 mm3, n = 5, vs. 239 ±15 mm3, n = 5, P = 0.39). Lesion volume progressively decreased with intravenous injection of increasing number of hTERT-MSCs (104 = 176 ±21 mm3; 105 = 138 ±36 mm3; 106 = 56 ±18 mm3; 107 = 23 ±31 mm3) (Fig. 2F). These reductions in lesion volume were also significant (P < 0.001) when compared to 1 week sham control.
Primary MSCs were also intravenously delivered in a separate experiment. Delivery of 106 primary MSCs showed an identical reduction in lesion volume as hTERT-MSCs of the same cell number (Fig. 2F) (61 ±18 mm3, n = 5, vs. 56 ±18 mm3, n = 5, P = 0.69). An additional sham control experiment was carried out using skin fibroblast (Fig. 2F). No reduction in lesion volume was observed with delivery of 106 skin fibroblasts (240 ±27 mm3, n = 5, P = 0.95).
Histological determination of infarction
After completion of the MRI analysis to estimate lesion volume, before and after cell delivery, the animals were perfused and stained with 2,3,5-triphenyltetrazolium chloride (TTC) to obtain a second independent measure of infarction volume (n = 5 for each group). Normal brain (gray matter) tissue typically stains with TTC, but infarcted lesions show no or reduced staining (Bederson et al., 1986). TTC-staining obtained 1 week after MCAO without cell transplantation is shown in Fig. 3A. Note the reduced staining on the lesion side primarily in the corpus striatum. Lesion volume was calculated by measuring the area of reduced TTC-staining in the forebrain (see Materials and methods). As with MRI analysis, there was a progressive reduction in infarction size with increasing number of transplanted cells. Intravenous delivery of 107 hTERT-MSCs resulted in very substantial reduction in lesion volume as estimated from TTC-staining (Figs. 3B, E).
Fig. 3.
Summary of the TTC-unstained lesion volumes in each group. Brain slices were stained with 2,3,5-triphenyltetrazolium chloride (TTC) to visualize the damaged lesions. A brain slice from the non-treated MCAO rats is shown for comparison (A). The TTC-stained brain slices from rats that were intravenously transplanted with 107 hTERT-MSCs 12 h after MCAO are shown in panel B. The sections were also counterstained with hematoxylin and eosin. Although the glial scar tissue and a large number of inflammatory cells were obvious in the lesion without cell transplantation (C), parenchymal brain tissue was greatly preserved in the treated group (D). Note that a small number of inflammatory cells were observed in the regions that may be heavily stressed by the ischemia. Panel E summarizes the TTC-unstained lesion volumes in each group. Scale bar = 3 mm (A, B), 20 μm (C, D).
The detailed histological examination with HE-staining indicated that the ischemic damage was greater and the glial scar tissue and a large number of inflammatory cells were observed in the damaged lesion in the non-treated group (218 ±21 mm3, n = 5) (Fig. 3C). On the other hand, most parenchymal brain tissue was protected in the treated group (12 ±15 mm3, n = 5), even though a small number of inflammatory cells were observed in the regions that may be heavily stressed by the ischemia (Fig. 3D).
