Abstract
Abstract—I.v. delivery of mesenchymal stem cells prepared from adult bone marrow reduces infarction size and ameliorates functional deficits in rat cerebral ischemia models. Administration of the brain-derived neurotrophic factor to the infarction site has also been demonstrated to be neuroprotective. To test the hypothesis that brain-derived neurotrophic factor contributes to the therapeutic benefits of mesenchymal stem cell delivery, we compared the efficacy of systemic delivery of human mesenchymal stem cells and human mesenchymal stem cells transfected with a fiber-mutant F/RGD adenovirus vector with a brain-derived neurotrophic factor gene (brain-derived neurotrophic factor–human mesenchymal stem cells). A permanent middle cerebral artery occlusion was induced by intraluminal vascular occlusion with a microfilament. Human mesenchymal stem cells and brain-derived neurotrophic factor– human mesenchymal stem cells were i.v. injected into the rats 6 h after middle cerebral artery occlusion. Lesion size was assessed at 6 h, 1, 3 and 7 days using MR imaging, and histological methods. Functional outcome was assessed using the treadmill stress test. Both human mesenchymal stem cells and brain-derived neurotrophic factor–human mesenchymal stem cells reduced lesion volume and elicited functional improvement compared with the control sham group, but the effect was greater in the brain-derived neurotrophic factor–human mesenchymal stem cell group. ELISA analysis of the infarcted hemisphere revealed an increase in brain-derived neurotrophic factor in the human mesenchymal stem cell groups, but a greater increase in the brain-derived neurotrophic factor–human mesenchymal stem cell group. These data support the hypothesis that brain-derived neurotrophic factor contributes to neuroprotection in cerebral ischemia and cellular delivery of brain-derived neurotrophic factor can be achieved by i.v. delivery of human mesenchymal stem cells.
Keywords: bone marrow, neuroprotection, regeneration, stroke, transplantation
Transplantation of bone marrow-derived cells several hours after ischemia onset has been shown to reduce infarction size and improve functional outcome in rodent cerebral ischemia models (Chen et al., 2001; Iihoshi et al., 2004). The post-injury time window for cell delivery and the prospect of preparation of large numbers of autologous cells from bone marrow aspirates, suggest the potential utility of this approach as a treatment in stroke.
The precise cell type within bone marrow responsible for the neuroprotection is not known, but is thought to reside within the marrow stromal or mesenchymal stem cell (MSC) population (Li et al., 2002; Iihoshi et al., 2004). MSCs can be isolated and expanded as plastic adherent cells having a flattened fibroblast-like morphology (Friedenstein, 1976; Woodbury et al., 2000) that are CD34−, CD45−, SH2+, and SH3+ (Majumdar et al., 1998; Kobune et al., 2003). MSCs have been suggested to differentiate into osteoblasts, chondrocytes, adipocytes and hepatocytes (Prockop, 1997; Pittenger et al., 1999; Sanchez-Ramos et al., 2000; Krause et al., 2001; Kobune et al., 2003). It has also been suggested that they can differentiate into cells of neuronal and glial lineage (Azizi et al., 1998; Kopen et al., 1999; Brazelton et al., 2000; Sanchez-Ramos et al., 2000; Woodbury et al., 2000; Sasaki et al., 2001; Iihoshi et al., 2004). Recent work indicates that under some conditions MSCs can fuse with other cells thereby making the distinction between cell fusion and transdifferentiation difficult (Castro et al., 2002; Alvarez-Dolado et al., 2003).
Independent of the cell fusion vs transdifferentiation issue, isolated cultured bone marrow-derived MSCs have been shown to secrete trophic factors such as brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), vascular endothelial growth factors (VEGF), and hepatocyte growth factor (HGF) (Hamano et al., 2000; Chen et al., 2002; Iihoshi et al., 2004; Kurozumi et al., 2004). Indeed, BDNF administration has neuroprotective effects in a rat ischemia model (Schabitz et al., 1997, 2000; Yamashita et al., 1997). The release of trophic factors from transplanted MSCs within the host brain may contribute to the reduction in infarction size and to the recovery of function following ischemia in recipient animals (Iihoshi et al., 2004). Additionally, direct intracranial injection of human mesenchymal stem cells transfected with the BDNF gene (BDNF-hMSCs) resulted in therapeutic benefits in the transient rat middle cerebral artery occlusion (MCAO) model (Kurozumi et al., 2004).
Although direct intracerebral injection of BDNF-hMSCs in the rat MCAO model showed efficacy, this approach would be limited in clinical applications because of the surgical requirement and the difficulty in distributing cells to large areas of brain by focal injection. Systemic delivery of bone marrow cells has been reported to distribute in the ischemic lesion in the rat MCAO model and is associated with functional improvement (Iihoshi et al., 2004). In this study, hMSCs and hypersecreting BDNF-hMSCs were i.v. delivered at 6 h after induction of unilateral permanent cerebral ischemia to investigate if cellular delivery of BDNF by MSCs could influence lesion volume and functional outcome.
