Abstract
Electrical synapses can undergo activity-dependent plasticity. The calcium/calmodulin-dependent kinase II (CaMKII) appears to play a critical role in this phenomenon, but the underlying mechanisms of how CaMKII affects the neuronal gap junction protein connexin36 (Cx36) are unknown. Here we demonstrate effective binding of 35S-labeled CaMKII to 2 juxtamembrane cytoplasmic domains of Cx36 and in vitro phosphorylation of this protein by the kinase. Both domains reveal striking similarities with segments of the regulatory subunit of CaMKII, which include the pseudosubstrate and pseudotarget sites of the kinase. Similar to the NR2B subunit of the NMDA receptor both Cx36 binding sites exhibit phosphorylation-dependent interaction and autonomous activation of CaMKII. CaMKII and Cx36 were shown to be significantly colocalized in the inferior olive, a brainstem nucleus highly enriched in electrical synapses, indicating physical proximity of these proteins. In analogy to the current notion of NR2B interaction with CaMKII, we propose a model that provides a mechanistic framework for CaMKII and Cx36 interaction at electrical synapses.
Keywords: brain, electrical synapse, gap junction, protein–protein interaction, synaptic plasticity
The glutamatergic synapse exhibits well-known long-term plasticity, where activity-dependent modulation has been attributed to the calcium/calmodulin (CaM)-dependent protein kinase CaMKII (in the following CaMKII refers to the chemical α-isoform) (for review see ref. 1). Electrical coupling has been reported to exhibit activity-dependent plasticity as well, in particular at the mixed-electrical synapse of the VIII nerve–Mauthner cell synapse of goldfish, which exhibits long-term potentiation of both electrical and chemical components after tetanic stimulation (2–4) and in response to activation of the cannabinoid type 1 receptor (5). Sustained changes of electrical activity have also been documented at electrical synapses in the reticular nucleus of the thalamus, where interneuronal gap junctions are frequent (6).
The gap junction protein involved in the electrical synapse of the fish Mauthner cell is connexin35 (Cx35) (7); the mammalian ortholog is Cx36 (8, 9), which is the major gap junctional component of electrical and mixed synapses in the CNS. Activation of the NMDA receptor and subsequent increase in intracellular Ca2+ were reported to be essential for the induction of changes in gap junction conductance at the mixed Mauthner cell synapse involving modifications of gap junction channels, and CaMKII was considered to play a key role in this electrical synaptic plasticity (4, 10). However, mechanistic aspects of this putative interaction and whether it exists in mammalian systems are unknown. We therefore have examined whether Cx36 may provide a functional target for changes occurring during activation of CaMKII.
CaMKII is a multimeric holoenzyme having 8–12 subunits. Each subunit has kinase activity. Central to the ability of CaMKII to generate sustained changes in postsynaptic efficacy after stimulation is interaction of the subunits with the calmodulin/Ca2+ complex (Ca2+/CaM) (see ref. 1 for a recent review). In the “off state,” the enzyme is inactive because an autoinhibitory regulatory domain of the enzyme binds to its catalytic region preventing autoactivation and substrate binding. Binding of Ca2+/CaM is the primary event for release from autoinhibition by exposing a target site (T-site) and a substrate binding site (S-site) of the enzyme (1, 11) This opening of the CaMKII generates an autonomous kinase by an intraholoenzyme, intersubunit phosphorylation of a threonine residue at position T(286) (1). Once phosphorylated at T(286) (see Fig. 5 for additional information) the autoinhibitory domain with its pseudotarget and pseudosubstrate sites can no longer interact with the corresponding domains of the catalytic site, allowing the catalytic domain to access and phosphorylate substrates (12, 13). Remarkably, sustained autonomous activity is independent of CaM/Ca2+ and keeps the kinase active after the initial Ca2+ stimulus has subsided (see ref. 1 for review). Interaction of CaMKII with 1 of its most prominent target proteins at postsynaptic sites, the NR2B subunit of the NMDA receptor, has been scrutinized within recent years (12, 14), identifying several amino acid residues in the autoinhibitory domain of CaMKII that are critical for the interaction with this target protein (14, 15).
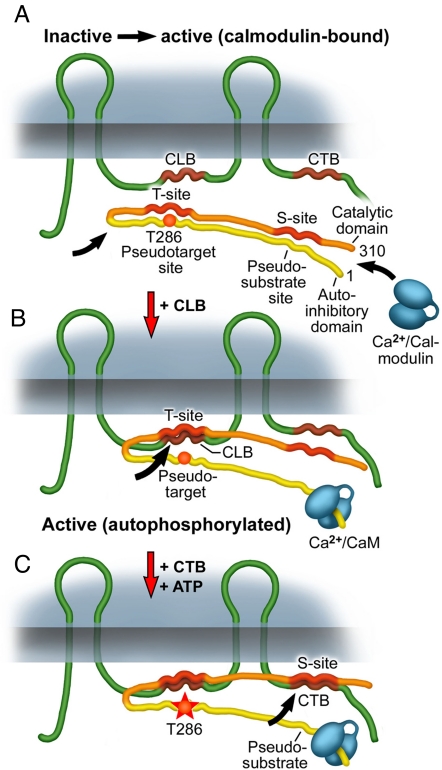
Fig. 5.