Identification and differentiation of donor cells in vivo
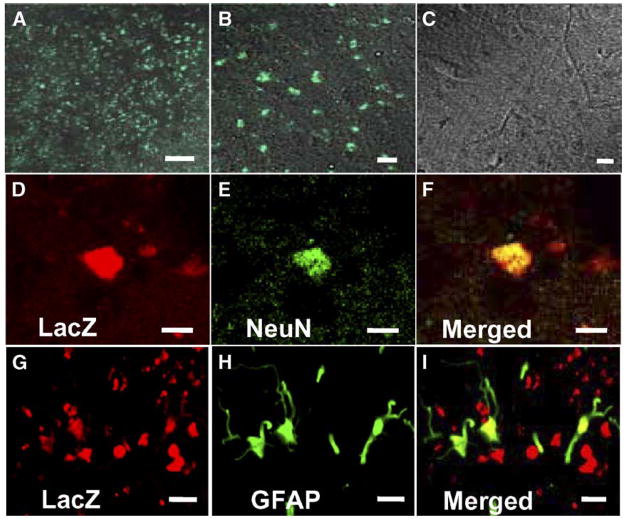
GFP-transfected or LacZ-transfected MSCs that had been intravenously administered (107 cells) 12 h after MCAO were identified in vivo (n = 10). The GFP-expressing hTERT-MSCs were found primarily in the corpus striatum; the area of greatest identifiable infarction in the non-transplanted animals. Merged confocal images showing GFP fluorescence and transmitted light images at low and high power in the striatum on the lesion side are shown in Figs. 4A and B, respectively. Note the abundance of GFP-positive cellular-like elements. While primarily clustered in the corpus striatum, some GFP-expressing cells were observed throughout the affected hemisphere. Images obtained from non-lesion contralateral striatum showed no GFP expression (Fig. 4C). These data suggest that systemic deliver of cells reached the lesion site. Immunohistochemical studies were carried out to identify neurons (NeuN) and astrocytes (GFAP) in the lesion zone in animals transplanted with LacZ-transfected hTERT-MSCs. Only small numbers of NeuN (9.31 ±0.34%, n = 5) and GFAP positive cells (7.98 ±0.42%, n = 5) were co-stained with LacZ (Figs. 4D–I), and other donor-derived cells seemed to be not differentiated into neural or glial cells.
Fig. 4.
Intravenously-administrated hTERT-MSCs accumulated in and around the ischemic lesions. Lower (A) and higher micrograph (B) demonstrating a large number of eGFP-positive cells in and around the lesion, but not in the non-treated rats (C). Confocal images show the differentiation of the transplanted hTERT-MSCs (LacZ in red; D, G) into neurons (NeuN in green; E) or astrocytes (GFAP in green; H). Panels F and I confirm the co-labeling of LacZ/NeuN or LacZ/GFAP in the cells, respectively. Scale bar = 250 μm (A), 10 mu;m (B, C), 5 mu;m (D–I).
Magnetic resonance spectroscopy
Magnetic resonance spectroscopy (MRS) was utilized to study NAA and lactate levels in the brain before and after cell transplantation (see Materials and methods). NAA signals in MRS have been shown to correlate with the presence of healthy neurons, and elevated lactate with neuronal damage (Barker et al., 1994). Twelve hours after MCAO induction, MRS analysis was carried out to access NAA and lactate levels in the lesioned and non-lesioned hemispheres. The non-lesioned hemisphere showed the high levels of NAA (NAA/Cr peak ratio: 1.12 ±0.10, n = 5) and no lactate signal (Figs. 5A, D). Conversely, the lesion side showed low NAA (NAA/Cr peak ratio: 0.81 ±0.08, n = 5) and high lactate signals (Lac/Cr peak ratio: 2.59 ±0.42, n = 5) (Figs. 5A, E). With no cell transplantation NAA signals were low (NAA/Cr peak ratio: 0.63 ±0.10, n = 5) and lactate signals were high (Lac/Cr peak ratio: 1.31 ±0.13, n = 5) 1 week after lesion induction (Figs. 5B, F). However, following intravenous delivery of 107 hTERT-MSCs at 12 h post lesion, MRS analysis 1 week later indicated that NAA signals were present (NAA/Cr peak ratio: 1.22 ±0.19, n = 5) and lactate signals were low (Lac/Cr peak ratio: 0.21 ±0.11, n = 5) (Figs. 5C, G), suggesting preservation of brain tissue by the transplantation procedure.
Fig. 5.
Evaluation of cell therapy on stroke model rats by Magnetic Resonance Spectroscopy (MRS). NAA and lactate levels in the brain before and after cell transplantation were studied. Twelve hours (A, D, E) and 1 week (B, C, F, G) after MCAO induction, MRS analysis was carried out to access NAA and lactate levels in the lesioned (E–G) and non-lesioned hemispheres (D). With no cell transplantation, NAA signals were low and lactate signals were high 1 week after lesion induction (B, F). However, following intravenous delivery of 107 hTERT-MSCs, NAA signals were present and lactate signals were low (C, G). Scale bar = 5 mm (A–C).