EXPERIMENTAL PROCEDURES
Preparation of hMSCs
Human bone marrow from healthy adult volunteers was obtained by aspiration from the posterior iliac crest after informed consent was obtained; this study was approved by the institutional review board at our university (Kobune et al., 2003). Bone marrow mono-nuclear cells were isolated, and were plated in 150-cm2 plastic tissue culture flasks and incubated overnight. After washing away the free cells, the adherent cells were cultured in mesenchymal stem cell basal medium (MSCBM, Cambrex, Walkersville, MD, USA) containing mesenchymal cell growth supplement (MCGS, Cambrex, Walkersville, MD, USA), 4 mM L-glutamine, in a humidified atmosphere of 5% CO2 at 37 °C. After reaching confluence, they were harvested and cryopreserved as primary MSCs or used for gene transduction.
Adenoviral vectors
Adenoviral vectors carrying a human BDNF cDNA were constructed as described previously (Kurozumi et al., 2004). Briefly, human BDNF cDNA was cloned using the reverse-transcription polymerase chain reaction (RT-PCR) method from the total RNA extracted from primary hMSC as the template. The identity of BDNF cDNA obtained in this manner was confirmed by sequencing and comparing it to the GeneBank sequence XM_006027.
The human BDNF primer sequence was forward, 5′-CGG-AATTCCACCATGACCATCCTTTTCCTTACTATGGTTA-3′, and reverse, 5′-CCAGATCTATCTTCCCCTTTTAATGGTCAATGTA-3′.
The BDNF cDNA was inserted between the EcoR I site and the BglII site in the pCAcc vector and the resulting plasmid was designated pCAhBDNF. The plasmid pCAhBDNF was digested with ClaI, and the fragment containing the BDNF cDNA expression unit was isolated by agarose gel electrophoresis. The adenoviral BDNF expression vector, pWEAxCAhBDNF-F/RGD, was prepared using LipofectAMINE 2000 (Invitrogen, Tokyo, Japan).
Before being used, the above viral vectors were evaluated for their viral concentration and titer, and viral stocks were examined for potential contamination with replication-competent viruses. To determine viral concentration (particle unit (pu)/ml), the viral solution was incubated in 0.1% sodium dodecyl sulfate and A260 was measured. The viral titers of AxCAhBDNF-F/RGD were 1.0×1012 pu/ml, respectively.
Adenovirus infection
Adenovirus-mediated gene transfection was performed as previously described (Tsuda et al., 2003; Kurozumi et al., 2004). Briefly, the cells were seeded at a density of 2×106 cells per 15 cm plate. MSCs were exposed to the infectious viral particles in 7.5 ml DMEM at 37 °C medium for 60 min; cells were infected with AxCAhBDNF-F/RGD at a multiplicity of infection (MOI) of 3.0×103 pu/cell. The medium was then removed, and the cells washed once with DMEM and then recultured with normal medium for 12 h, after which transplantation was performed.
Cerebral ischemic model
The rat MCAO model was used as a stroke model. We induced permanent MCAO by using a previously described method of intraluminal vascular occlusion (Longa et al., 1989; Iihoshi et al., 2004). Adult male Sprague–Dawley rats (n=80) weighing 250–300 g were initially anesthetized with 5% isoflurane and maintained under anesthesia with 1.5% isoflurane in a mixture of 70% N2O and 30% O2 with mechanical ventilation. Rectal temperature was maintained at 37° with an infrared heat lamp. The left femoral artery was cannulated for measuring blood pH, pO2, and pCO2 throughout the surgery. A length of 20.0–22.0 mm 4-0 surgical Dermalon suture with the tip rounded by heating near a flame was advanced from the external carotid artery into the lumen of the internal carotid artery until it blocked the origin of the MCA.
This study was approved by the Institutional Review Board at our university, and all experiments conformed to international guidelines on the ethical use of animals, minimizing the number of animals used and their suffering.
Transplantation procedures
Experiments consisted of three groups (n=66). In group 1 (control), rats were given medium alone (without donor cell administration) injected i.v. at 6 h after MCAO (just after the initial MRI measurement) (n=22). In group 2, rats were given hMSCs (1.0×107) in 1 ml total fluid volume (MSCBM) injected i.v. at 6 h after MCAO (n=22). In group 3, rats were given BDNF-hMSCs (1.0×107) injected i.v. at 6 h after MCAO (n=22). Seven rats in each group were used to calculate the infarct lesion volume, and the remaining rats were used for the additional histological analysis.
In some experiments, Adex1CAlacZ adenovirus was used to transduce the LacZ gene into the MSCs. Details of the construction procedures are described elsewhere (Nakamura et al., 1994; Nakagawa et al., 1998; Takiguchi et al., 2000; Iihoshi et al., 2004). For in vitro adenoviral infection, 1.0×107 hMSCs were placed with Adex1CAlacZ at 50 MOI for 1 h and incubated at 37 °C in DMEM containing 10% fetal calf serum.
MRI
Rats were anesthetized with ketamine (75 mg/kg) and xylazine (10 mg/kg) i.p. Each rat was placed in an animal holder/MRI probe apparatus and positioned inside the magnet. The animal’s head was held in place inside the imaging coil. All MRI measurements were performed using a 7-T, 18-cm-bore superconducting magnet (Oxford Magnet Technologies) interfaced to a UNITYINOVA console (Oxford Instruments, UK and Varian, Inc., Palo Alto, CA, USA). T2-weighted images were obtained from a 1.0-mm-thick coronal section using a 3 cm field of view, TR=3000 ms, TE=35 ms, and reconstructed using a 512×512 image matrix. Accurate positioning of the brain was performed to center the image slice 5 mm posterior to the rhinal fissure with the head of the rat held in a flat skull position. MRI measurements were obtained 6, 24, 72 h and 1 week after MCAO.
