Abstract
Objective
To test the potential adjuvant effect of repetitive transcranial magnetic stimulation (rTMS) on motor learning in a group of stroke survivors undergoing constraint-induced therapy (CIT) for upper-limb hemiparesis.
Design
This was a prospective randomized, double-blind, sham-controlled, parallel group study. Nineteen individuals, one or more years poststroke, were randomized to either a rTMS + CIT (n = 9) or a sham rTMS + CIT (n = 10) group and participated in the 2-wk intervention.
Results
Regardless of group assignment, participants demonstrated significant gains on the primary outcome measures: the Wolf Motor Function Test (WMFT) and the Motor Activity Log (MAL)–Amount of Use, and on secondary outcome measures including the Box and Block Test (BBT) and the MAL–How Well. Participants receiving rTMS failed to show differential improvement on either primary outcome measure.
Conclusions
Although this study provided further evidence that even relatively brief sessions of CIT can have a substantial effect, it provided no support for adjuvant use of rTMS.
Keywords: Stroke, Neuronal Plasticity, Transcranial Magnetic Stimulation, Rehabilitation, Hemiparesis
Since its introduction as a noninvasive method to stimulate the human brain,1 repetitive transcranial magnetic stimulation (rTMS) has provided a potential means to modulate cortical excitability and function. Depending on essential parameters of the stimulation frequency and number of trains of stimuli, rTMS can produce lasting up- or down-regulation of the corticospinal system. At higher frequencies (≥5 Hz) rTMS has been shown to increase excitability in the motor nervous system.2–4 The extent to which these effects persist over time may be related to the duration of stimulation. Whereas 900 stimuli delivered at 5 Hz resulted in a transient increase in corticospinal excitability lasting only a few minutes after rTMS, this same effect was extended to 30–40 min after 1800 stimuli.3 Some survivors of stroke may have abnormally low motor cortex excitability,5 a condition that may impair the relearning of functional daily skills. There is at least preliminary evidence that both skill acquisition in normal subjects6 and functional improvement in stroke survivors7 are associated with increases in cortical motor excitability. Artificially increasing cortical excitability with rTMS could facilitate motor learning and recovery after stroke. The mechanism by which high-frequency rTMS elicits sustained increases in cortical excitability is uncertain; one possibility is that by inducing coactivation of connected cortical neurons, it rapidly enhances the strength of at least some connections, a process referred to as fast Hebbian learning.8–10
The primary motor cortex is an essential part of the neural network involved in the acquisition and mastery of motor skills.11 Focused, massed practice leading to learning of motor skills is associated with changes in the functional organization and excitability of the motor cortex.11–14 Learning corresponds to increases in synaptic connection strengths.15 The relationship between the short-lived alterations in connection strengths associated with fast Hebbian learning and the more durable alterations associated with improvement in function is unknown. The use of rapid rTMS to facilitate learning in the poststimulus period is predicated on the hypothesis that short-duration connections achieved through fast Hebbian learning facilitate the establishment of more durable connections during training conducted immediately after rTMS. Some recent studies have suggested that high- or low-frequency rTMS can enhance motor learning in healthy subjects4, 16, 17; other studies have failed to demonstrate an effect.18 The evidence of a possible facilitative effect of rTMS on learning suggests that it may have value as a therapeutic adjuvant in individuals with movement deficits attributable to stroke.
Although rTMS may be capable of accelerating the development of neural connectivity underlying functional improvements, this technology cannot provide the brain with the new knowledge implicit in skill acquisition. For this reason, rTMS should be paired with an empirically supported behavioral intervention. The impact of rTMS as an adjuvant to stroke rehabilitation could easily be obscured if the therapeutic approach had only a modest effect, if it were applied to individuals with highly variable deficits, or if the approach was administered differently among therapists. Constraint-induced therapy (CIT) is an empirically supported movement therapy that addresses these concerns. By engaging the paretic arm and hand in massed practice of functional tasks, while constraining the use of the unaffected upper limb, both traditional CIT and modified versions of CIT (i.e., shorter duration of treatment sessions) increase the amount of use of the affected side.19–25 Furthermore, therapies structured like CIT are believed to reverse learned nonuse of the hemiparetic limb in the chronic stroke population. Because of its strict protocol and apparent success in remediating movement in the stroke-affected upper limb, CIT-based interventions meet the essential requirements of a “behavioral engine” to drive a study of rTMS as an adjuvant.
