Abstract
The blood-brain tumor barrier (BTB) significantly limits delivery of effective concentrations of chemotherapeutic drugs to brain tumors. Previous studies suggest that BTB permeability may be modulated via alteration in the activity of potassium channels. In this study, we studied the relationship of BTB permeability increase mediated by potassium channel agonists to channel expression in two rat brain tumor models. Intravenous infusion of KCO912 (KATP agonist), minoxidil sulfate (KATP agonist) or NS1619 (KCa agonist) increased tumor permeability more in the 9L allogeneic brain tumor model than in the syngeneic brain tumor model. Consistently, expression of both KATP and KCa channels in 9L tumors was increased to a significantly greater extent in Wistar rats (allogeneic) as compared to Fischer rats (syngeneic). Furthermore, as a preliminary effort to understand clinical implication of potassium channels in brain tumor treatment, we determined the expression of KATP in surgical specimens. KATP mRNA was detected in glioblastoma multiforme (GBM) from nineteen patients examined, with a wide range of expression levels. Interestingly, in paired GBM tissues from seven patients before and after vaccination therapy, increased levels of KATP were detected in five patients after vaccination that had positive response to chemotherapy after vaccination. The present study indicates that the effects of potassium channel agonists on BTB permeability are different between syngeneic and allogeneic models which have different expression levels of potassium channels. The expression of potassium channels in brain tumors is variable, which may be associated with different tumor permeability to therapeutic agents among patients.
Keywords: Blood-brain barrier, Potassium channel, chemotherapy, Brain tumor model
1. Introduction
Brain capillary endothelium and its contiguous cells, pericytes and astrocytes, form the blood-brain barrier (BBB) [4,7,25]. The blood-brain tumor barrier (BTB), which includes the microvessels supplying brain tumors, retains many characteristics of the normal BBB that significantly impedes the adequate delivery of chemotherapeutics into brain tumors [9,10,18]. However, BTB has also structural and functional characteristics that are different from those of normal BBB. In particular, there are receptors, ion channels and enzymes that are uniquely over-expressed in the tissue and microvessels of brain tumors that regulate vascular permeability [2,4,11,16,22]. Pharmacologically modulating these unique components of the BTB can selectively increase transport of chemotherapeutics across tumor capillaries and into brain tumors. Therefore, understanding the biochemical regulation of the BTB is critical for developing approaches to increase delivery of therapeutic compounds to brain tumors.
Widely distributed KATP channels are heterodimers of sulfonylurea receptors and inwardly rectifying potassium channel subunits (Kir6.x) with a (SUR-Kir6.x) 4 stoichiometry [15,23]. In the vasculature of the brain, KATP channels, by coupling intracellular metabolic changes to the electrical activity of the plasma membrane, regulate cerebral vascular tone and mediate the relaxation of cerebral vessels to diverse stimuli, including vasomodulators, in normal [5] and disease states [13]. Recently, intravenous infusion of minoxidil sulfate (MS) has been shown to increase tumor permeability via activation of KATP channels without any effect on permeability of normal brain capillaries in RG2 experimentally-induced gliomas [21]. Up-regulation of KATP channel expression was detected in and around brain tumors in a hypoxic environment [24] and also in ischemic conditions [13].
The calcium-activated potassium (KCa) channels represent a unique member of the six transmembrane domain potassium channel family that are triggered by depolarization and enhanced by an increase in cytosolic Ca2+ [6,8]. KCa channels of cerebral blood vessels participate in regulating cerebral blood vessel tone [13]. Our previous studies have shown that NS-1619, a KCa channel agonist, significantly enhances BTB permeability in the brain tumors of RG2 experimentally-induced glioma but not in normal brain tissue [20]. The selective increase in BTB permeability with NS-1619 was significantly attenuated by the KCa channel antagonist, iberiotoxin [20]. We have proposed that KCa channels over-expressed in tumor microvessels serve as a convergence point for BTB permeability modulation by molecules such as bradykinin, nitric oxide (NO) donors, and NS-1619 [20]. These findings suggest KCa channels are effective targets for BTB modulation, although it is conceivable that other types of potassium channels such as KATP channels may also contribute to BTB permeability regulation [21].
Although the involvement of multiple signals and their effectors such as KATP and KCa channels in BTB permeability regulation has been demonstrated, the contribution from each of them requires being determined under different pathphysiological conditions that affect their activity and/or expression. For brain tumor studies, there are different tumor models such as C6, RG2, 9L, GL26, and U87 which may be further developed as syngeneic or non-syngeneic animal models. Bradykinin (BK) and its analog, RMP-7 (Cereport), selectively increase brain tumor permeability and drug delivery across the BTB [1,11,17,22]. The B2 receptors of BK are found to be differentially expressed in C6, RG2 and 9L tumor models, and the expression patterns are consistent with different increases in BK-mediated BTB permeability [14]. In addition, in clinical trials, difference in B2 receptor expression in tumors has been regarded as a major reason for the significant variability in the effect of cereport on the BTB permeability in patients with malignant brain tumors [17]. These findings suggest that modulation of BTB permeability can be significantly affected by the activity and/or expression levels of the target protein(s) for a modulator drug.
In the present study, we sought to determine whether the effects of KATP and KCa channel agonists on BTB permeability are different between syngeneic and non-syngeneic rat brain tumor models and whether the differences, if any, are related to differential expression of KATP and/or KCa channels. The expression patterns of KATP and KCa channels and the effects of their agonists on tumor permeability were examined in experimentally-induced 9L gliosarcoma implanted in Fisher (syngeneic model) and Wistar (non-syngeneic model) rats. The mRNA levels of KATP channel were also examined for nineteen glioblastoma multiforme (GBM) surgical specimens. Furthermore, we have previously shown that vaccinated patients receiving subsequent chemotherapy exhibited significantly longer times to tumor recurrence relative to their own previous recurrence times after chemotherapy [26]. In this study, the mRNA levels of KATP channel were examined in the paired GBM tissues from seven patients before and after vaccination therapy that have been reported by Wheeler et al. [26].
2. Results
2.1. Effects of KATP or KCa channel agonists on tumor permeability in syngeneic and non-syngeneic rat brain tumor models
Our previous studies suggest that the agonists of KATP or KCa channels increase BTB permeability in certain rat brain tumor models [19–21]. Here we sought to determine whether the effects of potassium channel agonists on BTB permeability were different between syngeneic and non-syngeneic rat brain tumor models. 9L gliosarcomas is originally derived from Fisher rats. The cells were implanted into Fisher rat brain for our syngeneic model and Wistar rat brain for our non-syngeneic model. The tumor size of 9L gliosarcomas was similar and comparable between the two models (H&E staining; data not shown). The permeability of brain tumor was determined by autoradiography of [14C]-sucrose measured in the tumor, as describe before [11,22].