Functional analysis
To access behavioral performance in the lesioned and transplanted animals, two functional tests were used: the treadmill stress test and Morris water maze test (see Materials and methods). Behavioral testing began 1 week after lesion induction alone or with cell transplantation. In the treadmill stress test, control animals (no lesion) reach a maximum treadmill velocity of about 60 m/min (Iihoshi et al., 2004). One week after MCAO with or without transplantation, maximum velocity on the treadmill test was about 35 m/min (Fig. 6A). Non-treated animals showed increased treadmill velocity beginning 11 days post lesion with slow improvement up to 25 days (46.3 ±6.1 m/min, n = 10). In the cell transplantation group, the improvement in velocity was greater and approached control values (62.0 ±7.2 m/min, n = 10) at 25 days post injury.
Fig. 6.

The functional benefits following the intravenous administration of hTER-MSCs into the MCAO model rats were analyzed. The Treadmill test clearly demonstrates that the maximum speed at which the rats could run on a motor-driven treadmill was faster in the treated rats (A). The Morris water maze test also indicates that the learning and memory function was damaged in the MCAO model rats, but rescued and improved in the treated group (B). Days were plotted after MCAO.
The animals were also tested with more complex behavioral paradigm, Morris water maze test (see Materials and methods). Testing was carried out on alternate days for 26 days (Fig. 6B). Control rats learned to swim to mount the platform within a few seconds. The animals subjected to MCAO require about 140 s to perform the task. Non-transplanted animals show gradual improvement and on the 26th day of testing mount the platform by about 40 s. Animals with an MCAO lesion and intravenous hTERT-MSC injection also show progressive shortening of time for platform mounting, and by 26 days, platform mounting is achieved by a few seconds.
Behavioral improvement was observed for both tests. The Treadmill test showed that the maximum speed at which the treated rats could run on a motor-driven treadmill was faster than that of the non-treated rats, although the motor deficits were not obvious in both the non-transplanted and transplanted groups from casual observation. The mild motor dysfunction in the present study unlikely accounts for the deficiency in the Morris water maze test, although severe motor deficits might affect speed of swimming.
Discussion
We demonstrate that intravenous infusion of immortalized human mesenchymal stem cells 12 h after transient MCAO in the rat results in reduction in infarction volume and improvement in behavioral performance. These results are consistent with previous studies showing beneficial effects of bone marrow cell infusion in experimental cerebral ischemic models (Iihoshi et al., 2004; Kurozumi et al., 2004; Li et al., 2002). Because stromal cells represent a mixed cell population, the cell type conferring neuroprotection in stromal cell transplantation studies is uncertain. Use of hTERT-MSCs in our study suggests that the mesenchymal stem cell component of bone marrow may contribute to the therapeutic benefits of this procedure. Yet, we are cautious in making this interpretation in that development of human MSCs can show variability from culture to culture and from different bone marrow aspirates even from the same subject (Sekiya et al., 2003). However, our immortalized cells showed epitopes characteristic of MSCs (Kobune et al., 2003). Given that cell number is important to achieve maximum therapeutic benefits, identification and preparation of a single cell type will likely offer advantage in cell therapy approaches to treat neurologic diseases.
A concern with in vitro cell expansion and immortalization is that changes in cell phenotype may occur and the cells could potentially either lose their neuroprotective potential in vivo or may elicit deleterious effects. In this regard, it is important that the hTERT-MSCs used in this study reduced the infarction area, improved functional outcome and no obvious negative effects were observed. However, we have not as yet tested extensively expanded hTERT-MSCs and therefore do not know the extent of cell expansion that would be compatible with safe and efficacious in vivo transfer.
We estimated lesion volume in vivo before and after cell transplantation using MRI. Subsequent histological examination of the infarcted brain indicated a clear correspondence between in vivo MRI and histology. Moreover, MRS indicated expected changes in NAA and lactate in normal, ischemic, and transplanted animals.