METHODS
Subjects
All study procedures were approved by the local institutional review boards, and all participants provided written informed consent to participate. Subjects were recruited by convenience sample from inpatient and outpatient populations at the Malcolm Randall Department of Veterans Affairs (VA) Medical Center (MRDVAMC), the Shands Hospital and Brooks Hospital systems; and through newspaper articles and advertisements and contact with stroke support groups. The study was conducted at the VA Rehabilitation Research and Development Brain Rehabilitation Research Center at the MRDVAMC. Twenty stroke survivors (eight female; mean age, 67 ± 6.8 yrs; mean time since stroke, 3.8 ± 3.3 yrs; 10 left-cerebral vascular accident) were recruited for this study; one failed to complete it because of intercurrent health problems unrelated to the study. Inclusion criteria were
clinically diagnosed stroke at least 1 yr before
at least minimal motor function in the paretic arm, as defined by Wolf et al.21 including 10 degrees of active finger extension and 20 degrees of active wrist extension
magnetic resonance imaging or computed tomography performed within 2 wks of treatment
age not less than 18 yrs
All subjects scored below 2.5 on the MAL–Amount scale at baseline. This is a typically-used cutoff which ensures that subjects are not too high functioning to benefit from the CIT intervention.26
Exclusion criteria were
use of medications that may lower seizure threshold (e.g., Metronidazole)
history of epilepsy, brain tumor, learning disorder, mental retardation, drug or alcohol abuse, dementia, major head trauma, or major psychiatric illness
evidence of epileptiform activity on electroencephalography obtained before beginning treatment
history or radiographic evidence of arteriovenous malformation, intracortical hemorrhage, subarachnoid hemorrhage, or bilateral cerebrovascular disease
history of implanted pacemaker or medication pump, metal plate in skull, or metal objects in the eye or skull
Pregnancy
There were no restrictions related to sex, ethnicity, handedness, pain, or spasticity. Aphasia and other cognitive deficits did not preclude inclusion as long as subjects were sufficiently sentient to be able to understand the potential risks and benefits of the study, to personally provide informed consent, and to understand and cooperate with the treatment. The use of drugs that might potentially inhibit neuroplasticity (neuroleptics, α-1 noradrenergic antagonists, α-2 noradrenergic agonists, anticonvulsants, benzodiazepines, anticholinergics, and tricyclic antidepressants)27 constituted a relative contraindication to study entry; in general, subjects were not entered if they were on more than one of these drugs. On meeting all criteria, subjects were randomly assigned to either the rTMS (treatment, n = 9) or sham stimulation (control, n = 10) group by the neurologist who administered these interventions (WJT). Both the subject and treating therapist were blinded to group assignment.
In the sham group, six subjects had hemispheric large-vessel distribution infarcts (middle cerebral artery), and four subjects had hemispheric lacunar infarcts. In the treatment group, five subjects had hemispheric large-vessel distribution infarcts (middle cerebral artery), one had a deep hemispheric hemorrhage (i.e., nonintracortical or subarachnoid), and three had lacunar infarcts. In the sham group, two subjects were taking a benzodiazpine. In the treatment group, one subject was receiving a tricyclic antidepressant and three subjects were taking either an α-1 noradrenergic blocker or an α-2 noradrenergic agonists; one of these subjects was also taking a benzodiazepine. Further demographic data are presented in Table 1.
TABLE 1.
Baseline characteristics of groups
| rTMS (n = 9) | Sham rTMS (n = 10) | |
|---|---|---|
| Age at time of study (mean ± SD) | 68.4 ± 8.4 | 65.7 ± 5.1 |
| Education (mean ± SD) | 13.4 ± 2.8 | 13.4 ± 2.4 |
| Years since stroke (mean ± SD) | 3.9 ± 3.1 | 3.8 ± 3.7 |
| Females/males | 4/5 | 4/6 |
| Left cerebral vascular accident | 6 | 4 |
| Dominant-side hemiparesis | 6 | 4 |
| WMFT, sees | 15.5 ± 13.1 | 35.5 ± 33.9 |
| BBT, no. of blocks | 15.8 ± 7.9 | 15.4 ± 15.1 |
| MAL–amount of use | 1.1 ± 0.6 | 0.8 ± 0.6 |
| MAL–how well | 1.2 ± 0.7 | 0.8 ± 0.6 |
WMFT, Wolf Motor Function Test; BBT, Box and Block Test; MAL, Motor Activity Log.