The ischemic lesion area was calculated from T2-weighted images using imaging software (Scion Image, Version Beta 4.0.2, Scion Corporation), based on the previously-described method (Neumann-Haefelin et al., 2000). For each slice, the higher intensity lesions in T2-weighted images where the signal intensity was 1.25 times higher than the counterpart in the contra-lateral brain lesion were marked as the ischemic lesion area, and infarct volume was calculated taking slice thickness (1 mm/slice) into account.
Detection of BDNF in vitro and in vivo
Forty-eight hours after MSCs were transfected in vitro at various MOIs (pu/cell), culture supernatants were collected for analysis. Furthermore, seven days after MCAO, rats were anesthetized with ketamine (4.4~8 mg/100 g) and xylazine (1.3 mg/100 g) i.p., their brains were removed, and coronal sections (200mg) from −1.0–1.0 mm to bregma in the ischemic hemisphere were dissected on ice and were stored at −80 °C until use. Subsequently, each tissue sample was suspended in an equal weight of homogenate buffer (1 ml; 137 mM NaCl, 20 mM Tris, 1% NP40, 1 mM PMSF, 10 μg/ml aprotinin, 1 μg/ml leupeptin, 0.5 mM sodium vanadate) and homogenized with a Dounce homogenizer. The homogenate was centrifuged (10,000×g) for 10 min at 4 °C, and the supernatant (5 μg/μl) collected for analysis. Commercial BDNF ELISA kits (Promega, Madison, WI, USA) were used to quantify the concentration of BDNF in each of the samples.
TTC staining and quantitative analysis of infarct volume
One week after transplantation, the rats were deeply anesthetized with ketamine (4.4~8 mg/100 g) and xylazine (1.3 mg/100 g) i.p. The brains were removed carefully and dissected into coronal 1 mm sections using a vibratome. The fresh brain slices were immersed in a 2% solution of 2,3,5-triphenyltetrazolium chloride (TTC) in normal saline at 37 °C for 30 min. The cross-sectional area of infarction in each brain slice was examined with a dissection microscope and was measured using an image analysis software, NIH image. The total infarct volume for each brain was calculated by summation of the infarcted area of all brain slices.
Detection of donor hMSC cells and phenotypic analysis in vivo
One week after transplantation, phenotypic analysis of the transplanted cells in vivo was carried out using laser scanning confocal microscopy. Brains of the deeply-anesthetized rats were removed, fixed in 4% paraformaldehyde in phosphate-buffer, dehydrated with 30% sucrose in 0.1 M PBS for overnight, and frozen in powdered dry ice. Coronal cryostat sections (10 μm) were processed for immunohistochemistry. To identify the cell type derived from the donor bone marrow, double-labeling studies were performed with the use of antibodies to beta-galactosidase (rhodamine-labeled polyclonal rabbit anti-beta-galactosidase antibody, DAKO), neurons (FITC-labeled monoclonal mouse anti-NeuN, DAKO; FITC-labeled monoclonal mouse anti-neurofilament [NF], Sigma), and astrocytes (FITC-labeled monoclonal mouse anti-glial fibrillary acidic protein [GFAP], Sigma).
To excite the FITC fluorochrome (green), a 488-nm laser line generated by an argon laser was used, and for the rhodamine fluorochrome (red), a 543-nm laser line from a HeNe laser was used. Confocal images were obtained using a Zeiss laser scanning confocal microscope with the use of Zeiss software.
Treadmill stress test
Rats were trained 20 min per day for 2 days a week to run on a motor driven treadmill at a speed of 20 m/min with a slope of 0°. Rats were placed on a moving belt facing away from the electrified grid and induced to run in the direction opposite of the movement of the belt. Thus, to avoid foot-shocks (with intensity in 1.0 mA), the rats had to move forward. Only the rats that had leaned to avoid the mild electrical shock were included in this study (n=21). The maximum speed at which the rats could run on a motor driven treadmill was recorded.
Statistical analysis
The lesion volume and the behavior scores (treadmill stress test) recorded were statistically analyzed. Data are presented as mean values±S.D. Differences among groups were assessed by ANOVA with Scheffe’s post hoc test to identify individual group differences. Differences were deemed statistically significant at P=0.05.
RESULTS
Morphological characteristics of primary and BDNF gene-transduced hMSCs
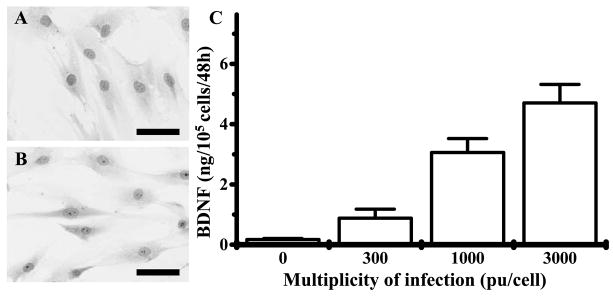
Primary hMSCs cultured as plastic adherent cells could be maintained in culture. A characteristic feature of MSCs is a CD34−, CD45−, SH2+ (CD105), SH3+ (CD73), CD117−, CD133−, CD166+, CD9+, CD157+, CD166+ (ALCAM) cell surface (Kobune et al., 2003). Fig. 1A shows the morphological features of these cells. Characteristic flattened and spindle-shaped-cells can be recognized. Fig. 1B is a photomicrograph of BDNF-hMSCs. Note the similar flattened and spindle shaped morphology of the genetically-engineering cells. These data are in agreement with previous work (Kurozumi et al., 2004).
