Abstract
Screening, diagnosis and monitoring of paediatric diseases relies on the measurement of a spectrum of disease biomarkers in clinical laboratories to guide important clinical decisions. Physicians rely on the availability of suitable and reliable reference intervals to accurately interpret laboratory test results with data collected during medical history and physical examination. However, critical gaps currently exist in accurate and up-to-date reference intervals (normal values) for accurate interpretation of laboratory tests performed in children and adolescents. These gaps in the available paediatric laboratory reference intervals have the clear potential of contributing to erroneous diagnosis or misdiagnosis of many diseases of childhood and adolescence. Most of the available reference intervals for laboratory tests were determined over two decades ago on older instruments and technologies, and are no longer relevant considering the current testing technology used by clinical laboratories. It is thus critical and of utmost urgency that a more acceptable and comprehensive database be established. There are however many challenges when attempting to establish paediatric reference intervals. Paediatric specimen collection is a major concern for health care providers as it is frequently difficult to obtain sufficient volumes of blood or urine from paediatric patients. Common reference intervals have not been widely implemented due to lack of harmonisation of methods and differences in patient populations. Consequently, clinical laboratory accreditation organisations and licensing agencies require that each laboratory verify or establish reference intervals for each method. To provide such reference intervals requires selection criteria for suitable reference individuals, defined conditions for specimen collection and analysis, method selection to determine reference limits and validation of the reference interval. The current review will provide a brief introduction to the current approach to establishment of reference intervals, will highlight the current gaps in data available in paediatric populations, and review a recent Canadian initiative, CALIPER (Canadian Laboratory Initiative on Paediatric Reference Intervals), to establish a comprehensive database for both traditional and emerging biomarkers of paediatric disease.
Paediatric Reference Intervals: Gaps and Challenges
It is well recognised that paediatricians and paediatric specialists have unique needs and requirements for the laboratory testing of their patients. Age-specific reference intervals and specimen collection issues are particular concerns in paediatric patient care and evaluation. Frequently, adult reference intervals are not appropriate for paediatric patients. To assist physicians in treating their paediatric patients, attempts have been made by many laboratories to establish age-specific reference intervals for many traditional disease biomarkers with limited success. Unfortunately, critical gaps currently exist in available paediatric reference intervals for accurate interpretation of laboratory tests (results of a recent gap analysis is discussed further below).1 These gaps may subject infants and children unnecessarily to extra blood collection, infectious risk, stressful diagnostic procedures or inappropriate treatment. Moreover, inadequate paediatric reference intervals may be costly and devastating potentially contributing to misclassification or erroneous/delayed diagnosis of many diseases of childhood and adolescence. Children are not small adults. Therefore, establishing paediatric reference intervals requires a more complex approach, considering not only age, gender and ethnicity but reflecting the significant differences that exist in nutritional status, growth, development, disease frequencies, specimen collection, test performance and test interpretation.
Age-specific reference intervals are important for all types of paediatric laboratory testing. Many endocrinology, chemistry, serology, coagulation and haematology markers are subject to age-specific variability. Sex hormones, growth hormones and bone alkaline phosphatase levels vary with age and gender during childhood growth/development and puberty. Serology test results are influenced by the transplacental passage of maternal IgG and by immunisation responses in infants and children. Coagulation tests are optimised for anti-coagulation monitoring and not for childhood genetic diseases such as haemophilia. In haematology, the automated differential uses algorithms in children that differ from those used in adults. Currently available laboratory information systems do not permit the automatic calculation of a patient’s age related to prematurity and cannot separate reference intervals by gestational age. Unfortunately, most of the available reference intervals are incomplete covering a limited paediatric age interval that does not always cover both genders. Clearly, determining age- and gender-specific reference intervals is crucial for screening, diagnosis and monitoring of many paediatric disorders.