rTMS Procedure
All participants were seated comfortably in a chair. Surface electromyographic electrodes were placed over the first dorsal interosseous muscles and connected to a Nicolet Viking III Electromyograph. Motor evoked potential (MEP) threshold was defined as the lowest stimulus intensity eliciting MEPs >20 mV in at least 3 of 6 consecutive stimulations. We measured MEP threshold before and after each rTMS session using a 9-cm-diameter circular magnetic coil centered at the scalp vertex and connected to a Magstim 200 high-power magnetic stimulator (Magstim Ltd, UK). rTMS was then administered using a Magstim Super Rapid Magnetic Stimulator and a 7-cm-diameter figure-8 coil centered over the hand area of affected motor cortex and fixed at the optimal location for eliciting MEPs in the affected target muscle. MEP threshold was remeasured in this location using the figure-8 coil and the Super Rapid stimulator. All subjects received 2000 stimulations daily for ten consecutive weekdays. Each daily treatment of 2000 stimuli was administered as 50 trains of 40 stimuli, stimulus rate of 20 Hz, stimulus train duration of 2 sees, with an intertrain interval of 28 sees. Stimulus intensity was 90% of motor threshold. In the event that MEPs could not be elicited at maximal stimulator output (two subjects randomized to the rTMS group and one subject randomized to the sham group), we referenced the location and stimulus intensity on the basis of responses obtained during stimulation of the undamaged hemisphere. When this occurred, the coil was fixed at a location over the damaged hemisphere that was homologous to the hand area of the motor cortex in the undamaged hemisphere, and the stimulus intensity was set to 90% of threshold for eliciting MEPs in the unaffected limb. All subjects received either rTMS or sham rTMS to the damaged hemisphere.
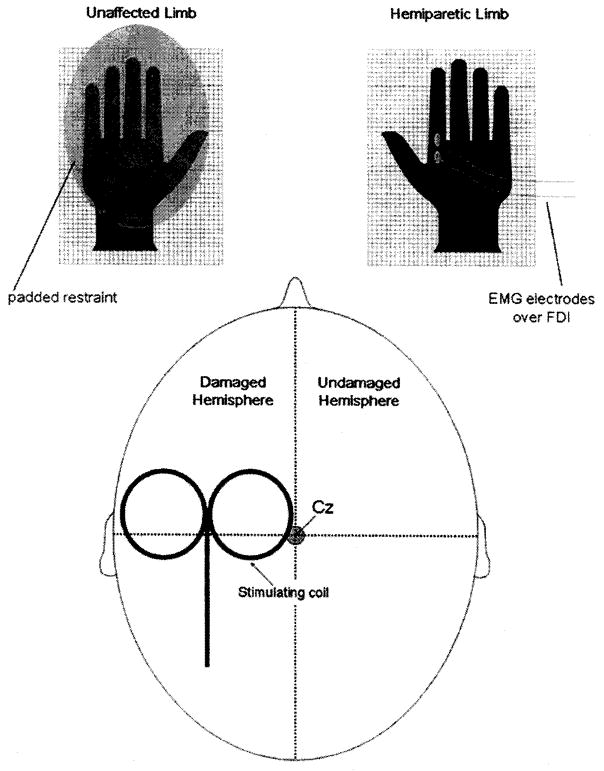
Sham rTMS (Fig. 1) was administered using a specially designed Magstim figure-eight coil (Magstim Ltd, UK). This coil generates a magnetic field that is more than 90% attenuated, but it does produce noise and vibration comparable with those of a real magnetic coil. In addition to obvious coil discharge noise, rTMS also is associated with transient contraction of scalp musculature during stimulus trains. We simulated this muscle contraction (and concomitant cutaneous and intramuscular neural sensory input) during sham rTMS by attaching surface electrodes underneath the magnetic coils and in contact with the scalp connected to the electromyograph. During sham rTMS, we simultaneously administered a train of 20 Hz electrical impulses to the scalp. The intensity of this stimulation (stimulus duration 0.1 msec) was adjusted to produce scalp muscle contraction discernible to the subject and a modest degree of scalp discomfort, comparable with that described by subjects receiving actual rTMS. Stimulus parameters for sham rTMS were identical to those for real rTMS (i.e., 50 trains of 40 sham stimuli administered at a rate of 20 Hz with intertrain intervals of 28 secs).
FIGURE 1.
Schematic of rTMS set-up. The magnetic stimulating coil was positioned over the motor cortex of the damaged hemisphere and 2000 stimuli were administered as 50 trains of 40 stimuli, stimulus rate of 20 Hz, stimulus train duration of 2 secs, with an intertrain interval of 28 secs. Passive bipolar electromyographic surface electrodes were applied over the FDI for the purpose of monitoring muscle activation during and between stimulations.
Surface electromyography from the FDI and a single electroencephalographic channel from a pair of electrodes placed over the forehead were monitored online during the 28-sec rest periods separating rTMS trains. Background electromyography remained stable during stimulation sessions, and we observed normal electroencephalographic activity.