Model for the interaction of CaMKII with Cx36. The model follows the “gate” concept developed for interaction of CaMKII with the NMDA receptor (see also text). (A) Upon local Ca2+ elevation Ca2+/calmodulin, either from cytosolic sources or Cx36 bound, binds to the calmodulin binding site of CaMKII that overlaps with its pseudosubstrate site. (B) Binding of Ca2+/CaM to CaMKII opens the gate and exposes the target site (T-site) and the substrate site (S-site) of the kinase. Exposure of the T-site allows binding of the cytoplasmic loop binding site (CLB) of Cx36, a process that does not require autophosphorylation of the kinase. (C) Autophosphorylation of CaMKII at T(286) leads to enhanced binding of the carboxyl-terminal binding site (CTB) of Cx36 to the S-site of the activated kinase. Increasing autophosphorylation of CaMKII may also facilitate shifting of Ca/CaM from a Cx36-bound position to kinase binding because of an increase in CaM affinity (trapping phenomenon) of CaMKII.
To elucidate mechanistic aspects of the interaction of the neuronal gap junction protein Cx36 with CaMKII, we first used in silico screens to identify putative binding and phosphorylation sites. These sites were further verified by pull-down experiments and in vitro phosphorylation assays. Two cytoplasmic CaMKII binding sequences of Cx36 were discovered. One site resides at the cytoplasmic loop of Cx36 (CLB), and another resides nearer to the carboxyl-terminal region of the protein (CTB). Surprisingly, both putative binding sites revealed sequences that are similar to those within the autoinhibitory domain of CaMKII. The CLB site contains core residues similar to a sequence surrounding the autoinhibitory domain around T(286) (pseudotarget site), and the CTB site reveals a conserved motif, which is central to the pseudosubstrate site of the kinase. In vitro binding and phosphorylation studies of Cx36-GST fusion proteins indicated striking similarities with those of carboxyl-terminal domains of the NR2B subunit of the NMDA receptor in terms of their dependence on autophosphorylation of CaMKII. In addition, fragments of the Cx36 cytoplasmic binding sites generate autonomous activation akin to residues of the carboxyl terminus of the NR2B subunit. In situ immunocytochemistry of the inferior olive, a brainstem nucleus highly enriched with Cx36 at sites of electrical synapses, indicated physical proximity of the 2 proteins. In accordance with these findings, we suggest that Cx36, the most common connexin at electrical synapses in mammals, interacts with and is phosphorylated by CaMKII in a way similar to CaMKII phosphorylation of glutamate receptors.
Results
In Vitro Analysis Indicates Binding of CaMKII to Cytoplasmic Domains of Cx36.
In silico analysis [supporting information (SI) Text] yielded 2 potential consensus motifs for CaM/CaMKII binding within the cytoplasmic domains of Cx36. The first site resides at a juxtamembrane position of the cytoplasmic loop (CLB, amino acids 175–195) between the second and third transmembrane domains of Cx36, and the second domain corresponds to a juxtamembrane position of the carboxyl terminus (CTB, amino acids 272–292) (Fig. 1A and SI Text). In addition to the predicted binding sites, 4 putative CaMKII phosphorylation motifs (SI Text and Table S1) were identified in 2 regions in the cytoplasmic loop.
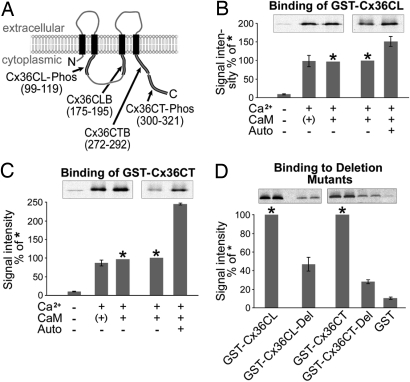
Fig. 1.
CaMKII binds effectively to cytoplasmic loop (GST-Cx36CL) and carboxyl-terminal (GST-Cx36CT) fusion proteins immobilized on GST agarose beads. (A) Schematic representation of the putative CaMKII binding (CB) and phosphorylation domains (C-Phos) of Cx36 as indicated by in silico screens. (B) Representative autoradiographs and quantitative densitometry of binding of in vitro translated [35S]-CaMKII to the GST-Cx36CL fusion protein. Binding is effective with translated CaMKII protein plus Ca2+ (second lane with CaM in brackets) because in vitro translated CaMKII contains sufficient amounts of calmodulin from the erythrocyte lysate as assessed by Western blotting (data not shown). Addition of Ca2+/CaMKII does not increase binding significantly. (See the third lane in B labeled with an asterisk. This band was used for quantification of signal intensity set to 100%; see also in C.) Autophosphorylation was achieved by incubation of CaMKII with Ca2+/CaM at 30 °C in the presence of ATP. In the control, ATP was added after incubation. Autophosphorylated CaMKII binds more effectively than the unphosphorylated CaMKII form. (C) Binding of [35S]-CaMKII to the carboxyl-terminal Cx36 fusion protein (GST-Cx36CT). Autophosphorylation leads to a 2.5-fold increase of CaMKII binding. (D) Deletion of the putative CaMKII binding domains (Del) of Cx36 leads to a clearly reduced affinity of CaMKII. Quantification of signal intensity was in relation to the respective wild-type constructs (100%, indicated with *). Incubation with GST alone served for control. Note that the autoradiograph shows duplicate lanes from the same experiment.