Children often acquire diseases that differ from adults and are lower in frequency. Newborns are “immunologically naïve”, have little if any infectious disease history and are exposed to relatively unique infections. They respond to infections in a different way and often require special testing. Premature birth can result in a set of diseases due to incomplete organ system development, especially those affecting the lungs and the central nervous system. Cancer in childhood comprises a set of neoplasms for which genetic alterations are much more important than environmental factors. Metabolic disorders and genetic diseases such as cystic fibrosis and sickle cell disease are examples of diseases diagnosed during childhood. Neonatal screening programs established in some countries are becoming more widespread with test menu expansion. Reference intervals are not available for new and emerging paediatric disease biomarkers. Cut-off ranges for health and disease are challenging and data are limited or difficult to find. Consensus guidelines established for adults may not apply to children, as is the case for cholesterol and hyperlipidaemias. Drugs-of-abuse testing in children is another area in which data are very limited but could have important implications for growth, development and overall patient care. Finally, metabolism differs in children and will affect therapeutic drug monitoring and therapy. For instance, different antibiotics are indicated for children and not adults.
Many of the problems associated with establishing good reference intervals for adults also exist for the paediatric population. Current paediatric reference intervals have been derived predominantly from samples collected on hospitalised infants and children of the Caucasian population and may not reflect levels in healthy multicultural populations.2 Collecting samples from healthy children is difficult, particularly from premature infants, term infants and toddlers. The influences of prematurity on laboratory test results are poorly understood and have not been assessed systematically. Different rates of organ maturation occur after premature birth; however, due to concerns about blood volume depletion in premature infants, it has been difficult to study reference intervals. Recruiting children as research subjects poses ethical dilemmas and protocols with institutional review board approval and informed consent are required for reference interval studies on normal, healthy children. Alternative ways of developing reference intervals for established tests require further study and could include mathematical approaches, extrapolation and establishment of decision limits. There is also a need to include a diversity of ethnic groups into paediatric reference interval databases. Application of reference ranges specific to other ethnic groups is clinically inappropriate for many biomarkers.
Another problem with most available paediatric reference intervals, is that they were determined over two decades ago using older/less accurate instrumentation and methodologies that are no longer relevant to testing technology used by clinical laboratories today.3 Changes in instrumentation and methodology are often not accompanied by reference interval modification and literature data are often adopted without critical appraisal. Manufacturer supplied reference intervals are often not accompanied with a detailed description of how reference intervals were derived including partitioning factors, sub-class differences, sample size, age, gender, ethnicity, race, percentiles used or traceability.
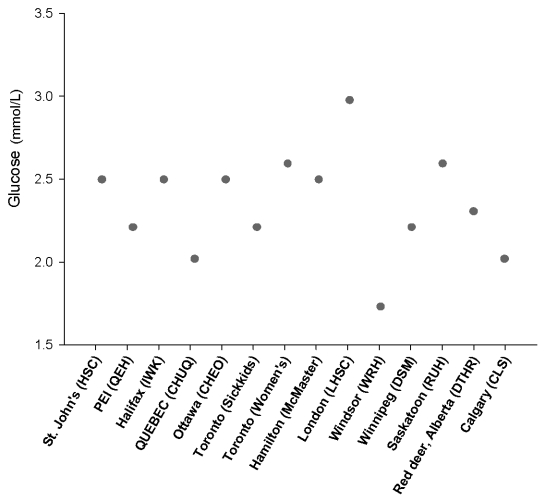
Different critical values and reference intervals for the same analyte exist between different laboratories and analytical platforms. In May 2008, the Hospital for Sick Children devised and mailed out a survey to collect critical values provided by paediatric hospitals across Canada. An example of the variability observed between laboratories for glucose critical values at the low end is shown in the Figure. Hypoglycaemia is the most common metabolic problem in neonates. Sustained or repetitive episodes of hypoglycaemia associated with neonatal hyperinsulinaemia, inborn errors of carbohydrate metabolism or diabetes mellitus in children has a major impact on normal brain development and function. Adequate glycaemic control in the paediatric population is also hampered by variability in analytical methodology and reference intervals for insulin (The Table provides a few examples of instrument specific reference intervals determined as part of the CALIPER pilot projects at the Hospital for Sick Children in Toronto). Despite use of a standardised calibrator across different analytical methods, a difference in paediatric age-specific reference intervals exists for insulin perhaps suggesting need for method-specific reference intervals to account for differences in antibody specificity. Inconsistency in paediatric reference intervals also extends to immunoassays for other hormones without standard reference material including oestradiol (Table). Sex hormones are present at substantially lower concentrations in infants and children compared to adolescents and adults. International efforts to improve paediatric hormone-testing standardisation by assuring traceability to sensitive reference methods are required. These efforts will facilitate establishment of appropriate method-specific reference intervals for neonatal and pubertal developmental stages. Common reference intervals may be applied to some assays such as total calcium, which appears to be well standardised (Table). Finally, the clinical utility of plasma or serum creatinine measurements for the identification of renal insufficiency is hindered by the sub-optimal quality of the non-specific Jaffe method used in several routine laboratories. Even with the international movement to standardise creatinine assays to the ID/MS reference method, the kinetic alkaline picrate method of the Abbott Architect ci8200 gave higher reference intervals than the enzymatic methods evaluated (Table). The challenge lies in developing method-specific reference intervals that have been aligned with high order reference standards and validation of the Modification of Diet in Renal Disease (MDRD) formula for the paediatric population.