CIT Procedure
Both rTMS- and sham-treated subjects participated in daily therapy sessions, approximating previously reported programs of CIT,19–21 for ten consecutive weekdays. We employed a modified CIT protocol consisting of both onsite training and structured home practice. Onsite training immediately followed the rTMS or sham stimulation. During these onsite sessions, participants wore a restraining mitt on the unaffected hand and were engaged in a variety of functional tasks directed at the affected upper limb. After each daily onsite CIT session, participants then performed 5 hrs of home practice of functional tasks using the affected upper limb. This structured home program was designed to ensure massed practice of upper-extremity activities as commonly performed in intensive therapies such as CIT. The treating therapist assigned home-practice tasks from a menu of commonly performed activities in the following broad areas of function: fine motor coordination, gross motor coordination, power, and endurance. This structured home program specified activities to be performed, how and for how long these were to be carried out, how to increase or decrease the difficulty level of the activity, and the number of repetitions or trials to be attempted. While at home, the participants wore the restraining mitt for 90% of their waking hours. Mitt-wearing compliance and adherence to the home practice program was monitored and ensured by use of a structured activity log, which the trainer and subject extensively reviewed on a daily basis.
Data Analysis
Outcome Measures
Our primary outcome measures were the Wolf Motor Function Test (WMFT)28 and the Motor Activity Log (MAL)–Amount,29 which are commonly used to assess change in upper-extremity function after intensive treatments like CIT.19 The WMFT is an upper-extremity functional capacity test, comprising a series of 15 timed tasks that require movement at all joints. Performance on the WMFT was scored as mean performance time (in seconds) of the affected arm and hand. The MAL is a structured interview during which subjects used a six-point scale to rate how much and how well they used their hemiparetic limb to perform 30 common functional activities.19 Secondary outcome measures included the MAL-How Well and the Box and Block Test (BBT). The BBT30 was used to assess grasp, transport, and release of small objects, with performance measured as the number of blocks moved in 1 min. These outcome measures were obtained before and immediately after the rTMS-CIT intervention, and again 6 mos later. Secondary outcome measures also included determination of MEP threshold (motor threshold) before and after each rTMS session.
Statistical Analysis
All data were analyzed with SAS version 8.2 (SAS Institute, Cary, NC). For each measure, the changes at 2 wks and at 6 mos from baseline were assessed in a single analysis with the use of PROC GLM, with a group and a time effect included in the model. The group effect tested differences in improvement between the rTMS and sham rTMS groups, the time effect studied whether changes at 6 mos remained the same compared with those at 2 wks, and the intercept parameter indicated whether the scores changed from baseline. We also ran paired t tests on change in score from baseline to 2 wks and from baseline to 6 mos on each of the assessment scores to determine whether the scores improved during these two time periods.
RESULTS
rTMS and sham rTMS were associated with scalp discomfort during stimulation. However, the stimulation procedures were well tolerated, and none of the subjects asked to withdraw their participation. There were no discernible adverse effects of rTMS beyond scalp discomfort. The baseline characteristics of both groups are presented in Table 1. Notably, the rTMS group tended to perform better on all four outcome measures at baseline, especially on the WMFT, although no differences were statistically significant.
Primary Outcome Measures
Wolf Motor Function Test
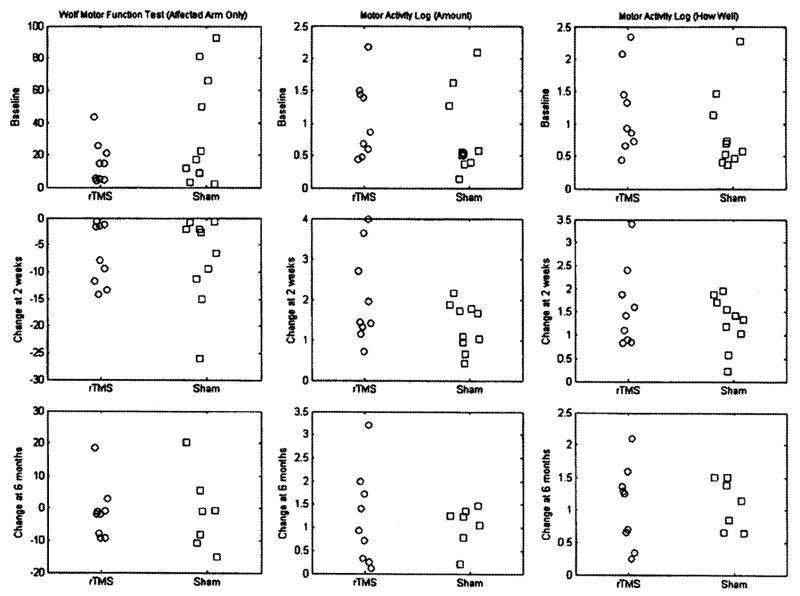
The results of the analysis of treatment effect are detailed in Table 2 and Figure 2. The mean of the WMFT scores for rTMS group was 20.0 points lower than the sham group at the baseline (P = 0.09). The group by time interaction was not significant; this implies that the two groups remained marginally different at 2 wks and 6 mos and that rTMS group did not improve more than the sham group. The overall WMFT scores decreased by 7.3 secs from baseline to 2 wks after (P = 0.01, d = 1.5,), but the difference disappeared at 6 mos. When comparing differences between the groups, the rTMS group decreased 0.85 secs less at 2 wks (P = 0.84, d = 0.06), and 0.21 secs less at 6 mos than did the sham group (P = 0.89, d = 0.04).
