Abstract
Transplantation of human mesenchymal stem cells (hMSCs) prepared from adult bone marrow has been reported to ameliorate functional deficits after cerebral artery occlusion in rats. Although several hypotheses to account for these therapeutic effects have been suggested, current thinking is that both neuroprotection and angiogenesis are primarily responsible. In this study, we compared the effects of hMSCs and angiopoietin-1 gene-modified hMSCs (Ang-hMSCs) intravenously infused into rats 6 h after permanent middle cerebral artery occlusion. Magnetic resonance imaging and histologic analyses revealed that rats receiving hMSCs or Ang-hMSCs exhibited comparable reduction in gross lesion volume as compared with the control group. Although both cell types indeed improved angiogenesis near the border of the ischemic lesions, neovascularization and regional cerebral blood flow were greater in some border areas in Ang-hMSC group. Both hMSC- and Ang-hMSC-treated rats showed greater improved functional recovery in the treadmill stress test than did control rats, but the Ang-hMSC group was greater. These results indicate the intravenous administration of genetically modified hMSCs to express angiopoietin has a similar effect on reducing lesion volume as hMSCs, but the Ang-hMSC group showed enhanced regions of increased angiogenesis at the lesion border, and modest additional improvement in functional outcome.
Keywords: angiogenesis, regeneration, stem and progenitor cells, stroke, transplantation
Introduction
Although transplantation of human mesenchymal stem cells (hMSCs) several hours after onset of ischemia can reduce infarction size and improve functional outcome in rodent cerebral ischemia models (Chen et al, 2001; Li et al, 2002; Iihoshi et al, 2004; Nomura et al, 2005; Honma et al, 2006; Horita et al, 2006; Liu et al, 2006), the detailed mechanisms by which MSCs promote functional recovery remain unclear. Angiogenesis is thought to be associated with improved neurologic recovery after ischemia (Krupinski et al, 1994; Chen et al, 2003).
Vascular endothelial growth factor has been reported to show strong angiogenetic effects in brain ischemia (Zhang and Chopp, 2002), limb ischemia (Baumgartner et al, 1998), and myocardial ischemia models (Kastrup, 2003). Vascular endothelial growth factor exerts its biologic functions via three related receptor tyrosine kinases, VEGFR-1, VEGFR-2, VEGFR-3 (Yancopoulos et al, 2000), and is required to initiate the formation of immature vessels by vasculogenesis or angiogenesis (Carmeliet and Collen, 1997). However, vascular endothelial growth factor (VEGF) is also a potent enhancer of vascular permeability to blood plasma proteins within minutes after ischemic insult, which often causes brain edema after the cerebral infarction (Wang et al, 1996; Bates et al, 2002).
Angiopoietin is involved in maturation, stabilization, and remodeling of vessels (Davis et al, 1996; Suri et al, 1996, 1998; Yancopoulos et al, 2000). Angiopoietin-1 is the best characterized member of this family and binds to Tie2, a receptor tyrosine kinase that is expressed on endothelial cells lining blood vessels and in the choroid plexus (Dumont et al, 1994; Nourhaghighi et al, 2003), and promotes angiogenesis in the brain (Ward and Lamanna, 2004). Furthermore, angiopoietin-1 (Ang-1) protects the peripheral vasculature from vascular leakage (Thurston et al, 1999), which may account for its anti-edematic effects after cerebral ischemia.
In the present study, hMSCs and Ang-1 gene-modified hMSCs (Ang-hMSCs), which had been transfected with the Ang-1 gene were intravenously delivered 6 h after induction of unilateral permanent cerebral ischemia to investigate if cellular delivery of Ang-1 by hMSCs could influence angiogenesis and functional outcome.
Materials and Methods
Preparation of Human Mesenchymal Stem Cells
Human mesenchymal stem cells were processed for cell culture as described previously (Nomura et al, 2005; Honma et al, 2006; Liu et al, 2006). Briefly, human bone marrow from healthy adult volunteers was obtained by aspiration from the posterior iliac crest after informed consent was obtained; the subject’s consent was obtained according to the Declaration of Helsinki Principles, and this study was approved by the Institutional Review Board at our university. Bone marrow mononuclear cells were isolated, and were plated in 150-cm2 plastic tissue culture flasks and incubated overnight. After washing away the free cells, the adherent cells were cultured in Mesenchymal Stem Cell Basal Medium (Cambrex, Walkersville, MD, USA) containing Mesenchymal Cell Growth Supplement (Cambrex), 4 mmol/L L-glutamine, in a humidified atmosphere of 5% CO2 at 37°C. After reaching confluency, they were harvested and cryopreserved as primary MSCs or used for gene transduction.
Adenoviral Vectors
Adenoviral vectors carrying a human Ang-1 cDNA were constructed as described previously with minor modifications (Takahashi et al, 2003; Kurozumi et al, 2004). Briefly, human Ang-1 cDNA was cloned using the reverse transcription polymerase chain reaction method using the total RNA extracted from primary MSC as the template. The identity of Ang-1 cDNA obtained in this manner was confirmed by sequencing and comparing it with the GeneBank sequence U83508. The Ang-1 cDNA was inserted between the EcoRI site and the BglII site in the pCAcc vector, and the resulting plasmid was designated pCAhAng1. The plasmid pCAhAng1 was digested with ClaI, and the fragment containing the Ang-1 cDNA expression unit was isolated by agarose gel electrophoresis. The adenoviral Ang-1 expression vector, pWEAxCAhAng1-F/RGD, was prepared using LipofectA-MINE 2000 (Invitrogen, Tokyo, Japan).
Before being used, the above viral vectors were evaluated for their viral concentration and titer, and viral stocks were examined for potential contamination with replication-competent viruses. To determine viral concentration (particle unit (pu)/mL), the viral solution was incubated in 0.1% sodium dodecyl sulfate and A260 was measured. The viral titers of AxCAhAng1-F/RGD were 1.0 × 1012 pu/mL, respectively.
Adenovirus Infection
Adenovirus-mediated gene transfection was performed as described previously (Takahashi et al, 2003; Kurozumi et al, 2004). Briefly, the cells were seeded at a density of 2 × 106 cells per 15cm plate. Human mesenchymal stem cells were exposed to the infectious viral particles in 7.5mL Dulbecco’s modified Eagle’s medium at 37°C medium for 60 mins; cells were infected with AxCA-hAng1-F/RGD or AxCALacZ-F/RGD at an multiplicity of infection (MOI) of 3.0 × 103 pu/cell. The medium was then removed, and the cells were washed once with Dulbecco’s modified Eagle’s medium and then recultured with normal medium for 24 h, after which transplantation was performed.