Although we did observe a very limited number of cells that showed features suggestive of neurons and glia in the lesion zone, the extent of infarction reduction could not be explained by neurogenesis. It is much more likely that a neuroprotective effect was operative. In this regard, a number of neurotrophic factors have been reported to have therapeutic effects on cerebral infarction (Hirouchi and Ukai, 2002). These include GDNF, BDNF, NGF, EGF, and bFGF (Kurozumi et al., 2004). Mechanisms proposed for the neuroprotective effect of these agents include free radical scavenging, anti-apoptotic activity, anti-inflammatory activity, and anti-glutamate excitotoxicity (Hirouchi and Ukai, 2002). Indeed, a recent report introduced the concept that stem cells may exert a therapeutic influence on abnormal hosts by exerting a protective “chaperone” effect (Ourednik et al., 2002). A recent report using olfactory ensheathing cell transplantation in the injured spinal cord, suggests that the cells may confer a neuroprotective effects on the injured spinal cord (Plant et al., 2003). Indeed, cell transplantation of a variety of cell types may operate through common mechanisms. Transplanted cells may provide neurotrophic support for endogenous cell survival (Chen et al., 2002), but additionally may contribute to neural repair by, for example, remyelination (Akiyama et al., 2001, 2002; Honmou et al., 1996; Kato et al., 2000). An advantage of bone marrow-derived MSCs is that they can be easily and safely obtained in large numbers from autologous bone marrow aspirates. While controversial (Alvarez-Dolado et al., 2003; Castro et al., 2002), it has been suggested that transdifferentiation of bone marrow cells into astrocytes (Azizi et al., 1998; Kopen et al., 1999), neurons (Brazelton et al., 2000; Iihoshi et al., 2004), and into myelin-forming cells (Akiyama et al., 2002; Inoue et al., 2003; Sasaki et al., 2001) can occur in the CNS after direct or intravenous delivery. The prospect that intravenous delivery of these cells could lead to a global neuroprotection with subsequent repair such as remyelination is intriguing and should be further explored.
In addition to neurotrophins, bone marrow cells secrete interleukins, macrophage colony-stimulating factor, Flt-3 ligand, and stem-cell factors (Eaves et al., 1991; Majumdar et al., 1998). Bone marrow cells also provide several angiogenic growth factors such as VEGF and bFGF (Hamano et al., 2000), which may prevent endothelial cells from ischemic damage or stimulate angiogenesis. These cells produce soluble mediators that down-regulate immune responses (Bernstein and Shearer, 1988) which could also contribute to neuroprotection. Our results indicate that the transplanted cells reach the lesion zone as indicated by GFP fluorescence. Given that the transplanted hTERT-MSCs reach the lesion site, they may through the various mechanisms described reduce the ischemic brain from secondary injury. In our study, a large number of the transplanted hTERT-MSCs were identified in areas of the cerebrum with little damage that may correspond to the penumbra region of untreated animals. These cells probably accessed the damaged lesion from the systemic circulation; ischemic stress results in a partial disruption of the blood–brain barrier (BBB) that increases at 3 h after the ischemic insults (Hatashita and Hoff, 1990). Our interpretation of these data is that the transplanted cells reach the site of infarction and have a significant neuroprotective effect through yet undefined mechanisms on brain areas that would be vulnerable to secondary ischemic injury.
A variety of immature cells such as embryonic stem cells and neural stem cells have been suggested as cell candidates for cell-based stroke therapies. Bone marrow cells are readily available and widely applied clinically for leukemia therapy. Moreover, autologous transplantation of these cells would circumvent potential ethical, infectious, and immunological concerns other cell types may present. The significant therapeutic effect of bone marrow transplantation in experimental cerebral ischemia models (Iihoshi et al., 2004; Li et al., 2002) even when applied many hours after infarction, and the prospect of preparation of large numbers of autologous cells from bone marrow aspirates, suggests the feasibility of this approach in clinical studies.
Acknowledgments
This work was supported in part by grants from the Japanese Science and Technology Agency (12207), the Japanese Ministry of Education, Science, Sports and Culture (16390414, 14370442, 09671434, 11770765, 12470295), the Japanese Ministry of Economy, Trade, and Industry, and NEDO, Mitsui Sumitomo Insurance Welfare Foundation, the National Multiple Sclerosis Society (U.S.A) (RG2135; CA1009A10), the National Institutes of Health (NS43432), and the Medical and Rehabilitation and Development Research Services of the Department of Veterans Affairs.