TABLE 2.
Outcome analysis of treatment effects
| Change from Baseline to 2 wks
|
Change from Baseline to 6 mos
|
||||||
|---|---|---|---|---|---|---|---|
| Measure | Baseline Mean (SD) | 2-wk Mean (SD) | 6-mo Mean (SD) | Mean, (SD) | Effect Size | Mean, (SD) | Effect Size |
| WMFT | |||||||
| Overall | 26.0 (27.5) | 18.8 (23.6) | 23.9 (26.7) | −7.3 (6.9)* | 1.56 | −1.3(9.8) | 0.18 |
| rTMS | 15.5 (13.1) | 8.7 (9.1) | 14.4 (19.5) | −6.8 (5.6)* | 1.72 | −1.2(8.6) | 0.19 |
| Sham | 35.5 (33.9) | 27.8 (29.1) | 36.3 (31.0) | −7.7(8.1)* | 1.34 | −1.4(11.9) | 0.18 |
| MAL–amount | |||||||
| Overall | 0.9 (0.6) | 2.6(1.1) | 2.1 (1.0) | 1.7 (0.9)* | 2.51 | 1.1 (0.8)* | 2.02 |
| rTMS | 1.1 (0.6) | 3.1 (1.0) | 2.3(1.1) | 2.0 (1.2)* | 2.50 | 1.2 (1.0)* | 1.66 |
| Sham | 0.8 (0.6) | 2.1(1.1) | 1.8 (1.0) | 1.3 (0.6)* | 3.20 | 1.1 (0.4)* | 3.4 |
| MAL–how well | |||||||
| Overall | 1.0 (0.6) | 2.5 (0.8) | 2.1 (0.8) | 1.4 (0.7)* | 2.86 | 1.1 (0.5)* | 3.00 |
| rTMS | 1.2 (0.7) | 2.8 (0.7) | 2.3 (0.8) | 1.6 (0.9)* | 2.64 | 1.1 (0.6)* | 2.45 |
| Sham | 0.8 (0.6) | 2.2 (0.8) | 2.0 (0.9) | 1.3 (0.6)* | 3.29 | 1.1 (0.4)* | 4.04 |
| BBT | |||||||
| Overall | 15.6 (11.9) | 18.5 (13.2) | 16.3 (13.3) | 2.9 (4.6)* | 0.90 | 1.6 (4.7) | 0.47 |
| rTMS | 15.8 (7.9) | 20.6 (9.6) | 20.3 (9.9) | 4.8 (5.3)* | 1.27 | 4.6 (2.5)* | 2.57 |
| Sham | 15.4 (15.1) | 16.6 (16.0) | 11.1 (16.1) | 1.2 (3.1) | 0.54 | −2.3 (4.0) | 0.81 |
| Motor threshold | |||||||
| Overall | 53.3 (13.9) | 51.8 (14.4) | — | −1.5(2.3)* | 0.95 | — | — |
| rTMS | 53.6 (18.3) | 50.3 (18.5) | — | −3.3 (1.9)* | 2.5 | — | — |
| Sham | 53.1 (10.6) | 53.0 (11.4) | — | −0.1 (1.9) | 0.13 | — | — |
P < 0.05. WMFT, Wolf Motor Function Test; BBT, Box and Block Test; MAL, Motor Activity Log.
FIGURE 2.
Baseline and change scores, by group, for the Wolf Motor Function Test (WMFT), Motor Activity Log (MAL)–Amount, and Motor Activity Log–How Well. The ordinate for WMFT is in seconds, and the ordinates for MAL–Amount and MAL–How Well are average scale scores.
MAL–Amount
There was no significant difference between the two groups for the MAL–Amount at the baseline (p = 0.54). However, there were marginally significant differences between the groups in change over time. Compared with the baseline, the sham group increased 1.3 points at 2 wks (P < 0.01, d = 3.28) and increased 1.1 points at 6 mos (P < 0.01, d = 3.43, B = 1.0). The change in the rTMS group was 0.7 points greater at 2 wks (P = 0.08, d = 0.57) and 0.1 points greater at 6 mos (P = 0.78, d = 0.09).