Figure.
Low critical value cut-offs for plasma/serum glucose reported by 14 different paediatric clinical laboratories across Canada.
Table.
Plasma/serum, age-specific paediatric reference intervals for total calcium, creatinine, insulin and oestradiol for various analytical platforms (determined as part of CALIPER pilot projects at the Hospital for Sick Children, Toronto).
| Abbott Diagnostics Architect ci8200 | Roche Diagnostics Cobas 6000 | Ortho Clinical Diagnostics (Fusion 5,1) | Siemens Immulite 2500 | DiaSorin Clinical Assays | |
|---|---|---|---|---|---|
| Calcium (mmol/L) | |||||
| Standardisation | NIST SRM 915 (Coulometric Titration) | NIST SRM 909b (ID/MS) | NIST SRM 915a (Flame AAS) | ||
| Methodology | Arsenazo III | O-cresolphthalein complexone | Arsenazo III | ||
| Reference intervals | |||||
| 0–12 months | F: 2.5 – 2.8 M: 2.3 – 2.8 | F: 2.29 – 3.08 M: 1.91 – 2.93 | <1 month: 2.00 – 2.75 | ||
| 1–5 years | 2.3 – 2.6 | F: 2.13 – 2.65 M: 2.19 – 2.77 | 1 mo – 11 mo: 2.17 – 2.70 | ||
| 6–10 years | 2.3 – 2.6 | 2.15 – 2.60 | 1 – 3 years: 2.17 – 2.65 | ||
| 11–14 years | 2.2 – 2.6 | 2.15 – 2.60 | ≥4 year: 2.19 – 2.60 | ||
| 15–20 years | 2.2 – 2.6 | 2.15 – 2.60 | |||
| Creatinine (μmol/L) | |||||
| Standardisation | NIST SRM 909 and 914 (IDMS) | IDMS | NIST SRM 914 (GC/IDMS) | ||
| Methodology | Kinetic Alkaline Picrate | Enzymatic | Enzymatic | ||
| Reference intervals | |||||
| 0–12 months | 30.2 – 47.7 | 15.7 – 35.1 | <6 days: 19 – 99 | ||
| 1–5 years | 30.9 – 52.2 | 16.4 – 43.0 | 7 days – 60 days: 10 – 56 | ||
| 6–10 years | F: 35.8 – 60.5 M: 45.7 – 63.2 | 25.8 – 55.3 | 2 months – 5 years: <36 | ||
| 11–14 years | F: 45.9 – 70.0 M: 46.3 – 72.2 | 29.4 – 72.1 | 6 – 9 years: <53 | ||
| 15–20 years | F: 51.3 – 80.9 M: 53.7 – 92.2 | F: 38.6 – 84.9 M: 35.6 – 95.6 | 10 – 13 years: <79 | ||
| ≥14 years: <98 | |||||
| Insulin (pmol/L) | |||||
| Standardisation | WHO NIBSC 1st IRP 66/403 | WHO NIBSC 1st IRP 66/403 | WHO NIBSC 1st IRP 66/403 | ||
| Methodology | One step chemiluminescent microparticle immunoassay | Biotinylated-Streptavidin chemiluminescent | Solid phase, two-site chemiluminescent | ||
| Reference intervals | |||||
| 0–12 months | 9.0 – 199.6 | 4.2 – 248.1 | <90 | ||
| 1–5 years | 4.8 – 205.6 | 1.59 – 233.4 | <118 | ||
| 6–10 years | 11.9 – 400.9 | 12.0 – 614.5 | <118 | ||
| 11–14 years | 18.3 – 754.3 | 12.0 – 614.5 | <118 | ||
| 15–20 years | 14.2 – 725.9 | 12.0 – 614.5 | <118 | ||
| Oestradiol (pmol/L) | |||||
| Standardisation | Abbott internal reference standard - verified against ID-GCMS | Standardised via ID-GCMS | Calibrated against in-house internal (99% purity) reference preparation | ||
| Methodology | Delayed one step chemiluminescent particle immunoassay | Biotinylated-Streptavidin chemiluminescent | Competitive Radioimmunoassay | ||
| Reference intervals | |||||
| 0–12 months | F: 28.2 – 155.9 M: 30.3 – 85.6 | F: 20.3 – 133.1 M: 11.8 – 77.9 | <11 month: 370 | ||
| 1–5 years | F: 31.7 – 97.8 M: 15.5 – 84.1 | F: 11.9 – 75.4 M: 14.2 – 68.1 | 1 year - adrenarche: <92 | ||
| 6–10 years | F: 30.3 – 137.8 M: 14.8 – 69.2 | F: 14.4 – 150.6 M: 13.0 – 77.1 | Adult: <165 | ||