Fig. 1.
May-Giemsa staining of primary hMSCs (A) and BDNF-hMSCs (B) (scale bar=20 μm). (C) Secreted BDNF levels in supernatant of hMSCs (ng/105 cells/48 h) transfected with AxCAhBDNF-F/RGD (BDNF-hMSC) at an MOI of 300, 1000, and 3000 pu/cell.
Detection of immunoreactive human BDNF and quantitative analysis in vitro
Levels of BDNF in the supernatant of cultured hMSCs and BDNF-hMSCs with different MOI levels are shown in Fig. 1C. hMSC transfected with AxCAhBDNF-F/RGD (MSC-BDNF) at an MOI of 300, 1000, and 3000 pu/cell secreted BDNF at a rate of 0.91±0.26, 3.09±0.43, and 4.73±0.59 ng/105 cells/48 h, respectively (n=4). Non-transfected MSC also produced BDNF protein (0.05± 0.02 ng/105 cells/48 h) (n=4). The level of BDNF production from BDNF-hMSC transfected at an MOI of 1000 pu/cell was 65-fold greater than that seen in non-infected MSC.
Characterization of ischemic lesion size by magnetic resonance image analysis
An estimate of lesion size was obtained using in vivo MRI (see Experimental Procedures). Brain images (T2-weighted) were collected from all experimental animals 6, 24, 72 h and 7 days after MCAO. The cells were i.v. delivered immediately after the 6 h MRI. The left hand column in Fig. 2 (A1–C1) shows single brain images obtained 6 h post-injury. These coronal forebrain sections were obtained at the level of caudato-putamen complex. Note the reduction in density in lesions on the right side of the brains that were subjected to ischemic injury. Lesion volume (mm3) was determined by analysis of high intensity areas on serial images collected through the cerebrum (see Experimental Procedures). The lesion volume at 6 h post-lesion-induction was 270.30±12.10 mm3 in the control group (Fig. 2A1) (n=7), 268.40±19.60 mm3 in the MSC transplant-group (Fig. 2B1) (n=7), 267.20± 2.84 mm3 in the BDNF-hMSC transplant-group (Fig. 2C1) (n=7).
Fig. 2.
Evaluation of the ischemic lesion volume with MRI T2-weighted images. hMSCs or BDNF-hMSCs were i.v. injected immediately after the initial MRI scanning (6 h). Images obtained 6, 24, 72 h and one week after MCAO in non-treated (A1–4), hMSC-treated (B1–4), and BDNF-hMSC-treated groups (C1–4). Scale bar=3 mm.
In order to determine the efficacy of hMSCs or BDNF-hMSCs transplantation in modulating the ischemic lesion volume, 1×107 cells were delivered i.v. at 6 h after lesion induction, and animals were re-imaged at 24, 72 h, and 7 days later.
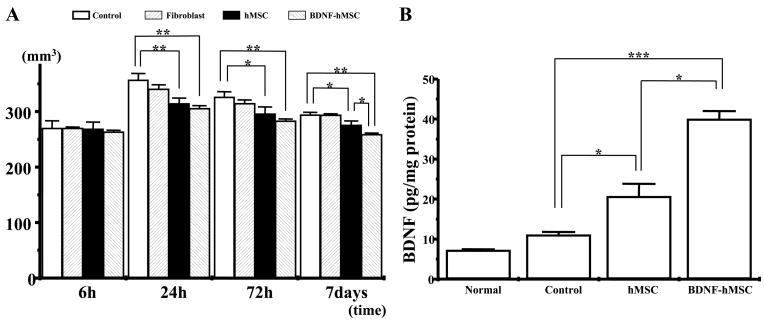
In sham control (vehicle alone) (Fig. 2A1–4), MRI-estimated lesion volume reached maximum at 24 h post-MCAO (Fig. 2A2) (357.30±11.50 mm3, n=7) and gradually decreased at 72 h and 7 days post-MCAO (326.50± 2.40 mm3, n=7; 294.50±4.10 mm3, n=7, respectively). Although lesion volume estimated 6 h after MCAO was almost equal among three groups, lesion volume dramatically decreased with i.v. injection of hMSCs at 24 (Fig. 2B2), 72 h (Fig. 2B3) and 7 days (Fig. 2B4) post-MCAO (310.20±10.30 mm3, n=7; 295.90±12.40 mm3, n=7; 275.40±7.64 mm3, n=7, respectively). Lesion volume reduction was also observed when BDNF-hMSCs were i.v. injected 6 h after MCAO. However, lesion volume in the BDNF-hMSC transplant-group was decreased to a greater degree than in the hMSC group (Fig. 2C2–4) (305.60± 5.10 mm3, n=7; 283.00±3.67 mm3, n=7; 258.80± 2.53 mm3, n=7, at 24 h, 72 h, and 7 days, respectively). These results are summarized in Fig. 3A.