Secondary Outcome Measures
MAL–How Well
There was no significant difference between the two groups for the MAL–How Well at baseline (P = 0.33). The group by time interaction was not found to be significant, indicating there was not a significant difference in score improvement between the treatment groups. However, both groups significantly improved over time. When comparing differences between the groups, the rTMS group increased 0.31 points more at 2 wks (P = 0.31, d = 0.32), and 0.02 points less at 6 mos than the sham group (P = 0.94, d = 0.03); neither was significant.
Box and Block Test
There was no significant difference between the two groups for the Box and Block test at baseline (P = 0.95). However, there were significant differences between the groups in change over time. When comparing differences between the groups, the rTMS group mean change was 3.6 points greater at 2 wks (P = 0.10, d = 0.54, B = 0.30), and 6.9 points greater at 6 mos (P < 0.01, d = 1.39, B = 0.90).
Motor Threshold
There was no significant difference in motor threshold between the two groups at baseline (P = 0.96). There was, however, a significant between-group difference for change in motor threshold from pre- to post-CIT (F = 12.5, P = 0.003, F = 0.95, B = 1.0), with the rTMS group demonstrating a larger reduction in this measure after the intervention (t = 5.4, P = 0.002, d = 1.71, B = 0.94).
DISCUSSION
Our study failed to demonstrate a significant effect of rTMS as an adjuvant to CIT. Furthermore, whereas CIT led to significant improvements in WMFT times at 2 wks, this improvement was not sustained at 6 mos. Significant improvements in MAL–Amount at 2 wks were sustained at 6 mos, suggesting that even though CIT did not induce a sustained improvement in motor performance, it did induce a sustained improvement in the predisposition to use the affected arm in daily life; that is, it did have a sustained effect on learned nonuse. The significantly greater variance in MAL–Amount change scores in the rTMS treated group at 2 wks (F = 3.93, P = 0.03) and at 6 mos (F = 5.52, P = 0.01) raises the possibility that there was a subgroup of subjects in this group who could be classified as rTMS responders, and that if we had means for predicting response to CIT, we could construct more powerful trials of adjuvant therapies by restricting recruitment to subjects with responder characteristics.
Possible reasons for our failure to observe an rTMS adjuvant effect include the following: (1) the transient effects in cortical networks induced by rTMS do not promote the establishment of more lasting changes through learning, as suggested by the results of our prior study18; (2) the dose or intensity of rTMS was insufficient to induce the desired effects, the locus of stimulation was incorrect, or the area of stimulation was insufficient; (3) because the effect of an adjuvant therapy is likely to be multiplicative of the effect of the behavioral therapy, if the behavioral therapy has minimal impact, one cannot expect to detect the impact of the adjuvant therapy. CIT, in the modified form given in this trial, failed to induce a lasting change in mean WMFT scores and, therefore, failed to meet the standards of a behavioral engine as defined in the introduction to this paper. It is possible that changes in motor cortex connectivity reflected in WMFT scores are more susceptible to rTMS effect than are the presumably more diffuse, largely frontal system changes involved in overcoming learned nonuse, which are likely to be reflected primarily in MAL scores; (4) there was an effect, but it was small, it may have been masked by differential responses to rTMS based on lesion location, and our trial was not adequately powered to detect it; (5) a larger percentage of the subjects in the rTMS group was on potentially antineuroplastic drugs; or (6) the cerebral cortex we stimulated with rTMS was too damaged to be effected by the treatment; this seems less likely because in our observation and those of others,31–34 among subjects who qualify for CIT, the motor and premotor cortex are typically substantially spared and paresis stems predominantly from damage to the posterior periventricular white matter, where corticobulbar and corticospinal tracts pass to the brainstem and spinal cord. Although we could not elicit MEPs in two subjects in the rTMS group and in one subject in the sham group, we chose to include these individuals in our analyses because it is not a foregone conclusion that inability to obtain an MEP would predict poor response to rTMS.
Although our CIT intervention failed to induce a persistent change in mean WMFT score for either of the treatment groups (Table 2), it did achieve substantial gains at 2 wks and was associated with long-lasting gains in several subjects. Therefore, these subjects had the opportunity to experience an adjuvant effect from rTMS. Our WFMT and MAL–How Well results were comparable with those reported in traditional CIT programs.19, 35 The MAL–Amount scores and effect sizes reported here were lower than findings from traditional CIT programs19, 35 but were similar to results of a modified CIT protocol of lesser daily training time.23 The optimal duration of CIT and method of delivery (i.e., on-site training vs. a combined on-site, home practice program) has yet to be established. Clearly, studies like ours could be improved if we had data on reliable predictors of response to CIT.