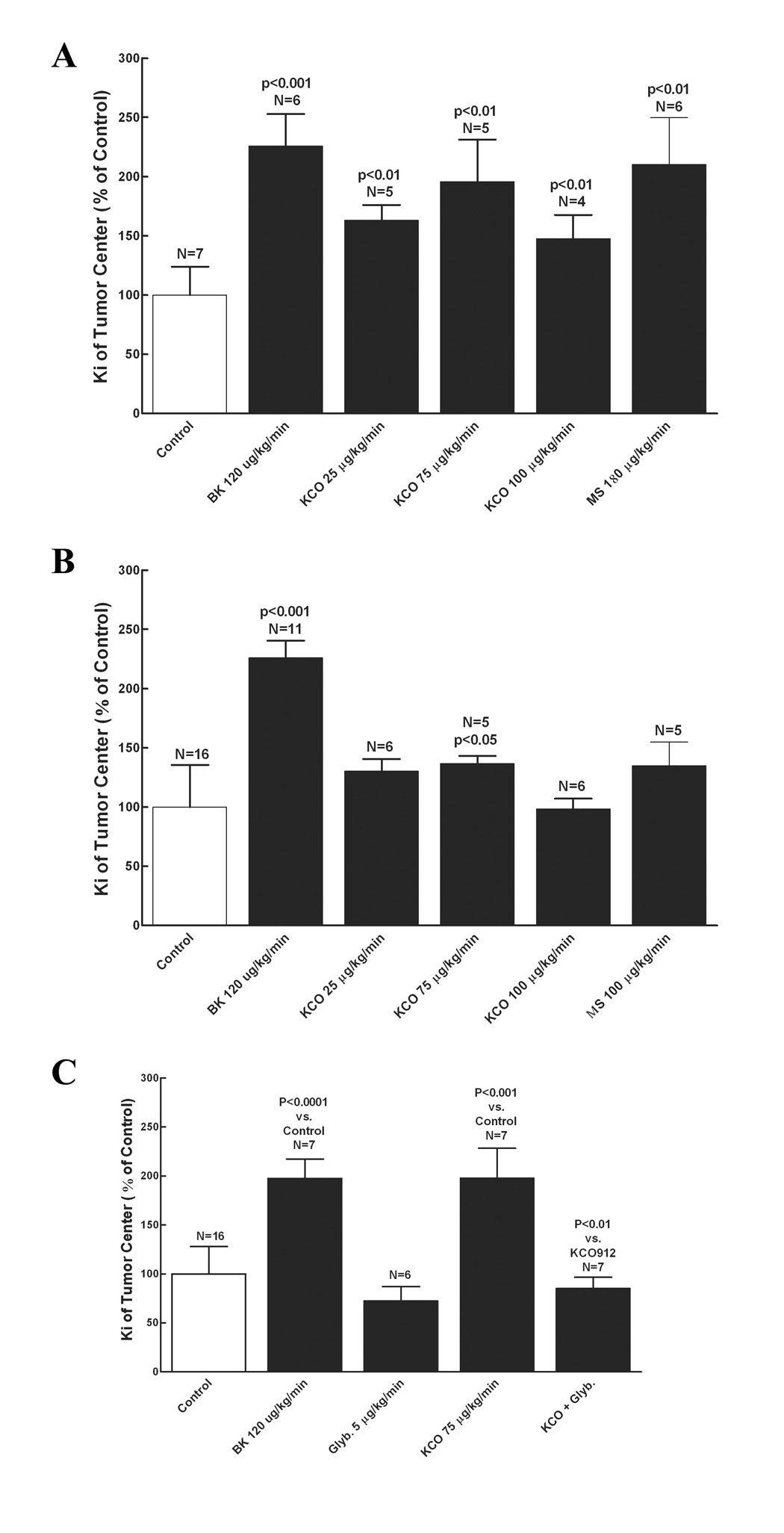
Six days following implantation of 9L gliosarcomas in Fisher and Wistar rats, the effects of KATP channel agonist (KCO912 and MS) on brain regional tumor permeability were examined. Intravenous infusion of KCO912 (75 µg/kg/min) (n = 5) resulted in a significant increase in 9L tumor permeability, Ki, over the PBS treatment (n = 7) in the non-syngeneic model (2-fold higher, p < 0.01, 10.9 ± 4.42 vs 5.56 ± 1.33 µl/g/min), while there was only a slight trend of Ki increase by KCO912 in the syngeneic model (1.4-fold higher, p = 0.038, 10.8 ± 1.17 vs 7.88 ± 2.79 µl/g/min) (Figure 1A and B). Co-infusion of glibenclamide, a KATP antagonist, abolished the increase in tumor permeability by KCO912 in the non-syngeneic model (Figure 1C). Consistent with the results of KCO912, another KATP channel agonist, minoxidil sulfate (180 µg/kg/min) (n = 6) also caused a significant increase in tumor Ki (2.1-fold higher, p < 0.01, 11.7 ± 2.21 vs 5.56 ± 1.33 µl/g/min) in the non-syngeneic model with no significant effect in the syngeneic model (1.3-fold higher, p = 0.053, 10.8 ± 1.17 vs 7.88 ± 2.79 µl/g/min) (Figure 1B).
Figure 1. Effect of KATP potassium channel agonists (KCO912, MS) on tumor permeability.
The regional Ki values in 9L tumors are calculated for different treatments and expressed as the percentage over that of control (% of control). The data represent mean ± SD. Refer to the results for Ki values. Potassium channel agonists and bradykinin were infused for 15 minutes with permeability determined at the 5 to 15-minute time interval, as described in the method. Bradykinin treatment served as the positive control. A, Treatments of KCO912 and MS in 9L/Wistar; C, Treatments of KCO912 and MS in 9L/Fischer; C, Treatments of KCO912 and glybenclamide in 9L/Wistar. BK, bradykinin; MS, minoxidil sulfate. The P values represent the comparisons to the control.