References
- Akiyama Y, Honmou O, Kato T, Uede T, Hashi K, Kocsis JD. Transplantation of clonal neural precursor cells derived from adult human brain establishes functional peripheral myelin in the rat spinal cord. Exp Neurol. 2001;167:27–39. doi: 10.1006/exnr.2000.7539. [DOI] [PubMed] [Google Scholar]
- Akiyama Y, Radtke C, Honmou O, Kocsis JD. Remyelination of the spinal cord following intravenous delivery of bone marrow cells. Glia. 2002;39:229–236. doi: 10.1002/glia.10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003 Oct. 30;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats—Similarities to astrocyte grafts. Proc Natl Acad Sci U S A. 1998;95:3908–3913. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker PB, Gillard JH, van Zijl PC, Soher BJ, Hanley DF, Agildere AM, Oppenheimer SM, Bryan RN. Acute stroke: evaluation with serial proton MR spectroscopic imaging. Radiology. 1994;192(3):723–732. doi: 10.1148/radiology.192.3.8058940. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–1308. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- Bernstein DC, Shearer GM. Suppression of human cytotoxic T lymphocyte responses by adherent peripheral blood leukocytes. Ann N Y Acad Sci. 1988;532:207–213. doi: 10.1111/j.1749-6632.1988.tb36339.x. [DOI] [PubMed] [Google Scholar]
- Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- Castro RF, Jackson KA, Goodell MA, Robertson CS, Liu H, Shine HD. Failure of bone marrow cells to transdifferentiate into neural cells in vivo. Science. 2002 Aug. 23;:297. doi: 10.1126/science.297.5585.1299. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001;189:49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- Chen X, Li Y, Wang L, Katakowski M, Zhang L, Chen J, Xu Y, Gautam SC, Chopp M. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002;22:275–279. doi: 10.1046/j.1440-1789.2002.00450.x. [DOI] [PubMed] [Google Scholar]
- Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000;28(8):875–884. doi: 10.1016/s0301-472x(00)00482-3. [DOI] [PubMed] [Google Scholar]
- Eaves CJ, Cashman JD, Kay RJ, Dougherty GJ, Otsuka T, Gaboury LA, Hogge DE, Lansdorp PM, Eaves AC, Humphries RK. Mechanisms that regulate the cell cycle status of very primitive hematopoietic cells in long-term human marrow cultures: II. Analysis of positive and negative regulators produced by stromal cells within the adherent layer. Blood. 1991;78:110–117. [PubMed] [Google Scholar]
- Friedenstein AJ. Precursor cells of mechanocytes. Int Rev Cytol. 1976;47:327–359. doi: 10.1016/s0074-7696(08)60092-3. [DOI] [PubMed] [Google Scholar]
- Hamano K, Li TS, Kobayashi T, Kobayashi S, Matsuzaki M, Esato K. Angiogenesis induced by the implantation of self-bone marrow cells: a new material for therapeutic angiogenesis. Cell Transplant. 2000;9:439–443. doi: 10.1177/096368970000900315. [DOI] [PubMed] [Google Scholar]
- Hatashita S, Hoff JT. Brain edema and cerebrovascular permeability during cerebral ischemia in rats. Stroke. 1990;21:582–588. doi: 10.1161/01.str.21.4.582. [DOI] [PubMed] [Google Scholar]
- Hirouchi M, Ukai Y. Current state on development of neuroprotective agents for cerebral ischemia. Nippon Yakurigaku Zasshi. 2002;120:107–113. doi: 10.1254/fpj.120.107. [DOI] [PubMed] [Google Scholar]
- Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci U S A. 2002;99:2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honmou O, Felts PA, Waxman SG, Kocsis JD. Restoration of normal conduction properties in demyelinated spinal cord axons in the adult rat by transplantation of exogenous Schwann cells. J Neurosci. 1996;16:3199–3208. doi: 10.1523/JNEUROSCI.16-10-03199.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iihoshi S, Honmou O, Houkin K, Hashi K, Kocsis JD. A therapeutic window for intravenous administration of autologous bone marrow after cerebral ischemia in adult rats. Brain Res. 2004;1007:1–9. doi: 10.1016/j.brainres.2003.09.084. [DOI] [PubMed] [Google Scholar]
