Abstract
The assembly of neural circuits in the vertebrate central nervous system depends on the organized generation of specific neuronal subtypes. Studies over recent years have begun to reveal the principles and elucidate some of the detailed mechanisms that underlie these processes. In general, exposure to different types and concentrations of signals directs neural progenitor populations to generate specific subtypes of neurons. These signals function by regulating the expression of intrinsic determinants, notably transcription factors, which specify the fate of cells as they differentiate into neurons. In this review, we illustrate these concepts by focusing on the generation of neurons in ventral regions of the spinal cord, where detailed knowledge of the mechanisms that regulate cell identity has provided insight into the development of a number of neuronal subtypes, including motor neurons. A greater knowledge of the molecular control of neural development is likely to have practical benefits in understanding the causes and consequences of neurological diseases. Moreover, recent studies have demonstrated how an understanding of normal neural development can be applied to direct differentiation of stem cells in vitro to specific neuronal subtypes. This type of rational manipulation of stem cells may represent the first step in the development of treatments based on therapeutic replacement of diseased or damaged nervous tissue.
Keywords: neuronal subtype identity, neural tube patterning, neurogenesis, Hedgehog signalling, transcription factors
1. Introduction
One of the fundamental goals in developmental neurosciences is to understand the mechanisms that control the generation of distinct classes of neurons in the vertebrate central nervous system (CNS). Within the developing CNS, hundreds of distinct subtypes of neurons are generated in spatially and temporally coordinated programmes, and this organized emergence of neuronal diversity is the foundation for the assembly of functional neuronal circuits. To understand the mechanisms that underlie this process, a greater knowledge of the molecular control of the acquisition of neuronal identity is required. This knowledge is not only important for understanding normal CNS development, but also key to uncovering the mechanisms of neurological diseases. Moreover, a greater understanding of the regulation of neuronal subtype identity may contribute to the development of effective treatments for the repair of damaged nervous systems, as the use of progenitor cell populations and/or stem cells in the treatment of neurodegenerative diseases is explored.
Considerable progress has been made in identifying the signals and elucidating the molecular mechanisms that regulate neural cell fate (for reviews see Edlund & Jessell 1999; Jessell 2000; Shirasaki & Pfaff 2002). In broad terms, initially uncommitted progenitor populations at distinct positions along the rostrocaudal and dorsoventral axes acquire their fate by exposure to different types and concentrations of signals. These signals regulate sets of intrinsic, cell-autonomous determinants, which specify cell fate by controlling the transcriptional programme of the cell. The vertebrate spinal cord represents a well-characterized region of the CNS, where our understanding of the mechanisms that regulate neuronal subtype identity are most advanced. Distinct neuronal subtypes that reside in characteristic, stereotypic positions perform the main functions of the spinal cord—controlling motor output and transmitting sensory information. The neurons that process and relay sensory input are found, predominantly, in the dorsal half of the spinal cord, whereas the neurons that participate in motor output, including motor neurons (MNs), are located ventrally (see Ramón y Cajal 1906). This positioning reflects, to a large extent, the developmental origin of each individual neuronal subtype. In this review, we will focus on neuronal development in ventral regions of the spinal cord and discuss recent work that has shed light on the molecular mechanisms controlling the generation of these neuronal subtypes.
2. Shh controls the pattern of generation of neuronal subtypes in the ventral spinal cord
The identification and use of molecular markers, notably transcription factors, has revealed the presence of at least five distinct neuronal subtypes in the ventral neural tube (Ericson et al. 1997a; Pierani et al. 1999). Each of these neuronal subtypes expresses a unique combination of transcription factors and comprises MNs and four groups of interneurons (V0–V3). Several lines of evidence indicate that these classes of neurons, although originally defined in developmental studies, represent separate functional groups of neurons with conserved and distinct roles in motor circuit activity. Moreover, the combination of transcription factors expressed by these differentiating neurons not only serves as useful markers for the different cell types, but also appears to be functionally relevant because at least some of the properties of the developing neurons are determined by these transcription factors (reviewed by Shirasaki & Pfaff 2002; Goulding & Pfaff 2005).
The five neuronal subtypes of the ventral neural tube emerge in precise spatial order from proliferating progenitor cells arrayed along the dorsoventral axis of the spinal cord (figure 1; Ericson et al. 1997b; Briscoe et al. 2000). This partition of the dorsoventral axis into distinct domains of progenitors that generate different neuronal classes takes place early during the neural development and depends on signals that derive from axial midline cells of the notochord (a mesodermal rod of cells underlying the ventral neural tube) and floor plate (the specialized group of cells that occupies the ventral midline of the spinal cord; figure 2; Placzek et al. 1991; Yamada et al. 1991). Observations and embryological manipulations of chick and amphibians first indicated the role for these structures in establishing the dorsoventral polarity of the spinal cord (e.g. Watterson et al. 1955; van Straaten et al. 1985), and subsequent studies confirmed and extended these ideas, raising the possibility that a single signal controlled neuronal subtype identity in the ventral neural tube.
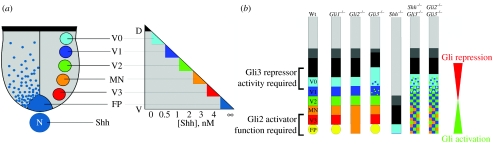
Figure 1.
The generation of neuronal subtypes in the ventral spinal cord by a gradient of Shh/Gli signalling. Distinct neuronal subtypes are generated along the dorsoventral axis in the ventral neural tube in response to graded Shh signalling. (a) Five distinct ventral neuronal subtypes arise from an equivalent number of progenitor domains in the ventricular zone of the ventral spinal cord. Progressively, more dorsal progenitor domains are exposed to a decreasing concentration of Shh protein and, in vitro, the concentration of Shh determines the neuronal subtype generated. (b) Dorsoventral spinal cord patterning defects in Gli mutant embryos can be explained by a gradient model of Gli activator and repressor activity. The diagrams illustrate the effects of Shh and Gli gene loss-of-function mutations on the patterning of the ventral and intermediate regions of the spinal cord. Gli1−/− mutants have no defect in ventral patterning. In Gli2−/− embryos, the most ventral cell types, the FP and V3 neurons, are absent and there is a concomitant expansion of the MN domain. By contrast, in Gli3−/− mutants, patterning of the intermediate region of the neural tube is disrupted. Embryos lacking both Shh and Gli3 functions demonstrate a substantial restoration in normal dorsoventral patterning, compared to Shh null mutants alone and a similar situation is observed in Gli2−/− and Gli3−/− mutants.
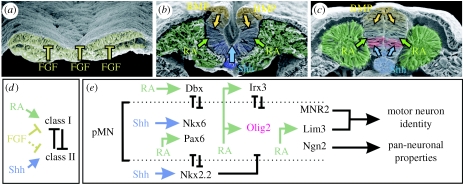
Figure 2.
Signalling pathways leading to motor neuron generation. Scanning electron micrographs of the developing chick neural tube at (a) neural plate, (b) neural folds and (c) neural tube stages (images generously provided by Dr Kathryn Tosney, University of Michigan). Images display sources of FGF (yellow), retinoic acid (RA, green), Shh (blue) and BMPs (orange) surrounding the neural tissue and influencing progenitor gene expression. (d) Influence of FGF, RA and Shh on class I and II protein expressions. FGF signalling prevents the emergence of dorsal–ventral pattern by blocking both class I and II protein expressions. RA signalling induces the expression of class I proteins, whereas Shh signalling appears to induce class II proteins. Once expressed, reciprocal pairs of class I and II proteins exhibit cross-repressive interactions to partition the ventral neural tube into discrete progenitor domains. (e) Shh and RA initiate the expression of the HD proteins Nkx6.1, Nkx6.2 (Nkx6) and Pax6 in ventral spinal cord progenitors. The repressor activities of Nkx6 and Pax6 prevent the expression of inhibitors of MN formation, such as Dbx1, Dbx2 (Dbx) and Nkx2.2, and permit ligand-bound retinoid receptors to activate Olig2 expression (red). Within MN progenitors, Olig2 directs MN differentiation by repressing Irx3 and other unidentified target genes that regulate the expression of the MN-specific transcription factors, MNR2, Lim3, Isl1, Isl2 and Hb9, and the pan-neuronal transcription factors, Ngn2 and NeuroM/Math3. This repressor activity of Olig2 works in conjunction with the activator function of ligand-bound retinoid receptors.
The search for the molecular identity of the signal responsible for patterning the ventral neural tube culminated with the discovery of the secreted protein Sonic hedgehog (Shh), the protein encoded by the vertebrate orthologue of the Drosophila segment polarity gene Hedgehog (Echelard et al. 1993; Krauss et al. 1993; Riddle et al. 1993; Chang et al. 1994; Roelink et al. 1994). Shh is produced by the notochord and floor plate at the times when these two groups of cells exhibit their influence over neural tube patterning. The importance of Shh has been confirmed by the loss of ventral neurons in embryos, in which Shh signalling had been blocked either biochemically or genetically (Chiang et al. 1996; Ericson et al. 1996). In addition, Shh signalling appears to be sufficient to specify the pattern of ventral neurogenesis (Marti et al. 1995; Roelink et al. 1995; Ericson et al. 1996, 1997b). In vitro experiments indicate that incremental two- to threefold changes in Shh concentration promote the generation of the distinct neuronal subtypes, such that the concentration of Shh necessary to induce each neuronal class in vitro corresponds to its distance from the source of Shh in vivo. Thus, neurons generated in progressively more ventral regions of the neural tube require correspondingly higher Shh concentrations for their induction. From these studies, the idea has emerged that Shh functions in a graded manner to pattern the ventral neural tube, directing the position of generation and subtype identity of the neurons at defined concentration thresholds.
