Abstract
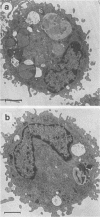
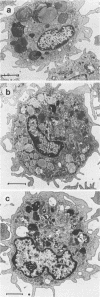
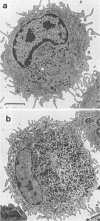
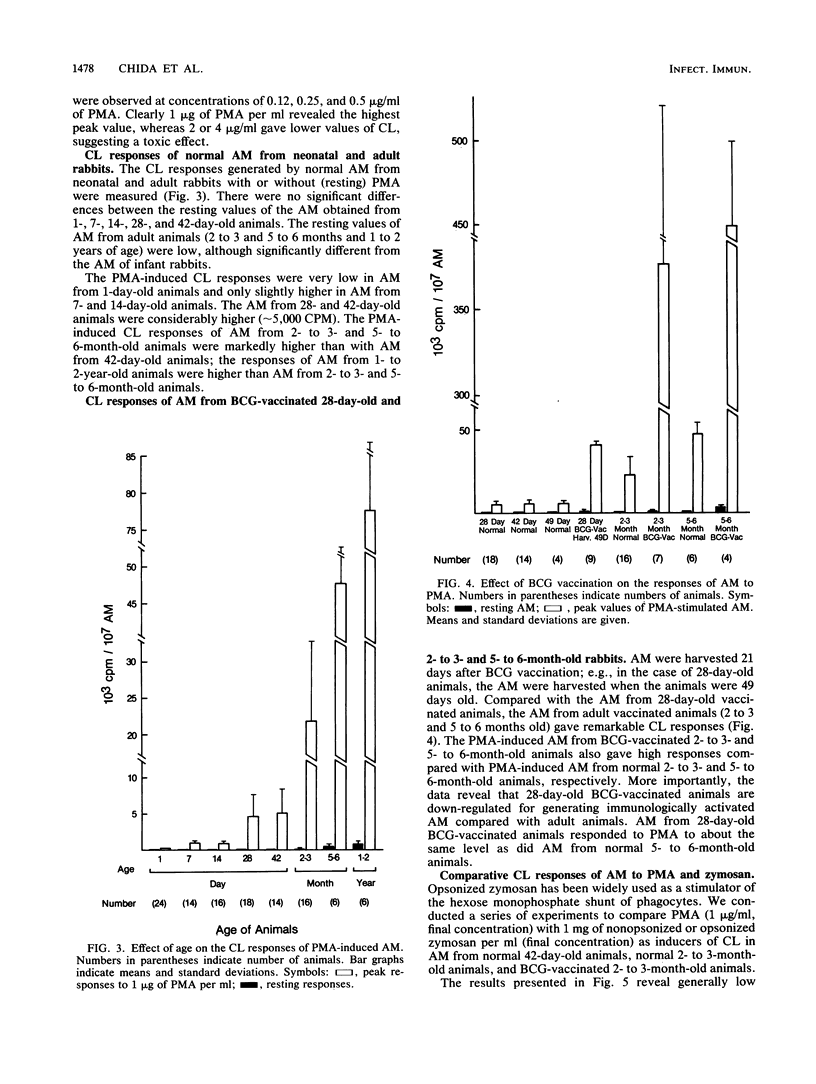
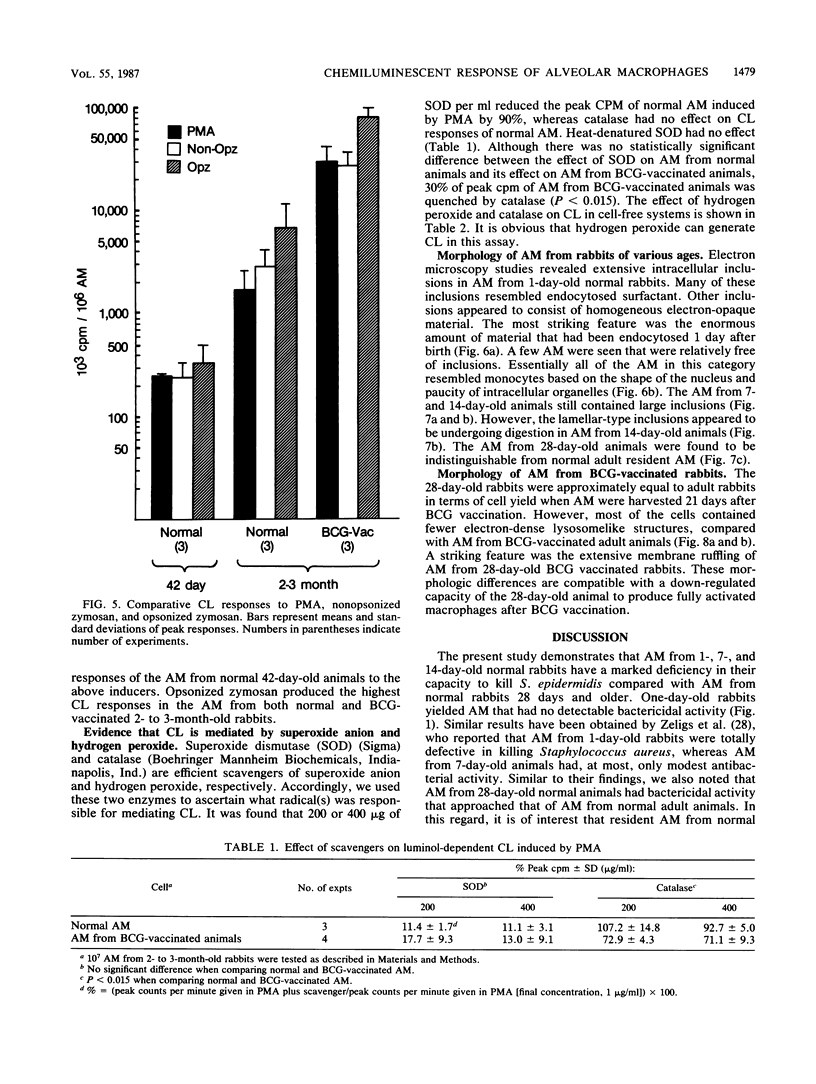
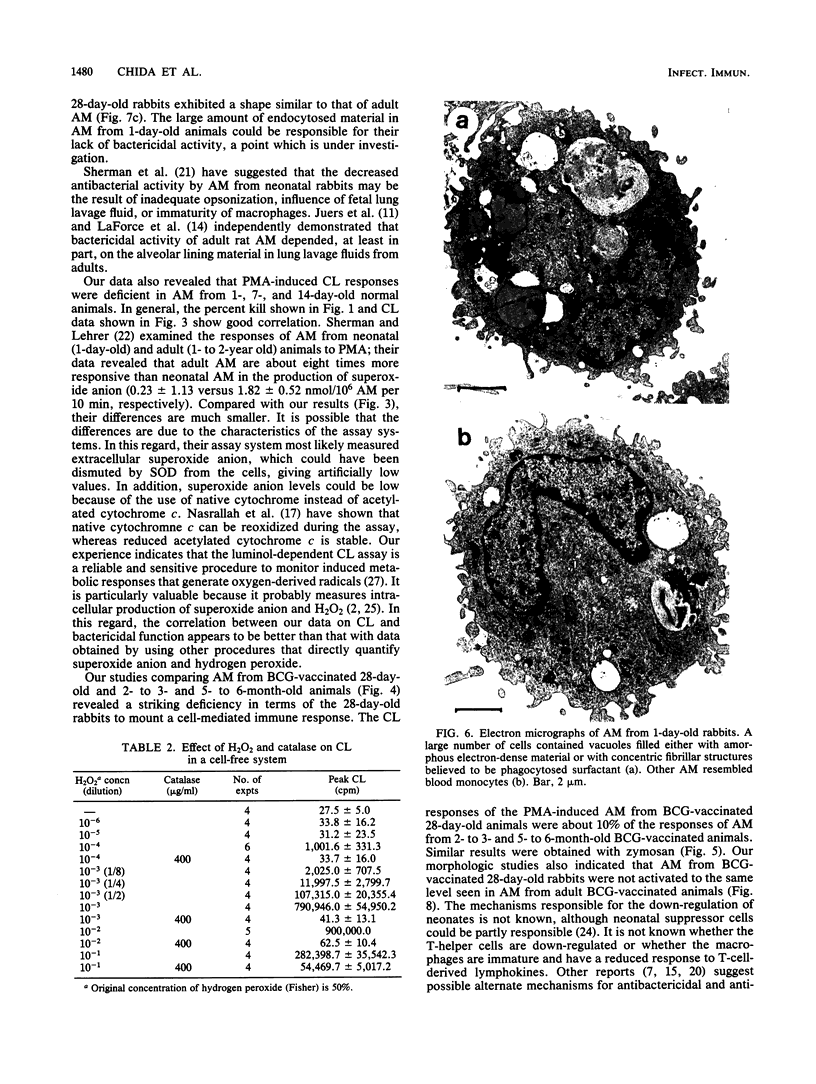
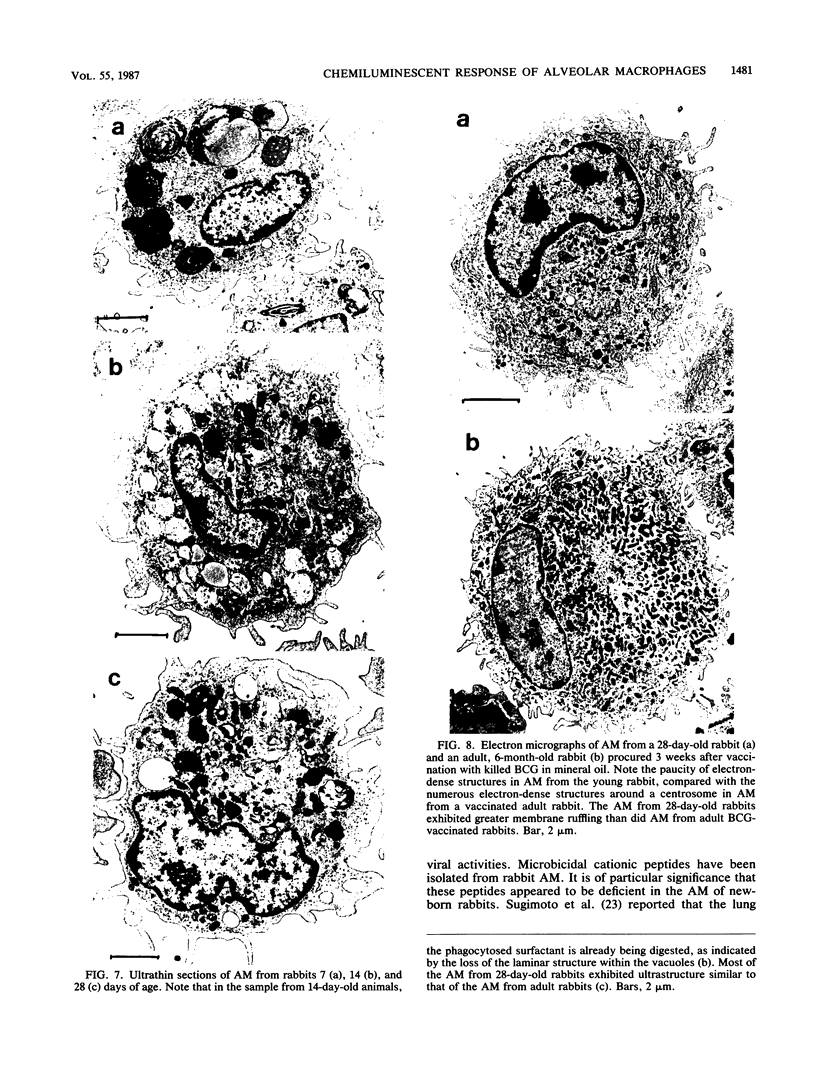
Luminol-dependent chemiluminescence (CL) responses of alveolar macrophages (AM) from normal and Mycobacterium bovis BCG-vaccinated infant and adult rabbits were compared. AM from 1-, 7-, and 14-day-old normal rabbits exhibited much lower peak CL responses than did AM from 28- and 42-day-old normal animals as well as rabbits 2 to 3 or 5 to 6 months and 1 to 2 years of age. The most striking differences among AM from infant and adult rabbits were noted when AM were obtained from 28-day-old and 5- to 6-month old rabbits 21 days after the rabbits were immunized with 200 micrograms of BCG intravenously. In this case, AM from 5- to 6-month-old animals gave peak counts per minute of 400,000 to 500,000 whereas AM from 28-day-old rabbits vaccinated with BCG (harvested at 49 days of age) gave peak counts per minute of only 40,715 +/- 2,688. These data reveal that AM from neonatal animals are grossly deficient as responders to phorbol myristate acetate-induced CL. This deficiency, which improved with age, is still apparent in AM from 28-day-old animals. The data also reveal that BCG vaccination of 28-day-old animals yields AM that are poor responders to phorbol myristate acetate compared with AM from BCG-vaccinated animals 