| 11–14 years | F: 26.9 – 354.6 M: 28.8 – 113.4 | F: 29.8 – 600.1 M: 4.7 – 127.1 | Follicular phase: 110 – 183 | ||
| 15–20 years | F: 34.0 – 953.4 M: 29.6 – 181.9 | F: 29.5 – 576.5 M: 48.8 – 135.7 | Luteal phase: 550 – 845 | ||
| Treated with synthetic oestrogen: <165 | |||||
Establishment of up-to-date reference intervals for current and emerging instrumentation and methodology using standardised pre-analytical and analytical phases is essential. Both the IFCC and CLSI are working together towards a more standardised method for establishing reference intervals. At the 2007 AACC meeting in San Diego a session was dedicated to the issue of reference intervals. The new CLSI guidelines (version C28-A3) were unveiled to the laboratory community. The main differences between the new C28-A3 versus the C28-A2 are as follows:
Decision limits as well as reference intervals should be used for certain analytes e.g. cholesterol, glycated haemoglobin and neonatal bilirubin.
Validation vs establishment of reference intervals by a laboratory is acceptable.
Establishment of reference intervals through multi-centre trials.
Using the “robust” statistical method as well as the nonparametric method to calculate reference intervals is valid.
Laboratories should make reports easier to understand with abnormal values highlighted.
Laboratories should quote either the decision limit or reference intervals, not both.
Establishing Population-Based Reference Intervals
Reference Individuals
Ideally, reference intervals should be determined by sampling a healthy population that spans the whole age range.3–5 Potential sources of healthy volunteers include the blood bank, hospital employees and students. Clinical Laboratory Standards Institute (CLSI) recommends a minimum of 120 individuals, the minimum sample size required to determine 90% confidence intervals for the 95th percentile reference limits (2.5th and 97.5th percentiles).6 There are two sampling methods: direct and indirect. Direct techniques involve selection of individuals from a population using defined criteria. A priori sampling uses well-defined inclusion/exclusion and partition criteria for reference individuals before specimens are collected whereas with a posteriori sampling the process of exclusion and partition of reference individuals occurs after specimen collection and analysis. Participation requires informed consent and completion of a questionnaire that ensures confidentiality. Individuals are selected based on analyte-dependent criteria and health assessment. Reference individuals are most commonly partitioned by age and gender. The direct technique agrees with IFCC recommendations and is preferred; however, the challenge and cost of obtaining a representative group of reference individuals (particularly difficult for paediatric populations) may be overcome with the indirect technique. The indirect technique involves application of statistical methods to analytical values in a laboratory database without selection of reference individuals. The indirect technique may have clinical utility in select situations including paediatrics and the elderly population where collection of sufficient numbers of reference samples may be difficult.