To validate the direct binding of CaMKII to Cx36, we used pull-down strategies with Cx36 fusion proteins as baits: For this purpose we incubated a GST fusion protein consisting of the cytoplasmic loop site (GST-Cx36CL, amino acids 100–201) or the carboxyl terminus (GST-Cx36CT, amino acids 272–321) with in vitro translated 35S-labeled CaMKII under conditions where Ca2+ and CaM were either present or absent. As shown in Fig. 1 B and C, Ca2+/CaM is sufficient to promote binding of CaMKII to GST-Cx36CL and GST-Cx36CT. Note that the addition of Ca2+ alone is effective presumably because of the presence of CaM in the erythrocyte lysate from which CaMKII was translated in vitro (as assessed by Western blotting; data not shown). Because autophosphorylation of CaMKII is crucial for its interaction with target receptor proteins (16), we also evaluated binding efficiency of both fusion proteins to autophosphorylated CaMKII. Autophosphorylation of [35S] CaMKII was achieved by incubation with ATP in the presence of Ca2+/CaM at 30 °C for 15 min (in the control sample ATP was added after incubation at 30 °C). Autophosphorylation increased affinity of CaMKII to GST-Cx36 fusion proteins of both cytoplasmic fragments with a more pronounced effect on GST-Cx36CT (Fig. 1 B and C, last 2 lanes each for original autoradiograms). Deletion mutants of the GST fusion proteins lacking the presumptive binding sites [GST-Cx36CL-Del (Δ175–195) and GST-Cx36CT-Del (Δ272–292)] showed significantly weaker binding to CaMKII (Δ175–195 by ≈40% and Δ272–292 by 25%) than did the full-length GST proteins (Fig. 1D), confirming the interaction of CaMKII with both binding domains.
Additionally, affinities of Cx36 domains to CaMKII were quantified by using surface plasmon resonance spectroscopy and peptides (20–22mers) containing the binding sites (see above) and the phosphorylation domains (amino acids 99–119 for the cytoplasmic loop site and amino acids 300–321 for the carboxyl-terminal domain). The two peptides containing the CaMKII binding sites showed a stronger affinity to CaMKII than those corresponding to the phosphorylation regions. Application of 25 μM peptides corresponding to the putative Cx36 binding and phosphorylation domains to immobilized CaMKII resulted in the following sequence of affinities: CTB > CLB > CLP > CTP (Fig. S1). Concentration/response curves for CTB and CLB peptides indicated EC50 values of ≈8 μM for CTB and >30 μM for CLB, respectively (data not shown).
Cx36 Is Phosphorylated by CaMKII.
We and others have recently shown that Cx36 and the fish ortholog Cx35 are targets for multiple kinases (17, 18). Because our in silico screen indicated 4 putative cytoplasmic phosphorylation motifs, we investigated whether the consensus sequences are actually targets for phosphorylation. To quantitatively assess the autoradiography, we immobilized GST-Cx36CL and GST-Cx36CT fusion proteins on a solid-phase medium coated with glutathione (glutathione-agarose beads). After clearing the solid phase from unbound fusion proteins, beads were incubated with either nonphosphorylated or autophosphorylated CaMKII in the presence or absence of Ca2+/CaM. Whereas without Ca2+/CaM induction of phosphorylation of either fusion protein did not achieve levels above background, Ca2+/CaM had a major effect on GST-Cx36CL (Fig. 2A) and a small effect on GST-Cx36CT (Fig. 2B). Autophosphorylation of CaMKII strongly increased the phosphorylation of the GSTCx36CT, indicating that phosphorylation is significantly more effective at the carboxyl-terminal region when CaMKII is autophosphorylated (Fig. 2B). To test whether phosphorylation requires both binding and phosphorylation sites, we performed competition experiments with peptides corresponding to the binding and phosphorylation sites of Cx36. GST-Cx36CL phosphorylation by CaMKII was almost completely inhibited by competing with the mimetic peptide corresponding to the cytoplasmic loop binding site. The effect was less pronounced when GST-Cx36CL was exposed to the peptide corresponding to the cytoplasmic loop phosphorylation site (Fig. 2A, last 2 lanes). In contrast, GST-Cx36CT phosphorylation could be substantially reduced by both cognate peptides of the binding and the phosphorylation domains of the carboxyl terminus of Cx36 (Fig. 2B, last 2 lanes). The minor effect of the CLP peptide to block phosphorylation of the GST-CL protein may be explained by steric hindrance within the cytoplasmic loop fragment, which does not allow access of the peptide to its target sequence. Alternatively, the CLP peptide may become phosphorylated and no longer prevent phosphorylation of the corresponding Cx36 site.
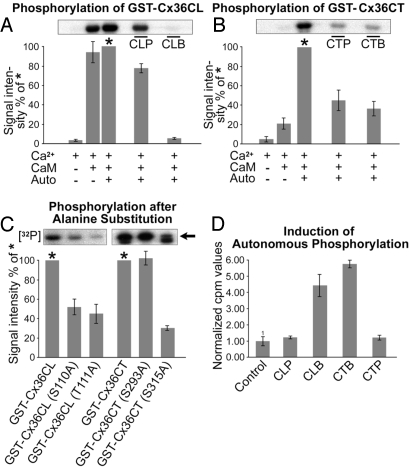
Fig. 2.