2 to 3 and 5 to 6 months of age. AM from animals vaccinated with BCG at 28 days of age contained fewer and smaller electron-dense lysosomelike structures than did AM from adult rabbits similarly vaccinated. These findings provide an explanation for the difficulties infants have in developing effective cell-mediated immune responses against intracellular parasites.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Loose L. D. Phagocytic activation of a luminol-dependent chemiluminescence in rabbit alveolar and peritoneal macrophages. Biochem Biophys Res Commun. 1976 Mar 8;69(1):245–252. doi: 10.1016/s0006-291x(76)80299-9. [DOI] [PubMed] [Google Scholar]
- Briheim G., Stendahl O., Dahlgren C. Intra- and extracellular events in luminol-dependent chemiluminescence of polymorphonuclear leukocytes. Infect Immun. 1984 Jul;45(1):1–5. doi: 10.1128/iai.45.1.1-5.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg Y., Pick E. Unsaturated fatty acids as second messengers of superoxide generation by macrophages. Cell Immunol. 1983 Jul 15;79(2):240–252. doi: 10.1016/0008-8749(83)90067-9. [DOI] [PubMed] [Google Scholar]
- Dahlgren C., Stendahl O. Effect of in vitro preincubation of polymorphonuclear leukocytes on formylmethionyl-leucyl-phenylalanine-induced chemiluminescence. Infect Immun. 1982 Jul;37(1):34–39. doi: 10.1128/iai.37.1.34-39.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drath D. B., Karnovsky M. L. Superoxide production by phagocytic leukocytes. J Exp Med. 1975 Jan 1;141(1):257–262. doi: 10.1084/jem.141.