- Inoue M, Honmou O, Oka S, Houkin K, Hashi K, Kocsis JD. Comparative analysis of remyelinating potential of focal and intravenous administration of autologous bone marrow cells into the rat demyelinated spinal cord. Glia. 2003;44:111–118. doi: 10.1002/glia.10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Honmou O, Uede T, Hashi K, Kocsis JD. Transplantation of human olfactory ensheathing cells elicits remyelination of demyelinated rat spinal cord. Glia. 2000;30:209–218. doi: 10.1002/(sici)1098-1136(200005)30:3<209::aid-glia1>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y, Kobune M, Yamaguchi M, Nakamura K, Ito Y, Sasaki K, Takahashi S, Nakamura T, Chiba H, Sato T, Matsunaga T, Azuma H, Ikebuchi K, Ikeda H, Kato J, Niitsu Y, Hamada H. Ex vivo expansion of human umbilical cord hematopoietic progenitor cells using a coculture system with human telomerase catalytic subunit (hTERT)-transfected human stromal cells. Blood. 2003;101(2):532–540. doi: 10.1182/blood-2002-04-1268. [DOI] [PubMed] [Google Scholar]
- Kobune M, Kawano Y, Ito Y, Chiba H, Nakamura K, Tsuda H, Sasaki K, Dehari H, Uchida H, Honmou O, Takahashi S, Bizen A, Takimoto R, Matsunaga T, Kato J, Kato K, Houkin K, Niitsu Y, Hamada H. Telomerized human multipotent mesenchymal cells can differentiate into hematopoietic and cobblestone area-supporting cells. Exp Hematol. 2003;31(8):715–722. doi: 10.1016/s0301-472x(03)00177-2. [DOI] [PubMed] [Google Scholar]
- Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, Honmou O, Houkin K, Date I, Hamada H. BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol Ther. 2004;9:189–197. doi: 10.1016/j.ymthe.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Ishii K, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, Honmou O, Houkin K, Date I, Hamada H. Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol Ther. 2005;11:96–104. doi: 10.1016/j.ymthe.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol. 1998;176:57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Morris RGM. Spatial localization does not depend upon the presence of local cues. Learn Motiv. 1981;12:239–260. [Google Scholar]
- Nakagawa I, Murakami M, Ijima K, Chikuma S, Saito I, Kanegae Y, Ishikura H, Yoshiki T, Okamoto H, Kitabatake A, Uede T. Persistent and secondary adenovirus-mediated hepatic gene expression using adenovirus vector containing CTLA4IgG. Hum Gene Ther. 1998;9:1739–1745. doi: 10.1089/hum.1998.9.12-1739. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Wakimoto H, Abe J, Kanegae Y, Saito I, Aoyagi M, Hirakawa K, Hamada H. Adoptive immunotherapy with murine tumor-specific T lymphocytes engineered to secrete interleukin 2. Cancer Res. 1994;54:5757–5760. [PubMed] [Google Scholar]
- Neumann-Haefelin T, Kastrup A, de Crespigny A, Yenari MA, Ringer T, Sun GH, Moseley ME. Serial MRI after transient focal cerebral ischemia in rats: dynamics of tissue injury, blood–brain barrier damage, and edema formation. Stroke. 2000;31(8):1965–1972. doi: 10.1161/01.str.31.8.1965. [DOI] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Ourednik J, Ourednik V, Lynch WP, Schachner M, Snyder EY. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat Biotech. 2002;20:1103–1110. doi: 10.1038/nbt750. [DOI] [PubMed] [Google Scholar]
- Plant GW, Christensen CL, Oudega M, Bunge MB. Delayed transplantation of olfactory ensheathing glia promotes sparing/regeneration of supraspinal axons in the contused adult rat spinal cord. J Neurotrauma. 2003;20(1):1–16. doi: 10.1089/08977150360517146. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Prockop DJ, Sekiya I, Colter DC. Isolation and characterization of rapidly self-renewing stem cells from cultures of human marrow stromal cells. Cytotherapy. 2001;3:393–396. doi: 10.1080/146532401753277229. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W, Patel N, Cooper DR, Sanberg PR. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164(2):247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Honmou O, Akiyama Y, Uede T, Hashi K, Kocsis JD. Transplantation of an acutely isolated bone marrow fraction repairs demyelinated adult rat spinal cord axons. Glia. 2001;35:26–34. doi: 10.1002/glia.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530–541. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- Takiguchi M, Murakami M, Nakagawa I, Saito I, Hashimoto A, Uede T. CTLA4IgG gene delivery prevents autoantibody production and lupus nephritis in MRL/lpr mice. Life Sci. 2000;66:991–1001. doi: 10.1016/s0024-3205(99)00664-5. [DOI] [PubMed] [Google Scholar]
- Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105(1):93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]