Abstract
Stem cells, although difficult to define, hold great promise as tools for understanding development and as therapeutic agents. However, as with any new field, uncritical enthusiasm can outstrip reality. In this review, we have listed nine common myths that we believe affect our approach to evaluating stem cells for therapy. We suggest that careful consideration needs to be given to each of these issues when evaluating a particular cell for its use in therapy. Data need to be collected and reported for failed as well as successful experiments and a rigorous scientific approach taken to evaluate the undeniable promise of stem cell biology.
Keywords: stem cell, homing, immortal, embryonic stem cells, adult stem cells
1. Introduction
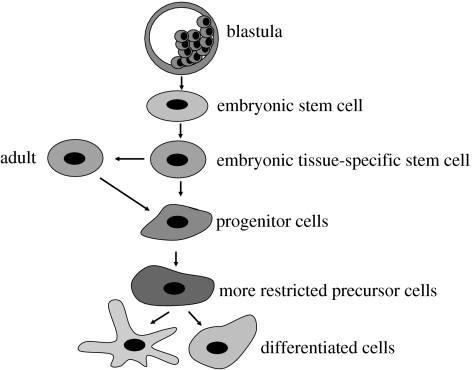
The related fields of stem cell biology and regenerative medicine are relatively young. Indeed, with the exception of bone marrow stem cells and possibly stromal stem cells, very little was known about tissue-specific stem cells in any other tissue until recently. The last decade, however, has seen an exponential increase in the number of articles on other stem cell types, including skin, liver, pancreas, brain, lung, intestine, skeletal muscle, cardiac, Wharton's jelly and cord blood. It is generally assumed that stem cells undergo maturation and progressive restriction in their developmental potential (figure 1). The ubiquitous nature of stem cells, their clear role in development, their presumed participation in repair and regeneration in some tissues and the undeniable success of bone marrow stem cell therapy have raised high expectations that we will be able to harness stem cells to cure diseases that have thus far proven resistant to conventional therapy in tissues such as the nervous system, heart and liver.
Figure 1.
A model of how pluripotent embryonic stem cells undergo maturation and progressive restriction in their developmental potential. Initially, tissue-specific stem cells, e.g. neural, haemopoietic, hepatic, etc., are generated. These stem cells proliferate and may mature during development but retain their differentiation ability. Tissue-specific stem cells do not generate fully differentiated cells directly, but do so by first generating intermediate precursors or blast cells that undergo limited proliferation and ultimately mature to generate fully differentiated progeny.
The promise of cell therapy/regenerative medicine has led to the California initiative to supplement federal funds, the establishment of several biotechnology companies focused on stem cells and the initiation of clinical trials to examine the utility of stem cells in a variety of disease models. Scientists from various specialties have been forced to consider results from outside their fields that are published in journals not common to their specialty and which use different and sometimes confusing abbreviations/terminology.
As is inevitable in any new and rapidly expanding field, misconceptions have arisen about the nature and possible uses of stem cells. In this review, we summarize some of these misconceptions, which we have termed myths, since they are based on experimental results that have been distorted (generally unintentionally), as accounts of the data have been transmitted from one laboratory to another. These distortions have acquired the status of well-established facts (dogma) and have been perpetuated as new investigators enter this expanding field. We emphasize that it is not our intention to downplay the importance of stem cells or to cast doubt on the validity of anyone's observations. Rather, we hope to underscore the importance of evaluating all of the available facts when planning experiments on particular cell types to be studied under particular conditions. Most importantly, we hope readers will come away with a reluctance to assume that what they have heard or what has worked in one system will necessarily be reproduced easily in another one.
2. Myth 1: stem cells are immortal
The idea that stem cells are immortal has been a key assumption underlying the belief that unlimited numbers of cells will be available for therapy. This idea is based on reliable observations in the haemopoietic system. In this system, it has been shown by sequential repopulation studies that the lifespan of bone marrow stem cells exceeds the lifespan of the donor animal by a significant fraction, thus leading to haemopoietic stem cells (HSCs) being described pragmatically as immortal cells. This finding supports the therapeutic use of HSCs, since transplanted stem cells should reasonably be expected to last the lifetime of an individual. HSCs express low levels of telomerase consistent with their prolonged self-renewal ability and studies following successful bone marrow transplant recipients have thus far not described failure due to premature HSC senescence. The idea that stem cells are similar to each other has led many researchers and commentators to assume that other stem cells will have the same ability for extensive self-renewal. These assumptions have been bolstered by reports that neural stem cells (NSCs) propagated in culture can undergo extensive expansion and that even cells isolated from adults or cadavers have an extensive self-renewal capability. Similar reports in some other stem cell populations, such as multipotential adult progenitor cells (MAPC; Reyes et al. 2002) and Wharton's jelly cells (Kogler et al. 2004), have provided further credence to these assumptions.
However, several reports challenge these assumptions. It has been suggested that NSCs cannot divide in culture for more than 12 passages without undergoing dramatic changes (Morshead et al. 2002). Whittemore and colleagues have shown that prolonged passaging alters the differentiation ability of NSCs (Cao et al. 2002). In addition, mesenchymal stem cells (MSCs) appear to have a limited self-renewal potential and generally cannot be expanded indefinitely (Kassem et al. 2004). The degree of expansion shows a correlation with the age of isolation.
One could perhaps argue that this may simply be a tissue culture issue, as HSCs cannot be propagated to any large extent in culture, but clearly have a prolonged self-renewing ability in vivo. Reports assessing the behaviour of directly harvested cells suggest, however, that even in vivo stem cells age and cannot be assumed to have a lifespan which is much greater than that of an individual. Pruitt and colleagues, for example, have examined karyotypic stability and found that ageing leads to a substantial mutational load within the NSC compartment, a change that is likely to affect the function of these cells (Bailey et al. 2004). More recent reports assessing telomerase levels also suggest a decline with the organism/stem cell age and many adult stem cell populations lack detectable levels of telomerase reverse transcriptase activity, consistent with the findings of limited self-renewal. For example, human neural progenitors typically downregulate telomerase activity to undetectable levels by 16 weeks of gestational age (Wright et al. 1996; Ulaner & Giudice 1997), or with early passage in vitro (Ostenfeld et al. 2000). Taken together, these results argue that not all stem cells have a lifespan that is extensive and that not all stem cells maintain their karyotypic integrity over the prolonged time period required for expansion and differentiation.
3. Myth 2: asymmetric divisions are required to define a stem cell
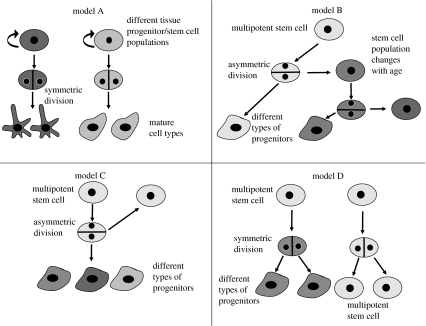
Experimental results suggest that differentiated cells must be derived from stem cells via a sequential process of differentiation. The process of stem cell differentiation into mature cells could occur through a variety of mechanisms and a variety of developmental models have been proposed (figure 2).
Figure 2.
Different models of stem cell division. In model A, each stem or progenitor cell gives rise to a different cell type requiring a different stem cell population for each tissue. Models B and C demonstrate the possibility of a multipotent stem cell generating different types of precursors by asymmetric division. Model B presupposes stem cell maturation over time in response to extrinsic signals, thus it does not generate truly identical daughter cells. Model D displays a model of symmetric cell division without the necessity of asymmetric division.
It appears that most stem cell biologists consider asymmetric division (model C) to be the hallmark of stem cells. Indeed, many have argued that asymmetric differentiation may be an essential property of stem cells that serves to distinguish them from other dividing populations, such as blast cells, restricted progenitors and dividing mature cells (reviewed by Lu et al. 1998, 2000).
The case for asymmetric division being an obligatory requirement of stem cells, however, does not appear to be strictly true on either theoretical or practical grounds. As described in figure 2, several alternative models can be proposed. At one extreme, any tissue may in fact consist of a mosaic of pre-committed progenitor cells. Each stem/progenitor cell type may give rise to multiple progeny of only one type (model A in figure 2). In this model, the multiplicity of mature cell types seen in a particular tissue or organ reflects the initial heterogeneity of the developing progenitor/stem cell population and as such no true multipotent stem cell population that can generate all cells in that tissue or organ actually exists.
Model D also differs from models B and C, in that it does not presuppose the existence of asymmetric cell divisions. Instead, two differing symmetric divisions generate either a self-renewing division into two daughter stem cells or a division into two differentiated cells. This model suggests that the population itself is self-renewing and multipotent, but that no individual cell must necessarily hold these properties. This model does not presuppose the existence of an identifiable stage of intermediate progenitors, or obligatory asymmetric division, and, in essence, the stem cells behave like a blast/intermediate precursor cell population only by undergoing symmetric differentiated cell divisions.