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easmon C. S., Cole P. J., Williams A. J., Hastings M. The measurement of opsonic and phagocytic function by Luminol-dependent chemiluminescence. Immunology. 1980 Sep;41(1):67–74. [PMC free article] [PubMed] [Google Scholar]
- Ganz T., Sherman M. P., Selsted M. E., Lehrer R. I. Newborn rabbit alveolar macrophages are deficient in two microbicidal cationic peptides, MCP-1 and MCP-2. Am Rev Respir Dis. 1985 Oct;132(4):901–904. doi: 10.1164/arrd.1985.132.4.901. [DOI] [PubMed] [Google Scholar]
- Hatch G. E., Gardner D. E., Menzel D. B. Chemiluminescence of phagocytic cells caused by N-formylmethionyl peptides. J Exp Med. 1978 Jan 1;147(1):182–195. doi: 10.1084/jem.147.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. B., McCracken G. H., Jr The spectrum of group B streptococcal infections in infancy. Am J Dis Child. 1974 Dec;128(6):815–818. doi: 10.1001/archpedi.1974.02110310063011. [DOI] [PubMed] [Google Scholar]
- Huang T. S., Hurd R. E., Chopra I. J., Stevens P., Solomon D. H., Young L. S. Inhibition of phagocytosis and chemiluminescence in human leukocytes by a lipid soluble factor in normal tissues. Infect Immun. 1984 Nov;46(2):544–551. doi: 10.1128/iai.46.2.544-551.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juers J. A., Rogers R. M., McCurdy J. B., Cook W. W. Enhancement of bactericidal capacity of alveolar macrophages by human alveolar lining material. J Clin Invest. 