Fig. 3.
(A) Summary of lesion volumes evaluated with MRI T2-weighted images in each group. (B) BDNF in vivo levels assayed with ELISA. Levels of BDNF were significantly increased in the ischemic hemisphere of hMSC and BDNF-hMSC compared with non-treated rats. In addition, BDNF levels were greater in the ischemic hemisphere of BDNF-hMSC-transplanted compared with rats that received hMSC. Assays were obtained 7 days after MCAO induction. (* P<0.05; ** P<0.01, *** P<0.0005.)
An additional sham control experiment was carried out using skin fibroblasts. No significant changes in lesion volume was observed with delivery of 106 skin fibroblasts at 6, 24, 72 h, and 7 days (269.90±2.23 mm3, n=5; 340.50±7.97 mm3, n=5; 314.50±6.51 mm3, n=5; 293.60± 2.16 mm3, n=5, respectively; Fig. 3A).
BDNF levels in vivo
BDNF levels in local brain tissue were measured by a sandwich ELISA 7 days after MCAO (see Experimental Procedures). Although BDNF levels in the non-transplant rat brain were not significantly different from those of the normal rat brain (7.23±0.22, n=4; 11.09±0.71 pg/mg protein, n=4, respectively), BDNF levels significantly increased in the ischemic hemisphere of MSC-treated group (20.68±3.12 pg/mg protein, n=4) compared with control group. In the BDNF-hMSC treated rats, BDMF increased in the ischemic hemisphere (40.01±1.99 pg/mg protein, n=4) compared with control rats and hMSC-treated rats. These results are summarized in Fig. 3B.
Histological determination of infarction volume
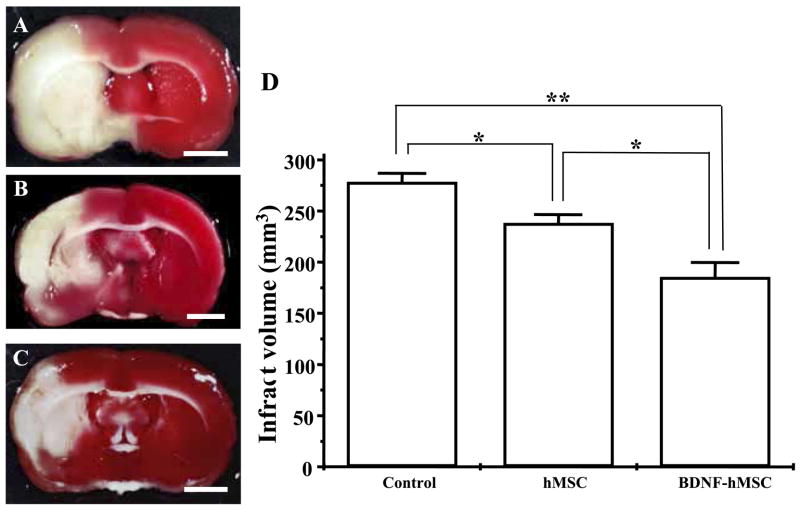
After completion of the MRI analysis to estimate lesion volume, before and after cell delivery, the animals were perfused and stained with TTC to obtain a second independent measure of infarction volume. Normal brain (gray matter) tissue typically stains with TTC, but infarcted lesions show no or reduced staining (Bederson et al., 1986). TTC-staining obtained one week after MCAO without cell transplantation is shown in Fig. 4A. Note the reduced staining on the lesion side primarily in the corpus striatum. Lesion volume was calculated by measuring the area of reduced TTC-staining in the forebrain (see Experimental Procedures). As with MRI analysis there was a progressive reduction in infarction size with MSC treatment (Fig. 4B). i.v. delivery of 107 BDNF-hMSCs resulted in very substantial reduction in lesion volume as estimated from TTC-staining (Fig. 4C, D).
Fig. 4.
(A–C) Brain slices stained with TTC to visualize lesions. TTC-stained brain slices from non-treated (A) and following i.v. delivery hMSCs or BDNF-hMSCs 6 h after MCAO are shown in B and C, respectively. (Scale bar=3 mm.) (D) Lesion volumes in each group. (* P<0.05; ** P<0.0005.)
Identification and characterization of donor cells in vivo
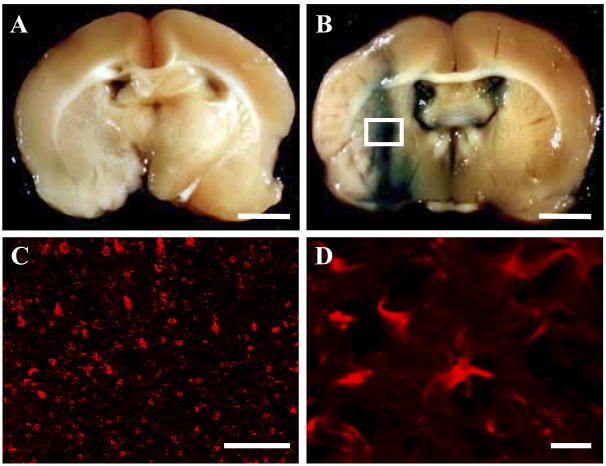
LacZ-transfected BDNF-hMSCs that had been i.v. administered (107 cells) 6 h after MCAO were identified in vivo (n=5). The LacZ-expressing BDNF-hMSCs were found primarily in the lesion penumbra. The transmitted light image in the LacZ-transfected group is shown in Fig. 5B. Note the abundance of LacZ-positive blue cellular-like elements in and around the affected lesion, indicating that systemic deliver of cells reached the lesion site. There was a paucity of blue staining in the non-infected group (Fig. 5A). Immunohistochemical studies were carried out to identify LacZ-positive cells in and around the lesion zone in animals transplanted with LacZ-transfected BDNF-hMSCs. Lower (C) and higher micrograph (D) demonstrated a large number of LacZ-positive cells in and around the lesion (458.6±42.1 cells/mm2, n=5), although virtually there is not any LacZ-positive cells in the non-damaged hemisphere and non-infected group. Small numbers of NeuN and GFAP positive cells were co-stained with LacZ (data not shown). A small number of the LacZ-positive donor cells expressed the NeuN (7.90±0.15%, n=5), NF (7.32±0.52%, n=5), or GFAP (7.65±0.17%, n=5).