Activated CaMKII phosphorylates Cx36 and exhibits autonomous kinase activity by interacting with binding fragments of Cx36. (A) GST fusion protein of the cytoplasmic loop site (GST-Cx36CL) was in vitro phosphorylated by CaMKII in the presence of Ca2+/CaM. Ca2+/CaM is sufficient to phosphorylate the cytoplasmic loop fusion protein. Autophosphorylation of CaMKII reveals no significant effect (set to 100% for quantification and labeled with an asterisk). Peptide inhibition using a sequence corresponding to the Cx36 binding site (CLB) inhibits phosphorylation almost completely, whereas peptide CLP corresponding to the phosphorylation domain of Cx36 shows a lower effect. (B) Autophosphorylation of CaMKII is essential for phosphorylation of the Cx36CT fusion protein (Cx36CT-GST). Phosphorylation is reduced by competition with both cognate peptides (CTP and CTB). (C) Point mutations, including (S110A) and (T111A) for the cytoplasmic loop and (S293A) and (S315A) of the carboxyl-terminal fusion proteins, were studied. Densitometric quantification revealed a significant reduction in phosphorylation at the cytoplasmic loop residues after alanine substitution. Note that the carboxyl-terminal GST-Cx36CT revealed a lower band in the autoradiogram, which is considered to represent a degradation product. The specific band is indicated by an arrow. In the carboxyl terminus, mutation of S293 had no effect on the phosphorylation efficacy. S315A mutation severely reduced phosphorylation. (D) Immobilized biotinylated peptides of cytoplasmic binding sites (CLB and CTB) and phosphorylation sites (CLP and CTP) were incubated with CaMKII without Ca2+ and calmodulin. Trapped CaMKII was then tested for activity to phosphorylate the CaMKII substrate autocamtide in the presence of [γ-32P]ATP. Both peptides derived from the Cx36 binding sites exhibited a profound effect on autonomous activity of CaMKII, whereas the peptides covering the phosphorylation sites did not.
Additionally, mass spectroscopy was applied on digested GST-Cx36CL and GST-Cx36CT proteins to determine the phosphorylated amino acid residues within the cytoplasmic loop and carboxyl-terminal phosphorylation motifs. Tryptic digests of the carboxyl terminus were found to be phosphorylated in vitro, at residues serine S293 and S315. Digests of the cytoplasmic loop fusion protein indicated phosphorylation of residues serine S110 and threonine T111 (SI Text and Fig. S2). To confirm the usage of the individual phosphorylation residues, GST fusion proteins carrying a point mutation of serine to alanine at the sites of interest were devised by using overlap extension PCR. Mutations of phosphorylation consensus motifs for GST-Cx36CL included (S110A) and (T111A), and those for GST-Cx36CT included (S293A) and (S315A). Mutations in the cytoplasmic loop, S110A and T111A, each led to ≈50% reduction in phosphorylation of GST-Cx36CL (Fig. 2C). In the carboxyl-terminal region, mutation of S293 had no effect on the phosphorylation efficacy, whereas it was strongly reduced after mutation at S315.
CaMKII Reveals Autonomous Activation by Cx36 Binding Sites.
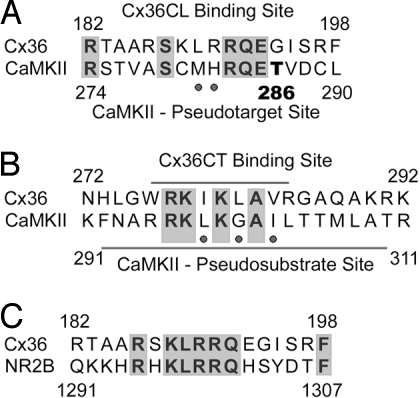
Sequence analyses of the CaMKII binding sites of Cx36CL and Cx36CT revealed striking similarities with the pseudotarget and pseudosubstrate binding sites of the kinase. When CLB was compared with the autoinhibitory region surrounding T(286) of CaMKII, a central core consisting of the R(283)QE285 motif, which has been shown to be necessary for target binding by CaMKII (14, 15), became apparent (Fig. 3A). Notably, in the carboxyl-terminal binding domain of Cx36 core residues R(277)KIKLAV(283) of the pseudosubstrate binding site of CaMKII are also similar (Fig. 3B). Even more remarkably, comparison of the Cx36CLB sequence to that of the CaMKII binding domain of the NMDA receptor subunit NR2B (Fig. 3C) revealed a greater similarity between these 2 proteins than described for any other CaMKII binding partner.
Fig. 3.
Sequence similarities between the Cx36 binding domains and the autoinhibitory region of CaMKII. (A) The cytoplasmic loop (CL) binding site of Cx36 reveals similarities to core residues of the pseudotarget site of CaMKII surrounding T(286). (B) The carboxyl-terminal binding site of Cx36 reveals a conserved motif, which is similar to the pseudosubstrate binding site of CaMKII. Notably, both the CT binding site of Cx36 and the pseudosubstrate binding site of CaMKII contain consensus motifs for calmodulin binding, indicated with underlying bars. (C) The CL binding site of Cx36 is also highly similar to the CaMKII binding sequence of the NR2B subunit of the NMDA receptor. Neutral amino acid exchanges are indicated by dots.