In Drosophila, some of the long-range wing patterning activity of Hedgehog appears to be exerted indirectly by the induction of secondary signal(s) (Zecca et al. 1995). This result raised the possibility that the patterning activity of Shh in the neural tube could be indirect, being mediated by intermediary signals. Thus, Shh signalling would act only at short range to initiate a relay signal that would act to pattern the neural tube. A variety of evidence, however, argues against this model and supports the idea that Shh signalling acts directly at long range to organize the pattern of neurogenesis in the ventral neural tube. Shh protein and the activity of Shh are detectable in the regions of the neural tube that are several cell diameters from its sources (Marigo & Tabin 1996; Gritli-Linde et al. 2001), and ectopic activation of Shh signalling cell autonomously induces ventral neuronal fates (Hynes et al. 2000). Conversely, the cell-autonomous blockade of Shh signalling, by either removal of an essential component of the Shh signalling pathway (Wijgerde et al. 2002) or ectopic expression of a dominant inhibitor of the signalling pathway (Briscoe et al. 2001), blocks the generation of ventral neurons, which instead develop with characteristics of neurons located in the dorsal neural tube.
Together, these studies support the view that the generation of the five ‘cardinal’ neuronal subtypes by graded Shh signalling represents a basic organizational structure of the ventral neural tube (figure 1). It is clear, however, that further subdivisions of these five neuronal classes will emerge as more molecular markers are characterized and additional assays are employed to study their function. In part, this is likely to reflect the diversification of individual neuronal fates following their initial generation—this is already apparent for MNs where mechanisms that subdivide somatic MNs into pools of neurons that innervate different muscles are under intensive study (Liu et al. 2001; Dasen et al. 2003). In addition, mechanisms that increase the initial diversity of neuronal subtypes are also likely to operate. For example, the expression of additional transcription factors divides the V2 neuron population into two spatially intermixed groups of neurons that appear to be generated in the same region of the spinal cord (Karunaratne et al. 2002; Smith et al. 2002). Nevertheless, examining the generation of MNs and V0–V3 neurons serves as a powerful experimental paradigm for exploring the mechanisms that control the spatial allocation of neuronal subtype identity.
3. Specification of progenitor cell identity by a transcription factor code
Graded Shh signalling controls neuronal subtype identity by influencing the behaviour of ventral progenitor cells. Progenitor cells respond to graded Shh signalling by regulating the expression of a series of transcription factors, which, with the exception of the basic helix-loop-helix (bHLH) protein Olig2, are members of the homeodomain protein family (figure 2; Briscoe et al. 2000; Briscoe & Ericson 2001; Novitch et al. 2001). Studies of both mouse and chick spinal cord suggest that the progenitor transcription factors act as intermediaries in the interpretation of graded Shh signalling (Ericson et al. 1997b; Briscoe et al. 1999; Briscoe et al. 2000; Novitch et al. 2001; Vallstedt et al. 2001; Gotz et al. 2005). On the basis of their mode of regulation by Shh signalling, progenitor transcription factors can be subdivided into two groups, termed class I and II proteins (figure 2). The expression of each class I protein is repressed at distinct thresholds of Shh activity; consequently, their ventral limits of expression are determined by Shh signalling. Conversely, neural expression of the class II proteins depends on Shh signalling, so their dorsal expression boundaries are defined by graded Shh signalling.
The response to Shh signalling is not, alone, sufficient to explain the domains of gene expression of the class I and II proteins. Strikingly, the ventral limit of most class I proteins corresponds to the dorsal limit of expression of a class II protein. Gain- and loss-of-function studies indicate that selective cross-repressive interactions between complementary pairs of class I and II proteins expressed in adjacent, abutting domains are important for establishing the boundaries of gene expression (figure 2; Ericson et al. 1997b; Briscoe et al. 1999, 2000; Novitch et al. 2001; Vallstedt et al. 2001; Gotz et al. 2005). These cross-repressive interactions ensure that each progenitor expresses a distinct combination of class I and II proteins. Moreover, they may also serve to consolidate progenitor domain identity by relieving a requirement for a prolonged period of Shh signalling to maintain gene expression domains. These reciprocal interactions also provide a mechanism to convert a gradient of Shh signalling into discrete all or none changes in gene expression. The principle of this mechanism resembles those observed in other developing tissues (Jäckle et al. 1986; Kraut & Levine 1991), perhaps suggesting a general strategy for the regional allocation of cell fate in response to graded inductive signals.
At the time neuronal differentiation is initiated, the combinatorial expression of the progenitor transcription factors divides the ventral spinal cord into five progenitor domains, each of which generates one of the five distinct neuronal subtypes. This subdivision is a primary requirement for the generation of distinct neurons. The five progenitor domains correspond to the position of generation of the five ventral neuronal subtypes, and the profile of the progenitor transcription factor expression appears to specify the identity of neurons generated from each progenitor domain. Consistent with this, the forced expression of a class I or II protein in the neural tube changes the fate and position of generation of individual neuronal subtypes in a manner predicted by the normal profile of homeodomain protein expression (Briscoe et al. 2000; Novitch et al. 2001). Conversely, the targeted inactivation of individual class I or II proteins in mice results in predictable switches of neuronal fate (Ericson et al. 1997b; Briscoe et al. 1999, 2000; Novitch et al. 2001; Vallstedt et al. 2001; Zhou & Anderson 2002; Gotz et al. 2005).
Gain-of-function studies revealed that the majority of the class I and II proteins acts as transcriptional repressors and directly interacts with the Groucho family of corepressors (Muhr et al. 2001). The exception to this rule being Pax6 that appears to repress Nkx2.2 indirectly, presumably via the induction of a yet to be identified transcriptional repressor. Together, these studies provided insight into the molecular basis of the control of neuronal subtype identity in the ventral neural tube and emphasize the importance of transcription repression, suggesting that the specific identity of each progenitor domain arises by suppressing all other possible fates (Muhr et al. 2001).
4. Gli proteins mediate graded Shh signalling
The pivotal role of graded Shh signalling in the ventral neural tube has focused attention on how Shh controls concentration-dependent differential gene expression of class I and II genes. The molecular mechanisms of Hedgehog (Hh) signalling are best understood in Drosophila, where the zinc-finger-containing transcription factor Cubitus interruptus (Ci) is critical to Hh-mediated control of gene expression (reviewed by Ingham & McMahon 2001; Lum & Beachy 2004). In the absence of Hh signalling, Ci is proteolytically processed into a truncated repressor form that inhibits Hh target genes. Hh signalling converts Ci into a transcriptional activator by, at least in part, inhibiting processing of Ci.
In vertebrates, all three Ci homologues, Gli1, Gli2 and Gli3, are expressed in the neural tube (Hui et al. 1994; Lee et al. 1997; Sasaki et al. 1997; Ruiz i Altaba 1998). However, Gli1, which appears to act solely as a transcriptional activator, is dispensable for normal mouse development and the generation of neurons in the spinal cord of Gli1 mutant mice proceeds without disturbance (figure 1; Park et al. 2000; Bai & Joyner 2001). In contrast, the analysis of mutant mice has identified specific functions for both Gli2 and Gli3 in the neural tube. Both Gli2 and Gli3 have C-terminal transcriptional activator and N-terminal repressor regions (Dai et al. 1999; Ruiz i Altaba 1999; Sasaki et al. 1999; Aza-Blanc et al. 2000), and Hh signalling appears to regulate the activity of these two proteins, enhancing the activator function of Gli2 and inhibiting repressor activity of Gli3 (Ruiz i Altaba 1999; Aza-Blanc et al. 2000; Wang et al. 2000; Bai et al. 2004; Lei et al. 2004). In the spinal cord of mice lacking Gli2, the development of the most ventral cell types is disrupted, i.e. the generation of the floor plate and adjacent V3 neurons are severely affected and there is a concomitant ventral expansion of MNs (figure 1; Matise et al. 1998). Other ventral neurons are, however, generated at their appropriate dorsoventral positions. The neural tube defects characteristic of Gli2 mutant embryos can be rescued by replacing Gli2 with a Gli1 cDNA, suggesting that the inductive effects of Gli2 are mediated through its function as a transcriptional activator (Bai & Joyner 2001). In embryos lacking Gli3, although patterning is normal in ventral regions, there is a dorsal expansion of cell types typical of the intermediate neural tube that results in progenitor cells responding as though exposed to a higher concentration of Shh (figure 1; Persson et al. 2002). These defects are rescued by a truncated allele of Gli3 that encodes a transcriptional repressor, suggesting that only repressor activity of Gli3 is normally required in the spinal cord (Persson et al. 2002). Activator function can, however, be revealed in compound Gli mutants (Bai et al. 2004; Lei et al. 2004).