Phenotypic Characterization of the Human Mesenchymal Stem and Angiopoietin-1 Gene-Modified Human Mesenchymal Stem Cells
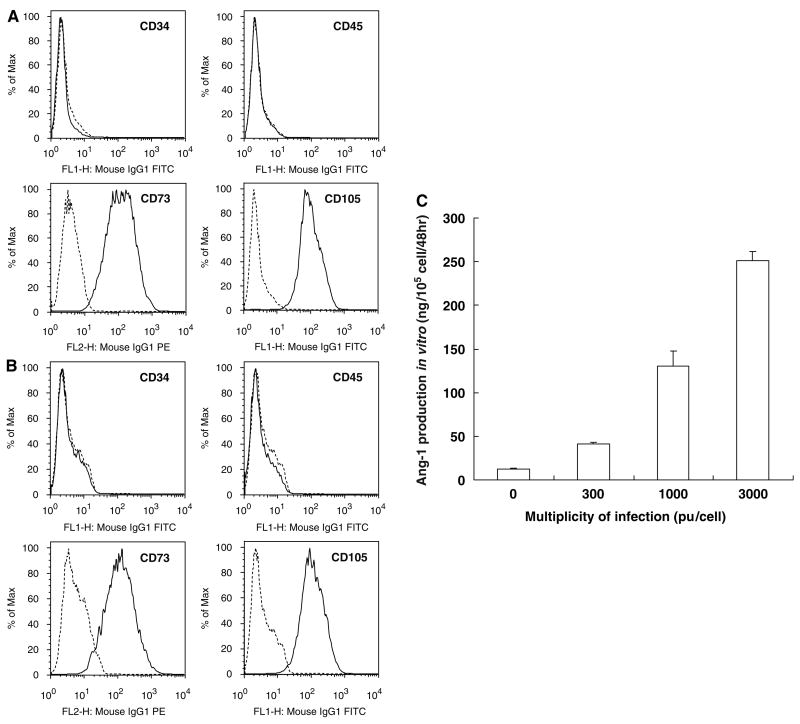
Flow cytometric analysis of primary hMSCs and Ang-hMSCs were performed as described previously (Honma et al, 2006; Liu et al, 2006). Briefly, cell suspensions were washed twice with phosphate-buffered saline containing 0.1% bovine serum albumin. For direct assays, aliquots of cells at a concentration of 1 × 106 cells/mL were immunolabeled at 4°C for 30 mins with the following anti-human antibodies: fluorescein isothiocyanate (FITC)-conjugated CD45, CD34, CD105 (Immunotech, Marseilles, France), and anti-human CD73 (SH-3; BD Biosciences Pharmingen, San Diego, CA, USA). As an isotype-matched control, mouse immunoglobulin G1-FITC (IgG1-FITC; Immunotech) was used. Labeled cells were analyzed by a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) with the use of CellQuest software. Dead cells were gated out with forward- versus side-scatter window and propidium iodide staining.
Cerebral Ischemic Model
The rat middle cerebral artery occlusion (MCAO) model was used as a stroke model. We induced permanent MCAO by using a previously described method of intraluminal vascular occlusion (Longa et al, 1989). Adult male Sprague–Dawley rats weighing 250 to 300 g were anesthetized with an intraperitoneal injection of ketamine (75 mg/kg) and xylazine (10 mg/kg). A length of 20.0 to 22.0mm 4-0 surgical dermalon suture with the tip rounded by heating near a flame was advanced from the external carotid artery into the lumen of the internal carotid artery until it blocked the origin of the MCA.
Transplantation Procedures
Experiments consisted of three groups: group 1, medium (Mesenchymal Stem Cell Basal Medium) alone (without donor cell administration) (n = 32); group 2, hMSCs (1.0 × 106) (n = 20); and group 3, Ang-hMSCs (1.0 × 106) (n = 21). All injections were made 6 h after MCAO and cells were suspended in 1mL of medium.
In some experiments, AxCALacZ-F/RGD adenovirus was used to transduce the LacZ gene into the hMSC (n = 8) and Ang-hMSC (n = 16). For in vitro adenoviral infection, 1.0 × 107 MSCs were placed with AxCALacZ-F/RGD at a MOI of 3.0 × 103 pu/cell for 1 h and incubated at 37°C in medium containing 10% fetal calf serum.
Magnetic Resonance Imaging Studies and Measurement of Infarct Volume
Rats were anesthetized intraperitoneally with ketamine (75 mg/kg) and xylazine (10 mg/kg). Each rat was placed in an animal holder/magnetic resonance imaging (MRI) probe apparatus and positioned inside the magnet. The animal’s head was held in place inside the imaging coil. All MRI measurements were performed using a 7-T, 18-cm-bore superconducting magnet (Oxford Magnet Technologies, Witney, Oxfordshire, UK) interfaced to a UNITYINOVA console (Oxford Instruments, UK and Varian, Palo Alto, CA, USA). T2-weighted images (T2WIs) were obtained from a 1.0-mm-thick coronal section with a 0.5mm gap using a 30mm × 30mm field of view, TR = 3000 ms, TE = 37 ms, b value = 0, and reconstructed using a 256 × 256 image matrix. Diffusion-weighted images (DWIs) were obtained under the same condition as T2WI except b value (1000). Accurate positioning of the brain was performed to center the image slice 5mm posterior to the rhinal fissure with the head of the rat held in a flat skull position. Magnetic resonance imaging measurements were obtained 6, 24 h, 3, and 7 days after MCAO.
The ischemic lesion area was calculated from both T2WI and DWI using imaging software (Scion Image, Version Beta 4.0.2, Scion Corporation, Frederick, MD, USA), based on the previously described method (Neumann-Haefelin et al, 2000). For each slice, the higher intensity lesions in both T2WI and DWI, where the signal intensity was 1.25 times higher than the counterpart in the contralateral brain lesion, were marked as the ischemic lesion area, and infarct volume was calculated taking slice thickness (1 mm/slice) into account.
Detection of Angiopoietin-1 In Vitro
In separate dishes, we cultured hMSCs (1.0 × 105) transfected in vitro at various MOI (0, 300, 1000, and 3000 pu/cell). Forty-eight hours after hMSCs were transfected, culture supernatants were collected for analysis. The culture supernatants were centrifuged for 10 mins. Commercial Ang-1 ELISA kits (R&D Systems, Minneapolis, MN, USA) were used to quantify the concentration of Ang-1 in each of the samples.
2,3,5-Triphenyltetrazolium Chloride Staining and Quantitative Analysis of Infarct Volume
One week after transplantation, the rats were deeply anesthetized intraperitoneally with ketamine (4.4 to 8 mg/kg) and xylazine (1.3 mg/100 g). The brains were removed carefully and dissected into coronal 1mm sections using a vibratome. The fresh brain slices were immersed in a 2% solution of 2,3,5-triphenyltetrazolium chloride (TTC) in normal saline at 37°C for 30 mins.
