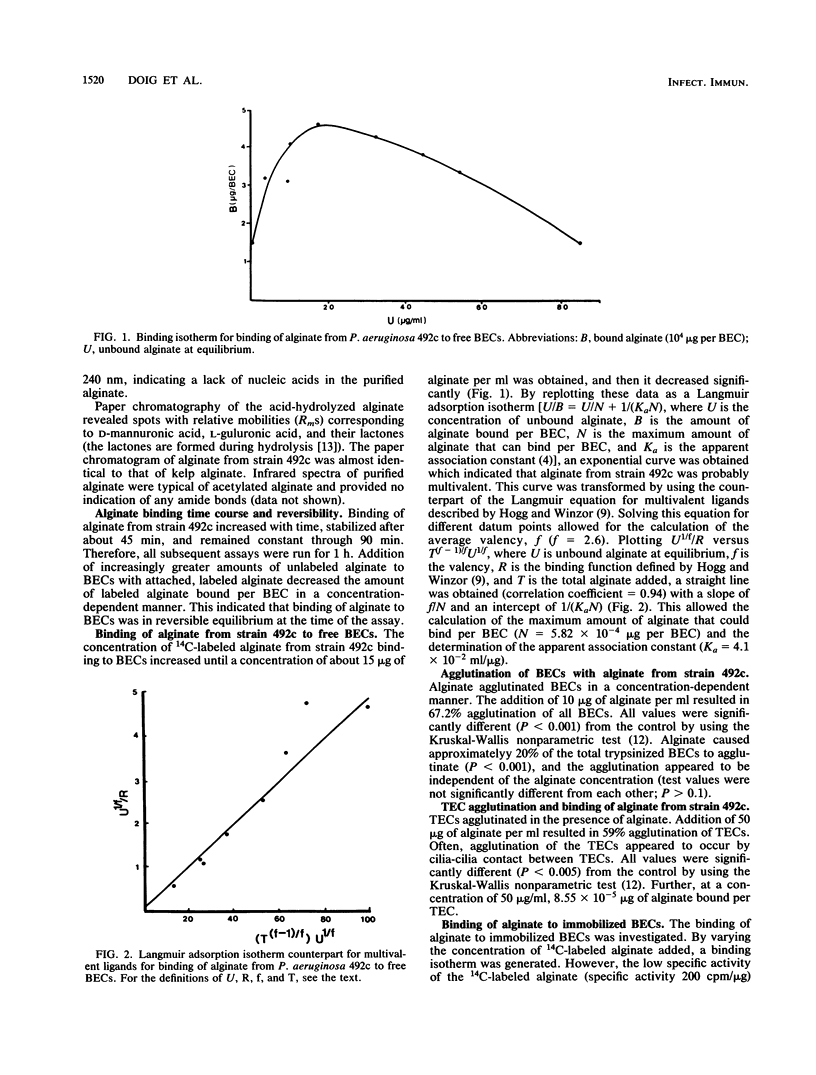
Abstract
The alginate produced by Pseudomonas aeruginosa has been reported to play a role in the adhesion of this bacterium to epithelial cell surfaces, although some controversy concerning this role exists. To clarify this controversy, we investigated the ability of alginate to bind to human buccal epithelial cells (BECs) and human tracheal epithelial cells (TECs). Alginate from P. aeruginosa 492c bound to both BECs and TECs. Alginate from strain 492c was found to be multivalent and thus capable of agglutinating both BECs and TECs. The multivalency of alginate complicated the determination of the number of alginate-specific receptors on the BEC and the apparent association constant (Ka). By using the analysis of Hogg and Winzor (Biochim. Biophys. Acta 843:159-163, 1985), an average valency of 2.6 BEC binding domains per alginate molecule was determined, and the maximum binding capacity per BEC was calculated to be 5.8 X 10(-4) micrograms, with a Ka of 4.1 X 10(-2) ml/micrograms. The binding of alginate to immobilized BECs (where only 50% of the BEC surface is exposed) yielded values of 2.52 X 10(-4) micrograms of alginate per BEC for the maximum binding capacity per BEC and a Ka of 3.30 X 10(-2) ml/micrograms. The alginate-specific site on the BEC surface was trypsin sensitive. Alginate from P. aeruginosa 492a did not bind to BECs, differing substantially from that of strain 492c. The data presented here demonstrate that alginate purified from some strains of P. aeruginosa may bind to TECs and BECs in a defined, specific manner, whereas alginate from other strains does not, reflecting structural diversity in P. aeruginosa alginates.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ceri H., McArthur H. A., Whitfield C. Association of alginate from Pseudomonas aeruginosa with two forms of heparin-binding lectin isolated from rat lung. Infect Immun. 1986 Jan;51(1):1–5. doi: 10.1128/iai.51.1.1-5.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. R., Linker A. Production and characterization of the slime polysaccharide of Pseudomonas aeruginosa. J Bacteriol. 1973 Nov;116(2):915–924. doi: 10.1128/jb.116.2.915-924.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin A. L., Todd T., Gurman G., Black D., Mankinen-Irvin P. M., Irvin R. T. Adherence of Pseudomonas aeruginosa to cilia of human tracheal epithelial cells. Infect Immun. 1987 Jun;55(6):1523–1525. doi: 10.1128/iai.55.6.1523-1525.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg P. J., Winzor D. J. Effects of ligand multivalency in binding studies: a general counterpart of the Scatchard analysis. Biochim Biophys Acta. 1985 Dec 13;843(3):159–163. doi: 10.1016/0304-4165(85)90134-5. [DOI] [PubMed] [Google Scholar]
- Irvin R. T., Govan J. W., Fyfe J. A., Costerton J. W. Heterogeneity of antibiotic resistance in mucoid isolates of Pseudomonas aeruginosa obtained from cystic fibrosis patients: role of outer membrane proteins. Antimicrob Agents Chemother. 1981 Jun;19(6):1056–1063. doi: 10.1128/aac.19.6.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson W. G., Jr, Woods D. E., Chaudhuri T. Association of respiratory tract colonization with adherence of gram-negative bacilli to epithelial cells. J Infect Dis. 1979 Jun;139(6):667–673. doi: 10.1093/infdis/139.6.667. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linker A., Jones R. S. A new polysaccharide resembling alginic acid isolated from pseudomonads. J Biol Chem. 1966 Aug 25;241(16):3845–3851. [PubMed] [Google Scholar]