Fig. 5.
I.v.-administrated BDNF-hMSCs accumulated in the ischemic lesion hemisphere. BDNF-hMSCs were transfected with the reporter gene LacZ. (B) Transplanted LacZ-positive cells (blue cells) were present in the ischemic lesion. Brain from control (BDNF-hMSCs without LacZ transfection) injected animals with comparable X-gal staining is shown in A. Confocal images (white square in panel B) at low (C) and high power (D) demonstrating a large number of LacZ-positive cells in the lesion hemisphere. Scale bar=3 mm (A, B), 100 μm (C), 10 μm (D).
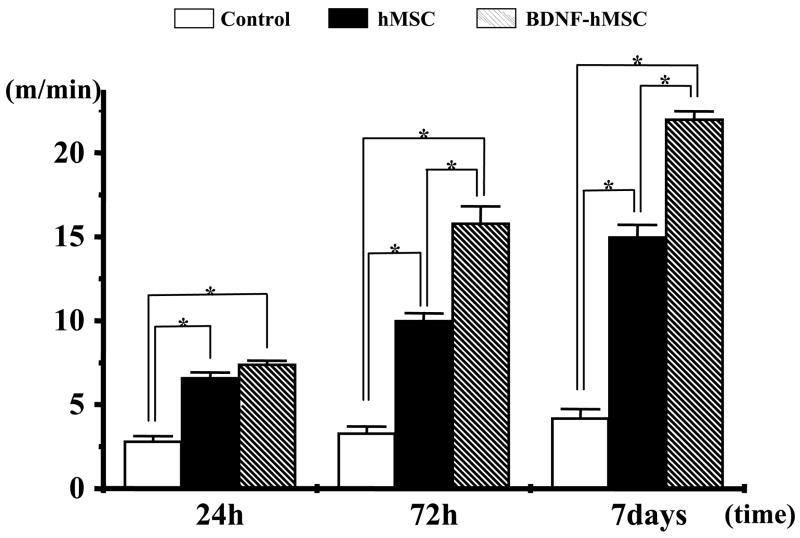
Functional analysis
To access behavioral performance in the lesioned and transplanted animals, the treadmill stress test was used (Fig. 6). Behavioral testing began 24 h after lesion induction alone or with cell transplantation. In the treadmill stress test control animals (no lesion) reach a maximum treadmill velocity of about 80 m/min (Iihoshi et al., 2004). Twenty-four hours after MCAO without transplantation maximum velocity on the treadmill test was 2.88±0.30 m/min (n=7). Non-treated animals showed increased treadmill velocity with slow improvement up to 7 days (4.13±0.23 m/min, n=7). In the hMSC transplantation group, the improvement in velocity was greater over the time course up to 7 days. The BDNF-hMSC-treated group showed increased treadmill velocity with dramatic improvement after 72 h compare with the MSC-treated group.
Fig. 6.
The treadmill stress test demonstrates that the maximum speed at which the rats could run on a motor driven treadmill was faster in the hMSC-and the BDNF-hMSC-treated rats than control. Moreover, the greatest velocity was achieved in the BDNF-hMSC group. Velocity is plotted for three times after MCAO induction. (* P<0.001.)
DISCUSSION
The present study demonstrates that i.v. infusion of either hMSCs or BDNF-hMSCs 6 h after permanent MCAO in the rat results in reduction in infarction volume, improvement in behavioral performance, and increases in BDNF levels in the infarcted cerebral hemisphere. These results are consistent with previous studies showing beneficial effects of bone marrow cell transplantation (Li et al., 2002; Iihoshi et al., 2004; Kurozumi et al., 2004) or BDNF delivery (Schabitz et al., 1997, 2000; Yamashita et al., 1997) in experimental cerebral ischemic models. While both hMSCs and BDNF-hMSCs showed efficacy, the effects, including brain BDNF levels, were greater in the BDNF-hMSC group.
Transplanted LacZ-positive BDNF-hMSCs were identified throughout the anticipated infarcted cerebrum. These cells likely accessed the damaged area from the systemic circulation (Iihoshi et al., 2004) through adjacent non-occluded or collateral arterial circulation. Given that the transplanted LacZ-positive BDNF-hMSCs distribute throughout the infarction zone, they likely migrated within the lesion domain from vasculature outside the lesion. LacZ-positive cells were located both in areas of severe damage and in surrounding less damaged areas. This suggests that tissue sparing, while not complete overlapped with deposition of transplanted cells. LacZ-positive cells were minimally observed in the contralateral non-infarcted hemisphere, suggesting that cells preferentially distributed to damaged CNS.