CONCLUSION
We found a significant decrease in motor threshold for subjects receiving rTMS, with no significant changes noted in those receiving sham treatment. These data provide evidence that rTMS produced a change in the excitability of the motor system. This change, however, did not translate into a clinically evident effect, which raises questions about the relationship between physiologic changes and improvements in motor function. Clearly, further study is warranted to address this issue, the overall utility of rTMS as an adjuvant to stroke rehabilitation, the effective stimulation parameters of rTMS, the influence of lesion location and type, and the role, if any, that antineuroplastic drugs have on response to rTMS. Studies denning reliable predictors of response to CIT could improve the power of CIT as a behavioral engine to test the value of adjuvant therapies.
Footnotes
Disclosures: This material is based on work supported by the Office of Academic Affairs and by the Rehabilitation Research and Development Service (grant F2182C to Leslie J. Gonzalez Rothi) of the Department of Veterans Affairs.
References
- 1.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–7. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 2.Di Lazzaro V, Oliviero A, Berardelli A, et al. Direct demonstration of the effects of repetitive transcranial magnetic stimulation on the excitability of the human motor cortex. Exp Brain Res. 2002;144:549–53. doi: 10.1007/s00221-002-1106-9. [DOI] [PubMed] [Google Scholar]
- 3.Peinemann A, Reimer B, Loer C, et al. Long-lasting increase in corticospinal excitability after 1800 pulses of subthreshold 5 Hz repetitive TMS to the primary motor cortex. Clin Neurophysiol. 2004;115:1519–26. doi: 10.1016/j.clinph.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Canete C, Catala MD. Study and modulation of human cortical excixility with transcranial magnetic stimulation. J Clin Neuropsychol. 1998;15:333–43. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Weber M, Eisen AA. Magnetic stimulation of the central and peripheral nervous systems. Muscle Nerve. 2002;25:160–75. doi: 10.1002/mus.10038. [DOI] [PubMed] [Google Scholar]
- 6.Pascualleone A, Grafman J, Hallett M. Modulation of cortical motor output maps during development of implicit and explicit knowledge. Science. 1994;263:1287–9. doi: 10.1126/science.8122113. [DOI] [PubMed] [Google Scholar]
- 7.Liepert J, Hamzei F, Weiller C. Lesion-induced and training-induced brain reorganization. Restor Neurol Neurosci. 2004;22:269–77. [PubMed] [Google Scholar]
- 8.Dinse HR, Godde B, Hilger T, Haupt SS, Spengler F, Zepka R. Short-term functional plasticity of cortical and thalamic sensory representations and its implication for information processing. Adv Neurol. 1997:159–78. [PubMed] [Google Scholar]
- 9.Lee L, Siebner HR, Rowe JB, et al. Acute remapping within the motor system induced by low-frequency repetitive transcranial magnetic stimulation. J Neurosci. 2003;23:5308–18. doi: 10.1523/JNEUROSCI.23-12-05308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pascualleone A, Vallssole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847–58. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- 11.Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377:155–8. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- 12.Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272:1791–4. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 13.Liepert J, Graef S, Uhde I, Leidner O, Weiller C. Training-induced changes of motor cortex representations in stroke patients. Acta Neurol Scand. 2000;101:321–6. doi: 10.1034/j.1600-0404.2000.90337a.x. [DOI] [PubMed] [Google Scholar]
- 14.Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E. Increased cortical representation of the fingers of the left hand in string players. Science. 1995;270:305–7. doi: 10.1126/science.270.5234.305. [DOI] [PubMed] [Google Scholar]
- 15.Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–86. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi M, Hutchinson S, Theoret H, Schlaug G, Pascual-Leone A. Repetitive TMS of the motor cortex improves ipsilateral sequential simple finger movements. Neurology. 2004;62:91–8. doi: 10.1212/wnl.62.1.91. [DOI] [PubMed] [Google Scholar]