The cross-sectional area of infarction in each brain slice was examined with a dissection microscope and was measured using an image analysis software, NIH image. The total infarct volume for each brain was calculated by summation of the infarcted area of all brain slices.
Detection of Donor Angiopoietin-1 Human Mesenchymal Stem Cells In Vivo
X-gal Staining
One week after transplantation, the LacZ-transfected Ang-hMSCs in vivo were detected by X-gal staining. Brains of the deeply anesthetized rats were removed and fixed in 0.5% glutaraldehyde in phosphate buffer for 1 h. Brains were removed and brain slices (1000 μm) were cut with a vibratome, and β-galactosidase-expressing cells were detected by incubating the sections at 37°C overnight with X-gal to a final concentration of 1mg/mL in X-Gal developer (35 mmol/L K3Fe(CN)6/35mmol/L K4Fe(CN)63H2O/2mmol/L MgCl2 in phosphate-buffered saline) to form a blue reaction product within the cell.
Immunohistochemistry
Two weeks after transplantation, the LacZ-transfected Ang-hMSCs in vivo were detected using laser-scanning confocal microscopy. Brains of the deeply anesthetized rats were removed, fixed in 4% paraformaldehyde in phosphate buffer, dehydrated with 30% sucrose in 0.1 mol/L phosphate-buffered saline for overnight, and frozen in powdered dry ice. Coronal cryostat sections (10 μm) were processed for immunohistochemistry. To identify the transplanted cells, LacZ-labeled Ang-hMSCs were visualized using the antibody to β-galactosidase (rhodamine-labeled polyclonal rabbit anti-β-galactosidase antibody, DAKO, Carpinteria, CA, USA). To identify the cell type derived from the donor Ang-hMSCs, double-labeling studies were performed with the use of antibody to endothelial cells (FITC-labeled polyclonal rabbit anti-von Willbrand factor (vWF); DAKO). To excite the FITC fluorochrome (green), a 488-nm laser line generated by an argon laser was used, and for the rhodamine fluorochrome (red), a 543-nm laser line from a HeNe laser was used. Confocal images were obtained using a Zeiss laser-scanning confocal microscope with the use of Zeiss software.
Three-Dimensional Image Acquisition for Angiogenesis
Seven and twenty-eight days after MCAO, rats were anesthetized and intravenously perfused with FITC-dextran (1 mL, Sigma). The brains were immediately removed, and were fixed in 4% paraformaldehyde at 4°C for 48 h. Coronal vibratome sections (100 μm) were analyzed with a laser-scanning confocal imaging system (LSM Pascal; Carl Zeiss, Jend, Germany). The sections were scanned in 512 × 512 pixel (651.5 μm × 651.5 μm) format in the x–y direction using a 5 × frame-scan average (2.18 μm interval and 2.4 μm thickness along the z axis) under a × 20 objective. Vessel volumes were measured in three dimensions using the Zeiss LSM software.
Dynamic Susceptibility Contrast-Enhanced Perfusion-Weighted Imaging
Perfusion-weighted imaging was acquired using T2*-weighted (TR = 13 ms, TE = 6.0 ms) gradient echo sequence (Ukai et al, 2007). A dynamic image series of 30 measurements resulted in a total scan time of 26 secs, with a fields of view of 30mm and image acquisition matrix of 128 × 64, which was interpolated by zero-filling to 512 × 512. During the dynamic series, a triple-dose (0.6 mL/kg) bolus injection of Magnevist (Schering AG, Berlin, Germany) was started after the fifth acquired volume to ensure a sufficient precontrast baseline. Images were reconstructed by an Inova Vision. Perfusion-weighted imaging measurements were obtained 6 h, 3, and 7 days after MCAO. For the perfusion-weighted imaging (PWI) and PWI-derived parameter maps, only one representative slice (involving cortex and stria terminalis) with the maximum lesion involving both cortex and striatum was chosen for cerebral blood flow quantification. The readout of abnormal regional cerebral blood flow (rCBF) from the regions of perfusion deficiency as a percentage of that measured in the contralateral brain was generated using Perfusion Solver software. Regions of interest (ROI) consists of four groups, based on the results of DWI, T2WI, and PWI. ROI-1 is defined as abnormal in all images, ROI-2 as normal in only T2WI and abnormal in others, ROI-3 as abnormal in only PWI and normal in others, and ROI-4 as normal in all images.
Treadmill Stress Test
Rats were trained 20 mins/day for 2 days a week to run on a motor-driven treadmill at a speed of 20 m/min with a slope of 0°C. Rats were placed on a moving belt facing away from the electrified grid and induced to run in the direction opposite to the movement of the belt. Thus, to avoid foot shocks (with intensity in 1.0 mA), the rats had to move forward. Only the rats that had learned to avoid the mild electrical shock were included in this study. The maximum speed at which the rats could run on a motor-driven treadmill was recorded.
Statistical Analysis
Data are presented as mean values±s.d., and were statistically analyzed. Differences among groups were assessed by ANOVA with Scheffes post hoc test and the Kruskal–Wallis test to identify individual group differences. Differences were deemed statistically significant at P < 0.05.
Results
Characteristics of Primary and Angiopoietin-1 Gene-Transduced Human Mesenchymal Stem Cells
Primary hMSCs were cultured as plastic adherent cells to subconfluency, which took approximately 1 week (see Materials and methods). A characteristic feature of hMSCs is a CD34−, CD45−, SH2+(CD105), and SH3+(CD73) cell surface phenotype (Kobune et al, 2003). Both flattened and spindle-shaped cells can be recognized in the culture. Angiopoietin-1 gene-modified human mesenchymal stem cells showed similar flattened and spindle-shaped morphology. Flow cytometric analysis of the Ang-hMSCs (Figure 1B) was essentially identical to primary hMSCs (Figure 1A).
Figure 1.
Flow cytometric analysis of surface antigen expression on primary hMSC (A) and Ang-hMSC (B). The cells were immunolabeled with FITC-conjugated monoclonal antibody specific for the indicated surface antigen. Dead cells were eliminated by forward and side scatter. Angiopoietin-1 production of hMSC, Ang-transfected hMSC were summarized (C). Levels of Ang-1 in the supernatant were shown.
Detection of Immunoreactive Human Angiopoietin-1 and Quantitative Analysis In Vitro
Levels of Ang-1 in the supernatant of cultured hMSCs and Ang-hMSCs with different MOI levels were studied. Human mesenchymal stem cell transfected with AxCAhAng1-F/RGD (Ang-hMSC) at an MOI of 300, 1000, and 3000 pu/cell secreted Ang-1 at a rate of 40.75±1.94, 130.08±17.78, and 251.37±10.31 ng/105 cells/48 h, respectively (n = 4). Non-transfected hMSC also produced Ang-1 protein (12.91±0.093 ng/105 cells/48 h) (n = 4). These data are summarized in Figure 1C. The level of Ang-1 production from Ang-hMSC transfected at an MOI of 3000 pu/cell was approximately 20-fold greater than that observed in non-infected.