MSCs secrete a variety of bioactive substances such as neurotrophins (including BDNF), interleukins, macrophage colony-stimulating factor, Flt-3 ligand, and stem-cell factors (Eaves et al., 1991; Majumdar et al., 1998). Intracranial infusion of BDNF using an osmotic mini-pump significantly reduced infarct volume following cerebral ischemia induction (Schabitz et al., 1997; Yamashita et al., 1997), suggesting that BDNF may provide a protective effect on ischemic cerebral tissue. To investigate the potential role of BDNF on the neuroprotective action of hMSC delivery in the MCAO model, we compared the effects of hMSCs to BDNF-hypersecreting hMSCs (BDNF-hMSCs). While numerous secreted trophic factors could contribute to the observed neuroprotection, we compared hMSCs that were constructed to differ only in their levels of BDNF secretion.
The BDNF gene was introduced into MSCs using Adv-F/RGD, and an elevated secretion of BDNF protein as compared with nontransgenic hMSCs was confirmed in vitro using ELISA. This relatively high secretory rate was achieved by using Ad-F/RGD which has a higher transfection rate (Nakamura et al., 2002; Tsuda et al., 2003). BDNF levels assayed by ELISA in the ischemic brain lesion increased after either hMSC or BDNF-MSC delivery, but levels were significantly higher in the BDNF-hMSC group. This suggests that the BDNF-MSCs could maintain high levels of BDNF during the critical post-ischemic period and that this elevated BDNF secretion contributes to the enhanced neuroprotection.
In a previous study we reported that direct injection of BDNF-hMSCs into the infarction site promoted tissue sparing and improved functional outcome, but hMSCs did not, although hMSCs can secrete BDNF in vitro (Kurozumi et al., 2004). This contrasts with results of the present study where transplantation of either cell type showed efficacy, but the BDNF-hMSCs response was greater. One possibility to account for this difference is that the i.v. delivery method allowed for a larger area of infarcted tissue to be reached by the transplanted cells. Following direct injection, the increased BDNF secretion by the transplanted BDNF-hMSCs may have contributed to the neuroprotective effects, but the BDNF secretion of hMSCs may have been insufficient. Another difference is that the hMSCs were delivered at 6 h post-infarction in the present study, as opposed to 24 h in the Kurozumi et al. (2004) study.
I.v. injection is less invasive, and can be more readily carried out in the clinic compared with the intracerebral injection. Certainly, many more cells would be needed to treat patients via i.v. injection as compared with the intracranial transplantation. It has been estimated that about 1% of i.v.-injected cells reach and survive in the lesion of rats (Chen et al., 2001). Targeting infarction lesions by intracerebral injection could cause significant surgical risks. For example, insertion of an injection needle for cell transplantation into the ischemic brain lesion in the acute phase would result in increased risk of the intracerebral hemorrhage. I.v. delivery of these cells could lead to a more global neuroprotection over the entire ischemia-related brain lesion with subsequent repair.
CONCLUSION
In summary, the i.v. injection of either hMSCs or BDNF-hMSCs transfected with the BDNF gene using a fiber-mutant adenovirus vector resulted in reduced ischemic damage and improved function in a rat MCAO model, but the BDNF-hMSCs showed greater efficacy. The therapeutic effect of BDNF-hMSC transplantation in the permanent rat MCAO model was observed even when applied six hours after infarction. Thus, cellular delivery of BDNF secreting hMSCs may have a therapeutic effect following cerebral ischemia.