The role of Gli3 as a repressor is further emphasized by the analysis of embryos that lack both Gli3 and Hh signalling. Mutation of either Shh or Smo (an essential transmembrane component of the Shh signalling pathway) results in a neural tube lacking ventral cell types (Chiang et al. 1996; Zhang et al. 2001; Wijgerde et al. 2002). In contrast, when Gli3 is removed in addition to Shh or Smo, there is a recovery of MN, V2, V1 and V0 neuron generation (figure 1; Litingtung & Chiang 2000; Wijgerde et al. 2002). This suggests that, in the absence of Shh signalling, Gli3 normally represses ventral neuronal fates. Therefore, in embryos lacking Gli3, Shh signalling is no longer required to remove the repressive activity of Gli3. Thus, an important role for Shh signalling in the ventral neural tube is the inhibition of Gli3 repressor activity. Although several ventral cell types are recovered in double mutants lacking Gli3 and Shh signalling, V3 neurons and floor plate cells are not rescued, indicating that active Hh signalling is required for the induction of these cell types. These two cell types are also lost in Gli2 mutants (Matise et al. 1998), indicating that the generation of floor plate cells and V3 neurons depends on the transcriptional activator function of Gli2 induced by Shh signalling.
The generation of MNs, V2, V1 and V0 neurons independent of Shh signalling and Gli activity has been confirmed by the analysis of double mutant mice, lacking both Gli2 and Gli3 (figure 1; Bai et al. 2004; Lei et al. 2004), and in single mutant mice that are unable to generate both Gli repressor and Gli activator function (Huangfu et al. 2003; Liu et al. 2005). In each case, these mutant embryos display a neural tube phenotype similar to Shh/Gli3 double mutants. These findings indicate that the Gli proteins mediate all patterning activity attributable to Shh signalling. Moreover, these data indicate that Gli3 represses ventral neural fates and Shh signalling acts by relieving this repression and promoting the transcriptional activity of Gli2.
Despite the generation of many ventral neuronal subtypes in the absence of Shh/Gli activity, the organization of these cells is severely disrupted (Litingtung & Chiang 2000; Persson et al. 2002; Wijgerde et al. 2002). In place of the spatially segregated domains of different neuronal fates, delimited by sharp boundaries in gene expression, the generation of V2, V1 and MNs in Shh/Gli3 double mutant embryos is intermingled over a wide area of the ventral spinal cord. Some intermixing between adjacent domains in the intermediate neural tube is also observed in single mutant embryos that lack Gli3 (Persson et al. 2002). In both Gli3 mutants and Shh/Gli3 double mutants, the intermingling of cell types is not a consequence of progenitors or neurons acquiring mixed identities, because individual cells retain discrete, appropriate gene expression profiles. Instead, the intermingling of cell types appears to reflect a loss of positional information within the ventral neural tube. Thus, cells acquire identities with little or no respect for their position along the dorsoventral axis. This suggests that the primary role of Shh/Gli signalling is the spatial regulation, but not the induction, of progenitor domain transcription factors and this spatial regulation organizes progenitors into distinct dorsoventral domains. In part, this may reflect a role of Shh/Gli activity in controlling the cell affinity of progenitors along the dorsoventral axis (Wijgerde et al. 2002; Lei et al. 2004). However, the loss of all ventral identities in embryos lacking Shh or Smo indicates that Gli proteins can directly influence the expression of progenitor genes.
How might Gli activity impart positional information to the neural tube? An attractive model that would account for the data is that Shh establishes a gradient of Gli activity by inhibiting Gli repressor activity and potentiating Gli activator function (figure 1). In the absence of Shh signalling, repressor activity dominates and suppresses all ventral fates. Shh signalling relieves Gli3 repression and, at the same time, promotes Gli2 activation. The distance from the source of Shh determines the balance of repression to activation, i.e. the closer a cell is to the source of Shh the less repression and higher the level of activation. This balance of Gli activity provides positional identity to a cell and influences the progenitor transcription factors it expresses. Consistent with this idea, providing different levels of Gli activity to neural cells is sufficient to recapitulate the activity of different concentrations of Shh (Stamataki et al. 2005), suggesting that the level of Gli activity harboured by a cell represents the intracellular correlate of extracellular Shh concentration. Thus, in this view, a gradient of Shh protein in the developing neural tube is transduced to a gradient of Gli activity, which, in turn, regulates the patterning of ventral progenitors. In the absence of Gli activity, for example in Gli2/Gli3 double mutant embryos, cells lack the positional information normally provided by the ratio of Gli activator and repressor. However, these cells remain able to express ventral progenitor transcription factors and, perhaps under the influence of other signals, initiate progenitor transcription factor expression. The network of cross-repressive interactions, described in figure 3, ensures that, in these circumstances, individual cells express a combination of genes appropriate for a discrete cell type. The lack of positional information, normally imposed by the balance of Gli activity, means that spatial organization is lost and, instead of the ordered generation of cell types, significant intermixing of neuronal production occurs.
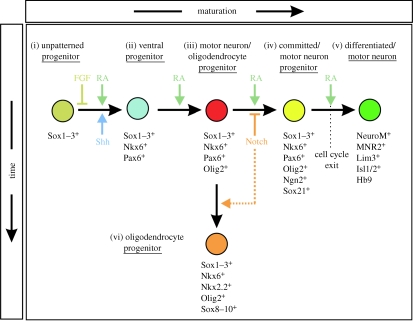
Figure 3.
Progressive changes in progenitor cells during motor neuron development. (i) Neural progenitors expressing Sox1–3 are held in an undifferentiated and unpatterned state through the actions of FGFs. (ii) The combined actions of Shh and RA signalling ventralizes neural progenitors through the induction of progenitor transcription factors, such as Nkx6. (iii) Continued exposure to Shh and RA leads to the induction of Olig2 in bipotential motor neuron/oligodendrocyte progenitors. (iv) In the presence of RA and absence of Notch signalling, Olig2+ cells express Ngn2 and Sox21 and begin to exit the cell cycle and differentiate into motor neurons. (v) The further differentiation of motor neurons requires the ongoing RA signalling, as well as the downregulation of Olig2. (vi) Activation of the Notch signalling pathway prevents the expression of Ngn2 in Olig2+ motor neuron/oligodendrocyte progenitors and maintains these cells in an undifferentiated state until additional temporal cues direct the formation of oligodendrocytes from these cells. Notch signalling may also positively promote oligodendrocyte development (dashed line). See text for more details.
5. Contributions of other signals to dorsoventral patterning
Though the importance of Shh signalling in ensuring the appropriate spatial pattern within the neural tube is evident, it is clear that as the neural tube forms, it encounters many other signals that provide cells with their early neural character and additionally contribute to dorsoventral patterning (figure 2). From the time of their formation, neural progenitors are surrounded with fibroblast growth factor (FGF) signals, emanating from the regressing node, adjacent presomitic mesoderm and within the neural plate itself (figure 2; reviewed by Diez del Corral & Storey 2004; Wilson & Maden 2005). FGF signalling appears to have two functions in neural progenitors. First, it can act as a general inhibitor of neural differentiation, maintaining cells in a primitive, stem cell-like state (Mathis et al. 2001; Diez del Corral et al. 2002; Diez del Corral et al. 2003). Second, it suppresses the expression of the class I and II proteins, and thus prevents the emergence of dorsoventral pattern (figure 2; Bertrand et al. 2000; Diez del Corral et al. 2003; Novitch et al. 2003). An important step in the maturation of neural progenitors is therefore evasion from FGF signals. Working in opposition to FGFs is the action of retinoic acid (RA) produced by the condensing paraxial mesoderm rostral to the node and adjacent to the developing neural tube (figure 2; reviewed by Wilson & Maden 2005). Retinoid signalling attenuates the proliferative effects of FGFs and is further required for the expression of neural progenitor transcription factors and neuronal differentiation (figures 2 and 3; Diez del Corral et al. 2003; Novitch et al. 2003; Wilson et al. 2004). In addition to these effects, retinoid signalling plays a major role in dorsoventral patterning through its ability to induce the expression of class I proteins and thereby offset the ventralizing effects of Shh (figure 2; Diez del Corral et al. 2003; Novitch et al. 2003; Wilson et al. 2004).
In addition, signals from the dorsal pole of the neural tube also influence patterning in ventral regions. Bone morphogenetic protein (BMP) family members, which have a prominent role in the specification of neuronal fate in the dorsal neural tube (Liem et al. 1997), appear to additionally inhibit the specification of ventral neural tube cell types. Zebrafish embryos mutant for BMP family members have expanded domains of ventral neuronal subtypes (Barth et al. 1999; Nguyen et al. 2000). Consistent with this, BMPs alter the in vitro response of neural progenitor cells to a fixed concentration of Shh, resulting in a dorsal shift in the identity of the neural progenitors and neuronal subtypes generated (Liem et al. 2000). Conversely, BMP antagonists sensitize neural cells to Shh signalling (Liem et al. 2000; Patten & Placzek 2002). These data suggest that the Shh and BMP signalling pathways intersect and that BMPs act to limit the ventralizing activity of Shh. In addition to BMPs, Wnt proteins emanating from the dorsal midline have also been found to affect neural progenitor behaviour and neuronal identity (Muroyama et al. 2002). Wnt signals appear to promote progenitor proliferation throughout the neural tube (Megason & McMahon 2002; Chesnutt et al. 2004), and have also been suggested to inhibit Shh signalling and, consequently, the generation of ventral cell types (Robertson et al. 2004).