Characterization of Ischemic Lesion Size by Magnetic Resonance Image Analysis
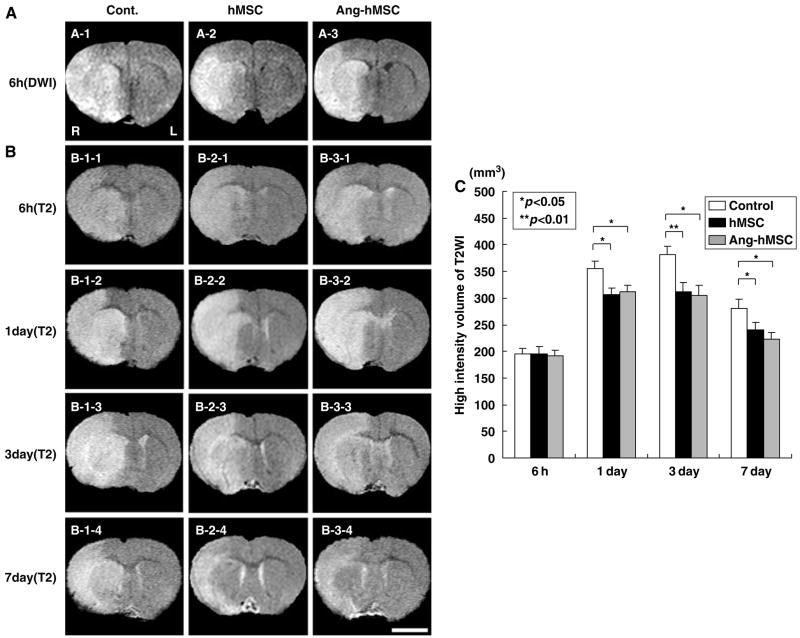
An estimate of lesion size was obtained using in vivo MRI (see Materials and methods). The cells were intravenously delivered immediately after the 6 h MRI. Both T2WI and DWI were carried. The reason is that the DWI is more useful for lesion volume detection in the acute infarction phase and the T2WI is more important for later time points. Therefore, DWI was used at only the time point of 6 h. Diffusion-weighted image and T2WI are shown in Figures 2A and 2B, respectively. These coronal forebrain sections were obtained at the level of caudato-putamen complex. T2-weighted images obtained at 6 h, 1, 3, and 7 days are shown for control (medium) (n = 15), hMSC (n = 10), and Ang-hMSC-injected groups (n = 8) (Figure 2B). Note the reduction in density in lesions on the right side of the brains of the cell-treated groups at 7 days. Lesion volume (mm3) was determined by analysis of high-intensity areas on serial images collected through the cerebrum (see Materials and methods).
Figure 2.
Evaluation of the ischemic lesion volume with DWIs and T2WIs. Human mesenchymal stem cells or Ang-hMSCs were intravenously injected immediately after the initial MRI scanning (6 h after MCAO). Diffusion-weighted image obtained 6 h MCAO in medium-injected (A-1), hMSC-treated (A-2), and Ang-hMSC-treated group (A-3). T2-weighted images obtained 6 h, 1, 3, and 7 days MCAO in medium-injected (B-1-1 to -4), hMSC-treated (B-2-1 to -4), and Ang-hMSC-treated group (B-3-1 to -4). Bar=5 mm. (C) A summary of lesion volumes evaluated with MRI (T2WI) were obtained 6 h, 1, 3, and 7 days after MCAO in rats treated with medium (control), hMSC, or Ang-hMSC.
To determine the efficacy of hMSCs or Ang-hMSCs transplantation in modulating the ischemic lesion volume, 1 × 106 cells were delivered intravenously at 6 h after MCAO, and animals were reimaged at 6, 24 h, 3, and 7 days later. In the sham control group, MRI-estimated lesion volume reached maximum at 3 days (Figure 2B-1–3) and gradually decreased at 7 days post-MCAO (Figure 2B-1–4). Although lesion volumes estimated 6 h after MCAO were the same among the three groups (Figures 2A and 2B) at 1, 3, and 7 days, lesion volume was less in both hMSC and Ang-hMSC groups as compared with control. The reduction in lesion volume showed no significant difference between the Ang-hMSC group and the hMSC group at 1, 3, and 7 days post-MCAO. These results are summarized in Figure 2C.
Histological Determination of Infarction Volume
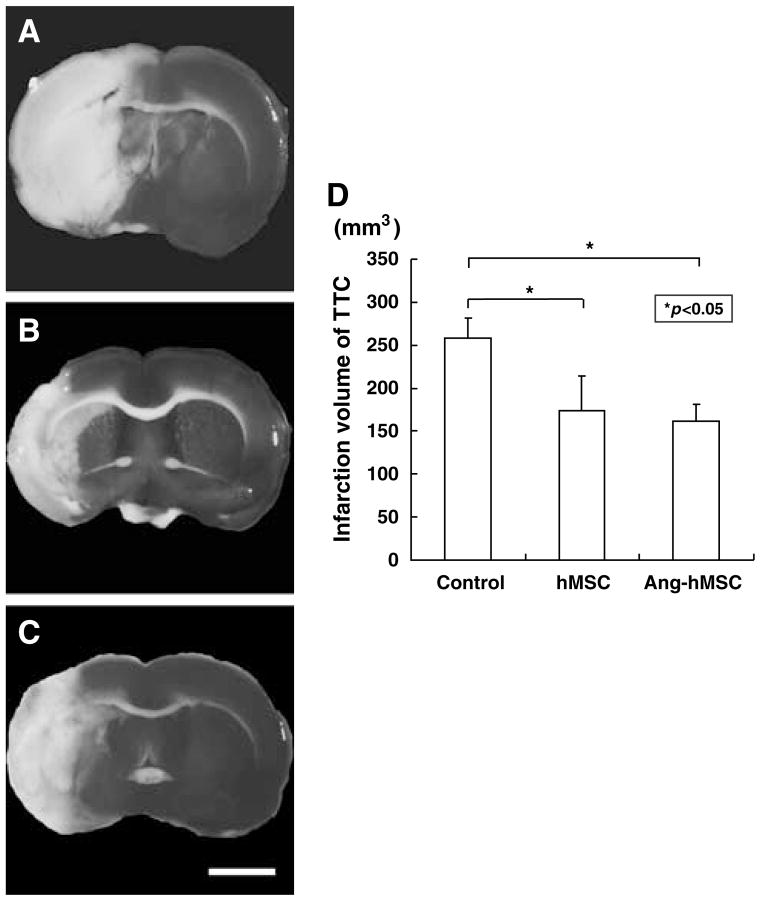
A second independent measure of infarction volume was performed. The brains were stained with TTC 7 days after MCAO (Figure 3). Normal brain (gray matter) tissue typically stains with TTC, but infarcted lesions show no or reduced staining (Bederson et al, 1986). 2,3,5-Triphenyltetrazolium chloride staining obtained 7 days after MCAO without cell transplantation is shown in Figure 3A. Note the reduced staining on the lesion side. Lesion volume was calculated by measuring the area of reduced TTC staining in the forebrain (n = 5) (see Materials and methods). As with MRI analysis there was a reduction in infarction volume with both hMSC (n = 5) (Figure 3B) and Ang-hMSC (n=5) (Figure 3C) treatment. Intravenous delivery of 1 × 106 hMSCs and Ang-hMSCs resulted in very substantial reduction in lesion volume as estimated from TTC staining. The reduction in lesion volume was not significantly different between the Ang-hMSC group and the hMSC group at 7 days post-MCAO (Figure 3D).