Models B and C, unlike the models discussed previously, do invoke asymmetric division but differ from each other, in that model B presupposes that a stem cell not only generates differentiated progeny, but also matures over time in response to extrinsic signals. Thus, it does not generate a daughter cell that is truly identical to itself, i.e. the properties, including differential potential, change. Such a model appears to be better than the classical asymmetric differentiation (model C) in describing retinal differentiation (Livesey & Cepko 2001).
Each of the non-exhaustive lists of models described can account for the majority of reported observations conventionally assumed to derive from the asymmetric division model.
In many systems, particularly the haemopoietic and neural systems, where this has been examined in some detail in vitro and in vivo (e.g. Desai & McConnell 2000), models with a stem cell undergoing asymmetric division best fit the available data. However, even in these systems, it is unclear whether asymmetric divisions are obligatory for stem cells and predictive of stem cell behaviour at all stages of development. Indeed, in early developmental stages, it is clear that only symmetric self-renewing divisions are apparent as the organs are forming. Even at later developmental stages in the nervous system, both symmetric and asymmetric divisions are observed. Even in the haemopoietic system, Quesenberry and others have shown that the ability of a stem cell to differentiate is related to its cell cycle state and one cannot presume to predict the properties of a population (Quesenberry et al. 2002). It is also important to remember that homogenous populations of stem cells such as NSCs and embryonic stem (ES) cell can only be maintained in culture if symmetric divisions can occur.
We believe the data indicate that the process of segregation of a multipotent stem cell into multiple derivatives could occur via multiple pathways. A set of sequential asymmetric binary/trinary fate choices or symmetric differentiation steps could explain central nervous system (CNS) as well as other tissue development.
4. Myth 3: adult stem cells are like foetal cells, only better
Given the political implications, it is important to be extremely precise regarding the potential of different stem cell populations. The idea that stem cells are present in the same tissue at all stages of development and that these potential stem cell populations appear similar to each other at least superficially has led to the suggestion that stem cells of a particular tissue or organ are the same and can be used interchangeably. This has also been extended to argue that while this is true, adult cells may be better than foetal cells, as they have had more time to adapt to the adult environment, and thus can be reasonably expected to fare better when transplanted when compared with foetal cells. This assumption seems reasonable at face value and, indeed, some investigators have performed a limited number of such studies and assumed this is true (Lie et al. 2002).
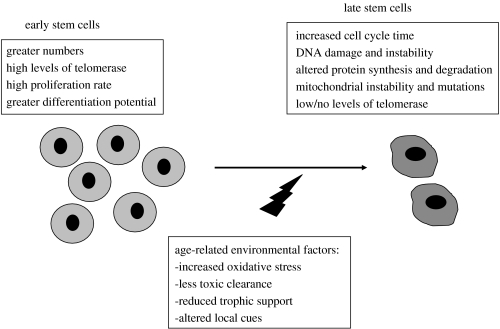
However, more detailed analysis of foetal and adult stem cell populations harvested from the same tissue has suggested that changes occur. During the normal process of ageing, somatic cells progressively show signs of DNA damage and instability, altered protein synthesis and degradation, mitochondrial instability and mutations, and elevated free radical production and decreased free radical scavenging, which lead to the accumulation of somatic mutations (figure 3). Similarly, it is not surprising that adult stem cells show similar signs of ageing. For example, the proliferative potential of HSCs and the size of the HSC compartment are under genetic control (Morrison et al. 1996; Geiger & Van Zant 2002). Genes that encode functions such as DNA repair or responsiveness to environmental signals and undergo age-related changes in expression or activity of their products could affect the quality of HSCs (reviewed by Linton & Dorshkind 2004). In addition, ageing also affects HSC's developmental potential, as demonstrated in many studies where bone marrow or purified HSCs from old mice did not efficiently generate lymphoid progenitors (Tyan 1977; Hirokawa et al. 1986; Sharp et al. 1990; Sudo et al. 2000).
Figure 3.
Age-related changes in stem cells. Although stem cells persist in different tissues and maintain certain traits throughout life, several changes can be found dependent upon the age at which the cells are examined. Environmental stressors and increasing failure of the support system can account for some of the observed changes.
Other stem cell types display age-related changes as well. For example, NSCs show decreased telomerase expression and activity, a loss of heterozygosity on one or more chromosomes or even a complete deletion of at least one chromosomal region with ageing (Bailey et al. 2004). These findings raise concerns about the potential for reactivating endogenous NSCs within the aged brain or the use of adult NSCs in transplantation.
In addition to age-related decline in function, stem cells isolated at different stages of development display different properties. For example, the continuous demand for rapidly increasing numbers of haemopoietic cells in foetal life requires an intrinsically different population of stem cells than in adult animals, where steady-state maintenance is important. Studies have shown that the ability of foetal liver HSCs to expand in culture is far greater than that of adult bone marrow HSCs (Mucenski et al. 1991; Lansdorp et al. 1993). Foetal cells have a higher turnover rate and show differences in cell cycle profile and engraftment capability (Tsai et al. 1994). Also, foetal and adult HSCs show molecular differences, including a developmental switch in the derived B- and T-cell lineage (Elliott et al. 1988; Bonifer et al. 1998).
NSCs likewise exhibit changes during development. For example, early neural tube progenitors (possibly NSCs) differentiate in response to ectopic inductive signals to adopt multiple neuronal phenotypes present along the dorsoventral axis of the neural tube (Artinger et al. 1995; Ye et al. 1998), while late neural tube progenitors lose the ability to adopt ectopic neuronal phenotypes (Olsson et al. 1997). Similarly, early neural crest stem cells (NCSCs) differentiate into noradrenergic and cholinergic neurons in sympathetic and parasympathetic ganglia, respectively, whereas late NCSCs exhibit little or no noradrenergic differentiation in sympathetic ganglia (White et al. 2001). This increasing lack of plasticity combined with a very different environment could result in a decrease in integration of adult NSCs. Indeed, decreased survival and migration of adult compared with newborn neural progenitors is seen when they are transplanted into a chick embryo (Durbec & Rougon 2001), and, in general, neural replacement in the adult system is mainly successful in areas of ongoing neurogenesis like the hippocampus and the olfactory bulb.
Overall, we note functional differences between foetal and adult stem cells, which preclude the assumption that stem cells from different ages will act alike.
5. Myth 4: cells similar to embryonic stem cells exist in adult tissue
ES cells are pluripotent cells, which can be maintained in culture for prolonged time periods while retaining their ability to contribute to an entire organism, including to the germ line. This ability has been harnessed in mice to generate modified animals, where every cell can be shown to be derived from a cell propagated in a dish. The recent discovery that equivalent cells can be derived from human blastocysts has sparked considerable ethical and moral debate and has led to a search for cells with embryonic stem (ES)-like properties obtainable from sources other than the inner cell mass.
Some investigators have argued that primordial germ cells could be such a source, while others have suggested that somatic nuclear transfer may achieve the same goal. Other investigators have suggested that ES-like cells persist in adult tissue. Reports range from small spore-like cells described by Vacanti et al. (2001), the Wharton's jelly pluripotent populations (Kogler et al. 2004), to pluripotent populations found in the inner ear (Li et al. 2003), multiple regional pluripotent cells described by Young & Black (2004) and MAPCs (Jiang et al. 2002). Each of these populations has been shown to differentiate into ectoderm, endoderm and mesoderm in vitro (and occasionally in vivo) or, in the case of MAPC, it has been shown to contribute to a chimera after blastocyst injection (table 1).
Table 1.
Comparison between ES and ES-like cells.
| properties | ES cells | ES-like cells | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Wharton jelly cells | VENT cells | neural crest stem cells | germ cells | multipotent adult progenitor cells (MAPC) | regional stem cells | spore like cells | |||
| pluripotency | differentiation into three germ layers | yes | yes | yes | yes | yes | yesb | yesc | yesc |
| teratoma formation | yes | not tested | not tested | no | yes | no | not tested | not tested | |
| chimera formation (germline) | yes | no | no | no | yes | yes (no germline transmission) | no | no | |
| immortality (don't undergo senescence) | yes | not tested | not tested | no | noa | not tested | not tested | not tested | |
| inner cell mass derived | yes | no | no | no | no | no | no | no | |
| expression of a characteristic set of markers | yes | some overlap | minimal overlap | minimal overlap | significant overlap | minimal overlap | not tested in detail | not tested in detail | |
| contact inhibition | no | yes | yes | yes | yes | yes | yes | yes | |
| cell cycle control | p53/Rb independent | no | no | no | ? | no | not tested | not tested | |
Human cells have not been propagated for really long periods.
In vivo data, but not as robust as ES cells.
In vitro data and generally not robust.