Pre-Analytical and Analytical Conditions
The quality of laboratory testing and valid reference intervals require careful consideration of pre-analytical and analytical variables.3–5 Inclusion/exclusion criteria, partitioning factors and the questionnaire must also consider biological variation and analytical interferences for each analyte. Depending on the analyte, subject preparation may include a specified diet (fasting or non-fasting), abstinence from pharmacologic agents, a prescribed drug regimen, sampling time in relation to biological rhythms, limited physical activity (rest period) or stress. Specimen collection considerations include environmental conditions during collection, specimen type, time of day, posture, sample volume, anti-coagulants, additives, collection site preparation, blood flow, equipment and phlebotomy technique. In the paediatric population, patient age is obviously a significant pre-analytical factor and specimen collection and handling may be markedly different compared to that in adults. Phlebotomy for infants and children is technically challenging and requires special training and skill, particularly for sites such as scalp, jugular veins and umbilical artery catheters. Paediatric patients have frequent blood draws and are at risk for anaemia necessitating small specimen volumes. Liquid anticoagulant in collection tubes may potentially dilute the specimen when sample volumes are small. Skin puncture specimens (capillary blood) are more commonly obtained from children and consist of a mixture of blood from arterioles, venules, and capillaries with interstitial and intracellular fluids. The results from various analytes deviate from those obtained with arterial or venous samples. Specimen handling and processing variables include time and temperature for transport, clotting, plasma/serum separation, storage and sample preparation for analysis. Since automated laboratory equipment frequently cannot handle small volume specimens or samples directly from paediatric-sized tubes, manual processing of paediatric samples is common in larger laboratories making it difficult to standardise specimen processing across laboratories and to maintain positive sample identification for paediatric specimens throughout all testing phases. Small paediatric samples may also preclude repeat testing to confirm abnormal results.
Analytical variables required when determining reference intervals are quality control procedures and analytical performance. The analytical method should be controlled in the same way as routine patient samples including equipment and instrument preventative maintenance and function checks, reagents, calibrators, controls and calculation methods. The analytical validity of the method is critical and the method chosen for analysis must clearly state traceability, precision, minimum detection limit, reportable range, recovery, and interference characteristics. Analytical interference is encountered more commonly in neonatal patients than in adults and represents a significant technical challenge. Specifically, high concentrations of bilirubin, lipids, and foetal haemoglobin are present in many neonatal specimens. Bilirubin absorbs light at wavelengths at which many spectrophotometric methods measure a variety of analytes and can cause spurious test results in both chemistry and haematology laboratories. Intravenous nutrition supplemented with fat emulsions can result in specimen turbidity, which interferes with both spectrophotometric and nephelometric methods based on changes in light scatter. Foetal haemoglobin, normally present in significant quantities only in newborns and infants, interferes with accurate measurement of several haemoglobin derivatives. Carboxyhaemoglobin and methaemoglobin are critical to oxygen transport in the newborn and are important parameters for the management of neonates in the intensive care unit, but large amounts of foetal haemoglobin invalidate their conventional spectrophotometric measurement. Instrumentation appropriate to the neonatal setting can eliminate these technical issues.
Statistical Evaluation of Reference Values and the Approach in Paediatrics
Visual inspection of a frequency distribution histogram of values helps determine whether the distribution is Gaussian (normal, symmetric) or non-Gaussian (skewed, kurtosis, bimodal, polymodal) and identifies outliers. Non-Gaussian distributions suggest that unhealthy individuals are included or the values require partitioning. The standard normal deviate test partitions by age and gender, one of which is the Harris and Boyd method for partitioning.7 A simple test for outlier exclusion is the Dixon/Reed rule. The suspected outlier is rejected if the distance between the outliers is >1/3 the range of all values. Another method of outlier detection, proposed by Tukey involves the labelling of extreme values by using the middle 50% of the sample, thus reducing, or eliminating, the possible masking effect of multiple outliers on one side of the distribution.8 Since the majority of analytes do not have normal Gaussian distributions, the non-parametric method is recommended by IFCC and CLSI with a minimum of 120 individuals required per partition. Reference limits can be easily calculated using non-parametric ascending rank order statistics. The conventional 95th percentile reference limits are determined by calculating the rank numbers for the 2.5th and 97.5th percentiles. If laboratories cannot establish reference intervals based on the requirement of 120 reference individuals, the bootstrap or robust methods may be applied to get a good estimate of reference intervals. The bootstrap method uses a computer to provide robust percentile estimates by re-sampling from a single subset of reference values.9 Horne and Pesce describe the robust method where more weight is given to central values of a distribution than to distant values for the calculation of reference intervals.10 The advantage of this approach is that it is more tolerant of outliers in the reference population data, it does not require as large a sample size as the non-parametric calculation method and it does not require reference data transformation to a Gaussian distribution. Normal values can be presented as reference intervals, decision limits or statistical cut-off values. For example, troponin uses a cut-off at the 99th percentile of the healthy population while lipid levels, haemoglobin A1c, and therapeutic/toxic drug levels employ medical decision limits based on outcomes or diagnostic performance.