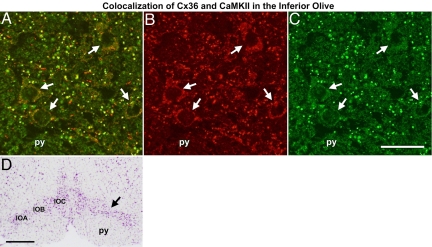
A prominent feature involved in the potentiation of CaMKII/NR2B interaction besides autophosphorylation is Ca2+/CaM-independent (autonomous) activation of CaMKII (13). Binding of the T site by NR2B prevents closure of the kinase and keeps the substrate binding site accessible (13). We therefore asked whether immobilized CLB and CTB peptides (and peptides corresponding to the phosphorylation consensus sequences) could activate CaMKII in the absence of Ca2+/CaM using the CaMKII substrate autocamtide. Measurements of phosphorylated autocamtide indicated that both peptides corresponding to the binding sites generated autonomous kinase activity, which did not require autophosphorylation or presence of CaM, whereas the peptides corresponding to the phosphorylation sites were ineffective (Fig. 2D). The CTB domain induced a stronger autonomous activation than the CLB fragment. One plausible explanation of this effect is that affinity of CTB to the substrate binding site is stronger than that of CLB to the target binding site under in vitro conditions, rendering the CaMKII in the more active autonomous state. This interpretation is supported by our surface plasmon resonance data (SI Text and Fig. S1), which indicate a 4-fold-higher affinity for CTB as compared with CLB. The observation of autonomous activation of CaMKII by both Cx36 binding sites suggests that these sequences represent heteromolecular catalytic domains that may function to amplify the activation of CaMKII and thereby potentiate its effect on Cx36, analogous to the effect described for the NR2B subunit of the NMDA receptor. To elucidate physical proximity of both proteins under in situ conditions similar to that described for CaMKII and NR2B interaction (19), we examined colocalization of Cx36 and CaMKII in brain tissue. We chose the inferior olive, which is a neuronal complex of the brainstem highly enriched in Cx36 (8, 20) and known to be extensively coupled by electrical synapses (21). Employing double immunolabeling with antibodies directed to the phosphorylated form of CaMKII and to Cx36, respectively, colocalized immunosignals were abundantly encountered in neurons of this brainstem nucleus (Fig. 4). Quantification of colocalization of both immunolabels (22) was done in both directions and indicated a highly significant overlap (Pearson correlation coefficient R ≈ 0.7) for Cx36 and CaMKII (SI Text and Fig. S3).
Fig. 4.
Cx36 and CaMKII are found to colocalize in immunostained inferior olive. (A) Merged image of anti-Cx36 (green) and CaMKII (red) immunolabeling depicts frequent overlapping localization of both proteins. Single neurons are labeled with white arrows. (B and C) Individual image pairs of CaMKII and Cx36 immunoreactivity. (D) Low magnification of a cresylviolet-stained cryostate section consecutive to the immunolabeled section shown in A–C. Black arrows show the area depicted in A–C. IOA, subnucleus A; IOB, subnucleus B; IOC, subnucleus C; py, pyramidal tract. (Scale bars: 40 μm in C for A–C and 500 μm in D.) Quantification of colocalization and pull-down experiments of CaMKII and Cx36 are indicated in Fig. S3.
To further confirm in situ physical association of these proteins we performed pull-down experiments of Cx36 from whole-brain homogenates using GST-Cx36CL and GST-Cx36CT proteins as baits and then performed Western blots of pulled-down extracts with a CaMKII-specific antibody. As shown in SI Text and Fig. S3, both Cx36 fusion proteins effectively precipitated the kinase. Together, the colocalization and pull-down experiments indicate interaction of these proteins under in situ conditions, the mechanistic basis for which was revealed in our in vitro studies.
Discussion
CaMKII is a key enzyme involved in activity-dependent plasticity of chemical synapses. Interaction with the postsynaptic NMDA receptor is central to the mechanism of receptor-dependent long-term potentiation, a widely studied model of learning and memory. A plethora of data exists on molecular aspects of the interaction of these proteins in neuronal tissues, which has led to the concept of CaMKII serving as a molecular switch that may underlie long-term memory storage (for reviews see refs. 1 and 12). Electrical synapses have long been considered to be less modifiable than chemical synapses, but recent evidence indicates that these synapses can undergo activity-dependent synaptic potentiation as well (2–4) and that CaMKII plays a central role in modulating junctional coupling (7). To understand the mechanisms by which CaMKII mediates these effects, we have examined the interaction of specific Cx36 domains with this kinase.