Figure 3.
TTC Brain sections slices stained with TTC to visualize the ischemic lesions 7 days after MCAO. 2,3,5-Triphenyl tetrazolium chloride-stained brain slices from (A) mediuminjected MCAO model rats, (B) following hMSC-treated, and (C) Ang-hMSC-treated groups. (D) Seven days after MCAO, there was a reduction in the lesion volume assayed using TTC staining for both hMSC and Ang-hMSC groups. Bar=3 mm.
Angiogenesis Induced by the Transplantation
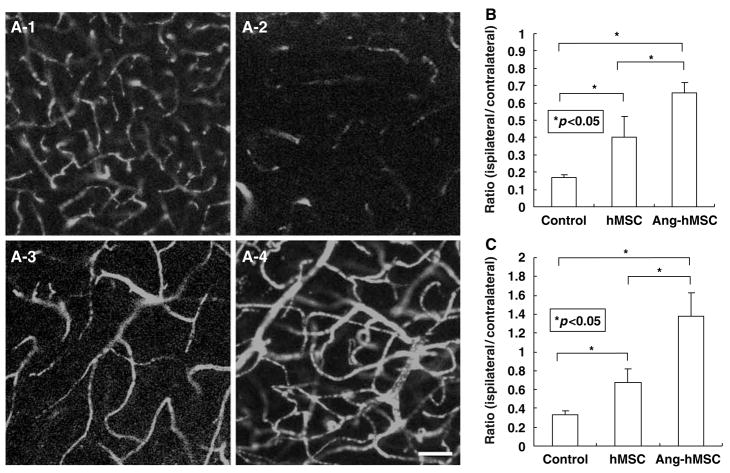
To examine whether the administration of hMSCs and Ang-hMSCs induces angiogenesis, three-dimensional analysis of capillary vessels in the lesion was performed using Zeiss LSM5 PASCAL software. Figure 4A-1 shows the three-dimensional capillary image in the normal rat brain (n = 4). The capillary vascular volume in the boundary region after MCAO was increased in the hMSC-treated group (n=4) (Figure 4A-3) compared with the medium-treated group (n = 4) (Figure 4A-2), and the angiogenesis was greater in the Ang-hMSC-treated group (n=4) (Figure 4A–4).
Figure 4.
Seven and twenty-eight days after MCAO, the angiogenesis in boundary zone was analyzed using a three-dimensional analysis system. (A-1) Three-dimensional capillary image with systemically perfused FITC-dextran in the normal rat brain is shown. The total volume of the microvessels in the sampled lesion site decreased (A-2) 28 days after MCAO, but was greater in (A-3) the hMSC-treated group, and (A-4) the Ang-hMSC-treated group. These results are summarized in (B) (7 days after MCAO) and (C) (28 days after MCAO). The ratio (ispilateral/contralateral) was significantly higher in both the hMSC- and the Ang-hMSC-treated groups as compared with the medium-treated group. Also the ratio of the Ang-hMSC-treated group was significantly higher than the ratio of the hMSC-treated group. Bar=150 μm.
The capillary vascular volume was expressed as a ratio by dividing that obtained from the ischemic hemisphere by that of the contralateral control hemisphere. At 7 days after MCAO, the ratio (ipsilateral/contralateral) was significantly higher in both the hMSC (0.17±0.07; P < 0.05) and the Ang-hMSC (0.46±0.05; P < 0.001)-treated groups as compared with the medium-treated group (0.13±0.01). The ratio of the Ang-hMSC-treated group was significantly higher than the ratio of the hMSCtreated group. These results are summarized in Figure 4B. At 28 days after MCAO, the ratio (ipsilateral/contralateral) of Ang-hMSC-treated group (1.1±0.29) was significantly higher than the ratio of hMSC-treated group (0.57±0.12) and medium- treated group (0.26±0.03). The ratio of the AnghMSC- treated group was significantly higher than the ratio of the hMSC-treated group. These results were summarized in Figure 4C.
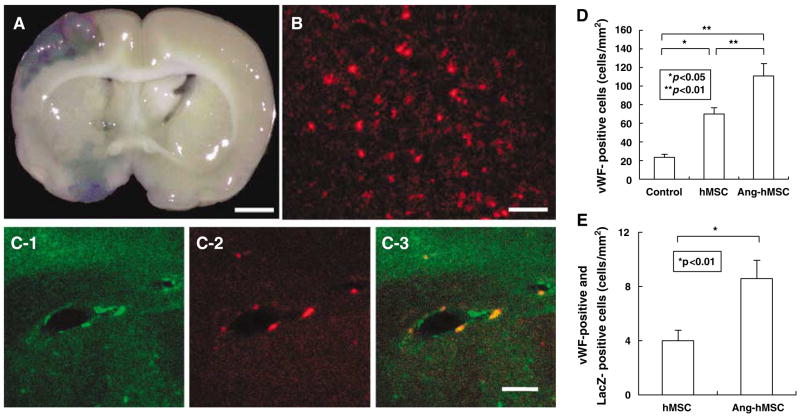
LacZ-transfected hMSCs and Ang-hMSCs that had been intravenously administered (1.0 × 106 cells) 6 h after MCAO were identified in vivo. The LacZexpressing MSCs were found primarily in the lesion. Note the abundance of β-gal-reaction product in and around the lesion, indicating that systemic delivery of the cells reached the lesion site (Figure 5A). There was a paucity of blue staining in the non-treated group.
Figure 5.