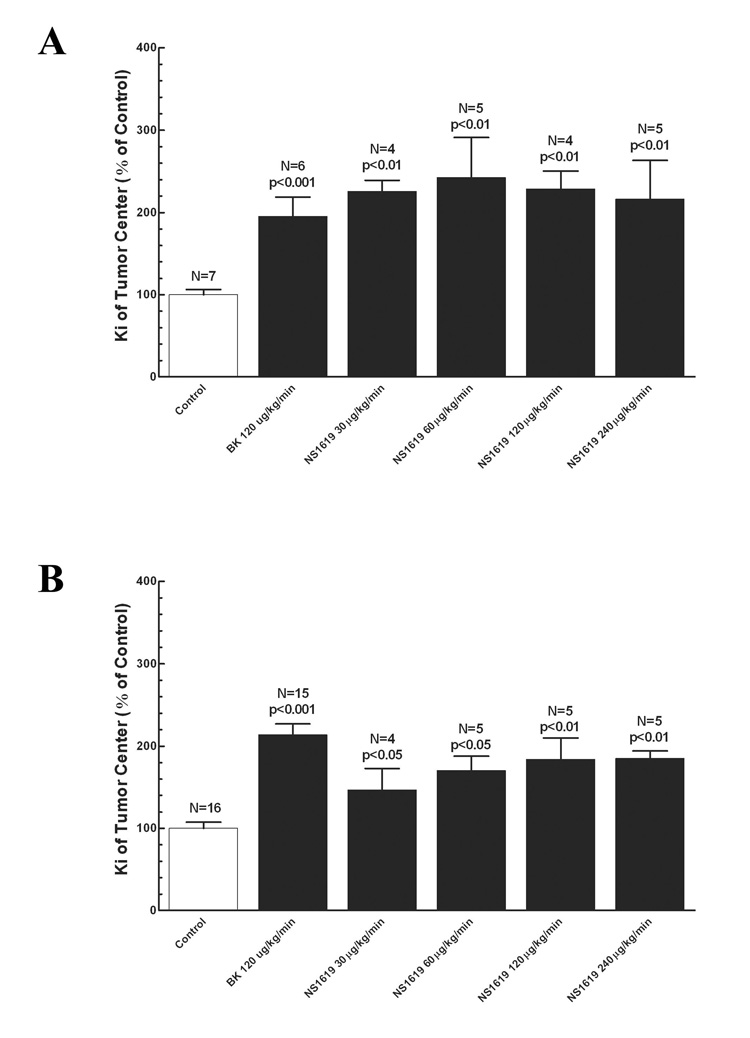
The effect of a KCa channel agonist, NS-1619, on tumor permeability was also compared between the two rat brain tumor models. In contrast to the effects by the KATP channel agonists, intravenous infusion of NS-1609 (60 µg/kg/min) significantly increased tumor permeability in both non-syngeneic and syngeneic animal models (p < 0.05 versus PBS-treated control). However, the increase in the non-syngeneic model was more significant than in the syngeneic model (2.4-fold increase in 9L tumor of Wistar rats versus 1.7-fold increase in 9L tumor of Fischer rats, p < 0.05) (Figure 2A and 2B). Bradykinin has been previously demonstrated to enhance tumor permeability in animal models [11,22]. In this study, it was noted that bradykinin increased Ki in our two models to a similar extent when compared with the PBS-treated control. The permeability in normal brain tissue (2 mm beyond the tumor border) and those of surrounding the tumor (within 2 mm of the border of the tumor) were not altered by the infusion of any of the vasomodulators (Data not shown). Bradykinin and the potassium channel agonists did not alter blood pressure in the animals. Under the experimental conditions in this study, brain and tumor tissue blood volumes should not significantly altered by bradykinin [3,12,14,22] and the potassium channel agonists. However, the exact effect from those potassium channel agonists requires further studying.
Figure 2. Effect of Kca potassium channel agoinist (NS1619) on tumor permeability.
The regional Ki values in 9L tumors are calculated for different treatments and then expressed as the percentage over that of control (% of control). The data represent mean ± SD. Refer to the results for Ki values. NS1619 and bradykinin were infused for 15 minutes with permeability determined at the 5 to 15-minute time interval, as described in the method. Bradykinin treatment served as the positive control. A, NS1619 treatment in 9L/Wistar; B, NS1619 treatment in 9L/Fischer. The P values represent the comparisons to the control.
2.2. Expression of KATP channels and KCa channels in non-syngeneic and syngeneic rat brain tumor models
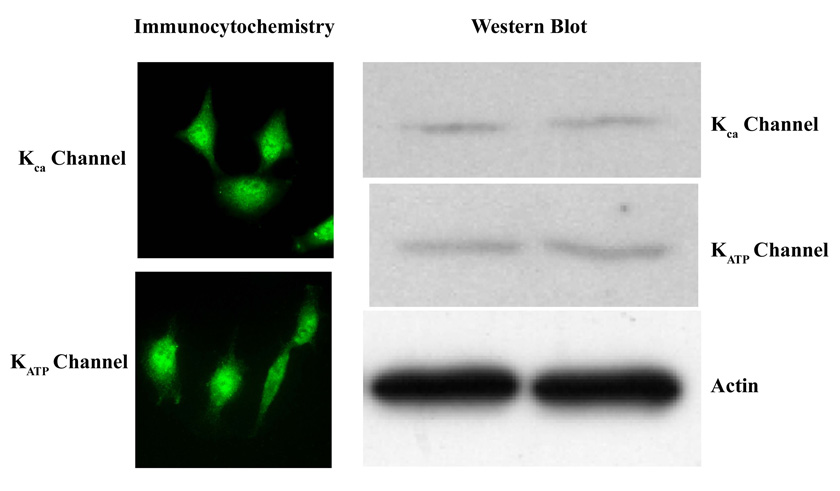
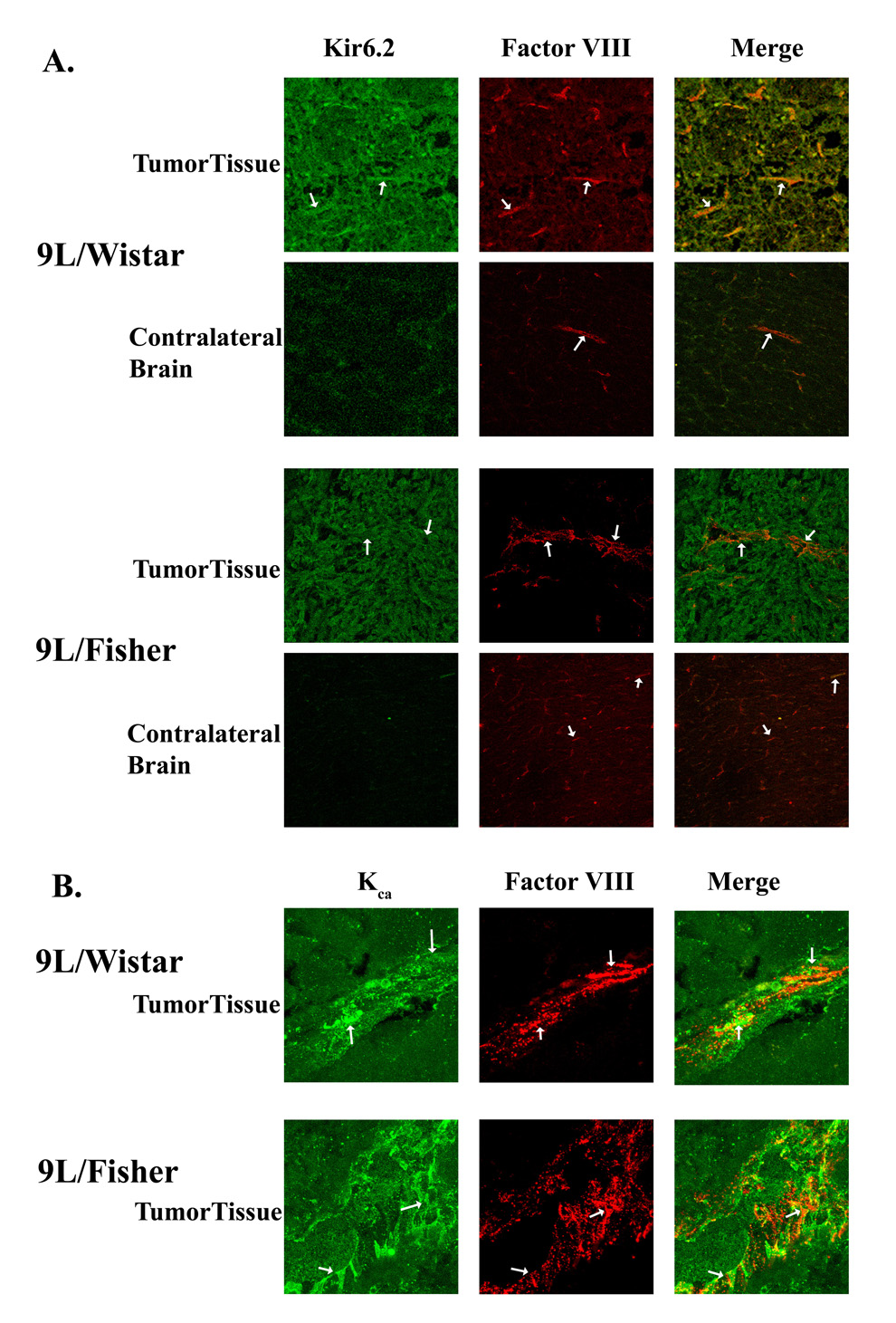