1976 Aug;58(2):271–275. doi: 10.1172/JCI108468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharazmi A., Høiby N., Döring G., Valerius N. H. Pseudomonas aeruginosa exoproteases inhibit human neutrophil chemiluminescence. Infect Immun. 1984 Jun;44(3):587–591. doi: 10.1128/iai.44.3.587-591.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaForce F. M., Kelly W. J., Huber G. L. Inactivation of staphylococci by alveolar macrophages with preliminary observations on the importance of alveolar lining material. Am Rev Respir Dis. 1973 Oct;108(4):784–790. doi: 10.1164/arrd.1973.108.4.784. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Selsted M. E., Szklarek D., Fleischmann J. Antibacterial activity of microbicidal cationic proteins 1 and 2, natural peptide antibiotics of rabbit lung macrophages. Infect Immun. 1983 Oct;42(1):10–14. doi: 10.1128/iai.42.1.10-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah V. N., Jr, Shirley P. S., Myrvik Q., Waite M. The use of acetylated cytochrome c in detecting superoxide anion production in rabbit alveolar macrophages. J Immunol. 1983 Nov;131(5):2104–2106. [PubMed] [Google Scholar]
- Nelson R. D., Herron M. J., Schmidtke J. R., Simmons R. L. Chemiluminescence response of human leukocytes: influence of medium components on light production. Infect Immun. 1977 Sep;17(3):513–520. doi: 10.1128/iai.17.3.513-520.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. D., Mills E. L., Simmons R. L., Quie P. G. Chemiluminescence response of phagocytizing human monocytes. Infect Immun. 