Thus, the activities of BMP, Wnt, FGF and RA together with Shh appear to choreograph the patterning of the ventral neural tube (figure 2; Liem et al. 2000; Diez del Corral et al. 2003; Novitch et al. 2003). During the early stages of neural differentiation, neural progenitors are exposed to high levels of FGF, and the levels of retinoid synthesis, BMP and Shh expression are low; consequently, the expression of class I and II proteins is absent. As the node regresses, the exposure of neural cells to FGF decreases and this coincides with the onset of retinoic acid synthesis in paraxial mesoderm and of Shh signalling from the notochord and floor plate. This switch is accompanied by the initiation of class I and II homeodomain (HD) protein expression. The presence of activated RA receptors presumably promotes the expression of class I and II proteins, whereas the positional information provided by the balance of BMP signalling and graded Shh signalling organizes the dorsoventral pattern of gene expression.
6. From neural induction to neurogenesis
Patterning of neural progenitors and the control of neuronal subtype identity must be coordinated with the processes that induce neural tissue and determine the timing of neuronal differentiation. The induction of neural tissue appears to require FGF signalling, together with the antagonism of BMP signalling and abrogation of Wnt signalling (Streit et al. 2000; Wilson et al. 2000; Wilson et al. 2001). Cell-intrinsic effectors of neural induction include Sox1–3, high mobility group box transcription factors of the SoxB1 family (reviewed by Pevny & Placzek 2005). Members of this family are expressed in ectodermal cells prior to neural induction, and their expression continues, or is upregulated, as cells become committed to a neural fate (Pevny & Placzek 2005). Moreover, studies in the chick embryo have identified regulatory elements within the Sox2 gene that respond to neural-inducing signals (Uchikawa et al. 2003), raising the possibility that the activation of SoxB1 gene expression is a primary response to these neuralizing signals.
Beyond their utility as markers of early neural tissue, the transcriptional activator function of SoxB1 proteins also appears to be involved in neural fate determination and the maintenance of the neural progenitor state. The forced expression of SoxB genes promotes the neural differentiation of multipotent embryonic stem cells and P19 embryonal carcinoma cells (Pevny et al. 1998). Conversely, in Xenopus, inhibition of SoxB signalling blocks neural differentiation (Kishi et al. 2000). Within the neural tube, SoxB1 proteins are expressed in the neural progenitors and expression is downregulated, as progenitors exit the cell cycle and become post-mitotic neurons (figure 3; Bylund et al. 2003; Graham et al. 2003). This progenitor expression of SoxB1 proteins maintains the undifferentiated state of progenitors. Ectopic expression of Sox2, Sox3 or dominant active versions of SoxB1 proteins in chick neural progenitors block neuronal differentiation, while the forced expression of dominant negative versions of SoxB1 proteins results in the precocious exit of progenitors from the cell cycle and the upregulation of post-mitotic markers in these cells (Bylund et al. 2003; Graham et al. 2003). Moreover, Sox21, a SoxB1 family member related to Sox1–3, but containing transcriptional repressor activity, counteracts Sox1–3 (Sandberg et al. 2005). Thus, the balance of activator functions of Sox1–3 and repressor functions of Sox21 appears to influence whether neural cells remain as progenitors or commit to differentiation (figure 4).
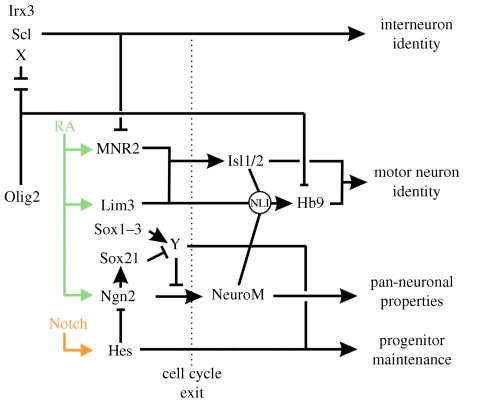
Figure 4.
Olig2 and the transcription factor network controlling motor neuron differentiation. Olig2 promotes motor neuron development through its ability to repress the expression of antagonists of motor neuron formation, such as Irx3, Scl and other unidentified target genes (X). The actions of ligand-bound retinoic acid receptors lead to the expression of transcription factors that act downstream of Olig2, including MNR2, Lim3 and Ngn2. The expression of Ngn2 is negatively regulated by Hes proteins that act downstream of the Notch signalling pathway. The balance of SoxB activator and repressor functions within motor neuron progenitors further regulates the activity of Ngn2 (Bylund et al. 2003; Sandberg et al. 2005). The activator properties of Sox1–3 appear to induce the expression of unknown inhibitors of Ngn2 function (Y). Sox21 acts downstream of Ngn2 and opposes the actions of Sox1–3 by reducing the expression of Y, thereby elevating Ngn2 activity and facilitating motor neuron differentiation. Through their ability to block Ngn2 expression and activity, Notch signalling, Hes genes and Sox1–3 maintain progenitors in an undifferentiated state. The release of Ngn2 from these regulatory constraints leads to the upregulation of NeuroM and the exit of progenitors from the cell cycle. NeuroM then forms a transcriptional activator complex with Lim3 and Isl1/2, mediated by NLI, that displaces an Olig2 repressor complex from the Hb9 promoter and activates Hb9 expression to drive motor neuron-specific gene expression (Lee et al. 2005).
A critical step in the progression of progenitors to differentiated neurons is the initiation of proneural bHLH gene expression (figures 3 and 4). These proneural genes play an essential role in the decision of progenitors to exit the cell cycle and in specifying the pan-neuronal characteristics of neurons, such as their formation of axons and dendrites. Among the best-studied members of this family of genes are Mash1, Neurogenin1 (Ngn1) and Neurogenin2 (Ngn2), whose expression is localized in discrete domains along the dorsoventral axis of the spinal cord and other regions of the CNS (Sommer et al. 1996; Ma et al. 1997). Both gain- and loss-of-function experiments have demonstrated that these bHLH proteins have common functions in promoting cell cycle exit and inducing general neuronal character (reviewed by Bertrand et al. 2002); moreover, recent data have provided evidence that Ngn2 is sufficient to induce Sox21 (Sandberg et al. 2005), establishing a possible link between the neuronal differentiation-inducing properties of these two classes of transcription factors.
The domain-restricted expression patterns of the bHLH proteins imply that they may be controlled by the signals that pattern the neural tube and that they may play a role in specifying the cell types which emerge from each progenitor domain. Evidence to support this has come from elegant genetic experiments analysing the role of Mash1 and Ngn2 in the ventral neural tube. Mash1 is expressed by progenitors that give rise to V2 interneurons and is required for their generation (Parras et al. 2002). In contrast, Ngn2 is more broadly expressed in progenitors of MNs, V1 and V3 neurons and loss of Ngn2 results in impaired generation of these cell types (Scardigli et al. 2001; Parras et al. 2002). The absence of V2 neurons in Mash1 mutant mice reflects a specific requirement for Mash1 in the formation of these cells, beyond its role in promoting neuronal differentiation because insertion of Ngn2 coding sequences into the Mash1 locus restores the overall generation of neurons, but not V2 characteristics (Parras et al. 2002). Conversely, the reciprocal replacement of Ngn2 with Mash1 similarly restores the deficit in the overall production of neurons by MN, V1 and V3 progenitors, but leads to the ectopic production of V2 neurons intermingled with MNs (Parras et al. 2002). These results indicate that while proneural bHLH proteins have a common function in promoting general aspects of neuronal differentiation, they also play a role in specifying specific cell fates. Some complications to this model are the observations that proneural bHLH proteins are typically expressed by multiple progenitor populations and that they contribute to the production of several different types of neurons (Scardigli et al. 2001b; Simmons et al. 2001; Helms et al. 2005). These observations indicate that, while proneural bHLH proteins may harbour fate-specifying functions, their activities must be coordinated with additional determinants that operate within a given progenitor domain.
7. Coordination of cell fate and neurogenic differentiation
Some insights into the mechanisms that link together cell-fate specification and pan-neuronal differentiation have emerged through the identification of the bHLH protein, Olig2, which appears to coordinate multiple steps in the formation of MNs: progenitor patterning; MN fate determination; and neurogenic differentiation (Mizuguchi et al. 2001; Novitch et al. 2001). Olig2 expression begins in the newly formed neural tube in response to both Shh and RA signals, and acts downstream of the class II proteins Nkx6.1 and Nkx6.2 (Lu et al. 2000; Novitch et al. 2001b; Novitch et al. 2003; Cai et al. 2005; Vallstedt et al. 2005). After its induction, Olig2 participates in the dorsoventral pattering of progenitors through its ability to repress the class I protein Irx3 from the ventral neural tube (Mizuguchi et al. 2001; Novitch et al. 2001). Subsequently, Olig2 serves as a unique marker of MN progenitors, and its transcriptional repressor function is both necessary and sufficient to stimulate the expression of a number of downstream homeodomain transcription factors that provide MNs with their unique character (Mizuguchi et al. 2001; Novitch et al. 2001b; Lu et al. 2002; Zhou & Anderson 2002; Takebayashi et al. 2002a). In addition, Olig2 elevates the expression of Ngn2 and the downstream proneural bHLH protein NeuroM within MN progenitors, causing these cells to exit the cell cycle and differentiate (Novitch et al. 2001b; Zhou & Anderson 2002).