Recent studies of paediatric reference intervals in our laboratory (under the CALIPER program) have employed non-parametric statistics to establish reference intervals. Examples are provided in the Table. Results are first reviewed to detect outliers using the Dixon’s test prior to estimating reference intervals. Any specimen that is deemed to be unacceptable on the basis of aberrant results for multiple analytes is excluded. Results that are deemed to be adversely affected by haemolysis, icterus and lipaemia interferences for any analyte are also excluded. A one way ANOVA analysis is performed to determine which age groups and gender groups could be combined. All partitioning decisions are checked by the Harris-Boyd method which is recommended for partitioning in the proposed C28-P3 guidelines of CLSI&IFCC. When the number of samples is less than the recommended 120, the Horn-Pesce robust method is used to estimate the 95% C.I. (confidence interval) and 95% R.I. (reference interval) of distribution as upper and lower normal reference intervals.
Validation of Reference Intervals
Transfer of reference intervals is not ideal given the lack of test method harmonisation and differences in patient populations. Because there are few analytes where test methods have been harmonised, it is often necessary to establish reference intervals for each test system. Laboratories establishing a reference interval must ensure comparability of the reference population and pre-analytical/analytical variables. The CLSI guidelines require sample collection from at least 20 reference individuals and performance of a formal outlier test. If no more than two (of 20) are outside the proposed reference interval, the proposed reference interval is acceptable for use. If greater than two are outside the proposed reference intervals, then samples for 20 additional individuals are collected and measured. However, if three or more again fall outside the limits (or if five or more in the original set fall outside the limits), the user should reexamine the analytical procedures used, consider possible differences in the biological characteristics of the two populations sampled, and consider whether the receiving laboratory should develop its own reference interval according to the full-scale study guidelines. Such validation studies are clearly more challenging when transferring paediatric reference intervals, as truly normal paediatric samples are difficult to obtain for most clinical laboratories.
Establishment of a National Collaborative Initiative on Paediatric Reference Intervals
The concept of common reference intervals was presented by the IFCC as a possible solution to the establishment of up-to-date and comprehensive reference intervals. Since individual laboratories do not have the resources to adequately reassess all paediatric reference intervals, multi-centre trials are required. The components of a successful multi-centre trial include: adequate numbers of reference individuals, goals for total allowable error, use of methods that are traceable to primary reference methods or commutable reference materials, comparable pre-analytical/analytical phases and an ad hoc quality control program. Improvement in method standardisation will potentially eliminate the need for method-specific reference intervals. With a good level of standardisation, only population differences justify different reference intervals and the introduction of common reference intervals might be feasible. Currently, there are few laboratory tests that are standardised against a reference method or material. Much work needs to be done before implementing common reference intervals. Do population differences exist? Does ethnicity or different lifestyles affect reference intervals? True population differences are demonstrated for only a few analytes; creatine kinase, creatinine and some proteins (orosomucoid, IgG, C3, C4 and CRP).11–13 For most common analytes the differences may be small or insignificant. All these questions can only be answered by multi-centre trials with large numbers of healthy reference individuals and the cooperation of international laboratory communities facilitated through an organisation such as the IFCC.
Despite the challenges that exist in establishing up-to-date, comprehensive paediatric reference intervals, an ambitious Canadian team of investigators from the paediatric focus group of the Canadian Society of Clinical Biochemists has recognised the urgent need and have assembled to lead the establishment of a reliable database across the country. This national research initiative, working under the name of Canadian Laboratory Initiative on Paediatric Reference interval database (CALIPER database),14 is hoped to fill important gaps in the clinical laboratory reference values database and benefit every Canadian and world-wide clinical laboratory serving a paediatric population. The objective of the proposed laboratory project is to develop a database of patient demographics and laboratory results for the paediatric biochemistry tests for the entire paediatric age range from birth to 18 years old.