Intravenously –administered Ang-hMSCs accumulated in and around the ischemic lesion hemisphere. Angiopoietin-1 gene-modified human mesenchymal stem cells were transfected with the reporter gene LacZ. (A) Transplanted LacZ-positive Ang-hMSCs (blue cells) were present in the ischemic lesion. (B) Confocal images demonstrating a large number of LacZ-positive cells in the lesion hemisphere. Ang-hMSCs differentiated into the endothelial lineage. (C-1 and C-2) Confocal images show the transplanted cells (C-2: LacZ in red) and endothelial cells (C-1: vWF in green). Panel C-3 confirms the co-labeling of LacZ/vWF in the cells. The cell density of vWF-positive cells are shown in D. Panel E demonstrates the cell density of vWF-positive cells co-localized with LacZ. Bar: (A)—3 mm; (B)—150 μm; and (C)—50 μm.
Immunohistochemical studies were performed to identify LacZ-positive cells in and around the lesion zone in animals transplanted with LacZ-transfected hMSCs. The photomicrographs of hMSCs and Angh-MSCs demonstrated a large number of LacZ-positive cells in and around the lesion (Figure 5B). Double staining was performed to show endothelial cell differentiation of LacZ-positive cells in the lesion zone in animals transplanted with LacZ-transfected Ang-hMSCs (Figure 5C). The vWF-positive cells in the boundary region 7 days after MCAO were increased in the hMSC-treated group (n = 4) compared with the medium-treated group (n = 4), which was the highest in the Ang-hMSCtreated group (n = 4) (Figure 5D). Double-positive cells of vWF and LacZ were also increased in the Ang-hMSC-treated group compared with the hMSC-treated group (Figure 5E).
Dynamic Susceptibility Contrast-Enhanced Perfusion-Weighted Image
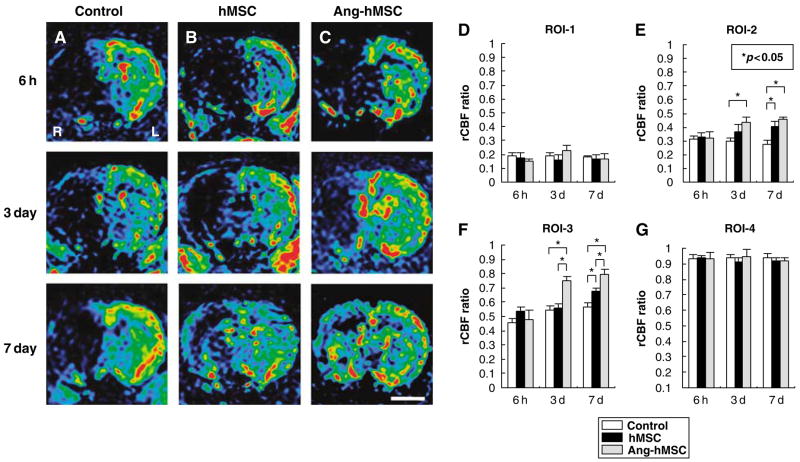
To assess regional cerebral blood flow, the PWI-derived parameter maps allowed further quantitative analysis for the hemodynamic changes of the lesions (see Materials and methods). Figures 6A–C shows images obtained at 6 h (row 1), 3 days (row 2), and 7 days (row 3). Control, hMSC- and Ang-hMSC-injected groups are in columns A, B, and C, respectively.
Figure 6.
Evaluation of hemodynamic state (rCBF maps) with PWIs. Human mesenchymal stem cells or Ang-hMSCs were intravenously injected immediately after the initial MRI scanning (6 h after MCAO). Images obtained 6 h, 3, and 7 days MCAO in (A) medium-injected, (B) hMSC-treated, and (C) Ang-hMSC-treated group. (D–G) Summary of rCBF evaluated with PWI in each groups. (D) ROI-1, (E) ROI-2, (F) ROI-3, and (G) ROI-4. Regional cerebral blood flow ratio (ischemic lesion/contralateral lesion) at 6 h, 3, and 7 days after MCAO are summarized in D–G. Bar=3 mm, *P<0.05.
The four regions of interest (ROI) for the analysis are defined in Materials and methods. The severity of the lesion was greatest in ROI-1 and progressively less in ROI-2 through ROI-4. A rCBF ratio was calculated at each ROI from PWI obtained in the infarction hemisphere divided by that of the non-infarcted hemisphere. In ROI-1, the rCBF ratio of control, hMSC-treated, and Ang-hMSC-treated groups were similar and decreased to <25% at 6 h post-MCAO, and remained low at 3 and 7 days (Figure 6D). The rCBF ratio in ROI-2 of the three groups was similar at 6 h post MCAO. However, the rCBF ratio of both hMSC- and Ang-hMSC-treated groups was increased at 7 days after MCAO as compared with control (Figure 6E). The rCBF ratio in ROI-3 was similar for the three groups at 6 h, but again both hMSC- and Ang-hMSC-treated groups had a greater rCBF ratio at 3 and 7 days (Figure 6F). In ROI-4, the rCBF ratio slightly decreased in all groups at all time points, but not more than 10% (Figure 6G).
Functional Analysis
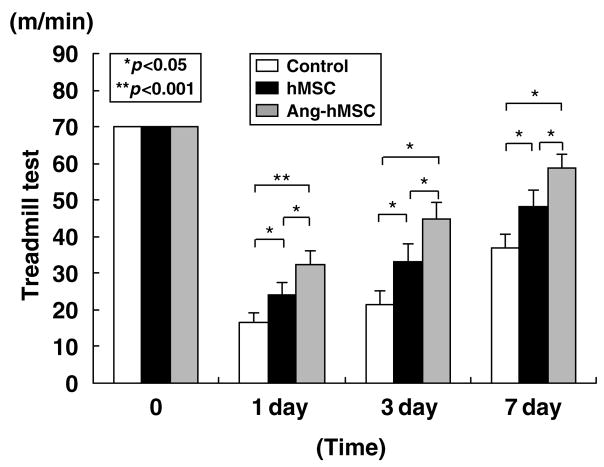
The maximum speed at which the rats could run on a motor-driven treadmill was recorded. Before MCAO, there was no significant difference among control (n = 27), hMSC-treated (n = 21), and Ang-hMSC- treated groups (n = 19) (Figure 7); and at 6 h post-MCAO there was comparable impairment among three groups. Twenty-four hours after MCAO maximum velocity on the treadmill test was at its maximum deficit. An increase in maximum velocity was observed for the Ang-hMSC group at 24 h. Both hMSC and Ang-hMSC groups had greater maximum velocity from 3 to 7 days than sham control, but the Ang-hMSC group attained a higher velocity than the hMSC group. These results are summarized in Figure 7.
Figure 7.