It is notable that MN progenitors are one of the first groups of progenitors in the spinal cord to differentiate (Altman & Bayer 1984), and their proliferative capacity appears to be very limited (B. G. Novitch 2005, unpublished data). It is probable that these features are a direct consequence of high levels of proneural bHLH gene expression within these cells, and suggest that an additional function of Olig2 may be to specify the timing at which MN progenitors differentiate. Through these actions, Olig2 appears to consolidate diverse aspects of pattern, cell identity, cell cycle control and neurogenesis into a coherent programme of differentiation (Mizuguchi et al. 2001; Novitch et al. 2001b). Since the coordination of these processes is equally important for the formation of other cell types in the spinal cord, an open question is how aspects of cell identity and neurogenesis are jointly regulated in other progenitor domains, and whether proteins related to Olig2 may be involved. An examination of the Olig2-related bHLH protein Olig3 in the dorsal spinal cord shows that Olig3 plays an essential role in the specification of different classes of dorsal interneurons and is additionally required for the patterned expression of proneural bHLH proteins within dorsal progenitors (Muller et al. 2005). Intriguingly, Olig3 appears to be expressed by several groups of cells in the ventral spinal cord (Takebayashi et al. 2002b), raising the possibility that the function of the Olig family of bHLH proteins in coordinating cell-fate choices and neurogenesis may be widespread.
Studies of the role of Olig2 and bHLH protein function in MN differentiation have led to insights into the mechanisms by which the temporal progression from dividing progenitors to differentiated MNs occurs. Though Olig2 orchestrates the expression of both the proneural bHLH proteins Ngn2 and NeuroM, as well as MN-specific transcription factors such as Lim3 (Lhx3), Isl1 and Isl2 in progenitors and newly formed neurons (Mizuguchi et al. 2001; Novitch et al. 2001), the sustained expression of Olig2 appears paradoxically to block the function of these transcription factors in activating downstream MN differentiation genes, including the homeodomain protein Hb9 (Lee et al. 2005). The activation of Hb9 expression within differentiated MNs results from a synergistic interaction between Lim3, Isl1 and either Ngn2 or NeuroM mediated by the nuclear Lim-interacting protein (NLI; Lee & Pfaff 2003; Lee et al. 2004). These proteins together form a transcriptional activation complex at a defined region within the Hb9 promoter, containing several Lim-HD protein binding sites, to which Lim3 and Isl1 bind, flanking an E-box, to which Ngn2 or NeuroM bind (Lee & Pfaff 2003). Strikingly, in immature MN progenitors, Olig2 competes with Ngn2 in the binding to this E-box within the Hb9 promoter and, when bound, Olig2 recruits a transcriptional repressor complex that prevents the expression of Hb9 (Lee et al. 2005). Thus, a critical step in the differentiation of MNs is the transition point at which the levels of Ngn2/NeuroM expression have risen above those of Olig2 to enable Ngn2/NeuroM to bind to the Hb9 promoter and activate its expression. Together, these findings indicate that the mechanisms that elevate the levels of Ngn2/NeuroM are vital for the development of MNs, and, conversely, pathways that reduce Ngn2/NeuroM are likely to hold Olig2+ progenitors in an undifferentiated state. Likewise, the downregulation of Olig2 is critical for the subsequent maturation of MNs.
8. Notch signalling and the transition of neural progenitors to glial progenitors
Though the majority of Olig2-expressing cells in the ventral spinal cord differentiates into MNs early in development, a residual population of Olig2+ cells persists and gives rise to oligodendrocytes later in development (Lu et al. 2000; Zhou et al. 2000, 2001). Thus, the ability to maintain a population of undifferentiated Olig2+ progenitor cells throughout the course of MN development is likely to be important for later gliogenesis. Since the development of MNs depends on the differentiation promoting function of proneural bHLH proteins such as Ngn2 (Mizuguchi et al. 2001; Novitch et al. 2001; Scardigli et al. 2001), mechanisms that antagonize proneural bHLH gene expression, such as the Notch signalling pathway, may contribute to the maintenance of Olig2+ progenitors and regulate the transition from MN progenitors to glial progenitors. In the spinal cord, Notch signalling activates the expression of the Hairy/Enhancer of split (Hes) family of bHLH repressors, most notably Hes1 and Hes5. These prevent the expression of proneural bHLH proteins, such as Ngn2, and maintain progenitor cells in an undifferentiated state (Hatakeyama et al. 2004; Fior & Henrique 2005). Loss of Notch signalling in the CNS, either by misexpression of dominant interfering signalling components or by deletion of Notch receptors, Notch ligands, Hes genes or other components of the Notch pathway leads to the precocious expression of proneural bHLH proteins and, consequently, premature neuronal differentiation and a depletion of progenitor cells (Wettstein et al. 1997; Ohtsuka et al. 1999, 2001; Handler et al. 2000; Park & Appel 2003; Hatakeyama et al. 2004). The reciprocal activation of the Notch pathway, through the misexpression of either the Notch1 intracellular domain or the Hes genes, appears to have different outcomes depending on the time at which these genes are expressed. In newly formed neural progenitors, ectopic activation of the Notch pathway leads to the marked downregulation of proneural bHLH gene expression and failed neural differentiation (Park & Appel 2003). This decrease in neurogenesis is reciprocated by an overall increase in the number of stem and progenitor cells that remain at later times in development (Park & Appel 2003).
Though gain-of-function experiments performed in the retina, telencephalon and neural crest have argued for an instructive role for Notch signalling in the formation of glia (Furukawa et al. 2000; Gaiano et al. 2000; Hojo et al. 2000; Morrison et al. 2000; Wakamatsu et al. 2000; Chambers et al. 2001; Scheer et al. 2001), an analysis of the effects of Notch signalling on oligodendrocyte formation in the spinal cord reveals that, at early stages, activation of the Notch pathway has little or no effect on Olig2 expression (Park & Appel 2003). However, as a probable consequence of their inability to differentiate into MNs, the number of Olig2+ cells is increased at later times following the activation of the Notch pathway, leading to an overall increase in production of oligodendrocytes (Park & Appel 2003). Interestingly, the timing of oligodendrocyte formation was not advanced by this manipulation, indicating that Notch signalling alone is not a sufficient stimulus for glial differentiation and that additional temporal cues are required. Together, these data indicate that, in the ventral spinal cord, Notch signalling does not play a role in directing cells to take on an oligodendrocyte fate. However, through its ability to limit the expression of Ngn2 within Olig2-expressing cells, Notch signalling can maintain a residual population of Olig2+ cells with the capacity to form oligodendrocytes later in development.
While progress has been made in understanding the mechanisms by which neural progenitors in the spinal cord transition into oligodendrocyte progenitors, much less is known about how astrocyte progenitors are specified. Insight into this process has recently been made through studies of the function of the bHLH protein Scl in the ventral spinal cord. Early in development, Scl is expressed by neural progenitors that give rise to a subset of V2 interneurons (Smith et al. 2002; Muroyama et al. 2005). However, at later times, the production of V2 interneurons ceases and Scl+ cells transform into astrocyte progenitors (Muroyama et al. 2005). In a manner analogous to Olig2, Scl appears to be both necessary and sufficient to direct the formation of V2 interneurons and astrocytes, which may be attributed to its ability to extinguish the expression of Olig2 and thereby suppress MN and oligodendrocyte generation (Muroyama et al. 2005). Thus, the opponent activities of Olig2 and Scl together with the Notch signalling pathway and other timing mechanisms may account for how distinct neuronal and glial cell types are generated in both a spatially and a temporally organized manner.
9. Concluding remarks
Through these studies of spinal cord development, a coherent description of the genetic networks that regulate the acquisition of neuronal subtype identity and neuronal differentiation has begun to emerge. At present, our understanding of the formation of MNs is the most complete (figures 3 and 4). In this case, the combined actions of Shh, RA, FGF and BMP signalling establish a group of progenitors for MNs in a discrete region of the ventral neural tube (figure 2). These progenitors are delineated by the expression and combinatorial patterning function of three homeodomain proteins, Nkx2.2, Nkx6.1 and Irx3, which act to restrict the generation of MNs to the appropriate region of the neural tube (Briscoe et al. 2000). This early patterning activity leads to the spatially restricted expression of factors essential for MN specification, most notably Olig2 (Mizuguchi et al. 2001; Novitch et al. 2001), which functions to coordinate the acquisition of pan-neuronal properties and subtype characteristics of differentiating MNs. Despite the advances made in our understanding of the pathways leading to MN development, many issues remain unresolved. For example, although in this review we have focused on the patterning of neurons along the dorsoventral axis, also apparent are anterior–posterior differences in the subtypes of neurons generated (Carpenter 2002). The details of how this aspect of neuronal identity is controlled have only recently begun to emerge. Remarkably, some of the signals known to contribute to anterior–posterior patterning include those that function in dorsoventral patterning such as FGF and RA (Liu et al. 2001), and the partitioning of groups of cells along the anterior–posterior axis appears to result from cross-repressive interactions between homeodomain proteins of the Hox family (Dasen et al. 2003). Thus, similarities between the patterning of cells along these axes appear to exist, and insights into mechanisms regulating dorsoventral pattern provide a foundation for unravelling the details of anterior–posterior patterning. A significant goal for the future will be to understand how positional information conveyed by signalling along these axes is integrated into specific programmes of cellular differentiation.