- 17.Kim YH, Park JW, Ko MH, Jang SH, Lee PKW. Facilitative effect of high frequency subthreshold repetitive transcranial magnetic stimulation on complex sequential motor learning in humans. Neurosci Lett. 2004;367:181–5. doi: 10.1016/j.neulet.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 18.Roth HL, Nadeau SE, Triggs WJ. Effect of repetitive transcranial magnetic stimulation on rate of memory acquisition. Neurology. 2004;63:1530–1. doi: 10.1212/01.wnl.0000142081.71440.6b. [DOI] [PubMed] [Google Scholar]
- 19.Taub E, Miller NE, Novack TA, et al. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74:347–54. [PubMed] [Google Scholar]
- 20.Miltner WH, Bauder H, Sommer M, Dettmers C, Taub E. Effects of constraint-induced movement therapy on patients with chronic motor deficits after stroke: a replication. Stroke. 1999;30:586–92. doi: 10.1161/01.str.30.3.586. [DOI] [PubMed] [Google Scholar]
- 21.Wolf SL, Barton LA. Forced use in hemiplegic upper extremities to reserve the effect of learned nonuse among chronic stroke and head-injured patients. Exp Neurol. 1989;104:125–32. doi: 10.1016/s0014-4886(89)80005-6. [DOI] [PubMed] [Google Scholar]
- 22.Page SJ, Sisto SA, Levine P. Modified constraint-induced therapy in chronic stroke. Am J Phys Med Rehabil. 2002;81:870–5. doi: 10.1097/00002060-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Sterr A, Elbert T, Berthold I, Kolbel S, Rockstroh B, Taub E. Longer versus shorter daily constraint-induced movement therapy of chronic hemiparesis: an exploratory study. Arch Phys Med Rehabil. 2002;83:1374–7. doi: 10.1053/apmr.2002.35108. [DOI] [PubMed] [Google Scholar]
- 24.Taub E, Lum PS, Hardin P, Mark VW, Uswatte G. Autocite— automated delivery of CI therapy with reduced effort by therapists. Stroke. 2005;36:1301–4. doi: 10.1161/01.STR.0000166043.27545.e8. [DOI] [PubMed] [Google Scholar]
- 25.Taub E, Uswatte G, Pidikiti R. Constraint-induced movement therapy: a new family of techniques with broad application to physical rehabilitation—a clinical review. J Rehabil Res Dev. 1999;36:237–51. [PubMed] [Google Scholar]
- 26.Kunkel A, Kopp B, Muller G, et al. Constraint-induced movement therapy for motor recovery in chronic stroke patients. Arch Phys Med Rehabil. 1999;80:624–8. doi: 10.1016/s0003-9993(99)90163-6. [DOI] [PubMed] [Google Scholar]
- 27.Goldstein LB. Potential effects of common drugs on stroke recovery. Arch Neurol. 1998;55:454–6. doi: 10.1001/archneur.55.4.454. [DOI] [PubMed] [Google Scholar]
- 28.Wolf SL, Thompson PA, Morris DA, et al. The EXCITE trial: attributes of the Wolf Motor Function Test in patients with subacute stroke. Neurorehabil Neural Repair. 2005;19:194–205. doi: 10.1177/1545968305276663. [DOI] [PubMed] [Google Scholar]
- 29.Uswatte G, Taub E, Morris D, Vignolo M, McCulloch K. Reliability and validity of the upper-extremity motor activity log-14 for measuring real-world arm use. Stroke. 2005;36:2493–6. doi: 10.1161/01.STR.0000185928.90848.2e. [DOI] [PubMed] [Google Scholar]
- 30.Desrosiers J, Bravo G, Hebert R, Dutil E, Mercier L. Validation of the box and block test as a measure of dexterity of elderly people—reliability, validity, and norms studies. Arch Phys Med Rehabil. 1994;75:751–5. [PubMed] [Google Scholar]
- 31.Feydy A, Carlier R, Roby-Brami A, et al. Longitudinal study of motor recovery after stroke—recruitment and focusing of brain activation. Stroke. 2002;33:1610–7. doi: 10.1161/01.str.0000017100.68294.52. [DOI] [PubMed] [Google Scholar]
- 32.Mohr JP, Foulkes MA, Polis AT, et al. Infarct topography and hemiparesis profiles with cerebral convexity infarction— the stroke data-bank. J Neurol Neurosurg Psychiatry. 1993;56:344–51. doi: 10.1136/jnnp.56.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knopman DS, Rubens AB. The validity of computed tomographic scan findings for the localization of cerebral functions—the relationship between computed-tomography and hemiparesis. Arch Neurol. 1986;43:328–32. doi: 10.1001/archneur.1986.00520040016011. [DOI] [PubMed] [Google Scholar]
- 34.Nadeau SE, Crosson B. Subcortical aphasia. Brain Lang. 1997;58:355–402. doi: 10.1006/brln.1997.1707. [DOI] [PubMed] [Google Scholar]
- 35.Kunkel A, Kopp B, Muller G, et al. Constraint-induced movement therapy for motor recovery in chronic stroke patients. Arch Phys Med Rehabil. 1999;80:624–8. doi: 10.1016/s0003-9993(99)90163-6. [DOI] [PubMed] [Google Scholar]