We first sought to determine if 9L gliosarcoma cells grown in vitro express both KCa and KATP channels by immunoflourescent labeling and western blot analysis. As shown in Figure 3, both KCa and KATP channels were expressed in 9L gliosarcoma cells. The expression of these channels was then examined in experimentally-induced 9L gliosarcoma in Fischer and Wistar rats. We also sought to determine if both KCa and KATP channels were present in endothelial cells within and surrounding the tumor. KCa and KATP channels had similar expression patterns within the tumor and were co-expressed in endothelial cells with Factor-VIII, a marker for vascular endothelia (Figure 4A and 4B). There were a few KCa or KATP channel-immunopositive cells in the brain parenchyma surrounding the tumor and in normal brain tissue (Figure 4A and data not shown). The immunochemistry staining indicated robust expression of both KCa and KATP channels in the tumors from both animal models, however, no difference in the expression pattern of either KATP or KCa channels in tumors was found between the two animal models (Figure 4).
Figure 3. Expression of potassium channels (KATP andKca) in cultured 9L tumor cells.
The expression of KATP and Kca were determined by immunohistochemistry (left panels) and Western blot analysis (right panels). Actin serves as the internal standard for Western analysis.
Figure 4. Expression of potassium channels (KATP andKca) in 9L tumor tissue from different models (Wistar and Fisher rats).
The expression of potassium channels and Factor VIII, a marker for endothelial cells were determined by immunohistochemical staining and imagined under confocal microscopy. A, KATP channel (Kir6.2) and Factor VIII staining; B, KCa channel and Factor VIII staining. The white arrows indicate microvessels in the tumor. Note tumor cells also express the channels. The image represents a field size of 500 × 500 µm. We use a HC PL APO 20× 0.7 N.A. lens.
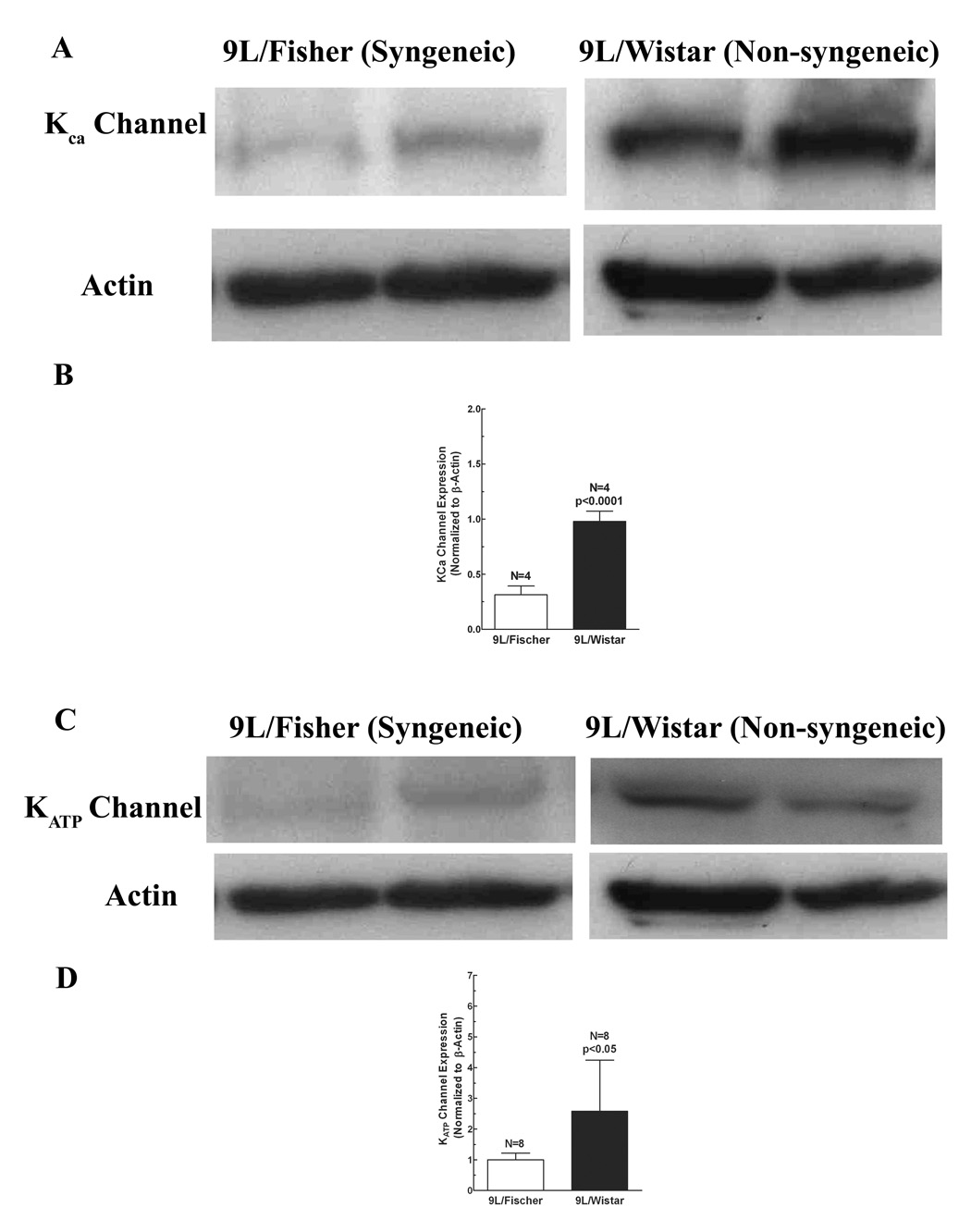
In order to quantify if there is a difference in the level of protein expression of KATP or KCa channels in brain tumors between the two animal models, we performed western blot analysis on tumor tissues. As shown in Figure 5, the protein expression levels for both KCa and KATP channels were significantly higher in Wistar rats than those in Fischer rats (KCa channels, 9L/Wistar vs. 9L/Fischer, 0.98 ± 0.05 vs. 0.31 ± 0.04, p < 0.0001, Figure 5A and 5B; and KATP channels, 2.5 ± 1.02 vs. 0.84 ± 0.10, p < 0.05, Figure 5C and 5D). These results, in combination with those from above permeability studies, indicate a positive correlation between expression levels of potassium channels including KCa and KATP and the BTB permeability induced by those channel agonists.