The treadmill stress test demonstrates that the maximum speed at which the rats could run on a motor-driven treadmill was faster in the hMSC- and the Ang-hMSC-treated rats than control. One day after MCAO, rats that received Ang-hMSC showed higher running speed compared with the medium alone and the hMSC group. From 3 days on after MCAO, the hMSC and Ang-hMSC groups attained higher velocities but the Ang-hMSC group was greater. The highest level of functional improvement was attained at 7 days.
Discussion
This study demonstrates that intravenous infusion of either hMSCs or Ang-hMSCs 6 h after permanent MCAO in the rat results in reduction in infarction volume, an induce in angiogenesis, and improvement in behavioral performance. These results are consistent with previous studies showing beneficial effects of bone marrow cell transplantation in experimental cerebral ischemic models (Chen et al, 2001; Iihoshi et al, 2004; Nomura et al, 2005; Honma et al, 2006; Horita et al, 2006; Kim et al, 2006; Liu et al, 2006; Ukai et al, 2007). Both hMSCs and Ang-hMSCs comparably reduced gross lesion volume as assayed with MRI and histology. Angiogenesis and rCBF were increased after infusion of either cell type, but were greater in small border areas after Ang-hMSC infusion. Functional recovery was also improved for both cell types, but modestly greater in the Ang-hMSC group. Thus, Ang-hMSCs do not effect lesion volume any more than hMSCs, but may facilitate small pockets of regional neovascularization and modest augumented functional recovery.
Both VEGF and Ang-1 play important roles in forming new vessels. Vascular endothelial growth factor has a role in enlarging small vessels (Pettersson et al, 2000). Although VEGF can initiate vessel formation, on its own it promotes formation of leaky, immature, and unstable vessels. Such destabilized vessels would be prone to regression in the absence of associated growth factors. In contrast, Ang-1 induces vessel sprouting and branching (Davis et al, 1996; Koblizek et al, 1998), and seemingly further stabilizes and protects the adult vasculature, making it resistant to the damage and leak by VEGF or inflammatory challenges (Yancopoulos et al, 2000).
Vascular endothelial growth factor displays other effects such as an increase in the endothelium permeability (Bates et al, 2002). This effect could lead to vasogenic edema, particularly deleterious in cerebral ischemia. Indeed, early administration of VEGF after MCAO exacerbates blood–brain barrier leakage, causing massive brain edema (Zhang et al, 2000). Systemic delivery of Ang-1 by adenoviral gene delivery causes resistance to vascular leakage induced by VEGF in the brain (Zhang et al, 2002) and in the skin (Thurston et al, 1999).
Upregulation of endogenous Ang-1 in ischemic brain tissue was reported (Beck et al, 2000; Zhang et al, 2002), suggesting that Ang-1 might play an important role in endogenous repair in the ischemic lesion. However, therapeutic effects of exogenously applied Ang-1 on ischemic brain had not been studied. In this study, we applied Ang-1 by delivering hMSCs that hypersecrete Ang-1 to determine if Ang-1 would augment neovascularization in the post-ischemic brain and possibly improve functional outcome. Although in three-dimensional image analysis for angiogenesis, the Ang-hMSC-transplanted group showed small border areas of increased angiogenesis as compared with the hMSC transplanted and control groups, the effects were small and regional. Similarly, both hMSCs and Ang-hMSCs transplantation groups showed improvement of rCBF in the lesion, and only small areas of increased rCBF were observed in the Ang-hMSC transplantation group as compared with the hMSC group. No increase in cerebral edema was observed for either cell type as assayed by both in vivo MRI and histologic analysis. Thus, cellular delivery of Ang-1 to the ischemic brain had only modest effects on angiogenesis. One possibility to account for the modest effects is that the endogenous upregulation of Ang-1 in ischemic brain (Beck et al, 2000; Zhang et al, 2002) may be sufficient to support the observed angiogenesis after hMSC infusion, and additional Ang-1 is unnecessary. Our immunohistochemical data suggest that Ang-hMSCs do not result in extensive or dynamic angiogenesis. However, in studies using genetically modified hMSC to express trophic factors including brain-derived neurotrophic factor (Nomura et al, 2005), placental growth factor (PlGF) (Liu et al, 2006), or glial cell line-derived neurotrophic factor (Horita et al, 2006) lesion volume was reduced over that observed after non-modified hMSCs. Thus, a neuroprotective effect from hMSC delivery may be an important if not the dominant mechanism to account for reduction in lesion size and improved functional outcome.
Cell-based therapeutic approaches are being considered for a number of neurologic diseases (Chopp et al, 2000; Pluchino et al, 2003; Iihoshi et al, 2004; Nomura et al, 2005; Pluchino et al, 2005; Zhang et al, 2005; Honma et al, 2006; Horita et al, 2006, Liu et al, 2006). Suggested mechanisms include reduction of inflammatory infiltration, elevation of trophic factors that may be neuroprotective, and structural repair such as remyelination (Radtke et al, 2007). A cell-based therapy may have the advantage of exerting multiple therapeutic effects at various sites and times within the lesion as the cells respond to a particular pathologic microenvironment.
Our results indicate that infusion of non-genetically modified hMSCs results in significant reduction in ischemic lesion volume and improved functional outcome, and Ang-hMSC had a modest additional influence on angiogenesis and functional outcome. Thus, hMSCs without genetic modification to induce additional neovascularization may be a useful source of cells for a cell-based therapeutic approach in clinical studies.
Acknowledgments
This work was supported in part by grants from the Japanese Ministry of Education, Science, Sports and Culture (16390414), Mitsui Sumitomo Insurance Welfare Foundation, the National Multiple Sclerosis Society (USA) (RG2135; CA1009A10), the National Institutes of Health (NS43432), and the Medical and Rehabilitation and Development Research Services of the Department of Veterans Affairs.