Acknowledgments
This work was supported in part by grants from the Japanese Ministry of Education, Science, Sports and Culture (16390414, 14370442), Mitsui Sumitomo Insurance Welfare Foundation, the National Multiple Sclerosis Society (USA) (RG2135; CA1009A10), the National Institutes of Health (NS43432), and the Medical and Rehabilitation and Development Research Services of the Department of Veterans Affairs.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- GFAP
glial fibrillary acidic protein
- hMSC
human mesenchymal stem cell
- MCAO
middle cerebral artery occlusion
- MOI
multiplicity of infection
- MSC
mesenchymal stem cell
- MSCBM
mesenchymal stem cell basal medium
- NF
neurofilament
- pu
particle unit
- TTC
2,3,5-triphenyltetrazolium chloride
References
- Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats-similarities to astrocyte grafts. Proc Natl Acad Sci U S A. 1998;95:3908–3913. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–1308. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- Castro RF, Jackson KA, Goodell MA, Robertson CS, Liu H, Shine HD. Failure of bone marrow cells to transdifferentiate into neural cells in vivo. Science. 2002;23:297. doi: 10.1126/science.297.5585.1299. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001;189:49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- Chen X, Li Y, Wang L, Katakowski M, Zhang L, Chen J, Xu Y, Gautam SC, Chopp M. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002;22:275–279. doi: 10.1046/j.1440-1789.2002.00450.x. [DOI] [PubMed] [Google Scholar]
- Eaves CJ, Cashman JD, Kay RJ, Dougherty GJ, Otsuka T, Gaboury LA, Hogge DE, Lansdorp PM, Eaves AC, Humphries RK. Mechanisms that regulate the cell cycle status of very primitive hematopoietic cells in long-term human marrow cultures. II. Analysis of positive and negative regulators produced by stromal cells within the adherent layer. Blood. 1991;78:110–117. [PubMed] [Google Scholar]
- Friedenstein AJ. Precursor cells of mechanocytes. Int Rev Cytol. 1976;47:327–359. doi: 10.1016/s0074-7696(08)60092-3. [DOI] [PubMed] [Google Scholar]
- Hamano K, Li TS, Kobayashi T, Kobayashi S, Matsuzaki M, Esato K. Angiogenesis induced by the implantation of self-bone marrow cells: a new material for therapeutic angiogenesis. Cell Transplant. 2000;9:439–443. doi: 10.1177/096368970000900315. [DOI] [PubMed] [Google Scholar]
- Iihoshi S, Honmou O, Houkin K, Hashi K, Kocsis JD. A therapeutic window for intravenous administration of autologous bone marrow after cerebral ischemia in adult rats. Brain Res. 2004;8:1–9. doi: 10.1016/j.brainres.2003.09.084. [DOI] [PubMed] [Google Scholar]
- Kobune M, Kawano Y, Ito Y, Chiba H, Nakamura K, Tsuda H, Sasaki K, Dehari H, Uchida H, Honmou O, Takahashi S, Bizen A, Takimoto R, Matsunaga T, Kato J, Kato K, Houkin K, Niitsu Y, Hamada H. Telomerized human multipotent mesenchymal cells can differentiate into hematopoietic and cobblestone area-supporting cells. Exp Hematol. 2003;31:715–722. doi: 10.1016/s0301-472x(03)00177-2. [DOI] [PubMed] [Google Scholar]
- Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, Honmou O, Houkin K, Date I, Hamada H. BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol Ther. 2004;9:189–197. doi: 10.1016/j.ymthe.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol. 1998;176:57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Nakagawa I, Murakami M, Ijima K, Chikuma S, Saito I, Kanegae Y, Ishikura H, Yoshiki T, Okamoto H, Kitabatake A, Uede T. Persistent and secondary adenovirus-mediated hepatic gene expression using adenovirus vector containing CTLA4IgG. Hum Gene Ther. 1998;9:1739–1745. doi: 10.1089/hum.1998.9.12-1739. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Wakimoto H, Abe J, Kanegae Y, Saito I, Aoyagi M, Hirakawa K, Hamada H. Adoptive immunotherapy with murine tumor-specific T lymphocytes engineered to secrete interleukin 2. Cancer Res. 1994;54:5757–5760. [PubMed] [Google Scholar]
- Nakamura T, Sato K, Hamada H. Effective gene transfer to human melanomas via integrin-targeted adenoviral vectors. Hum Gene Ther. 2002;13:613–626. doi: 10.1089/10430340252837215. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin T, Kastrup A, de Crespigny A, Yenari MA, Ringer T, Sun GH, Moseley ME. Serial MRI after transient focal cerebral ischemia in rats: dynamics of tissue injury, blood-brain barrier damage, and edema formation. Stroke. 2000;31:1965–1972. doi: 10.1161/01.str.31.8.1965. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W, Patel N, Cooper DR, Sanberg PR. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Honmou O, Akiyama Y, Uede T, Hashi K, Kocsis JD. Transplantation of an acutely isolated bone marrow fraction repairs demyelinated adult rat spinal cord axons. Glia. 2001;35:26–34. doi: 10.1002/glia.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabitz WR, Schwab S, Spranger M, Hacke W. Intraventricular brain-derived neurotrophic factor reduces infarct size after focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1997;17:500–506. doi: 10.1097/00004647-199705000-00003. [DOI] [PubMed] [Google Scholar]
- Schabitz WR, Sommer C, Zoder W, Kiessling M, Schwaninger M, Schwab S. Intravenous brain-derived neurotrophic factor reduces infarct size and counterregulates Bax and Bcl-2 expression after temporary focal cerebral ischemia. Stroke. 2000;31:2212–2217. doi: 10.1161/01.str.31.9.2212. [DOI] [PubMed] [Google Scholar]
- Takiguchi M, Murakami M, Nakagawa I, Saito I, Hashimoto A, Uede T. CTLA4IgG gene delivery prevents autoantibody production and lupus nephritis in MRL/lpr mice. Life Sci. 2000;66:991–1001. doi: 10.1016/s0024-3205(99)00664-5. [DOI] [PubMed] [Google Scholar]
- Tsuda HWT, Ito Y, Uchida H, Dehari H, Nakamura K, Sasaki K, Kobune M, Yamashita T, Hamada H. Efficient BMP2 gene transfer and bone formation of mesenchymal stem cells by a fiber-mutant adenoviral vector. Mol Ther. 2003;7:354–365. doi: 10.1016/s1525-0016(02)00062-x. [DOI] [PubMed] [Google Scholar]
- Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Wiessner C, Lindholm D, Thoenen H, Hossmann KA. Post-occlusion treatment with BDNF reduces infarct size in a model of permanent occlusion of the middle cerebral artery in rat. Metab Brain Dis. 1997;12:271–280. doi: 10.1007/BF02674671. [DOI] [PubMed] [Google Scholar]