Figure 5. Protein levels of potassium channels (KATP and Kca) in 9L tumor tissue from different models (Wistar and Fisher rats).
The protein levels of potassium channels and actin (internal standard) cells were determined by Western blot analysis. A, Representative immunoblots of KATP channel in 9L/Wistar and 9L/Fischer; B, quantitative analysis of KATP channel protein in 9L/Wistar and 9L/Fischer; C, Representative immunoblots of KCa channel in 9L/Wistar and 9L/Fischer; D, quantitative analysis of KCa channel protein in 9L/Wistar and 9L/Fischer.
2.3. Expression of KATP channels in GBM of patients
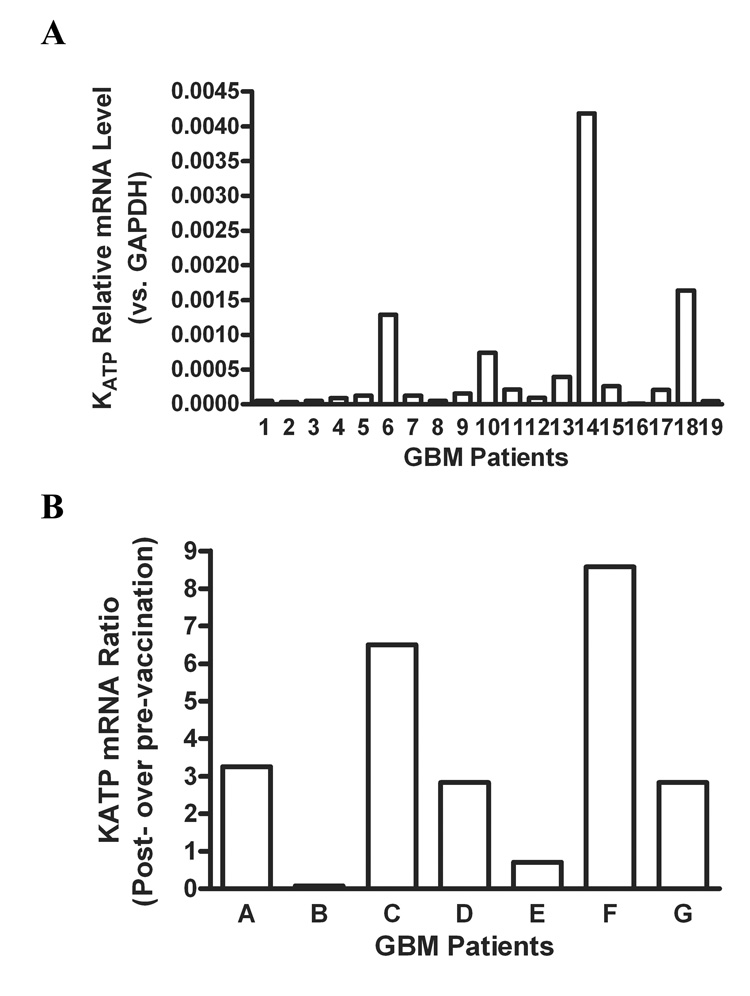
In order to determine if KATP channels are expressed in GBM tissues from patients, we studied the mRNA levels of KATP channels by Real-Time PCR. We first examined the mRNA levels of KATP channels in GBM of nineteen patients, and found that there was significant variation in the mRNA levels of KATP channels among GBM patients (Figure 6A). Next, in order to determine the effect of immunoresponse on expression of KATP channels in GBM patitents, we studied the mRNA levels of KATP channels in paired GBM tissues from seven patients before and after dendritic cell vaccination. Of seven patients, increased mRNA levels of KATP channel in GBM were detected in five patients who had positive response to chemotherapy after vaccination [26], whereas decreased levels of KATP channels for the other two patients (Figure 6B). It would be interesting to examine the expression of Kca channel in the surgical specimens in the future as well.
Figure 6. mRNA levels of KATP channels in GBM patients were determined by RT-PCR.
A, mRNA levels of KATP channels in GBM of 19 patients without vaccination therapy; B, Relative change in mRNA levels of KATP channels in GBM of 7 patients before versus after dendritic cell vaccination. The Y axis in B represents the ratio of mRNA levels of KATP channels after vaccination to those before vaccination for the same individual patients.
3. Discussion
The BTB prevents the delivery of chemotherapeutics into brain tumors. Pharmacological modulation is a novel approach to increase BTB permeability for chemotherapeutics. We have previously reported that the agonists of potassium channels including KATP and KCa increase drug transport across BTB [19–21]. However, the magnitude of the effect by those agonists was different among different rat tumor models during our preliminary observation. In this study, we particularly studied if the agonists of KATP and KCa channels cause differential effects on BTB permeability between syngeneic and allogeneic rat tumor models. We also examined the expression level of a potassium channel, KATP, in surgical GBM specimens from patients.