References
- Bates DO, Hillman NJ, Williams B, Neal CR, Pocock TM. Regulation of microvascular permeability by vascular endothelial growth factors. J Anat. 2002;200:581–97. doi: 10.1046/j.1469-7580.2002.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner I, Pieczek A, Manor O, Blair R, Kearney M, Walsh K, Isner JM. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation. 1998;97:1114–23. doi: 10.1161/01.cir.97.12.1114. [DOI] [PubMed] [Google Scholar]
- Beck H, Acker T, Wiessner C, Allegrini PR, Plate KH. Expression of angiopoietin-1, angiopoietin-2, and tie receptors after middle cerebral artery occlusion in the rat. Am J Pathol. 2000;157:1473–83. doi: 10.1016/S0002-9440(10)64786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–8. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Collen D. Molecular analysis of blood vessel formation and disease. Am J Physiol. 1997;273:2091–104. doi: 10.1152/ajpheart.1997.273.5.H2091. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001;189:49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92:692–9. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- Chopp M, Zhang XH, Li Y, Wang L, Chen J, Lu D, Lu M, Rosenblum M. Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. Neuroreport. 2000;11:3001–5. doi: 10.1097/00001756-200009110-00035. [DOI] [PubMed] [Google Scholar]
- Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–9. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8:1897–909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- Honma T, Honmou O, Iihoshi S, Harada K, Houkin K, Hamada H, Kocsis JD. Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Exp Neurol. 2006;199:56–66. doi: 10.1016/j.expneurol.2005.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horita Y, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Intravenous administration of glial cell line-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in the adult rat. J Neurosci Res. 2006;84:1495–504. doi: 10.1002/jnr.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iihoshi S, Honmou O, Houkin K, Hashi K, Kocsis JD. A therapeutic window for intravenous administration of autologous bone marrow after cerebral ischemia in adult rats. Brain Res. 2004;1007:1–9. doi: 10.1016/j.brainres.2003.09.084. [DOI] [PubMed] [Google Scholar]
- Kastrup J. Therapeutic angiogenesis in ischemic heart disease: gene or recombinant vascular growth factor protein therapy? Curr Gene Ther. 2003;3:197–206. doi: 10.2174/1566523034578366. [DOI] [PubMed] [Google Scholar]
- Kim S, Honmou O, Kato K, Nonaka T, Houkin K, Hamada H, Kocsis JD. Neural differentiation potential of peripheral blood- and bone-marrow-derived precursor cells. Brain Res. 2006;1123:27–33. doi: 10.1016/j.brainres.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koblizek TI, Weiss C, Yancopoulos GD, Deutsch U, Risau W. Angiopoietin-1 induces sprouting angiogenesis in vitro. Curr Biol. 1998;8:529–32. doi: 10.1016/s0960-9822(98)70205-2. [DOI] [PubMed] [Google Scholar]
- Kobune M, Kawano Y, Ito Y, Chiba H, Nakamura K, Tsuda H, Sasaki K, Dehari H, Uchida H, Honmou O, Takahashi S, Bizen A, Takimoto R, Matsunaga T, Kato J, Kato K, Houkin K, Niitsu Y, Hamada H. Telomerized human multipotent mesenchymal cells can differentiate into hematopoietic and cobblestone area-supporting cells. Exp Hematol. 2003;31:715–22. doi: 10.1016/s0301-472x(03)00177-2. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–8. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, Honmou O, Houkin K, Date I, Hamada H. BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol Ther. 2004;9:189–97. doi: 10.1016/j.ymthe.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514–23. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- Liu H, Honmou O, Harada K, Nakamura K, Houkin K, Hamada H, Kocsis JD. Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain. 2006;129:2734–45. doi: 10.1093/brain/awl207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin T, Kastrup A, de Crespigny A, Yenari MA, Ringer T, Sun GH, Moseley ME. Serial MRI after transient focal cerebral ischemia in rats: dynamics of tissue injury, blood-brain barrier damage, and edema formation. Stroke. 2000;31:1965–72. doi: 10.1161/01.str.31.8.1965. [DOI] [PubMed] [Google Scholar]
- Nomura T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Intravenous infusion of BDNF gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Neuroscience. 2005;136:161–9. doi: 10.1016/j.neuroscience.2005.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourhaghighi N, Teichert-Kuliszewska K, Davis J, Stewart DJ, Nag S. Altered expression of angiopoietins during blood-brain barrier breakdown and angiogenesis. Lab Invest. 2003;83:1211–22. doi: 10.1097/01.lab.0000082383.40635.fe. [DOI] [PubMed] [Google Scholar]
- Pettersson A, Nagy JA, Brown LF, Sundberg C, Morgan E, Jungles S, Carter R, Krieger JE, Manseau EJ, Harvey VS, Eckelhoefer IA, Feng D, Dvorak AM, Mulligan RC, Dvorak HF. Heterogeneity of the angiogenic response induced in different normal adult tissues by vascular permeability factor/vascular endothelial growth factor. Lab Invest. 2000;80:99–115. doi: 10.1038/labinvest.3780013. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–94. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalini A, Bergami A, Furlan R, Comi G, Constantin G, Martino G. Neurosphere-derived multipotent precursors promote neuro-protection by an immunomodulatory mechanism. Nature. 2005;436:266–71. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- Radtke C, Spies M, Sasaki M, Vogt PM, Kocsis JD. Demyelinating diseases and potential repair strategies. Int J Dev Neurosci. 2007;25:149–53. doi: 10.1016/j.ijdevneu.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–80. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, Sato TN, Yancopoulos GD. Increased vascularization in mice overexpressing angiopoietin- 1. Science. 1998;282:468–71. doi: 10.1126/science.282.5388.468. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Ito Y, Morikawa M, Kobune M, Huang J, Tsukamoto M, Sasaki K, Nakamura K, Dehari H, Ikeda K, Uchida H, Hirai S, Abe T, Hamada H. Adenoviral-delivered angiopoietin-1 reduces the infarction and attenuates the progression of cardiac dysfunction in the rat model of acute myocardial infarction. Mol Ther. 2003;8:584–92. doi: 10.1016/s1525-0016(03)00230-2. [DOI] [PubMed] [Google Scholar]
- Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically over-expressing angiopoietin-1. Science. 1999;286:2511–4. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- Ukai R, Honmou O, Harada K, Kiyohiro H, Hamada H, Kocsis JD. Mesenchymal stem cells derived from peripheral blood protects against ischemia. J Neuro-tauma. 2007;24:508–20. doi: 10.1089/neu.2006.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Merrill MJ, Borchardt RT. Vascular endothelial growth factor affects permeability of brain microvessel endothelial cells in vitro. Am J Physiol. 1996;271:C1973–80. doi: 10.1152/ajpcell.1996.271.6.C1973. [DOI] [PubMed] [Google Scholar]
- Ward NL, Lamanna JC. The neurovascular unit and its growth factors: coordinated response in the vascular and nervous systems. Neurol Res. 2004;26:870–83. doi: 10.1179/016164104X3798. [DOI] [PubMed] [Google Scholar]
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–8. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li Y, Chen J, Cui Y, Lu M, Elias SB, Mitchell JB, Hammill L, Vanguri P, Chopp M. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp Neurol. 2005;195:16–26. doi: 10.1016/j.expneurol.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Chopp M. Vascular endothelial growth factor and angiopoietins in focal cerebral ischemia. Trends Cardiovasc Med. 2002;12:62–6. doi: 10.1016/s1050-1738(01)00149-9. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–38. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Tsang W, Soltanian-Zadeh H, Morris D, Zhang R, Goussev A, Powers C, Yeich T, Chopp M. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002;22:379–92. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]