A more ambitious challenge, however, will be to rigorously test whether knowledge of the signals and genetic pathways that generate diverse cell types in the central nervous system during development can be applied to direct the identity and differentiation of stem cells and neural progenitors in vitro. By understanding which combinations of growth factors and genes drive the formation of specific cell types, it may be possible to design cell culture strategies that recapitulate the in vivo sequence of events and permit the generation of a cell type of interest on demand. The recent demonstration that the addition of the signalling factors that induce MN formation in vivo, Shh and RA, can also induce embryonic stem cells to generate MN progenitor cells that express the correct profile of intrinsic transcription factors, and subsequently to differentiate into functional MNs (Wichterle et al. 2002), is an elegant demonstration that it is indeed possible to recapitulate in vivo developmental pathways. These studies provide a proof of principle for this general approach and raise the possibility of exploiting stem cells for research into and treatment of a number of neurodegenerative diseases.
Acknowledgments
We thank Kathryn Tosney for the EM images in figure 2. J.B. is supported by the MRC (UK). B.G.N. is supported by grants from the Whitehall Foundation, the March of Dimes Foundation, the NINDS and the University of Michigan Biomedical Scholars Program.
Footnotes
One contribution of 14 to a Theme Issue ‘Stem cells and brain repair’.
References
- Altman J, Bayer S.A. The development of the rat spinal cord. Adv. Anat. Embryol. Cell Biol. 1984;85:1–164. doi: 10.1007/978-3-642-69537-7. [DOI] [PubMed] [Google Scholar]
- Aza-Blanc P, Lin H, Ruiz I.A.A, Kornberg T.B. Expression of the vertebrate gli proteins in Drosophila reveals a distribution of activator and repressor activities. Development. 2000;127:4293–4301. doi: 10.1242/dev.127.19.4293. [DOI] [PubMed] [Google Scholar]
- Bai C.B, Joyner A.L. Gli1 can rescue the in vivo function of Gli2. Development. 2001;128:5161–5172. doi: 10.1242/dev.128.24.5161. [DOI] [PubMed] [Google Scholar]
- Bai C.B, Stephen D, Joyner A.L. All mouse ventral spinal cord patterning by Hedgehog is Gli dependent and involves an activator function of Gli3. Dev. Cell. 2004;6:103–115. doi: 10.1016/s1534-5807(03)00394-0. doi:10.1016/S1534-5807(03)00394-0 [DOI] [PubMed] [Google Scholar]
- Barth K.A, Kishimoto Y, Rohr K.B, Seydler C, Schulte-Merker S, Wilson S.W. Bmp activity establishes a gradient of positional information throughout the entire neural plate. Development. 1999;126:4977–4987. doi: 10.1242/dev.126.22.4977. [DOI] [PubMed] [Google Scholar]
- Bertrand N, Medevielle F, Pituello F. FGF signalling controls the timing of Pax6 activation in the neural tube. Development. 2000;127:4837–4843. doi: 10.1242/dev.127.22.4837. [DOI] [PubMed] [Google Scholar]
- Bertrand N, Castro D.S, Guillemot F. Proneural genes and the specification of neural cell types. Nat. Rev. Neurosci. 2002;3:517–530. doi: 10.1038/nrn874. doi:10.1038/nrn874 [DOI] [PubMed] [Google Scholar]
- Briscoe J, Ericson J. Specification of neuronal fates in the ventral neural tube. Curr. Opin. Neurobiol. 2001;11:43–49. doi: 10.1016/s0959-4388(00)00172-0. doi:10.1016/S0959-4388(00)00172-0 [DOI] [PubMed] [Google Scholar]
- Briscoe J, Sussel L, Serup P, Hartigan-O'Connor D, Jessell T.M, Rubenstein J.L, Ericson J. Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature. 1999;398:622–627. doi: 10.1038/19315. doi:10.1038/19315 [DOI] [PubMed] [Google Scholar]
- Briscoe J, Pierani A, Jessell T.M, Ericson J. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell. 2000;101:435–445. doi: 10.1016/s0092-8674(00)80853-3. doi:10.1016/S0092-8674(00)80853-3 [DOI] [PubMed] [Google Scholar]
- Briscoe J, Chen Y, Jessell T.M, Struhl G. A Hedgehog-insensitive form of patched provides evidence for direct long-range morphogen activity of Sonic hedgehog in the neural tube. Mol. Cell. 2001;7:1279–1291. doi: 10.1016/s1097-2765(01)00271-4. doi:10.1016/S1097-2765(01)00271-4 [DOI] [PubMed] [Google Scholar]
- Bylund M, Andersson E, Novitch B.G, Muhr J. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat. Neurosci. 2003;6:1162–1168. doi: 10.1038/nn1131. doi:10.1038/nn1131 [DOI] [PubMed] [Google Scholar]
- Cai J, Qi Y, Hu X, Tan M, Liu Z, Zhang J, Li Q, Sander M, Qiu M. Generation of oligodendrocyte precursor cells from mouse dorsal spinal cord independent of Nkx6 regulation and Shh signaling. Neuron. 2005;45:41–53. doi: 10.1016/j.neuron.2004.12.028. doi:10.1016/j.neuron.2004.12.028 [DOI] [PubMed] [Google Scholar]
- Carpenter E.M. Hox genes and spinal cord development. Dev. Neurosci. 2002;24:24–34. doi: 10.1159/000064943. doi:10.1159/000064943 [DOI] [PubMed] [Google Scholar]
- Chambers C.B, Peng Y, Nguyen H, Gaiano N, Fishell G, Nye J.S. Spatiotemporal selectivity of response to Notch1 signals in mammalian forebrain precursors. Development. 2001;128:689–702. doi: 10.1242/dev.128.5.689. [DOI] [PubMed] [Google Scholar]
- Chang D.T, Lopez A, von Kessler D.P, Chiang C, Simandl B.K, Zhao R, Seldin M.F, Fallon J.F, Beachy P.A. Products, genetic linkage and limb patterning activity of a murine hedgehog gene. Development. 1994;120:3339–3353. doi: 10.1242/dev.120.11.3339. [DOI] [PubMed] [Google Scholar]
- Chesnutt C, Burrus L.W, Brown A.M.C, Niswander L. Coordinate regulation of neural tube patterning and proliferation by TGFβ and WNT activity. Dev. Biol. 2004;274:334–347. doi: 10.1016/j.ydbio.2004.07.019. doi:10.1016/j.ydbio.2004.07.019 [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young K.E, Corden J.L, Westphal H, Beachy P.A. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. doi:10.1038/383407a0 [DOI] [PubMed] [Google Scholar]
- Dai P, Akimaru H, Tanaka Y, Maekawa T, Nakafuku M, Ishii S. Sonic hedgehog-induced activation of the Gli1 promoter is mediated by GLI3. J. Biol. Chem. 1999;274:8143–8152. doi: 10.1074/jbc.274.12.8143. doi:10.1074/jbc.274.12.8143 [DOI] [PubMed] [Google Scholar]
- Dasen J.S, Liu J.P, Jessell T.M. Motor neuron columnar fate imposed by sequential phases of Hox-c activity. Nature. 2003;425:926–933. doi: 10.1038/nature02051. doi:10.1038/nature02051 [DOI] [PubMed] [Google Scholar]
- Diez del Corral R, Storey K.G. Opposing FGF and retinoid pathways: a signalling switch that controls differentiation and patterning onset in the extending vertebrate body axis. Bioessays. 2004;26:857–869. doi: 10.1002/bies.20080. doi:10.1002/bies.20080 [DOI] [PubMed] [Google Scholar]
- Diez del Corral R, Breitkreuz D.N, Storey K.G. Onset of neuronal differentiation is regulated by paraxial mesoderm and requires attenuation of FGF signalling. Development. 2002;129:1681–1691. doi: 10.1242/dev.129.7.1681. [DOI] [PubMed] [Google Scholar]
- Diez del Corral R, Olivera-Martinez I, Goriely A, Gale E, Maden M, Storey K. Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron. 2003;40:65–79. doi: 10.1016/s0896-6273(03)00565-8. doi:10.1016/S0896-6273(03)00565-8 [DOI] [PubMed] [Google Scholar]
- Echelard Y, Epstein D.J, St-Jacques B, Shen L, Mohler J, McMahon J.A, McMahon A.P. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. doi:10.1016/0092-8674(93)90627-3 [DOI] [PubMed] [Google Scholar]
- Edlund T, Jessell T.M. Progression from extrinsic to intrinsic signaling in cell fate specification: a view from the nervous system. Cell. 1999;96:211–224. doi: 10.1016/s0092-8674(00)80561-9. doi:10.1016/S0092-8674(00)80561-9 [DOI] [PubMed] [Google Scholar]
- Ericson J, Morton S, Kawakami A, Roelink H, Jessell T.M. Two critical periods of Sonic hedgehog signaling required for the specification of motor neuron identity. Cell. 1996;87:661–673. doi: 10.1016/s0092-8674(00)81386-0. doi:10.1016/S0092-8674(00)81386-0 [DOI] [PubMed] [Google Scholar]