Our results demonstrate that both KATP and KCa channel agonists enhance tumor permeability more in allogeneic (9L glioma cells implanted in Wistar rats) than in syngenic (9L glioma cells implanted in Fisher rats) brain tumor models. This is consistent with our observation of higher expression of KATP and KCa channels in tumor tissue from the allogeneic rat model. The approach of pharmacological modulation takes advantage of the structural and functional characteristics of BTB that are different from those of the normal BBB. In this study, the expression of both KATP and KCa channels was robustly detected in tumor capillary endothelium and tumor cells with much less expression in the brain parenchyma surrounding the tumor and in normal brain tissue. As a result, the agonists of both KATP and KCa channels increased the permeability of tumors but not that of normal brain tissue. We and others have previously demonstrated that BTB permeability can be modulated via receptors, ion channels and enzymes that are uniquely overexpressed in the tissue and microvessels of brain tumors [4,7–10,18–21,25]. However, the expression and activity of those molecules involved in the regulation of BTB permeability can be altered by a variety of pathophysiological conditions including tumor type, tumor grade and potentially the host immune response.
It remains unclear why there is the differential tumor permeability and expression levels of KATP and KCa channels between the syngeneic and non-syngeneic rat brain tumor models. The expression of KATP and KCa channels was detected in cultured 9L gliosarcoma cells which are derived from Fisher rats. However, after implantation, 9L gliosarcoma tissue, including capillary endothelium and tumor cells, expressed different levels of both channels, with higher levels in allogeneic (Wistar rat) and lower levels in syngeneic (Fisher rat) models. In view of the fundamental differences between syngeneic and allogeneic models, the immune status may be an important mechanism acounting for differential expression of KATP and KCa channels.
Interestingly, clinical studies suggest that immunotherapy by vaccines in brain tumor patients enhances their sensitivity to chemotherapy [26,27]. The mechanism remains unclear. It has been shown that after vaccination with tumor lysate-pulsed dendritic cells, patients with malignant gliomas have significant infiltration of CD4+ and CD8+ T cells in tumor tissues [27]. Although it is quite speculative, the underlying mechanism may also be related to alteration of gene expression of tumor permeability-related molecules by immunotherapy. We have previously shown that the activity of KATP channels can regulate BTB permeability in rat brain tumor models [21]. Our animal results from syngeneic and allogeneic models imply that KATP channels may be a molecule of which expression is altered by immunotherapy in brain tumor patients. This is consistent with our findings in surgical GBM specimens that the mRNA levels of KATP channels increased after vaccination therapy for the majority of the patients (5 out of 7 patients). More interestingly, the patients with the increased KATP expression turned out to have positive response to chemotherapy after vaccination. Further studies will be required to test our hypothesis that immune status modulates the expression of the critical molecules such as KATP and KCa channels associated with brain tumor permeability. In this study, we also found that the expression of KATP channels is very variable among GBM patients. Further studies are also required to address whether the variation accounts for any inter-individual difference in response to chemotherapy.
In conclusion, the present study presents evidence that the variable increases in BTB permeability induced by potassium channel agonists between syngeneic and allogeneic rat models are directly related to potassium channel expression levels, suggesting the importance of an appropriate animal model to study the pharmacological modulation of BTB permeability. Clinical studies of BTB permeability should also evaluate the variability in potassium channel expression among patients and its role in determining individual differences in response to chemotherapy.
4. Experimental Procedure
4.1. Materials
All animal experiments were conducted in accordance with policies set by the Institutional Animal Care and Use Committee in Cedars-Sinai Medical Center and by NIH guidelines. Female Fischer and Wistar rats, weighting 150–180 g, were used for this study..Bradykinin, minoxidil sulfate (MS), and NS-1619 were obtained from the Sigma Co. (St. Louis, MO), and KCO912 from Novartis Pharma (Switzerland). [14C]-Sucrose (363 mCi/mmol; MW 342) was obtained from ICN (Dupont New England Nuclear, Boston, MA).
4.2. Intracerebral Tumor Implantation
9L gliosarcoma cells were kept frozen until use, and then thawed and maintained in a monolayer culture in DMEM medium with 10% FBS. The rats were anesthetized with intraperitoneal injections of ketamine (50 mg/kg)/xylazine (6 mg/kg), and immobilized in a stereotactic frame. A Hamilton syringe was lowered into the right basal ganglia to a depth of 4.5 mm from the dural surface, 3 mm lateral and 1 mm anterior to the bregma, and was then withdrawn 0.5 mm to create a space for tumor cell accumulation. Intracerebral injections of 1×105 9L glioma cells in 5 µl of medium with 1.2% methylcellulose were then administered over a period of 10 minutes. The skin incision was closed using sterile surgical staples.
4.3. Animal Preparation
For permeability studies the tumor-implanted rats were divided into five groups as follows: (1) control group: treated with intravenous infusion of saline; (2) BK group: treated with intravenous infusion of bradykinin (120 µg/kg/min, dissolved in 0.9% saline); BK is used as a positive control for BTB permeability studies; (3) KCO912 group: treated with intravenous infusion of KCO912; (4) MS group: treated with intravenous infusion of MS; (5) NS-1619 group: treated with intravenous infusion of NS-1619. Six days after tumor implantation, the rats were again anesthetized with ketamine/xylazine. One femoral vein was cannulated for administration of the appropriate drugs and the radiotracer; one femoral artery was cannulated to withdraw arterial blood, and the other was cannulated to monitor systemic blood pressure. Body temperature was maintained at 37°C, and arterial blood gases, blood pressure and hematocrit were monitored. Animals that showed any abnormal physiologic parameters were eliminated from the study.
4.4. Tumor Permeability Study
The radiotracer used in this study was [14C] sucrose. Either a drug or saline was infused into the femoral vein at a rate of 66.7 µl/min for 15 minutes. Five minutes after the start of the intravenous infusion, 50 µCi/kg of [14C] sucrose was injected as an intravenous bolus. A peristaltic withdrawal pump was used to withdraw femoral artery blood at a constant rate of 0.083 ml/min, immediately after injection of the tracer. This blood was used for determination of serum radioactivity. Additional experiments were performed in Wistar rats with 9L tumor by infusing glibenclamide (5 µg/kg/min) to investigate whether inhibition of KATP channels by glibenclamide attenuates permeability increase induced by KCO912. After completion of the experiments (15 minutes from the beginning of drug/saline infusion or 10 minutes after tracer injection), the animals were euthanized by decapitation and the brains immediately removed and frozen.