1976 Jul;14(1):129–134. doi: 10.1128/iai.14.1.129-134.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted M. E., Brown D. M., DeLange R. J., Lehrer R. I. Primary structures of MCP-1 and MCP-2, natural peptide antibiotics of rabbit lung macrophages. J Biol Chem. 1983 Dec 10;258(23):14485–14489. [PubMed] [Google Scholar]
- Sherman M. P., Lehrer R. I. Superoxide generation by neonatal and adult rabbit alveolar macrophages. J Leukoc Biol. 1984 Jul;36(1):39–50. doi: 10.1002/jlb.36.1.39. [DOI] [PubMed] [Google Scholar]
- Sherman M., Goldstein E., Lippert W., Wennberg R. Neonatal lung defense mechanisms: a study of the alveolar macrophage system in neonatal rabbits. Am Rev Respir Dis. 1977 Sep;116(3):433–440. doi: 10.1164/arrd.1977.116.3.433. [DOI] [PubMed] [Google Scholar]
- Takata I., Gordon M. R., Myrvik Q. N. Demonstration of a macrophage migration enhancement factor in the sera of young rabbits. Cell Immunol. 1985 Oct 1;95(1):137–145. doi: 10.1016/0008-8749(85)90302-8. [DOI] [PubMed] [Google Scholar]
- Webb D. S., Jeska E. L. Enhanced luminol-dependent chemiluminescence of stimulated rat alveolar macrophages by pretreatment with alveolar lining material. J Leukoc Biol. 1986 Jul;40(1):55–64. doi: 10.1002/jlb.40.1.55. [DOI] [PubMed] [Google Scholar]
- Zeligs B. J., Nerurkar L. S., Bellanti J. A. Maturation of the rabbit alveolar macrophage during animal development. III. Phagocytic and bactericidal functions. Pediatr Res. 1977 Dec;11(12):1208–1211. doi: 10.1203/00006450-197712000-00008. [DOI] [PubMed] [Google Scholar]