The long-term aims of the CALIPER project are to:
Establish a Canada-wide, comprehensive database for both traditional as well as emerging biomarkers of paediatric disease.
Establish age-specific paediatric reference intervals from neonatal age to adolescence.
Establish gender-specific paediatric reference intervals from neonatal age to adolescence.
Establish paediatric reference intervals in major ethnic groups representing the current Canadian diverse population including native Canadians.
The database will include traditional as well as emerging biomarkers of paediatric disease and will be utilised to derive comprehensive age- and gender-specific reference intervals that encompass the major ethnic groups of Canada’s diverse population. The reference intervals will be shared among paediatric hospital laboratories across Canada. The major benefit of the study is an accurate and reliable determination of what is “normal” considering a child’s age and gender in clinical diagnosis. Healthy children are currently being recruited in several sites across Canada from hospital nurseries, day cares, schools and families of hospital volunteers to ensure a representative sampling distribution. Samples are sent to a central location to monitor specimen collection and to assess the gap in reference ranges. Developed guidelines are applied for all tests where on-site analysis is required to preserve specimen stability, to avoid delays in analyte measurement and to achieve accurate results. Following specimen collection, analyte levels are measured to establish reference ranges. Analysis is carried out at specified centres using a number of different instruments commonly used in clinical laboratories across Canada. The goal is to generate instrument-specific reference intervals and determine variations in methods across laboratories. Following data collection, reference intervals are established using parametric and non-parametric methods in collaboration with a clinical biostatistician. Based on a detailed gap analysis (published in the June 2006 issue of the “Clinical Biochemistry” journal),15–18 a series of cardiac/metabolic markers, bone markers, genetic metabolic markers, thyroid hormones, the growth hormone-insulin axis, creatinine and C-reactive protein have been selected for the initial phase of reference interval determination. CALIPER has recently finished several pilot studies in collaboration with in vitro diagnostics (IVD) industries.19–21 Data generated from these pilot projects has been found to be beneficial for the laboratory community. The last stage of the project is to implement standardised age-specific reference ranges in various paediatric healthcare centres across Canada. The CALIPER program is now fully implemented in three paediatric medical centres and plans are underway to initiate recruitment in other Canadian centres. The program is led by three principal investigators (K. Adeli, Toronto; N. Lepage, Ottawa; and V.J. Grey, Hamilton) and several other-coinvestigators, who hold regular investigator meetings to design, implement and monitor different phases of the program. Data generated by each centre is reviewed by the research team to ensure harmonisation across various study centres. Statistical approaches to data analysis and establishment of specific reference intervals will be standardised based on the recent version of the CLSI/IFCC guidelines. The reference interval database will be published on the CALIPER website as CALIPER strives to extend this valuable database worldwide as significant gaps in paediatric reference intervals exist in both developed and developing countries. In the long-term, the project seeks to improve the health care of children and to promote academic, clinical and industrial collaborations among paediatric laboratory medicine specialists, paediatricians and others caring for children.
Footnotes
Competing Interests: None declared.
References
- 1.Schnabl K, Chan MK, Adeli K. [Accessed 30 September 2008];Pediatric Reference Intervals: Critical Gap Analysis and Establishment of a National Initiative, eJIFCC . 19(2) http://www.ifcc.org/ejifcc/vol19no2/190201200804.htm. [PMC free article] [PubMed]
- 2.Soldin SJ, Brugnara C, Wong EC, editors. Pediatric Reference Intervals. 6th ed. Washington: AACC Press; 2007. [Google Scholar]
- 3.Endres DB. Reference Intervals. In: Clarke W, Dufour DR, editors. Contemporary Practice in Clinical Chemistry. Washington: AACC Press; 2006. pp. 13–19. [Google Scholar]
- 4.Solberg HE. Establishment and Use of Reference Values. In: Burtis CA, Ashwood ER, Bruns DE, editors. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 4th ed. St. Louis Missouri: Elsevier Saunders; 2006. pp. 425–46. [Google Scholar]
- 5.Coffin CM, Hamilton MS, Pysher TJ, Bach P, Ashwood E, Schweiger J, et al. Pediatric laboratory medicine: current challenges and future opportunities. Am J Clin Pathol. 2002;117:683–90. doi: 10.1309/NYA1-V9KQ-NVF8-MA8M. [DOI] [PubMed] [Google Scholar]
- 6.Clinical Laboratory Standards Institute. Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Proposed Guideline. [Accessed June 18 2008];Proposed Guideline. (Third Edition). C28-P3. http://www.clsi.org/source/orders/free/c28-p3.pdf.