- Ericson J, Briscoe J, Rashbass P, van Heyningen V, Jessell T.M. Graded Sonic hedgehog signaling and the specification of cell fate in the ventral neural tube. Cold Spring Harb. Symp. Quant. Biol. 1997a;62:451–466. [PubMed] [Google Scholar]
- Ericson J, Rashbass P, Schedl A, Brenner-Morton S, Kawakami A, van Heyningen V, Jessell T.M, Briscoe J. Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell. 1997b;90:169–180. doi: 10.1016/s0092-8674(00)80323-2. doi:10.1016/S0092-8674(00)80323-2 [DOI] [PubMed] [Google Scholar]
- Fior R, Henrique D. A novel hes5/hes6 circuitry of negative regulation controls Notch activity during neurogenesis. Dev. Biol. 2005;281:318–333. doi: 10.1016/j.ydbio.2005.03.017. doi:10.1016/j.ydbio.2005.03.017 [DOI] [PubMed] [Google Scholar]
- Furukawa T, Mukherjee S, Bao Z.Z, Morrow E.M, Cepko C.L. rax, Hes1, and notch1 promote the formation of Muller glia by postnatal retinal progenitor cells. Neuron. 2000;26:383–394. doi: 10.1016/s0896-6273(00)81171-x. doi:10.1016/S0896-6273(00)81171-X [DOI] [PubMed] [Google Scholar]
- Gaiano N, Nye J.S, Fishell G. Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron. 2000;26:395–404. doi: 10.1016/s0896-6273(00)81172-1. doi:10.1016/S0896-6273(00)81172-1 [DOI] [PubMed] [Google Scholar]
- Gotz K, Briscoe J, Ruther U. Homozygous Ft embryos are affected in floor plate maintenance and ventral neural tube patterning. Dev. Dyn. 2005;233:623–630. doi: 10.1002/dvdy.20354. [DOI] [PubMed] [Google Scholar]
- Goulding M, Pfaff S.L. Development of circuits that generate simple rhythmic behaviors in vertebrates. Curr. Opin. Neurobiol. 2005;15:14–20. doi: 10.1016/j.conb.2005.01.017. doi:10.1016/j.conb.2005.01.017 [DOI] [PubMed] [Google Scholar]
- Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. doi:10.1016/S0896-6273(03)00497-5 [DOI] [PubMed] [Google Scholar]
- Gritli-Linde A, Lewis P, McMahon A.P, Linde A. The whereabouts of a morphogen: direct evidence for short- and graded long-range activity of hedgehog signaling peptides. Dev. Biol. 2001;236:364–386. doi: 10.1006/dbio.2001.0336. doi:10.1006/dbio.2001.0336 [DOI] [PubMed] [Google Scholar]
- Handler M, Yang X, Shen J. Presenilin-1 regulates neuronal differentiation during neurogenesis. Development. 2000;127:2593–2606. doi: 10.1242/dev.127.12.2593. [DOI] [PubMed] [Google Scholar]
- Hatakeyama J, Bessho Y, Katoh K, Ookawara S, Fujioka M, Guillemot F, Kageyama R. Hes genes regulate size, shape and histogenesis of the nervous system by control of the timing of neural stem cell differentiation. Development. 2004;131:5539–5550. doi: 10.1242/dev.01436. doi:10.1242/dev.01436 [DOI] [PubMed] [Google Scholar]
- Helms A.W, Battiste J, Henke R.M, Nakada Y, Simplicio N, Guillemot F, Johnson J.E. Sequential roles for Mash1 and Ngn2 in the generation of dorsal spinal cord interneurons. Development. 2005;132:2709–2719. doi: 10.1242/dev.01859. doi:10.1242/dev.01859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo M, Ohtsuka T, Hashimoto N, Gradwohl G, Guillemot F, Kageyama R. Glial cell fate specification modulated by the bHLH gene Hes5 in mouse retina. Development. 2000;127:2515–2522. doi: 10.1242/dev.127.12.2515. [DOI] [PubMed] [Google Scholar]
- Huangfu D, Liu A, Rakeman A.S, Murcia N.S, Niswander L, Anderson K.V. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. doi:10.1038/nature02061 [DOI] [PubMed] [Google Scholar]
- Hui C.C, Slusarski D, Platt K.A, Holmgren R, Joyner A.L. Expression of three mouse homologs of the Drosophila segment polarity gene cubitus interruptus, Gli, Gli-2, and Gli-3, in ectoderm- and mesoderm-derived tissues suggests multiple roles during postimplantation development. Dev. Biol. 1994;162:402–413. doi: 10.1006/dbio.1994.1097. doi:10.1006/dbio.1994.1097 [DOI] [PubMed] [Google Scholar]
- Hynes M, Ye W, Wang K, Stone D, Murone M, Sauvage F, Rosenthal A. The seven-transmembrane receptor smoothened cell-autonomously induces multiple ventral cell types. Nat. Neurosci. 2000;3:41–46. doi: 10.1038/71114. doi:10.1038/71114 [DOI] [PubMed] [Google Scholar]
- Ingham P.W, McMahon A.P. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. doi:10.1101/gad.938601 [DOI] [PubMed] [Google Scholar]
- Jäckle H, Tautz D, Schuh R, Seifert E, Lehmann R. Cross-regulatory interactions among the gap genes of Drosophila. Nature. 1986;324:668–670. doi:10.1038/324668a0 [Google Scholar]
- Jessell T.M. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat. Rev. Genet. 2000;1:20–29. doi: 10.1038/35049541. doi:10.1038/35049541 [DOI] [PubMed] [Google Scholar]
- Karunaratne A, Hargrave M, Poh A, Yamada T. GATA proteins identify a novel ventral interneuron subclass in the developing chick spinal cord. Dev. Biol. 2002;249:30–43. doi: 10.1006/dbio.2002.0754. doi:10.1006/dbio.2002.0754 [DOI] [PubMed] [Google Scholar]
- Kishi M, Mizuseki K, Sasai N, Yamazaki H, Shiota K, Nakanishi S, Sasai Y. Requirement of Sox2-mediated signaling for differentiation of early Xenopus neuroectoderm. Development. 2000;127:791–800. doi: 10.1242/dev.127.4.791. [DOI] [PubMed] [Google Scholar]
- Krauss S, Concordet J.P, Ingham P.W. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell. 1993;75:1431–1444. doi: 10.1016/0092-8674(93)90628-4. doi:10.1016/0092-8674(93)90628-4 [DOI] [PubMed] [Google Scholar]
- Kraut R, Levine M. Mutually repressive interactions between the gap genes giant and Kruppel define middle body regions of the Drosophila embryo. Development. 1991;111:611–621. doi: 10.1242/dev.111.2.611. [DOI] [PubMed] [Google Scholar]
- Lee S.K, Pfaff S.L. Synchronization of neurogenesis and motor neuron specification by direct coupling of bHLH and homeodomain transcription factors. Neuron. 2003;38:731–745. doi: 10.1016/s0896-6273(03)00296-4. doi:10.1016/S0896-6273(03)00296-4 [DOI] [PubMed] [Google Scholar]
- Lee J, Platt K.A, Censullo P, Ruiz i Altaba A. Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development. 1997;124:2537–2552. doi: 10.1242/dev.124.13.2537. [DOI] [PubMed] [Google Scholar]
- Lee S.K, Jurata L.W, Funahashi J, Ruiz E.C, Pfaff S.L. Analysis of embryonic motoneuron gene regulation: derepression of general activators function in concert with enhancer factors. Development. 2004;131:3295–3306. doi: 10.1242/dev.01179. doi:10.1242/dev.01179 [DOI] [PubMed] [Google Scholar]
- Lee S.K, Lee B, Ruiz E.C, Pfaff S.L. Olig2 and Ngn2 function in opposition to modulate gene expression in motor neuron progenitor cells. Genes Dev. 2005;19:282–294. doi: 10.1101/gad.1257105. doi:10.1101/gad.1257105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Q, Zelman A.K, Kuang E, Li S, Matise M.P. Transduction of graded hedgehog signaling by a combination of Gli2 and Gli3 activator functions in the developing spinal cord. Development. 2004;131:3593–3604. doi: 10.1242/dev.01230. doi:10.1242/dev.01230 [DOI] [PubMed] [Google Scholar]
- Liem K.F, Jr, Tremml G, Jessell T.M. A role for the roof plate and its resident TGFbeta-related proteins in neuronal patterning in the dorsal spinal cord. Cell. 1997;91:127–138. doi: 10.1016/s0092-8674(01)80015-5. doi:10.1016/S0092-8674(01)80015-5 [DOI] [PubMed] [Google Scholar]
- Liem K.F, Jessell T.M, Briscoe J. Regulation of the neural patterning activity of Sonic hedgehog by secreted BMP inhibitors expressed by notochord and somites. Development. 2000;127:4855–4866. doi: 10.1242/dev.127.22.4855. [DOI] [PubMed] [Google Scholar]
- Litingtung Y, Chiang C. Specification of ventral neuron types is mediated by an antagonistic interaction between shh and gli3. Nat. Neurosci. 2000;3:979–985. doi: 10.1038/79916. doi:10.1038/79916 [DOI] [PubMed] [Google Scholar]
- Liu J.P, Laufer E, Jessell T.M. Assigning the positional identity of spinal motor neurons: rostrocaudal patterning of Hox-c expression by FGFs, Gdf11, and retinoids. Neuron. 2001;32:997–1012. doi: 10.1016/s0896-6273(01)00544-x. doi:10.1016/S0896-6273(01)00544-X [DOI] [PubMed] [Google Scholar]