The data, while controversial, are also limited in that most researchers have tested only a limited set of markers and performed experiments primarily in vitro. More importantly, only one aspect of ES cell behaviour has been looked at, i.e. the ability to differentiate into somatic tissue. However, the ability to differentiate into ectoderm, endoderm and mesoderm in culture is a property that is not unique to ES cells (e.g. Labosky et al. 1994; Shamblott et al. 1998; Rathjen & Rathjen 2003). ES cells are developmentally close to germ cells, which are pluripotent and migrate extensively to reach their final site and could possibly persist in multiple locations. It is plausible that adult pluripotent ES-like cells may be more closely related to germ cells and perhaps germ cell markers should be carefully examined in these populations. However, irrespective of what ultimately turns out to be the case, it is important not to prematurely assume that pluripotency is sufficient to call a cell ES-like.
ES cells are unique among all stem cell populations (reviewed by Burdon et al. 2002; Draper & Fox 2003) and have a well-defined core identity. Specifically, the ES cell cycle does not appear to be regulated by the Rb and p53 regulatory pathways. Human ES cells can generate trophoblast and also, more importantly, germ cells. ES cells appear to lack contact inhibition and density-dependent growth inhibition, and they readily form teratomas upon transplantation into an immune-compromised host.
To our knowledge, none of the adult pluripotent cells (APCs) share these key features of ES cells. APCs do not appear to express ES cell markers, do not form teratomas and, when injected into blastocysts, do not develop reliable germ lines. Their contribution to various germ layers, if shown at all, has been unimpressive. Thus, one must carefully specify which aspect of similarity is being considered when comparing APCs and ES cells.
It is important to remember that other candidate non-ES cells displaying this ability to differentiate into more than one germ layer exist. These include ventrally emigrating neural tube (VENT) cells, NCSCs and cells in the kidney and other organs that undergo an epithelial-to-mesenchymal transition. While one would not argue that these are ES cells, they would mimic other ES-like cells in being able to contribute to more than one germ layer, to migrate to multiple tissue sites when transplanted and, in the case of crest or VENT cells, to participate in normal tissue formation.
We believe that calling APCs ‘ES-like’ is inaccurate and misleading and may contribute to ascribing properties to the APCs that they do not possess. We would urge that unless APCs contribute to the germ line, have a characteristic cell cycle (a property relatively unique to ES cells) and have the ability to contribute to organ formation and substantial functional reconstitution in blastocyst injection experiments, they should be considered ‘adult multipotent cells’, since pluripotency generally requires germ-line contribution. The term ‘ES-like’ should probably be reserved for germ cells, embryoid body-derived pluripotent cells and epiblast cells. We do not believe that available data allow any adult-derived stem cell population to be reasonably classified as ‘ES-like’.
6. Myth 5: stem cells are more plastic than other cells
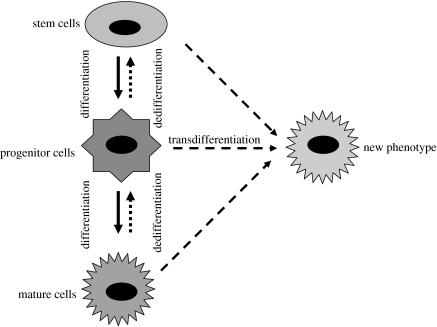
New results have indicated that stem cells may be much more plastic than originally thought. Stem cells taken from one tissue seem to have the ability to differentiate into cells from another tissue. This is believed to occur through two different mechanisms: transdifferentiation and dedifferentiation (figure 4; Liu & Rao 2003).
Figure 4.
Transdifferentiation and dedifferentiation. A change in phenotype could happen in two ways: a direct transformation (transdifferentiation) or by a regression to earlier developmental stages (dedifferentiation).
For example, it appears that bone marrow contains a population of pluripotent cells that can differentiate into a variety of cell lineages, including astrocytes, oligodendrocytes and neurons (Kopen et al. 1999; Brazelton et al. 2000; Mezey et al. 2000; Woodbury et al. 2000; Akiyama et al. 2002). In different injury model systems, these cells were found to be integrated into the brain parenchyma and, in part, were associated with a functional improvement (Hofstetter et al. 2002). Other examples include the generation of pneumocytes following infusion of bone marrow-derived cells into the lung, and cardiac myocytes in models of myocardial infarction (Orlic et al. 2001a,b) or limb regeneration in newts through transdifferentiation of muscle and connective tissue cells into blastema (Brockes & Kumar 2005). It has been shown that both mouse and human NSCs transplanted into the bone marrow of irradiated mice will generate cells of the haemopoietic lineage (Bjornson et al. 1999; Shih et al. 2001), or following transplantation into adult animals even generate skeletal myotubes (Galli et al. 2000). The underlying mechanism proposed is that changes in stem cell gene expression led to the generation of tissue-specific cells in response to the tissue microenvironment.
Transdifferentiation does not seem to be unique to stem cells (figure 4). Other investigators have noted the ability to transdifferentiate in more restricted populations of cells. For example, pancreatic cells can be converted into hepatocytes and vice versa (Shen et al. 2000; Horb et al. 2003). In the CNS, oligodendrocyte precursors, astrocytes and radial glia have all been shown to be capable of becoming mature neurons (Kondo & Raff 2000; Laywell et al. 2000; Malatesta et al. 2000). Even cells thought to be terminally differentiated, such as post-mitotic neurons, have been reported to re-enter the cell cycle and generate dividing progenitor cells (Brewer 1999; Alexanian & Nornes 2001). These and other examples suggest that if transdifferentiation does exist, it is a property of many cells and should not be considered unique to stem cells.
The most convincing demonstrations of transdifferentiation also suggest that such events are rare (Wagers et al. 2002). For example, when several million cells were infused into the venous circulation, at best a few hundred transdifferentiated cells could be identified (Choi et al. 2003; Lechner et al. 2004). Furthermore, substantial long-term survival and integration of these transdifferentiated cells have not been demonstrated. Many of the more dramatic results originally attributed to transdifferentiation have provoked re-examination, yielding alternative hypotheses for the mechanisms responsible. Some reports have suggested that the best explanation for their results is an effect that occurs outside the nervous system (Pluchino et al. 2005) or via modulation of neurotrophic factors, rather than direct tissue contribution (Mahmood et al. 2004).
We are not arguing that transdifferentiation does not occur, or that it has not been convincingly demonstrated by numerous researchers. But it is a myth to assume that transdifferentiation is a property unique to stem cells and that stem cells transdifferentiate in a robust and reliable fashion. Indeed, we believe that transdifferentiation, even when it can occur, is normally tightly regulated, since uncontrolled, ectopic or otherwise-inappropriate differentiation is likely to be selected against during evolution. Subverting this normal tight regulation with precision and permanence will be difficult, ensuring that this subversion is heritable may be impossible.
7. Myth 6: the most primitive stem cell for a tissue or organ is the best stem cell to use
This belief is based on bone marrow transplants, where a case can be made for identifying the most primitive stem cell population. More rapidly proliferating precursors do not home or engraft with any efficiency and contribute to early, but not persistent, reconstitution (Lepore et al. 2004). This truism for HSC transplants has become a dogma for other somatic stem cells (SSCs), but we believe that the available data do not support such a conclusion. We have argued that the HSC is a special case owing to the necessarily ongoing cell replacement (Cai & Rao 2002). Such large-scale self-renewal is not seen in most solid tissues or organs and, indeed, in the nervous system (a perhaps extreme example) there is a limited role for stem cells in the adult. In general, an intermediate or restricted blast or progenitor cell will often repair such tissues.
This lack of stem cell involvement in an ongoing repair or regeneration process has several critical implications. Perhaps the most important one is that there are only very limited niches for stem cells in the adult. In addition, cues to direct differentiation and migration most probably no longer exist in mature tissues. For example, stem cells transplanted into the brain do not readily differentiate into dopaminergic neurons (Yang et al. 2004). In addition, endogenous stem cells cannot target sites of injury in any robust fashion (Lepore et al. 2004) and, in instances where the extent of differentiation of stem cells compared with progenitors has been examined, progenitor cells have shown better and more robust differentiation (Lepore et al. 2004). We note that even ES cell biologists have suggested using dopaminergic neurons derived from ES cells or oligodendrocytes derived from ES cells rather than NSCs (an obligatory intermediate in the differentiation process) for therapy. Even in the haemopoietic system, in cases where short-term replacement is required or a single lineage is affected, transplants of short-term progenitors or cells restricted to a particular lineage may be a more efficacious option (Na et al. 2002; Paquette & Dorshkind 2002). Thus, in several cases, cells with some or even no self-renewal capability may be as good or preferable to a cell with lifetime self-renewal capability. Individual therapeutic need and will dictate the preferred cell type with regard to self-renewal and differential potential, and hence determine how primitive the chosen cell needs to be.