- 7.Horn PS, Pesce AJ. Reference intervals: an update. Clin Chim Acta. 2003;334:5–23. doi: 10.1016/s0009-8981(03)00133-5. [DOI] [PubMed] [Google Scholar]
- 8.Solberg HE, Lahti A. Detection of outliers in reference distributions: performance of Horn's algorithm. Clin Chem. 2005;51:2326–32. doi: 10.1373/clinchem.2005.058339. [DOI] [PubMed] [Google Scholar]
- 9.Henderson AR. The bootstrap: a technique for datadriven statistics. Using computer-intensive analyses to explore experimental data. Clin Chim Acta. 2005;359:1–26. doi: 10.1016/j.cccn.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Horn PS, Pesce AJ, Copeland BE. A robust approach to reference interval estimation and evaluation. Clin Chem. 1998;44:622–31. [PubMed] [Google Scholar]
- 11.Harris EK, Wong ET, Shaw ST., Jr Statistical criteria for separate reference intervals: race and gender groups in creatine kinase. Clin Chem. 1991;37:1580–2. [PubMed] [Google Scholar]
- 12.Johnson AM, Hyltoft Petersen P, Whicher JT, Carlström A, MacLennan S. International Federation of Clinical Chemistry and Laboratory Medicine, Committee on Plasma Proteins. Reference intervals for serum proteins: similarities and differences between adult Caucasian and Asian Indian males in Yorkshire, UK. Clin Chem Lab Med. 2004;42:792–9. doi: 10.1515/CCLM.2004.132. [DOI] [PubMed] [Google Scholar]
- 13.Ceriotti F. Prerequisites for use of common reference intervals. Clin Biochem Rev. 2007;28:115–21. [PMC free article] [PubMed] [Google Scholar]
- 14.Editorial, Pediatric reference intervals: Critical gap analysis and establishment of a national initiative. Clin Biochem. 2006;39:559–60. doi: 10.1016/j.clinbiochem.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Mansoub S, Chan MK, Adeli K. Gap analysis of pediatric reference intervals for risk biomarkers of cardiovascular disease and the metabolic syndrome. Clin Biochem. 2006;39:569–87. doi: 10.1016/j.clinbiochem.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Yang L, Grey V. Pediatric reference intervals for bone markers. Clin Biochem. 2006;39:561–8. doi: 10.1016/j.clinbiochem.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Delvin EE, Laxmi Grey V, Vergee Z. Gap analysis of pediatric reference intervals related to thyroid hormones and the growth hormone–insulin growth factor axis. Clin Biochem. 2006;39:588–94. doi: 10.1016/j.clinbiochem.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Lepage N, Li D, Kavsak PA, Bamforth F, Callahan J, Dooley K, et al. Incomplete pediatric reference intervals for the management of patients with inborn errors of metabolism. Clin Biochem. 2006;39:595–9. doi: 10.1016/j.clinbiochem.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Chan MK, Aytekin M, Adeli K. Paediatric Reference Intervals for 14 Chemistries and Immunoassays on the Roche Cobas 6000 System. Clin Chem. 2008;54(6 Suppl):E115. doi: 10.1016/j.clinbiochem.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Chan MK, Quinn F, Preston N, Ravalico T, Armbruster D, Adeli K. Paediatric Reference Intervals for 14 Chemistries and Immunoassays on the Abbott Architect ci8200 System. Clin Chem. 2008;54(6 Suppl):E114. [Google Scholar]
- 21.Chan MK, Seiden-Long I, Armbruster D, Adeli K. Age Specific Paediatric Reference Intervals for 18 Chemistry Assays on the Abbott Architect c8000 Analyzer: the Canadian Laboratory Initiative on Paediatric Reference Interval Database (CALIPER) Clin Chem. 2007;53(Suppl):D166. doi: 10.1016/j.clinbiochem.2009.01.014. [DOI] [PubMed] [Google Scholar]