- Marcus H., Baker N. R. Quantitation of adherence of mucoid and nonmucoid Pseudomonas aeruginosa to hamster tracheal epithelium. Infect Immun. 1985 Mar;47(3):723–729. doi: 10.1128/iai.47.3.723-729.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur H. A., Ceri H. Interaction of a rat lung lectin with the exopolysaccharides of Pseudomonas aeruginosa. Infect Immun. 1983 Nov;42(2):574–578. doi: 10.1128/iai.42.2.574-578.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEachran D. W., Irvin R. T. Adhesion of Pseudomonas aeruginosa to human buccal epithelial cells: evidence for two classes of receptors. Can J Microbiol. 1985 Jun;31(6):563–569. doi: 10.1139/m85-105. [DOI] [PubMed] [Google Scholar]
- Nelson J. D. Management of acute pulmonary exacerbations in cystic fibrosis: a critical appraisal. J Pediatr. 1985 Jun;106(6):1030–1034. doi: 10.1016/s0022-3476(85)80264-x. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Matthews W. J., Jr, Eardley D. D. Immunochemical characterization of the mucoid exopolysaccharide of Pseudomonas aeruginosa. J Infect Dis. 1983 Mar;147(3):494–503. doi: 10.1093/infdis/147.3.494. [DOI] [PubMed] [Google Scholar]
- Pier G. B. Pulmonary disease associated with Pseudomonas aeruginosa in cystic fibrosis: current status of the host-bacterium interaction. J Infect Dis. 1985 Apr;151(4):575–580. doi: 10.1093/infdis/151.4.575. [DOI] [PubMed] [Google Scholar]
- Ramphal R., Pier G. B. Role of Pseudomonas aeruginosa mucoid exopolysaccharide in adherence to tracheal cells. Infect Immun. 1985 Jan;47(1):1–4. doi: 10.1128/iai.47.1.1-4.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Pyle M. Adherence of mucoid and nonmucoid Pseudomonas aeruginosa to acid-injured tracheal epithelium. Infect Immun. 1983 Jul;41(1):345–351. doi: 10.1128/iai.41.1.345-351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Sadoff J. C., Pyle M., Silipigni J. D. Role of pili in the adherence of Pseudomonas aeruginosa to injured tracheal epithelium. Infect Immun. 1984 Apr;44(1):38–40. doi: 10.1128/iai.44.1.38-40.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera M., Nicotra M. B. Pseudomonas aeruginosa mucoid strain. Its significance in adult chest diseases. Am Rev Respir Dis. 1982 Nov;126(5):833–836. doi: 10.1164/arrd.1982.126.5.833. [DOI] [PubMed] [Google Scholar]
- Sherbrock-Cox V., Russell N. J., Gacesa P. The purification and chemical characterisation of the alginate present in extracellular material produced by mucoid strains of Pseudomonas aeruginosa. Carbohydr Res. 1984 Dec 15;135(1):147–154. doi: 10.1016/0008-6215(84)85012-0. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Bass J. A., Johanson W. G., Jr, Straus D. C. Role of adherence in the pathogenesis of Pseudomonas aeruginosa lung infection in cystic fibrosis patients. Infect Immun. 1980 Dec;30(3):694–699. doi: 10.1128/iai.30.3.694-699.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Bryan L. E. Studies on the ability of alginate to act as a protective immunogen against infection with Pseudomonas aeruginosa in animals. J Infect Dis. 1985 Apr;151(4):581–588. doi: 10.1093/infdis/151.4.581. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Straus D. C., Johanson W. G., Jr, Berry V. K., Bass J. A. Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect Immun. 1980 Sep;29(3):1146–1151. doi: 10.1128/iai.29.3.1146-1151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