8. Myth 7: stem cells can home and migrate to sites of injury
The concept of stem cell homing has largely been developed in the haemopoietic system. HSC therapy relies on the ability of such cells to successfully home to, and persist in, the marrow niche following delivery into the bloodstream. This process for HSCs in the bone marrow is complex and requires the orchestration of different adhesion molecules, chemokines and their receptors (Papayannopoulou et al. 1995; Frenette et al. 1998; Peled et al. 1999). In addition, the bone marrow niche is a heterogeneous mixture of cells, including adipocytes, endothelial cells, reticular cells, fibroblastic cells and smooth muscle cells, which provide growth factors, cytokines and chemokines, as well as a physical matrix for the well-being of HSCs. Other examples of such specialized niches include: (i) the germ-line stem cells (GSCs) in the Drosophila testis that attach to a cluster of hub cells and the loss of contact result in a commitment of the GSC to differentiate into a gonialblast, (ii) the subventricular zone (SVZ) in rodents (Luskin 1993; Alvarez-Buylla & Lois 1995) that provides a niche with an extracellular matrix-rich basal lamina for NSCs, (iii) the multipotent stem cells surrounded by a basement lamina found in hair follicles (Tumbar et al. 2004), and (iv) the stem cells in the intestine that reside near the base of each crypt (Potten 1998).
Homing has been observed not only in normal stem cell niches, but also in different injury sites. Transplanted stem cells can circulate for a limited time and are enriched in numbers at sites of injury when inflammatory- and wound-response stimuli are generated within the tissue (Whetton & Graham 1999). This process especially pertains to MSCs that have been demonstrated to home to sites of injury in a variety of models. During this process, different adhesion molecules seemed to be important (Pluchino et al. 2003). Additionally, NSCs express the marker CXCR4, which enables them to migrate along an SDF-1 gradient (Imitola et al. 2004). These cells may participate in the injury response and may also transdifferentiate.
It is difficult to imagine, however, that stem cells will target to an injury if they do not naturally do so in vivo and that all necessary homing signals would exist in an injured environment. It is therefore not surprising that the turnout and survival of transplanted stem cells is so low, even given the relatively large number of transplanted cells. In addition, there are other reasons why a sufficient homing of stem cells might fail (figure 5). First, not all stem cells (or just a small portion within the transplanted stem cell population) might have the appropriate receptors to react to the given cues (e.g. Wynn et al. 2004). Second, access to stem cell niches and tissues varies depending on the organ (e.g. the blood–brain barrier), and it therefore is unsurprising that tissues with close contact to the blood compartment (like the bone marrow) or with the greatest breakdown of vessel wall integrity show the best results. The third reason is most probably the local environment, which is generally not supportive of stem cell survival and self-renewal. Considering the complexity of the different stem cell niches (as indicated previously), which is needed to keep stem cells undifferentiated and dividing, it is not surprising that stem cells outside the niche quickly differentiate and abruptly change properties (Fuchs et al. 2004). Furthermore, an injured environment, as compared with the stem cell niche, lacks trophic support and stem cell survival will therefore be limited. Finally, transplanted stem cells have a competitive disadvantage in reaching and repopulating a niche; even in marrow transplants, depletion of a niche needs to be performed prior to transplantation. Even in an ideal situation, where stem cells could be delivered into an ‘empty’ stem cell niche, it might be important to choose the fitting stem cell and its specific niche. For example, even though SSCs can take advantage of an emptied GSC niche, the niche microenvironment does not appear to convert them into well-functioning GSCs (Kai & Spradling 2003), indicating that stem cell niches are geared towards their particular stem cell population.
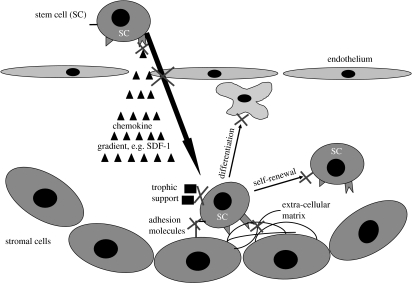
Figure 5.
Homing in the stem cell niche. The process of homing to a stem cell niche involves a complex orchestration of different soluble, cellular and extracellular signals, which eventually lead to a stable stem cell population capable of self-renewal and differentiation. Stem cells follow a chemokine gradient across an endothelial cell barrier to home to a niche provided by many different cell types. These niches in the adult are usually limited to very distinct areas (e.g. the SVZ). Under less than ideal circumstances, this process can fail at multiple levels (some of them indicated by crosses).
We predict that, depending on the tissue, homing of stem cells will be difficult and survival will be limited. For tissues such as the nervous system, other cell types (see §7) that are less dependent on environmental cues may be more helpful.
9. Myth 8: stem cells do not provoke an immune response
Stems cells are generally considered to provoke little immune response. Clinically, HSCs are widely used (e.g. to treat all kinds of blood cancers). Even under immune suppressive treatment, however, autologous transplantation bears the chance of immunological reactions (Spitzer 2001).
One might argue that transplantation into the CNS will be different, since the brain is considered an immunologically privileged site. This idea is based on the observation that tissue transplants in the CNS are not commonly rejected by the immune system (e.g. Medawar 1948; Barker & Billingham 1977). An anti-inflammatory and, with regard to invading immune cells, pro-apoptotic environment in the brain, the limited access of brain-derived antigens to the lymphoid organs, the presence of the blood–brain barrier, low major histocompatibility complex (MHC) expression in the brain parenchyma and the absence of dendritic cells have been used to explain the lack of an effective immune response to antigens in the brain. Numerous studies have challenged this view, however, by showing that potent immune reactions can and do occur in the CNS (Hickey 2001). Since even the uninjured CNS is constantly patrolled by activated T-lymphocytes, which in the context of a foreign MHC molecule would cause a host-versus-graft reaction, surgical disruption of the blood–brain barrier during transplantation increases the chance of a graft rejection. In addition, NSCs express increased levels of MHC classes I and II after stimulation with pro-inflammatory cytokines or after exposure to a foreign environment (McLaren et al. 2001; Modo et al. 2002, 2003; Al Nimer et al. 2004; Mammolenti et al. 2004). Intracerebral neural xenografts have been shown to trigger responses involving host cytotoxic T-lymphocytes, microglia/macrophages, immunoglobulin M and complement (Armstrong et al. 2001), and it is therefore not surprising that rejections of neural allografts and xenografts were observed in several studies (Sloan et al. 1991). Furthermore, it is probable that studies of transplanted inbred animals do not provide us with a correct picture of the expected immune reaction in humans, where rejections are more likely to occur, i.e. in contrast to rodent NSCs, human NSCs, express high levels of MHC class I (Klassen et al. 2001). This suggests that the immune response will perhaps be an even more significant problem in human-to-human transplants. Immune suppressive treatment would be necessary, even if MHC compatibility between donor and recipient had been optimized. The necessity of strong immune suppression during transplantation of human cells increases the probability of tumour growth originating from the transplanted cells and lowers the chances of the transplant to survive.
These observations may apply to other stem cell populations and thus far the data support this, as far as pancreatic islets, olfactory ensheathing cells or other stem and progenitor populations are concerned. The question of MHC mismatch may even be important for foetal cells, as suggested by cord blood studies (Rubinstein & Stevens 2000) and is a problem that most probably increases with the age of the recipient (Nash et al. 1992).
There is one population that has been suggested not to elicit immune responses, the MSCs. MSCs can differentiate into multiple cell types present in several tissues, including bone, fat, cartilage and muscle, making them candidates for a variety of cell-based therapies. There is in vitro evidence that human MSCs, by virtue of their distinct immunophenotype (associated with the absence of histocompatibility leukocyte antigen (HLA) class II expression) and low expression of co-stimulatory molecules (Majumdar et al. 2003), may be non-immunogenic or hypoimmunogenic and suppress T-cell responses (Di Nicola et al. 2002). In one clinical trial, patients with osteogenesis imperfecta were infused with MSCs from HLA-identical or single-antigen-mismatched siblings after patients had received ablative conditioning therapy. Since these patients also received a bone marrow transplantation, a chemoprophylaxis against graft-versus-host disease consisting of intravenous cyclosporine starting 2 days before transplantation (Horwitz et al. 1999, 2001, 2002), it is not possible to predict the exact in vivo immune response that MSCs may generate in a non-immune-suppressed patient.
Today, it is difficult to say if histocompatibility in stem cells is as critical as it is in bone marrow and organ transplants or is an irrelevant issue. However, the lack of available relevant evidence highlights the importance of additional experiments to clarify this issue.
10. Myth 9: therapy will be straightforward and will mimic strategies used in bone marrow therapy
The oldest and arguably the best-characterized stem cell is the HSC. It is also, thus far, the only clinically useful stem cell. The transplant strategy built on many years of work has suggested a conceptually straightforward process. Current strategies for cell replacement therapy appear to model this strategy, with the idea that any stem cell injected into the circulatory system would home to a suitable cellular niche, find the appropriate cues to survive, proliferate, differentiate and then repopulate the missing population of cells in a particular disease.