4.5. Quantitative Autoradiography
The frozen brains were mounted onto pedestals with M1 embedding matrix, after which 20 µm coronal sections were cut with a cryostat. The sections were thaw-mounted onto slides, and autoradiographs were generated by exposing the sections along tissue-calibrated 14C standards on a phosphor screen for 5 days. The sequential sections were then stained with hematoxylin and eosin (H&E) for histological correlation of areas of tumor with autoradiographs. The regional radioactivity was measured in tumor, in brain surrounding the tumor (BST; within 2 mm of the border of the tumor), in right cortex (ipsilateral to tumor), left cortex, right white matter, left white matter, right basal ganglia, and left basal ganglia. Quantitative analysis of the regional radioactivity was performed using a computer (Power Macintosh 7100) and Image 1.55 software (National Institutes of Health, Bethesda, MD).
4.6. Ki Value Calculation
Regional permeability in normal brain and in the tumor tissues was expressed by the unidirectional transfer constant, Ki value (µl/g/min). The initial rate for blood-to-brain transfer was calculated using the following equation:
Where Cbr is the brain or tumor concentration (dpm/g) of the tracer at the end of the experiment, Cbl is the blood concentration (dpm/g) of the tracer at the end of experiment, T (min) is the duration of the experiment, and Cpl is the arterial plasma concentration of radiotracer (dpm/ml). Vo is the regional cerebral blood volume in the tissue (µl/g), and dt denotes integration over time. Blood volumes (Vo) of the 9L tumors were derived from previously published studies [3,12,14,22].
4.7. Immunohistochemistry
The brain was removed immediately after decapitation and fixed in ice-cold fixative solution of 4% freshly prepared paraformaldehyde containing 10% sucrose. Coronal section (20 µm) was cut on a cryostat, mounted to geletin-coated slides and processed for immunohistochemistry. KCa and KATP channels in tumor sections were localized with anti-KCa or anti- KATP channel antibody (against a kir6.2 subunit). To determine whether KCa or KATP channels are present on capillary endothelial cells, the brain tumor sections were also incubated with polyclonal anti- Factor VIII primary antibodies. The colocalization of KCa or KATP channels and Factor VIII was accomplished with anti-rabbit IgG conjugated with fluorescent (FICT) and anti-mouse IgG conjugated with rhodamine (tetramethylrhodamine isothiocyanate), respectively. The negative control was performed by excluding the primary antibodies in incubations. The immunostaining was analyzed under a confocal-laser-scanning microscope as described below.
4.8. Confocal Microscopy
Immunoreactive visualization of KCa or KATP channels and vWF was performed as described previously [20]. In brief, confocal images were captured using a Leica True Confocal Scanner Spectrophotometer laser scanning confocal microscope (inverted) equipped with argon (488 nm) and krypton (568 nm) lasers (Heidelberg, Germany). Fluorescent signals for KCa or KATP channels expressed on brain sections were visualized using the 488-nm argon laser line, and fluorescent signals from Factor VIII were visualized using the 568-nm krypton laser line. Fluorescence signals for flour-488 or −568 were displayed as green and red pseudocolor projections respectively or merged as overlay projections to visualize possible colocalization of two different antigens within a specific subcellular structure.
4.9. Western Blot Analysis
Tumor cells, tumor tissue and normal brain were rapidly homogenized in 200 µl of lysis buffer [1% SDS, 1.0mM sodium vanadate, 10 mM Tris (pH 7.4)]. After centrifugation for 10 min at 16,000 g, the supernatant was separated by electrophoresis on a 10% SDS-polyacrylamide gel, and electrotrandferred to a nitrocellulose membrane (Imobilon; Millipore, Bedford, MA). Non-specific binding sites were blocked in TBS with 5% BSA and 0.1% Tween-20 for 1 hour. Membranes were rinsed for 30 minutes in washing buffer (0.1% Tween-20 in TBS) and then incubated with anti-KCa (1:1000) and anti-Kir6.2 (1:1000), followed by anti-rabbit IgG horseradish peroxidase-conjugate; or with anti-actin (1:1000), followed by anti-mouse IgG horseradish peroxidase-conjugate. All primary and secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). After rinsing with the washing buffer, the immunocomplexes were visualized by chemiluminescence using the ECL kit (Amersham Biotech Inc., Piscataway, NJ) according to the manufacturer’s instructions. The signals in the film were digitally scanned and then quantified using NIH Image software.
4.10. GBM Tissues and Vaccination Therapy in Patients
The GBM tissue collection and clinical trial were conducted and approved in accordance with policies set by the IRB in Cedars-Sinai Medical Center. The detail clinical trial for vaccination therapy in GBM patients were described elsewhere [26]. In brief, seven patients suffered from de novo GBM were provided informed consent to treatments and associated monitoring. Patients underwent craniotomy before receiving the vaccine therapy. Vaccinated patients were steroid-free during blood collection. The patients received three vaccines, 2 weeks apart, of 10–40 × 106 autologous dendritic cells loaded with either HLA-eluted peptides from cultured tumor cells or 150 µg/ml autologous tumor freeze-thaw lysate, starting approximately 15 weeks after surgery. Serial magnetic resonance imaging (MRI) scans were performed every 2–3 months for all patients for continuous monitoring. After vaccination, these patients underwent another craniotomy at the time of recurrence of GBM. GBM tissues obtained in the surgery before and after vaccination were processed for real time PCR study.
4.11. Real-time PCR
Total cellular RNA was extracted from glioblastoma multiforme (GBM) of patients using TRIzol Reagent (Invitrogen, Carlsbad, CA) according to the manufacture’s protocol. One microgram (1 µg) of total RNA was reverse-transcribed into cDNA using iScript™ cDNA Synthesis kit (Bio-Rad Laboratories, Hercules, CA). cDNA was stored at −20°C until use. Targeting gene primers (KATP channel, PPH01409A.) were purchased from SuperArray (Frederick, MD). GAPDH is used as the internal control. Dural-color, real time quantitative RT-PCR was performed using the method of SYBR Green (Bio-Rad Laboratories, Hercules, CA). The mRNA expression levels of target genes were normalized by GAPDH expression in different samples.
4.12. Statistical Analysis
Results are expressed as mean ± standard deviation (SD), where applicable. The statistical analyses of Ki, KCa or KATP expression between different groups, with or without drug treatment, were performed using ANOVA, followed by either unpaired Student’s t test of parametric analysis or the Mann-Whitney U test of nonparametric analysis. A p-value of < 0.05 was considered statistically significant.