We have identified 2 juxtamembrane sequences that serve as potential binding sites for CaMKII and the amino acid residues S110 and T111 within the cytoplasmic loop and S315 at the carboxyl-terminal domain as potential phosphorylation residues. Binding and extent of phosphorylation of both cytoplasmic domains varied according to the autophosphorylated state of CaMKII and thus resemble behavior of fragments of the NR2B subunits of the NMDA receptor (13). Whereas autophosphorylation of CaMKII proved less effective at the cytoplasmic loop site in terms of binding and phosphorylation, the carboxyl-terminal fragment exhibited higher efficacy toward both effects when CaMKII was autophosphorylated. Additionally, a striking sequence similarity of both binding sites of Cx36 with domains of the autoinhibitory region of CaMKII became obvious. Whereas the central core of the CLB domain reveals amino acid residues resembling the pseudotarget binding site of CaMKII, the CTB site shows core residues similar to the pseudosubstrate binding region. We assume these similarities as a result of convergence because the intron/exon boundaries of CaMKII and Cx36 are different indicating that the domains may not have developed from a single ancestral gene. Apparently, the Cx36 cytoplasmic domain is endowed with 2 peptide sequences, which have been suggested to be crucial for the regulation of CaMKII activity (14, 15). According to similarities of interaction of the Cx36 binding sites with properties of the NR2B (13) subunit and the striking sequence similarity of both target binding sites we suggest a model where Cx36 may function in a bipartite fashion similar to the interaction of the NR2B subunit with CaMKII (1, 11). This model (Fig. 5) entails the autoinhibitory “gate” hypothesis of the regulatory and catalytic domains of CaMKII as described above. With respect to interactions with Cx36 the sequences of events may follow the following pathway: binding of Ca2+/CaM to the autoinhibitory domains displaces the gate and allows the CLB domain with its very similar pseudosubstrate binding site to interact with the target site of the kinase. Autophosphorylation of CaMKII is not required for this initial step, as indicated by the properties of the CLB fragment. Subsequent autophosphorylation at T(286), which enables the kinase to interact with further targets and/or substrate proteins (14, 15, 23), may then facilitate the binding of CTB to its corresponding substrate recognition motif at the catalytic segment of the kinase. Of note, the carboxyl-terminal binding site of Cx36 overlaps entirely with a sequence identified as a CaM binding site (24). This fact offers a further argument for a subsequential processing of both Cx36 segments during interaction with the kinase. Because CaM bound to the carboxyl terminus of Cx36 would inhibit the interaction with the substrate binding site of the kinase, the increasing affinity of the enzyme to Ca2+/CaM, which was found to increase 1,000 times (25) after autophosphorylation of T286 (so-called Ca2+/CaM trapping), could compete for CaM with Cx36. When CaM is displaced from the CTB site, it is released from inhibition and able to bind to the kinase.
It is interesting that both binding sites reveal autonomous activation of CaMKII, a feature that has been described for a peptide fragment of the NR2B subunit homologous to the autoinhibitory domain of the kinase (13). Autonomous activation of CaMKII is thought to convey a further activity-dependent mechanism that works synergistically with binding and autophosphorylation of the kinase and seems to be an essential feature responsible for sustained synaptic strength (for a review see refs. 1 and 12). In this context, Cx36 binding sites seem to share mechanisms with the NR2B subunit that have been proven to be essential for enhancing synaptic efficacy. Interspecies comparison shows that the CLB and CTB motifs of Cx36 are evolutionarily highly conserved among the Cx36 orthologs (data not shown), including the fish Cx35, for which most of the data on functional interaction of CaMKII with this neuronal gap junction protein have been obtained (26, 27). Protein–protein interaction, in the described way, requires physical association of the interacting partners. CaMKII is known to display translocation from the cytosol to postsynaptic sites in response to Ca2+ increase through activation of NMDA receptors (28). Subcellular association of Cx35 and CaMKII has recently been demonstrated in the mixed synapses of the Mauthner neuron of goldfish, implying a physical interaction of both proteins (29). Our finding of a colocalization of CaMKII and Cx36 in the inferior olive is a first hint that the electrical synapse constitutes a subcellular complex where dynamically regulated interaction with CaMKII could play a role in tuning electrical synapse activity. A feasible functional effect could be an increase in synaptic efficacy as has previously described by us (30) in the form of “run-up” of junctional conductance in N2A cells expressing Cx36.
In conclusion, the data described in this paper provide clues regarding mechanistic aspects underlying molecular interaction of CaMKII and the electrical synaptic protein Cx36. We uncovered similarities between this mechanism and regulation at chemical synapses, which have been described for the CaMKII/NR2B interaction (1, 11, 13). We thus suggest that both modes of interneuronal transmission may share common molecular features in modulating activity-dependent functional efficacy.
Materials and Methods
Binding of in Vitro Translated CaMKII to Fusion Proteins and Peptides.
CaMKII was in vitro translated in the presence of [35S]methionine (Amersham) by using pCMV-SPORT6-CaMKII (RZPD) and TNT T7 quick coupled transcription–translation kit (Promega). Labeled [35S] CaMKII was used directly in CaMK buffer without Ca2+/CaM, with Ca2+ and Ca2+/CaM (New England Biolabs), or after 15 min of incubation at 30 °C with calmodulin and Ca2+-containing CaMK buffer resulting in autophosphorylation of CaMKII. Fifty micrograms of GST Cx36 fusion proteins [GST-Cx36CL (aa100–201) and GST-Cx36CT (aa272–321)] were immobilized on 30 μL of GST-agarose beads (Pierce). Beads were washed 3 times with PBS containing Ca2+/Mg2+ and once in PBS without Ca2+/Mg2. Both washing solutions contained 1% BSA and 1% fish gelatin (Sigma). Immobilized GST fusion proteins were incubated for 2 h with CaMKII at 4 °C. After 30 min, BSA and fish gelatin were added to a final concentration of 1%. Subsequently, the beads were washed 4 times with the CaMK buffers as described for autophosphorylation (see below). Beads were incubated with SDS sample buffer for 5 min at 65 °C, and supernatants were separated via SDS/PAGE. Binding and phosphorylation experiments (see below) were all done in triplicate. Data are presented as means ± SD.