This belief has led to experiments such as injecting MSCs or NSCs into the arterial or venous circulation in a variety of injury paradigms. It has been shown that cells apparently target the injury site, home, sense the tissue they are in, identify the type and extent of damage, replicate and differentiate into the appropriate phenotype (e.g. Pluchino et al. 2003). Indeed, there has been so much excitement based on these results that several clinical studies are using MSCs to treat congestive cardiac failure, which is refractory to all other therapy. Initial results have been uniformly positive and presumably have lent credence to what we term the ‘smart stem cell strategy’. Indeed, some researchers have added the assumption that since stem cells are plastic, any stem cell type can be used and it will also transdifferentiate appropriately (the ‘very smart stem cell strategy’).
There are several reasons why transplantations of HSCs might be more successful than stem cell transplantation in other organs (table 2). In the haemopoietic system, we treat stem cell diseases in a system where ongoing replacement is occurring. It is possible to collect enough of the most primitive cell population and provide a competitive advantage to the transplanted cells to repopulate the existing niche. There is an ongoing differentiation and the existence of cues to direct appropriate differentiation. In addition, HSCs do not have to integrate into solid tissue and establish close connections with other cell types. Importantly, even HSCs are not very smart. Only a fraction ever reaches the marrow: if a competitive advantage is not provided, most cells fail to effectively repopulate, cells do not home except over very limited distances and they only respond appropriately if the correct environmental cues exist. Even more critically, it appears that endogenous stem cells (including HSCs) cannot respond to cues in other systems to mount an effective repair.
Table 2.
Relevant differences between haemopoietic and neural stem cells regarding treatment.
| haemopoietic stem cells | neural stem cells |
|---|---|
| most primitive cells are available | most primitive cells are not available |
| stem cell niches are easily accessible | stem cell niches are limited and not readily accessible |
| homing has been proven | homing is difficult |
| ongoing differentiated cell turnover exist including regulatory feedback loops | ongoing differentiated cell turnover is limited including regulatory feedback loops |
| positional information, connectivity and integration are unimportant | positional information, connectivity and integration are important |
| route of delivery, cell markers and cell numbers are known | route of delivery, cell markers and cell numbers are unknown |
It is hard to understand conceptually how stem cells in other systems could adopt the ‘HSC strategy’, or how we can extend this model of therapy to other stem cells without making many unproven assumptions or ignoring existing data. Diseases in most organs are not diseases of stem cells and therapy must be directed towards cellular replacement of post-mitotic cells that are fundamentally different from the HSC system. In most organs, unlike HSCs, we cannot provide transplanted stem cells a competitive advantage by creating a suitable niche, as is routinely done for bone marrow transplants by irradiation and chemotherapy. Indeed, in many organs, no niche may exist, because the number of stem cells may be vanishingly small. Even though some experiments, for example in the CNS, indicated that after specific injuries cues for neuronal differentiation were provided (Magavi et al. 2000), it is difficult to imagine that we can provide adequate differentiation cues, since there is normally a limited ongoing replacement. There will be no regulatory feedback or cues to direct differentiation. NSCs, for example, when transplanted into the spinal cord, will lack appropriate positional and differential cues and will differentiate stochastically and often inappropriately (Cao et al. 2001; Magnuson et al. 2001). Indeed, transplantation and culture evidence have clearly shown that the region from which a stem cell is isolated determines the identity of the differentiated progeny (Englund et al. 2002), and this cannot be overcome by cues in the site. It is difficult in a solid tissue to avoid issues of migration and connectivity, which are not faced by HSCs in the bone marrow. While such cues exist during development, these probably do not persist in the adult brain, since there is little ongoing repair and regeneration, and it does not appear that such cues are activated to direct appropriate differentiation (Sheen et al. 1999). Thus, there seems little basis for assuming that therapy in other organs can simply mimic the strategies that work so successfully in the HSC field.
We would suggest that while data suggesting that smart stem cells can perform extremely well exist, other less promising data can also be found. Although results suggesting that stem cells cannot home, can cause microemboli when injected into arteries, do not transdifferentiate appropriately and frequently cannot reach a target site, survive and integrate in significant numbers exist, they generally do not get the same level of attention as the other, more exciting, data. We strongly urge that stem cell researchers evaluate how ‘smart’ our cells really are, determine whether there is a strong factual basis for the assumptions we make and understand that because the field is still young, not all findings can be extrapolated to all systems.
11. Conclusions
We strongly believe in the potential utility of stem cells for therapy. We have learnt an incredible amount in a very short period of time and current data fairly indicate that stem cell-based therapies will soon be worthy additions to our therapeutic armamentarium. As in any new field, however, progress is often stalled by the necessity to backtrack, re-examine assumptions, test hypotheses and understand critically the rationale for a particular approach to therapy. We suggest that some of the assumptions made on the basis of the available successes of stem cell therapy cannot be generalized and that there is not one approach that will suit all therapeutic situations.
Which cell one uses, how one uses them and what outcome can be predicted reliably from existing data need to be critically evaluated. We believe that transplant experiments that share features with the HSC model will have higher probability of success. Cell therapy in diabetes (Soria et al. 2001) or the use of MSCs for connective tissue diseases (Caterson et al. 2001) will follow the haemopoietic model, given that ongoing tissue remodelling does occur and therefore regulatory cues presumably exist. Additionally, positional information appears irrelevant (Muschler & Midura 2002) and MSCs have shown the tendency to home to the bone marrow. On the other hand, in more complex organs with little ongoing cell replacement and repair, where stem cell niches do not appear to exist and positional information is much more critical, cell replacement will be much more challenging. Examples of such systems include liver, lung, heart and skeletal muscles. In these systems, the most primitive stem cell may not be as appropriate to transplant as a more differentiated cell, since lifetime replacement may not be an absolute requirement and delivery may be local rather than via the circulatory system.
We suggest that some assumptions have undeservedly acquired the status of facts, and that such myths need to be critically evaluated as we move towards more applied experiments. We ask readers to carefully evaluate all the available facts and determine whether they are applicable to their particular population of cells in their particular circumstances. Simply stated, researchers should not assume what still needs to be proven before safe and effective stem cell therapy can become a reality for most organs.
Acknowledgments
This work was supported by the NIH, the NIA, the Packard Center and the CNS foundation. We thank all the members of our laboratories for their constant stimulating discussions. M.S.R. acknowledges the contributions of Dr S. Rao that made undertaking this project possible.
Footnotes
One contribution of 14 to a Theme Issue ‘Stem cells and brain repair’.
References