Acknowledgement
This work was supported by grants from the National Institutes of Health (NIH, NS32103, NS25554, RR13707, and a Jacob Javits Award (KLB)).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Black KL, Chio CC. Increased opening of blood-tumour barrier by leukotriene C4 is dependent on size of molecules. Neurol Res. 1992;14:402–404. doi: 10.1080/01616412.1992.11740093. [DOI] [PubMed] [Google Scholar]
- 2.Black KL, Cloughesy T, Huang SC, Gobin YP, Zhou Y, Grous J, Nelson G, Farahani K, Hoh CK, Phelps M. Intracarotid infusion of RMP-7, a bradykinin analog, and transport of gallium-68 ethylenediamine tetraacetic acid into human gliomas. J Neurosurg. 86;1997:603–609. doi: 10.3171/jns.1997.86.4.0603. [DOI] [PubMed] [Google Scholar]
- 3.Black KL, King WA, Ikezaki K. Selective opening of the blood-tumour barrier by intracarotid infusion of leukotriene C4. Acta Neurochir Suppl (Wien) 1990;51:140–141. doi: 10.1007/978-3-7091-9115-6_47. [DOI] [PubMed] [Google Scholar]
- 4.Black KL, Ningaraj NS. Modulation of brain tumor capillaries for enhanced drug delivery selectively to brain tumor. Cancer Control. 2004;11:165–173. doi: 10.1177/107327480401100304. [DOI] [PubMed] [Google Scholar]
- 5.Brayden DJ. Controlled release technologies for drug delivery. Drug Discov Today. 2003;8:976–978. doi: 10.1016/s1359-6446(03)02874-5. [DOI] [PubMed] [Google Scholar]
- 6.Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J Biol Chem. 2000;275:6453–6461. doi: 10.1074/jbc.275.9.6453. [DOI] [PubMed] [Google Scholar]
- 7.Cloughesy TF, Black KL. Pharmacological blood-brain barrier modification for selective drug delivery. J Neurooncol. 1995;26:125–132. doi: 10.1007/BF01060218. [DOI] [PubMed] [Google Scholar]
- 8.Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- 9.Fenstermacher JD, Cowles AL. Theoretic limitations of intracarotid infusions in brain tumor chemotherapy. Cancer Treat Rep. 1977;61:519–526. [PubMed] [Google Scholar]
- 10.Groothuis DR, Fischer JM, Lapin G, Bigner DD, Vick NA. Permeability of different experimental brain tumor models to horseradish peroxidase. J Neuropathol Exp Neurol. 1982;41:164–185. doi: 10.1097/00005072-198203000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Inamura T, Black KL. Bradykinin selectively opens blood-tumor barrier in experimental brain tumors. J Cereb Blood Flow Metab. 1994;14:862–870. doi: 10.1038/jcbfm.1994.108. [DOI] [PubMed] [Google Scholar]
- 12.Inamura T, Nomura T, Bartus RT, Black KL. Intracarotid infusion of RMP-7, a bradykinin analog: a method for selective drug delivery to brain tumors. J Neurosurg. 1994;81:752–758. doi: 10.3171/jns.1994.81.5.0752. [DOI] [PubMed] [Google Scholar]
- 13.Kitazono T, Faraci FM, Taguchi H, Heistad DD. Role of potassium channels in cerebral blood vessels. Stroke. 1995;26:1713–1723. doi: 10.1161/01.str.26.9.1713. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Hashizume K, Chen Z, Samoto K, Ningaraj N, Asotra K, Black KL. Correlation between bradykinin-induced blood-tumor barrier permeability and B2 receptor expression in experimental brain tumors. Neurol Res. 2001;23:379–387. doi: 10.1179/016164101101198596. [DOI] [PubMed] [Google Scholar]
- 15.Masia R, Enkvetchakul D, Nichols CG. Differential nucleotide regulation of KATP channels by SUR1 and SUR2A. J Mol Cell Cardiol. 2005;39:491–501. doi: 10.1016/j.yjmcc.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Matsukado K, Inamura T, Nakano S, Fukui M, Bartus RT, Black KL. Enhanced tumor uptake of carboplatin and survival in glioma-bearing rats by intracarotid infusion of bradykinin analog, RMP-7. Neurosurgery. 1996;39:125–133. doi: 10.1097/00006123-199607000-00025. discussion 133-4. [DOI] [PubMed] [Google Scholar]
- 17.Matsukado K, Sugita M, Black KL. Intracarotid low dose bradykinin infusion selectively increases tumor permeability through activation of bradykinin B2 receptors in malignant gliomas. Brain Res. 1998;792:10–15. doi: 10.1016/s0006-8993(97)01502-3. [DOI] [PubMed] [Google Scholar]
- 18.Neuwelt EA, Barnett PA, Bigner DD, Frenkel EP. Effects of adrenal cortical steroids and osmotic blood-brain barrier opening on methotrexate delivery to gliomas in the rodent: the factor of the blood-brain barrier. Proc Natl Acad Sci U S A. 1982;79:4420–4423. doi: 10.1073/pnas.79.14.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ningaraj NS, Rao M, Black KL. Calcium-dependent potassium channels as a target protein for modulation of the blood-brain tumor barrier. Drug News Perspect. 2003;16:291–298. doi: 10.1358/dnp.2003.16.5.878815. [DOI] [PubMed] [Google Scholar]
- 20.Ningaraj NS, Rao M, Hashizume K, Asotra K, Black KL. Regulation of blood-brain tumor barrier permeability by calcium-activated potassium channels. J Pharmacol Exp Ther. 2002;301:838–851. doi: 10.1124/jpet.301.3.838. [DOI] [PubMed] [Google Scholar]
- 21.Ningaraj NS, Rao MK, Black KL. Adenosine 5'-triphosphate-sensitive potassium channel-mediated blood-brain tumor barrier permeability increase in a rat brain tumor model. Cancer Res. 2003;63:8899–8911. [PubMed] [Google Scholar]
- 22.Nomura T, Inamura T, Black KL. Intracarotid infusion of bradykinin selectively increases blood-tumor permeability in 9L and C6 brain tumors. Brain Res. 1994;659:62–66. doi: 10.1016/0006-8993(94)90863-x. [DOI] [PubMed] [Google Scholar]
- 23.Rosenblum WI. ATP-sensitive potassium channels in the cerebral circulation. Stroke. 2003;34:1547–1552. doi: 10.1161/01.STR.0000070425.98202.B5. [DOI] [PubMed] [Google Scholar]
- 24.Ruoslahti E. Specialization of tumour vasculature. Nat Rev Cancer. 2002;2:83–90. doi: 10.1038/nrc724. [DOI] [PubMed] [Google Scholar]
- 25.Terasaki T, Pardridge WM. Targeted drug delivery to the brain; (blood-brain barrier, efflux, endothelium, biological transport) J Drug Target. 2000;8:353–355. doi: 10.3109/10611860008997911. [DOI] [PubMed] [Google Scholar]
- 26.Wheeler CJ, Das A, Liu G, Yu JS, Black KL. Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res. 2004;10:5316–5326. doi: 10.1158/1078-0432.CCR-04-0497. [DOI] [PubMed] [Google Scholar]
- 27.Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]