In Vitro Phosphorylation of Fusion Proteins by CaMKII.
Fifty micrograms of fusion protein (GST-Cx36CL, GST-Cx36CT) was immobilized on 30 μL of GST-agarose beads (Pierce) and washed 4 times with PBS. Two-hundred-fifty units of CaMKII were incubated in CaMKII buffer containing Ca2+ or Ca2+/CaM (New England Biolabs). To activate the CaMKII via autophosphorylation, the enzyme was incubated at 30 °C in the presence of 150 μM ATP. To the nonautophosphorylated probes, ATP was added after incubation at 30 °C. The immobilized fusion proteins were incubated with either nonphosphorylated or autophosphorylated CaMKII in the presence of [γ-32P]ATP for 30 min at 30 °C. One hundred μg of peptides (see SI Text) containing the predicted phosphorylation sites and binding regions of Cx36 were added to autophosphorylated probes to test competition for binding and phosphorylation of CaMKII. Beads were washed 5 times with PBS and incubated with SDS sample buffer for 5 min at 65 °C. The supernatants were separated via SDS/PAGE. [32P]-Phosphorylated fusion proteins were visualized via autoradiography.
Induction of CaMKII Autonomous Activity by Cx36 Binding Peptides.
Twenty micrograms of peptides (CLB, CTB, CLP, and CTP) were biotinylated as described previously (31) and bound to 15 μL of neutravidin beads (Pierce) according to the instructions of the manufacturer. A control sample without peptide was also prepared. The beads were washed 3 times with CaMKII reaction buffer (New England Biolabs) and incubated with 250 units of CaMKII (New England Biolabs). After 30 min at 4 °C, 300 μM autocamtide 2 (New England Biolabs), 100 μM [γ-32P]ATP (Amersham Biosciences), and 0.5 mM EGTA were added and incubated for 8 min at 30 °C for phosphorylation. Thereafter, 2× SDS sample buffer was added and the beads were heated (65 °C) for 5 min to stop the reaction. The supernatants were spotted on phosphocellulose squares (Upstate), and the washed squares were measured in a Beckman scintillation counter. Values were normalized with respect to the control sample.
Acknowledgments.
We thank Dr. Takayuki Asahara from the Laboratory for Stem Cell Translational Research and Kaori Shinmyozu from the Mass Spectrometry Analysis Subunit of the RIKEN Center for Developmental Biology for their constructive advice and kind support. We thank Dr. Elisabeth Petrasch-Parwez for help with the histology of the inferior olive. We thank the Ichiro Kanehara Foundation for a grant to C.A. This work was supported by Deutsche Forschungsgemeinschaft Grants SFB509, De 292/11-3, and Wi 270/31-1 (to R.D., G.Z., and K.W.) and National Institutes of Health Grant DK4191819 (to D.C.S.). C.A. was a fellow of the International Graduate School of Neuroscience (Bochum, Germany) when performing the experimental work.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805408105/DCSupplemental.
References
- 1.Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- 2.Yang XD, Korn H, Faber DS. Long-term potentiation of electrotonic coupling at mixed synapses. Nature. 1990;348:542–545. doi: 10.1038/348542a0. [DOI] [PubMed] [Google Scholar]
- 3.Pereda AE, Faber DS. Activity-dependent short-term enhancement of intercellular coupling. J Neurosci. 1996;16:983–992. doi: 10.1523/JNEUROSCI.16-03-00983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pereda AE, et al. Ca2+/calmodulin-dependent kinase II mediates simultaneous enhancement of gap-junctional conductance and glutamatergic transmission. Proc Natl Acad Sci USA. 1998;95:13272–13277. doi: 10.1073/pnas.95.22.13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cachope R, Mackie K, Triller A, O'Brien J, Pereda AE. Potentiation of electrical and chemical synaptic transmission mediated by endocannabinoids. Neuron. 2007;56:1034–1047. doi: 10.1016/j.neuron.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landisman CE, Connors BW. Long-term modulation of electrical synapses in the mammalian thalamus. Science. 2005;310:1809–1813. doi: 10.1126/science.1114655. [DOI] [PubMed] [Google Scholar]
- 7.Pereda A, et al. Connexin35 mediates electrical transmission at mixed synapses on Mauthner cells. J Neurosci. 2003;23:7489–7503. doi: 10.1523/JNEUROSCI.23-20-07489.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Condorelli DF, et al. Cloning of a new gap junction gene (Cx36) highly expressed in mammalian brain neurons. Eur J Neurosci. 1998;10:1202–1208. doi: 10.1046/j.1460-9568.1998.00163.x. [DOI] [PubMed] [Google Scholar]
- 9.Söhl G, Degen J, Teubner B, Willecke K. The murine gap junction gene connexin36 is highly expressed in mouse retina and regulated during brain development. FEBS Lett. 1998;428:27–31. doi: 10.1016/s0014-5793(98)00479-7. [DOI] [PubMed] [Google Scholar]