- Akiyama Y, Radtke C, Kocsis J.D. Remyelination of the rat spinal cord by transplantation of identified bone marrow stromal cells. J. Neurosci. 2002;22:6623–6630. doi: 10.1523/JNEUROSCI.22-15-06623.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Nimer F, Wennersten A, Holmin S, Meijer X, Wahlberg L, Mathiesen T. MHC expression after human neural stem cell transplantation to brain contused rats. Neuroreport. 2004;15:1871–1875. doi: 10.1097/00001756-200408260-00007. doi:10.1097/00001756-200408260-00007 [DOI] [PubMed] [Google Scholar]
- Alexanian A.R, Nornes H.O. Proliferation and regeneration of retrogradely labeled adult rat corticospinal neurons in culture. Exp. Neurol. 2001;170:277–282. doi: 10.1006/exnr.2001.7705. doi:10.1006/exnr.2001.7705 [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lois C. Neuronal stem cells in the brain of adult vertebrates. Stem Cells. 1995;13:263–272. doi: 10.1002/stem.5530130307. [DOI] [PubMed] [Google Scholar]
- Armstrong R.J, et al. Porcine neural xenografts in the immunocompetent rat: immune response following grafting of expanded neural precursor cells. Neuroscience. 2001;106:201–216. doi: 10.1016/s0306-4522(01)00273-1. doi:10.1016/S0306-4522(01)00273-1 [DOI] [PubMed] [Google Scholar]
- Artinger K.B, Fraser S, Bronner-Fraser M. Dorsal and ventral cell types can arise from common neural tube progenitors. Dev. Biol. 1995;172:591–601. doi: 10.1006/dbio.1995.8038. doi:10.1006/dbio.1995.8038 [DOI] [PubMed] [Google Scholar]
- Bailey K.J, Maslov A.Y, Pruitt S.C. Accumulation of mutations and somatic selection in aging neural stem/progenitor cells. Aging Cell. 2004;3:391–397. doi: 10.1111/j.1474-9728.2004.00128.x. doi:10.1111/j.1474-9728.2004.00128.x [DOI] [PubMed] [Google Scholar]
- Barker C.F, Billingham R.E. Immunologically privileged sites. Adv. Immunol. 1977;25:1–54. [PubMed] [Google Scholar]
- Bjornson C.R, Rietze R.L, Reynolds B.A, Magli M.C, Vescovi A.L. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999;283:534–537. doi: 10.1126/science.283.5401.534. doi:10.1126/science.283.5401.534 [DOI] [PubMed] [Google Scholar]
- Bonifer C, Faust N, Geiger H, Muller A.M. Developmental changes in the differentiation capacity of haematopoietic stem cells. Immunol. Today. 1998;19:236–241. doi: 10.1016/s0167-5699(98)01259-6. doi:10.1016/S0167-5699(98)01259-6 [DOI] [PubMed] [Google Scholar]
- Brazelton T.R, Rossi F.M, Keshet G.I, Blau H.M. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. doi:10.1126/science.290.5497.1775 [DOI] [PubMed] [Google Scholar]
- Brewer G.J. Regeneration and proliferation of embryonic and adult rat hippocampal neurons in culture. Exp. Neurol. 1999;159:237–247. doi: 10.1006/exnr.1999.7123. doi:10.1006/exnr.1999.7123 [DOI] [PubMed] [Google Scholar]
- Brockes J.P, Kumar A. Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science. 2005;310:1919–1923. doi: 10.1126/science.1115200. doi:10.1126/science.1115200 [DOI] [PubMed] [Google Scholar]
- Burdon T, Smith A, Savatier P. Signalling, cell cycle and pluripotency in embryonic stem cells. Trends Cell Biol. 2002;12:432–438. doi: 10.1016/s0962-8924(02)02352-8. doi:10.1016/S0962-8924(02)02352-8 [DOI] [PubMed] [Google Scholar]
- Cai J, Rao M.S. Stem cell and precursor cell therapy. Neuromol. Med. 2002;2:233–249. doi: 10.1385/NMM:2:3:233. doi:10.1385/NMM:2:3:233 [DOI] [PubMed] [Google Scholar]
- Cao Q.L, Zhang Y.P, Howard R.M, Walters W.M, Tsoulfas P, Whittemore S.R. Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp. Neurol. 2001;167:48–58. doi: 10.1006/exnr.2000.7536. doi:10.1006/exnr.2000.7536 [DOI] [PubMed] [Google Scholar]
- Cao Q, Benton R.L, Whittemore S.R. Stem cell repair of central nervous system injury. J. Neurosci. Res. 2002;68:501–510. doi: 10.1002/jnr.10240. doi:10.1002/jnr.10240 [DOI] [PubMed] [Google Scholar]
- Caterson E.J, Nesti L.J, Albert T, Danielson K, Tuan R. Application of mesenchymal stem cells in the regeneration of musculoskeletal tissues. Med. Gen. Med. 2001;E1 [PubMed] [Google Scholar]
- Choi J.B, et al. Little evidence of transdifferentiation of bone marrow-derived cells into pancreatic beta cells. Diabetologia. 2003;46:1366–1374. doi: 10.1007/s00125-003-1182-9. doi:10.1007/s00125-003-1182-9 [DOI] [PubMed] [Google Scholar]
- Desai A.R, McConnell S.K. Progressive restriction in fate potential by neural progenitors during cerebral cortical development. Development. 2000;127:2863–2872. doi: 10.1242/dev.127.13.2863. [DOI] [PubMed] [Google Scholar]
- Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni P.D, Matteucci P, Grisanti S, Gianni A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. doi:10.1182/blood.V99.10.3838 [DOI] [PubMed] [Google Scholar]
- Draper J.S, Fox V. Human embryonic stem cells: multilineage differentiation and mechanisms of self-renewal. Arch. Med. Res. 2003;34:558–564. doi: 10.1016/j.arcmed.2003.08.006. doi:10.1016/j.arcmed.2003.08.006 [DOI] [PubMed] [Google Scholar]
- Durbec P, Rougon G. Transplantation of mammalian olfactory progenitors into chick hosts reveals migration and differentiation potentials dependent on cell commitment. Mol. Cell Neurosci. 2001;17:561–576. doi: 10.1006/mcne.2000.0951. doi:10.1006/mcne.2000.0951 [DOI] [PubMed] [Google Scholar]
- Elliott J.F, Rock E.P, Patten P.A, Davis M.M, Chien Y.H. The adult T-cell receptor delta-chain is diverse and distinct from that of fetal thymocytes. Nature. 1988;331:627–631. doi: 10.1038/331627a0. doi:10.1038/331627a0 [DOI] [PubMed] [Google Scholar]
- Englund U, Fricker-Gates R.A, Lundberg C, Bjorklund A, Wictorin K. Transplantation of human neural progenitor cells into the neonatal rat brain: extensive migration and differentiation with long-distance axonal projections. Exp. Neurol. 2002;173:1–21. doi: 10.1006/exnr.2001.7750. doi:10.1006/exnr.2001.7750 [DOI] [PubMed] [Google Scholar]
- Frenette P.S, Subbarao S, Mazo I.B, von Andrian U.H, Wagner D.D. Endothelial selectins and vascular cell adhesion molecule-1 promote hematopoietic progenitor homing to bone marrow. Proc. Natl Acad. Sci. USA. 1998;95:14 423–14 428. doi: 10.1073/pnas.95.24.14423. doi:10.1073/pnas.95.24.14423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. doi:10.1016/S0092-8674(04)00255-7 [DOI] [PubMed] [Google Scholar]
- Galli R, et al. Skeletal myogenic potential of human and mouse neural stem cells. Nat. Neurosci. 2000;3:986–991. doi: 10.1038/79924. doi:10.1038/79924 [DOI] [PubMed] [Google Scholar]
- Geiger H, Van Zant G. The aging of lympho–hematopoietic stem cells. Nat. Immunol. 2002;3:329–333. doi: 10.1038/ni0402-329. doi:10.1038/ni0402-329 [DOI] [PubMed] [Google Scholar]
- Hickey W.F. Basic principles of immunological surveillance of the normal central nervous system. Glia. 2001;36:118–124. doi: 10.1002/glia.1101. doi:10.1002/glia.1101 [DOI] [PubMed] [Google Scholar]
- Hirokawa K, Kubo S, Utsuyama M, Kurashima C, Sado T. Age-related change in the potential of bone marrow cells to repopulate the thymus and splenic T cells in mice. Cell Immunol. 1986;100:443–451. doi: 10.1016/0008-8749(86)90043-2. doi:10.1016/0008-8749(86)90043-2 [DOI] [PubMed] [Google Scholar]
- Hofstetter C.P, Schwarz E.J, Hess D, Widenfalk J, El Manira A, Prockop D.J, Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc. Natl Acad. Sci. USA. 2002;99:2199–2204. doi: 10.1073/pnas.042678299. doi:10.1073/pnas.042678299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horb M.E, Shen C.N, Tosh D, Slack J.M. Experimental conversion of liver to pancreas. Curr. Biol. 2003;13:105–115. doi: 10.1016/s0960-9822(02)01434-3. doi:10.1016/S0960-9822(02)01434-3 [DOI] [PubMed] [Google Scholar]
- Horwitz E.M, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat. Med. 1999;5:309–313. doi: 10.1038/6529. doi:10.1038/6529 [DOI] [PubMed] [Google Scholar]
- Horwitz E.M, et al. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood. 2001;97:1227–1231. doi: 10.1182/blood.v97.5.1227. doi:10.1182/blood.V97.5.1227 [DOI] [PubMed] [Google Scholar]
- Horwitz E.M, Gordon P.L, Koo W.K, Marx J.C, Neel M.D, McNall R.Y, Muul L, Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc. Natl Acad. Sci. USA. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. doi:10.1073/pnas.132252399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imitola J, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1α/CXC chemokine receptor 4 pathway. Proc. Natl Acad. Sci. USA. 2004;101:18 117–18 122. doi: 10.1073/pnas.0408258102. doi:10.1073/pnas.0408258102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. doi:10.1038/nature00870 [DOI] [PubMed] [Google Scholar]
- Kai T, Spradling A. An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proc. Natl Acad. Sci. USA. 2003;100:4633–4638. doi: 10.1073/pnas.0830856100. doi:10.1073/pnas.0830856100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassem M, Kristiansen M, Abdallah B.M. Mesenchymal stem cells: cell biology and potential use in therapy. Basic Clin. Pharmacol. Toxicol. 2004;95:209–214. doi: 10.1111/j.1742-7843.2004.pto950502.x. doi:10.1111/j.1742-7843.2004.pto950502.x [DOI] [PubMed] [Google Scholar]