- 10.Smith M, Pereda AE. Chemical synaptic activity modulates nearby electrical synapses. Proc Natl Acad Sci USA. 2003;100:4849–4854. doi: 10.1073/pnas.0734299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulman H. Activity-dependent regulation of calcium/calmodulin dependent protein kinase II localization. J Neurosci. 2004;24:8399–8403. doi: 10.1523/JNEUROSCI.3606-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hudman A, Schulman H. Neuronal Ca2+/calmodulin-dependent protein kinase II: The role of structure and autoregulation in cellular function. Annu Rev Biochem. 2002;71:473–510. doi: 10.1146/annurev.biochem.71.110601.135410. [DOI] [PubMed] [Google Scholar]
- 13.Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–805. doi: 10.1038/35081080. [DOI] [PubMed] [Google Scholar]
- 14.Yang E, Schulman H. Structural examination of autoregulation of multifunctional calcium/calmodulin-dependent protein kinase II. J Biol Chem. 1999;274:26199–26208. doi: 10.1074/jbc.274.37.26199. [DOI] [PubMed] [Google Scholar]
- 15.Strack S, McNeill RB, Colbran RJ. Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 2000;275:23798–23806. doi: 10.1074/jbc.M001471200. [DOI] [PubMed] [Google Scholar]
- 16.Colbran RJ. Inactivation of Ca2+/calmodulin-dependent protein kinase II by basal autophosphorylation. J Biol Chem. 1993;268:7163–7170. [PubMed] [Google Scholar]
- 17.Urschel S, et al. Protein kinaseA-mediated phosphorylation of connexin36 in mouse retina results in decreased gap junctional communication between AII amacrine cells. J Biol Chem. 2006;281:33163–33171. doi: 10.1074/jbc.M606396200. [DOI] [PubMed] [Google Scholar]
- 18.Kothmann WW, Li X, Burr GS, O'Brien J. Connexin35/36 is phosphorylated at regulatory sites in the retina. Visual Neurosci. 2007;24:363–375. doi: 10.1017/S095252380707037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen K, Teruel MN, Connor JH, Shenolikar S, Meyer T. Molecular memory by reversible translocation of calcium/calmodulin-dependent protein kinase II. Nat Neurosci. 2000;3:881–886. doi: 10.1038/78783. [DOI] [PubMed] [Google Scholar]
- 20.Meier C, et al. Immunohistochemical detection of the neuronal connexin36 in the mouse central nervous system in comparison to connexin36-deficient tissues. Histochem Cell Biol. 2002;117:461–471. doi: 10.1007/s00418-002-0417-z. [DOI] [PubMed] [Google Scholar]
- 21.Kistler WM, De Zeeuw CI. Gap junctions synchronize synaptic input rather than spike output of olivary neurons. Prog Brain Res. 2005;148:189–197. doi: 10.1016/S0079-6123(04)48016-9. [DOI] [PubMed] [Google Scholar]
- 22.Li Q, et al. A syntaxin 1, G{alpha}o, and N-type calcium channel complex at a presynaptic nerve terminal: Analysis by quantitative immunocolocalization. J Neurosci. 2004;24:4070–4081. doi: 10.1523/JNEUROSCI.0346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strack S, Colbran RJ. Autophosphorylation-dependent targeting of calcium/calmodulin-dependent protein kinase II by the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 1998;273:20689–20692. doi: 10.1074/jbc.273.33.20689. [DOI] [PubMed] [Google Scholar]
- 24.Burr GS, Mitchell CK, Keflemariam YJ, Heidelberger R, O'Brien J. Calcium-dependent binding of calmodulin to neuronal gap junction proteins. Biochem Biophys Res Commun. 2005;335:1191–1198. doi: 10.1016/j.bbrc.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer T, Hanson PI, Stryer L, Schulman H. Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science. 1992;256:1199–1202. doi: 10.1126/science.256.5060.1199. [DOI] [PubMed] [Google Scholar]
- 26.Bennett MV, Zukin RS. Electrical coupling and neuronal synchronization in the mammalian brain. Neuron. 2004;41:495–511. doi: 10.1016/s0896-6273(04)00043-1. [DOI] [PubMed] [Google Scholar]
- 27.Söhl G, Maxeiner S, Willecke K. Expression and functions of neuronal gap junctions. Nat Rev Neurosci. 2005;6:191–200. doi: 10.1038/nrn1627. [DOI] [PubMed] [Google Scholar]
- 28.Shen K, Meyer T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science. 1999;284:162–166. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- 29.Rash J, et al. High-resolution proteomic mapping in the vertebrate central nervous system: Close proximity of connexin35 to NMDA glutamate receptor clusters and co-localization of connexin36 with immunoreactivity for zonula occludens protein-1 (ZO-1) J Neurocytol. 2004;33:131–151. doi: 10.1023/B:NEUR.0000029653.34094.0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zoidl G, et al. Evidence for a role of the N terminal domain in subcellular localization of the neuronal connexin36 (Cx36) J Neurosci Res. 2002;69:448–465. doi: 10.1002/jnr.10284. [DOI] [PubMed] [Google Scholar]
- 31.Duffy HS, et al. pH-dependent intramolecular binding and structure involving Cx43 cytoplasmic domains. J Biol Chem. 2002;277:36706–36714. doi: 10.1074/jbc.M207016200. [DOI] [PubMed] [Google Scholar]