- Klassen H, Schwartz M.R, Bailey A.H, Young M.J. Surface markers expressed by multipotent human and mouse neural progenitor cells include tetraspanins and non-protein epitopes. Neurosci. Lett. 2001;312:180–182. doi: 10.1016/s0304-3940(01)02215-7. doi:10.1016/S0304-3940(01)02215-7 [DOI] [PubMed] [Google Scholar]
- Kogler G, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J. Exp. Med. 2004;200:123–135. doi: 10.1084/jem.20040440. doi:10.1084/jem.20040440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. doi:10.1126/science.289.5485.1754 [DOI] [PubMed] [Google Scholar]
- Kopen G.C, Prockop D.J, Phinney D.G. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc. Natl Acad. Sci. USA. 1999;96:10 711–10 716. doi: 10.1073/pnas.96.19.10711. doi:10.1073/pnas.96.19.10711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labosky P.A, Barlow D.P, Hogan B.L. Mouse embryonic germ (EG) cell lines: transmission through the germline and differences in the methylation imprint of insulin-like growth factor 2 receptor (Igf2r) gene compared with embryonic stem (ES) cell lines. Development. 1994;120:3197–3204. doi: 10.1242/dev.120.11.3197. [DOI] [PubMed] [Google Scholar]
- Lansdorp P.M, Dragowska W, Mayani H. Ontogeny-related changes in proliferative potential of human hematopoietic cells. J. Exp. Med. 1993;178:787–791. doi: 10.1084/jem.178.3.787. doi:10.1084/jem.178.3.787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laywell E.D, Rakic P, Kukekov V.G, Holland E.C, Steindler D.A. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc. Natl Acad. Sci. USA. 2000;97:13 883–13 888. doi: 10.1073/pnas.250471697. doi:10.1073/pnas.250471697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner A, Yang Y.G, Blacken R.A, Wang L, Nolan A.L, Habener J.F. No evidence for significant transdifferentiation of bone marrow into pancreatic beta-cells in vivo. Diabetes. 2004;53:616–623. doi: 10.2337/diabetes.53.3.616. [DOI] [PubMed] [Google Scholar]
- Lepore A.C, Han S.S, Tyler-Polsz C, Cai J, Rao M.S, Fischer I. Differential fate of multipotent and lineage-restricted neural precursors following transplantation into the adult CNS. Neuron Glia Biol. 2004;1:113–126. doi: 10.1017/s1740925x04000213. doi:10.1017/S1740925X04000213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Liu H, Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat. Med. 2003;9:1293–1299. doi: 10.1038/nm925. doi:10.1038/nm925 [DOI] [PubMed] [Google Scholar]
- Lie D.C, Dziewczapolski G, Willhoite A.R, Kaspar B.K, Shults C.W, Gage F.H. The adult substantia nigra contains progenitor cells with neurogenic potential. J. Neurosci. 2002;22:6639–6649. doi: 10.1523/JNEUROSCI.22-15-06639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton P.J, Dorshkind K. Age-related changes in lymphocyte development and function. Nat. Immunol. 2004;5:133–139. doi: 10.1038/ni1033. doi:10.1038/ni1033 [DOI] [PubMed] [Google Scholar]
- Liu Y, Rao M.S. Transdifferentiation—fact or artifact. J. Cell Biochem. 2003;88:29–40. doi: 10.1002/jcb.10281. doi:10.1002/jcb.10281 [DOI] [PubMed] [Google Scholar]
- Livesey F.J, Cepko C.L. Vertebrate neural cell-fate determination: lessons from the retina. Nat. Rev. Neurosci. 2001;2:109–118. doi: 10.1038/35053522. doi:10.1038/35053522 [DOI] [PubMed] [Google Scholar]
- Lu B, Jan L.Y, Jan Y.N. Asymmetric cell division: lessons from flies and worms. Curr. Opin. Genet. Dev. 1998;8:392–399. doi: 10.1016/s0959-437x(98)80108-1. doi:10.1016/S0959-437X(98)80108-1 [DOI] [PubMed] [Google Scholar]
- Lu B, Jan L, Jan Y.N. Control of cell divisions in the nervous system: symmetry and asymmetry. Annu. Rev. Neurosci. 2000;23:531–556. doi: 10.1146/annurev.neuro.23.1.531. doi:10.1146/annurev.neuro.23.1.531 [DOI] [PubMed] [Google Scholar]
- Luskin M.B. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. doi:10.1016/0896-6273(93)90281-U [DOI] [PubMed] [Google Scholar]
- Magavi S.S, Leavitt B.R, Macklis J.D. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. doi:10.1038/35016083 [DOI] [PubMed] [Google Scholar]
- Magnuson D.S, Zhang Y.P, Cao Q.L, Han Y, Burke D.A, Whittemore S.R. Embryonic brain precursors transplanted into kainate lesioned rat spinal cord. Neuroreport. 2001;12:1015–1019. doi: 10.1097/00001756-200104170-00030. doi:10.1097/00001756-200104170-00030 [DOI] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Chopp M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J. Neurotrauma. 2004;21:33–39. doi: 10.1089/089771504772695922. doi:10.1089/089771504772695922 [DOI] [PubMed] [Google Scholar]
- Majumdar M.K, Keane-Moore M, Buyaner D, Hardy W.B, Moorman M.A, McIntosh K.R, Mosca J.D. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J. Biomed. Sci. 2003;10:228–241. doi: 10.1007/BF02256058. doi:10.1007/BF02256058 [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hartfuss E, Gotz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000;127:5253–5263. doi: 10.1242/dev.127.24.5253. [DOI] [PubMed] [Google Scholar]
- Mammolenti M, Gajavelli S, Tsoulfas P, Levy R. Absence of major histocompatibility complex class I on neural stem cells does not permit natural killer cell killing and prevents recognition by alloreactive cytotoxic T lymphocytes in vitro. Stem Cells. 2004;22:1101–1110. doi: 10.1634/stemcells.22-6-1101. doi:10.1634/stemcells.22-6-1101 [DOI] [PubMed] [Google Scholar]
- McLaren F.H, Svendsen C.N, Van der M.P, Joly E. Analysis of neural stem cells by flow cytometry: cellular differentiation modifies patterns of MHC expression. J. Neuroimmunol. 2001;112:35–46. doi: 10.1016/s0165-5728(00)00410-0. doi:10.1016/S0165-5728(00)00410-0 [DOI] [PubMed] [Google Scholar]
- Medawar P.B. Immunity to homologous grafted skin. III. The fate of skin homografts transplanted to the brain, to subcutansous tissue and to the anterior chamber of the eye. Br. J. Exp. Pathol. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- Mezey E, Chandross K.J, Harta G, Maki R.A, McKercher S.R. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. doi:10.1126/science.290.5497.1779 [DOI] [PubMed] [Google Scholar]
- Modo M, Mellodew K, Rezaie P. In vitro expression of major histocompatibility class I and class II antigens by conditionally immortalized murine neural stem cells. Neurosci. Lett. 2003;337:85–88. doi: 10.1016/s0304-3940(02)01301-0. doi:10.1016/s0304-3940(02)01301-0 [DOI] [PubMed] [Google Scholar]
- Modo M, Rezaie P, Heuschling P, Patel S, Male D.K, Hodges H. Transplantation of neural stem cells in a rat model of stroke: assessment of short-term graft survival and acute host immunological response. Brain Res. 2002;958:70–82. doi: 10.1016/s0006-8993(02)03463-7. doi:10.1016/S0006-8993(02)03463-7 [DOI] [PubMed] [Google Scholar]
- Morrison S.J, Wandycz A.M, Akashi K, Globerson A, Weissman I.L. The aging of hematopoietic stem cells. Nat. Med. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. doi:10.1038/nm0996-1011 [DOI] [PubMed] [Google Scholar]
- Morshead C.M, Benveniste P, Iscove N.N, van der K.D. Hematopoietic competence is a rare property of neural stem cells that may depend on genetic and epigenetic alterations. Nat. Med. 2002;8:268–273. doi: 10.1038/nm0302-268. doi:10.1038/nm0302-268 [DOI] [PubMed] [Google Scholar]
- Mucenski M.L, McLain K, Kier A.B, Swerdlow S.H, Schreiner C.M, Miller T.A, Pietryga D.W, Scott W.J, Jr, Potter S.S. A functional c-myb gene is reqired for normal murine fetal hepatic hematopoiesis. Cell. 1991;65:677–689. doi: 10.1016/0092-8674(91)90099-k. doi:10.1016/0092-8674(91)90099-K [DOI] [PubMed] [Google Scholar]
- Muschler G.F, Midura R.J. Connective tissue progenitors: practical concepts for clinical applications. Clin. Orthop. Relat. Res. 2002;395:66–80. doi: 10.1097/00003086-200202000-00008. doi:10.1097/00003086-200202000-00008 [DOI] [PubMed] [Google Scholar]
- Na N.T, Traver D, Weissman I.L, Akashi K. Myeloerythroid-restricted progenitors are sufficient to confer radioprotection and provide the majority of day 8 CFU-S. J. Clin. Invest. 2002;109:1579–1585. doi: 10.1172/JCI15272. doi:10.1172/JCI200215272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash R.A, et al. Acute graft-versus-host disease: analysis of risk factors after allogeneic marrow transplantation and prophylaxis with cyclosporine and methotrexate. Blood. 1992;80:1838–1845. [PubMed] [Google Scholar]
- Olsson M, Campbell K, Turnbull D.H. Specification of mouse telencephalic and mid-hindbrain progenitors following heterotopic ultrasound-guided embryonic transplantation. Neuron. 1997;19:761–772. doi: 10.1016/s0896-6273(00)80959-9. doi:10.1016/S0896-6273(00)80959-9 [DOI] [PubMed] [Google Scholar]
- Orlic D, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001a;410:701–705. doi: 10.1038/35070587. doi:10.1038/35070587 [DOI] [PubMed] [Google Scholar]
- Orlic D, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc. Natl Acad. Sci. USA. 2001b;98:10 344–10 349. doi: 10.1073/pnas.181177898. doi:10.1073/pnas.181177898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostenfeld T, Caldwell M.A, Prowse K.R, Linskens M.H, Jauniaux E, Svendsen C.N. Human neural precursor cells express low levels of telomerase in vitro and show diminishing cell proliferation with extensive axonal outgrowth following transplantation. Exp. Neurol. 2000;164:215–226. doi: 10.1006/exnr.2000.7427. doi:10.1006/exnr.2000.7427 [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T, Craddock C, Nakamoto B, Priestley G.V, Wolf N.S. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc. Natl Acad. Sci. USA. 1995;92:9647–9651. doi: 10.1073/pnas.92.21.9647. doi:10.1073/pnas.92.21.9647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette R, Dorshkind K. Optimizing hematopoietic recovery following bone marrow transplantation. J. Clin. Invest. 2002;109:1527–1528. doi: 10.1172/JCI15916. doi:10.1172/JCI200215916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peled A, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. doi:10.1126/science.283.5403.845 [DOI] [PubMed] [Google Scholar]
- Pluchino S, et al. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. doi: 10.1038/nature01552. doi:10.1038/nature01552 [DOI] [PubMed] [Google Scholar]
- Pluchino S, et al. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436:266–271. doi: 10.1038/nature03889. doi:10.1038/nature03889 [DOI] [PubMed] [Google Scholar]
- Potten C.S. Stem cells in gastrointestinal epithelium: numbers, characteristics and death. Phil. Trans. R. Soc B. 1998;353:821–830. doi: 10.1098/rstb.1998.0246. doi:10.1098/rstb.1998.0246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesenberry P.J, Colvin G.A, Lambert J.F. The chiaroscuro stem cell: a unified stem cell theory. Blood. 2002;100:4266–4271. doi: 10.1182/blood-2002-04-1246. doi:10.1182/blood-2002-04-1246 [DOI] [PubMed] [Google Scholar]
- Rathjen J, Rathjen P.D. Lineage specific differentiation of mouse ES cells: formation and differentiation of early primitive ectoderm-like (EPL) cells. Methods Enzymol. 2003;365:3–25. doi: 10.1016/s0076-6879(03)65001-9. [DOI] [PubMed] [Google Scholar]
- Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker P.H, Verfaillie C.M. Origin of endothelial progenitors in human postnatal bone marrow. J. Clin. Invest. 2002;109:337–346. doi: 10.1172/JCI14327. doi:10.1172/JCI200214327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein P, Stevens C.E. Placental blood for bone marrow replacement: the New York Blood Center's program and clinical results, Baillieres Best. Pract. Res. Clin. Haematol. 2000;13:565–584. doi: 10.1053/beha.2000.0106. doi:10.1053/beha.2000.0106 [DOI] [PubMed] [Google Scholar]
- Shamblott M.J, Axelman J, Wang S, Bugg E.M, Littlefield J.W, Donovan P.J, Blumenthal P.D, Huggins G.R, Gearhart J.D. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc. Natl Acad. Sci. USA. 1998;95:13 726–13 731. doi: 10.1073/pnas.95.23.13726. doi:10.1073/pnas.95.23.13726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp A, Kukulansky T, Globerson A. In vitro analysis of age-related changes in the developmental potential of bone marrow thymocyte progenitors. Eur. J. Immunol. 1990;20:2541–2546. doi: 10.1002/eji.1830201203. [DOI] [PubMed] [Google Scholar]
- Sheen V.L, Arnold M.W, Wang Y, Macklis J.D. Neural precursor differentiation following transplantation into neocortex is dependent on intrinsic developmental state and receptor competence. Exp. Neurol. 1999;158:47–62. doi: 10.1006/exnr.1999.7104. doi:10.1006/exnr.1999.7104 [DOI] [PubMed] [Google Scholar]
- Shen C.N, Slack J.M, Tosh D. Molecular basis of transdifferentiation of pancreas to liver. Nat. Cell Biol. 2000;2:879–887. doi: 10.1038/35046522. doi:10.1038/35046522 [DOI] [PubMed] [Google Scholar]
- Shih C.C, Weng Y, Mamelak A, LeBon T, Hu M.C, Forman S.J. Identification of a candidate human neurohematopoietic stem-cell population. Blood. 2001;98:2412–2422. doi: 10.1182/blood.v98.8.2412. doi:10.1182/blood.V98.8.2412 [DOI] [PubMed] [Google Scholar]
- Sloan D.J, Wood M.J, Charlton H.M. The immune response to intracerebral neural grafts. Trends Neurosci. 1991;14:341–346. doi: 10.1016/0166-2236(91)90159-r. doi:10.1016/0166-2236(91)90159-R [DOI] [PubMed] [Google Scholar]
- Soria B, Skoudy A, Martin F. From stem cells to beta cells: new strategies in cell therapy of diabetes mellitus. Diabetologia. 2001;44:407–415. doi: 10.1007/s001250051636. doi:10.1007/s001250051636 [DOI] [PubMed] [Google Scholar]
- Spitzer T.R. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27:893–898. doi: 10.1038/sj.bmt.1703015. doi:10.1038/sj.bmt.1703015 [DOI] [PubMed] [Google Scholar]
- Sudo K, Ema H, Morita Y, Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. J. Exp. Med. 2000;192:1273–1280. doi: 10.1084/jem.192.9.1273. doi:10.1084/jem.192.9.1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai F.Y, Keller G, Kuo F.C, Weiss M, Chen J, Rosenblatt M, Alt F.W, Orkin S.H. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371:221–226. doi: 10.1038/371221a0. doi:10.1038/371221a0 [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry W.E, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. doi:10.1126/science.1092436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyan M.L. Age-related decrease in mouse T cell progenitors. J. Immunol. 1977;118:846–851. [PubMed] [Google Scholar]
- Ulaner G.A, Giudice L.C. Developmental regulation of telomerase activity in human fetal tissues during gestation. Mol. Hum. Reprod. 1997;3:769–773. doi: 10.1093/molehr/3.9.769. doi:10.1093/molehr/3.9.769 [DOI] [PubMed] [Google Scholar]
- Vacanti M.P, Roy A, Cortiella J, Bonassar L, Vacanti C.A. Identification and initial characterization of spore-like cells in adult mammals. J. Cell Biochem. 2001;80:455–460. doi:10.1002/1097-4644(20010301)80:3<455::AID-JCB180>3.0.CO;2-Z [PubMed] [Google Scholar]
- Wagers A.J, Sherwood R.I, Christensen J.L, Weissman I.L. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. doi:10.1126/science.1074807 [DOI] [PubMed] [Google Scholar]
- Whetton A.D, Graham G.J. Homing and mobilization in the stem cell niche. Trends Cell Biol. 1999;9:233–238. doi: 10.1016/s0962-8924(99)01559-7. doi:10.1016/S0962-8924(99)01559-7 [DOI] [PubMed] [Google Scholar]
- White P.M, Morrison S.J, Orimoto K, Kubu C.J, Verdi J.M, Anderson D.J. Neural crest stem cells undergo cell-intrinsic developmental changes in sensitivity to instructive differentiation signals. Neuron. 2001;29:57–71. doi: 10.1016/s0896-6273(01)00180-5. doi:10.1016/S0896-6273(01)00180-5 [DOI] [PubMed] [Google Scholar]
- Woodbury D, Schwarz E.J, Prockop D.J, Black I.B. Adult rat and human bone marrow stromal cells differentiate into neurons. J. Neurosci. Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. doi:10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C [DOI] [PubMed] [Google Scholar]
- Wright W.E, Piatyszek M.A, Rainey W.E, Byrd W, Shay J.W. Telomerase activity in human germline and embryonic tissues and cells. Dev. Genet. 1996;18:173–179. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. doi:10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3 [DOI] [PubMed] [Google Scholar]
- Wynn R.F, Hart C.A, Corradi-Perini C, O'Neill L, Evans C.A, Wraith J.E, Fairbairn L.J, Bellantuono I. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643–2645. doi: 10.1182/blood-2004-02-0526. doi:10.1182/blood-2004-02-0526 [DOI] [PubMed] [Google Scholar]
- Yang M, Donaldson A.E, Marshall C.E, Shen J, Iacovitti L. Studies on the differentiation of dopaminergic traits in human neural progenitor cells in vitro and in vivo. Cell Transplant. 2004;13:535–547. doi: 10.3727/000000004783983729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W, Shimamura K, Rubenstein J.L, Hynes M.A, Rosenthal A. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell. 1998;93:755–766. doi: 10.1016/s0092-8674(00)81437-3. doi:10.1016/S0092-8674(00)81437-3 [DOI] [PubMed] [Google Scholar]
- Young H.E, Black A.C., Jr Adult stem cells. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2004;276:75–102. doi: 10.1002/ar.a.10134. doi:10.1002/ar.a.10134 [DOI] [PubMed] [Google Scholar]