- Liu A, Wang B, Niswander L.A. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development. 2005;132:3103–3111. doi: 10.1242/dev.01894. doi:10.1242/dev.01894 [DOI] [PubMed] [Google Scholar]
- Lu Q.R, Yuk D, Alberta J.A, Zhu Z, Pawlitzky I, Chan J, McMahon A.P, Stiles C.D, Rowitch D.H. Sonic hedgehog—regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000;25:317–329. doi: 10.1016/s0896-6273(00)80897-1. doi:10.1016/S0896-6273(00)80897-1 [DOI] [PubMed] [Google Scholar]
- Lu Q.R, Sun T, Zhu Z, Ma N, Garcia M, Stiles C.D, Rowitch D.H. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. doi:10.1016/S0092-8674(02)00678-5 [DOI] [PubMed] [Google Scholar]
- Lum L, Beachy P.A. The hedgehog response network: sensors, switches, and routers. Science. 2004;304:1755–1759. doi: 10.1126/science.1098020. doi:10.1126/science.1098020 [DOI] [PubMed] [Google Scholar]
- Ma Q, Sommer L, Cserjesi P, Anderson D.J. Mash1 and neurogenin1 expression patterns define complementary domains of neuroepithelium in the developing CNS and are correlated with regions expressing notch ligands. J. Neurosci. 1997;17:3644–3652. doi: 10.1523/JNEUROSCI.17-10-03644.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marigo V, Tabin C.J. Regulation of patched by Sonic hedgehog in the developing neural tube. Proc. Natl Acad. Sci. USA. 1996;93:9346–9351. doi: 10.1073/pnas.93.18.9346. doi:10.1073/pnas.93.18.9346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti E, Bumcrot D.A, Takada R, McMahon A.P. Requirement of 19K form of Sonic hedgehog for induction of distinct ventral cell types in CNS explants. Nature. 1995;375:322–325. doi: 10.1038/375322a0. doi:10.1038/375322a0 [DOI] [PubMed] [Google Scholar]
- Mathis L, Kulesa P.M, Fraser S.E. FGF receptor signalling is required to maintain neural progenitors during Hensen's node progression. Nat. Cell Biol. 2001;3:559–566. doi: 10.1038/35078535. doi:10.1038/35078535 [DOI] [PubMed] [Google Scholar]
- Matise M.P, Epstein D.J, Park H.L, Platt K.A, Joyner A.L. Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons in the mouse central nervous system. Development. 1998;125:2759–2770. doi: 10.1242/dev.125.15.2759. [DOI] [PubMed] [Google Scholar]
- Megason S.G, McMahon A.P. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129:2087–2098. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- Mizuguchi R, Sugimori M, Takebayashi H, Kosako H, Nagao M, Yoshida S, Nabeshima Y, Shimamura K, Nakafuku M. Combinatorial roles of olig2 and neurogenin2 in the coordinated induction of pan-neuronal and subtype-specific properties of motoneurons. Neuron. 2001;31:757–771. doi: 10.1016/s0896-6273(01)00413-5. doi:10.1016/S0896-6273(01)00413-5 [DOI] [PubMed] [Google Scholar]
- Morrison S.J, Perez S.E, Qiao Z, Verdi J.M, Hicks C, Weinmaster G, Anderson D.J. Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell. 2000;101:499–510. doi: 10.1016/s0092-8674(00)80860-0. doi:10.1016/S0092-8674(00)80860-0 [DOI] [PubMed] [Google Scholar]
- Muhr J, Andersson E, Persson M, Jessell T.M, Ericson J. Groucho-mediated transcriptional repression establishes progenitor cell pattern and neuronal fate in the ventral neural tube. Cell. 2001;104:861–873. doi: 10.1016/s0092-8674(01)00283-5. doi:10.1016/S0092-8674(01)00283-5 [DOI] [PubMed] [Google Scholar]
- Muller T, Anlag K, Wildner H, Britsch S, Treier M, Birchmeier C. The bHLH factor Olig3 coordinates the specification of dorsal neurons in the spinal cord. Genes Dev. 2005;19:733–743. doi: 10.1101/gad.326105. doi:10.1101/gad.326105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroyama Y, Fujihara M, Ikeya M, Kondoh H, Takada S. Wnt signaling plays an essential role in neuronal specification of the dorsal spinal cord. Genes Dev. 2002;16:548–553. doi: 10.1101/gad.937102. doi:10.1101/gad.937102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroyama Y, Fujiwara Y, Orkin S.H, Rowitch D.H. Specification of astrocytes by bHLH protein SCL in a restricted region of the nerual tube. Nature. 2005;438:360–363. doi: 10.1038/nature04139. doi:10.1038/nature04139 [DOI] [PubMed] [Google Scholar]
- Nguyen V.H, Trout J, Connors S.A, Andermann P, Weinberg E, Mullins M.C. Dorsal and intermediate neuronal cell types of the spinal cord are established by a BMP signaling pathway. Development. 2000;127:1209–1220. doi: 10.1242/dev.127.6.1209. [DOI] [PubMed] [Google Scholar]
- Novitch B.G, Chen A.I, Jessell T.M. Coordinate regulation of motor neuron subtype identity and pan-neuronal properties by the bHLH repressor Olig2. Neuron. 2001;31:773–789. doi: 10.1016/s0896-6273(01)00407-x. doi:10.1016/S0896-6273(01)00407-X [DOI] [PubMed] [Google Scholar]
- Novitch B.G, Wichterle H, Jessell T.M, Sockanathan S. A requirement for retinoic acid-mediated transcriptional activation in ventral neural patterning and motor neuron specification. Neuron. 2003;40:81–95. doi: 10.1016/j.neuron.2003.08.006. doi:10.1016/j.neuron.2003.08.006 [DOI] [PubMed] [Google Scholar]
- Ohtsuka T, Ishibashi M, Gradwohl G, Nakanishi S, Guillemot F, Kageyama R. Hes1 and Hes5 as notch effectors in mammalian neuronal differentiation. EMBO J. 1999;18:2196–2207. doi: 10.1093/emboj/18.8.2196. doi:10.1093/emboj/18.8.2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka T, Sakamoto M, Guillemot F, Kageyama R. Roles of the basic helix-loop-helix genes Hes1 and Hes5 in expansion of neural stem cells of the developing brain. J. Biol. Chem. 2001;276:30 467–30 474. doi: 10.1074/jbc.M102420200. doi:10.1074/jbc.M102420200 [DOI] [PubMed] [Google Scholar]
- Park H.C, Appel B. Delta-Notch signaling regulates oligodendrocyte specification. Development. 2003;130:3747–3755. doi: 10.1242/dev.00576. doi:10.1242/dev.00576 [DOI] [PubMed] [Google Scholar]
- Park H.L, Bai C, Platt K.A, Matise M.P, Beeghly A, Hui C.C, Nakashima M, Joyner A.L. Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development. 2000;127:1593–1605. doi: 10.1242/dev.127.8.1593. [DOI] [PubMed] [Google Scholar]
- Parras C.M, Schuurmans C, Scardigli R, Kim J, Anderson D.J, Guillemot F. Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity. Genes Dev. 2002;16:324–338. doi: 10.1101/gad.940902. doi:10.1101/gad.940902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten I, Placzek M. Opponent activities of Shh and BMP signaling during floor plate induction in vivo. Curr. Biol. 2002;12:47–52. doi: 10.1016/s0960-9822(01)00631-5. doi:10.1016/S0960-9822(01)00631-5 [DOI] [PubMed] [Google Scholar]
- Persson M, Stamataki D, te Welscher P, Andersson E, Bose J, Ruther U, Ericson J, Briscoe J. Dorsal–ventral patterning of the spinal cord requires Gli3 transcriptional repressor activity. Genes Dev. 2002;16:2865–2878. doi: 10.1101/gad.243402. doi:10.1101/gad.243402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevny L, Placzek M. SOX genes and neural progenitor identity. Curr. Opin. Neurobiol. 2005;15:7–13. doi: 10.1016/j.conb.2005.01.016. doi:10.1016/j.conb.2005.01.016 [DOI] [PubMed] [Google Scholar]
- Pevny L.H, Sockanathan S, Placzek M, Lovell-Badge R. A role for SOX1 in neural determination. Development. 1998;125:1967–1978. doi: 10.1242/dev.125.10.1967. [DOI] [PubMed] [Google Scholar]
- Pierani A, Brenner-Morton S, Chiang C, Jessell T.M. A Sonic hedgehog-independent, retinoid-activated pathway of neurogenesis in the ventral spinal cord. Cell. 1999;97:903–915. doi: 10.1016/s0092-8674(00)80802-8. doi:10.1016/S0092-8674(00)80802-8 [DOI] [PubMed] [Google Scholar]
- Placzek M, Yamada T, Tessier-Lavigne M, Jessell T, Dodd J. Control of dorsoventral pattern in vertebrate neural development: induction and polarizing properties of the floor plate. Development. 1991;(Suppl. 2):105–122. [PubMed] [Google Scholar]
- Ramón y Cajal S. In Nobel lectures, physiology or medicine 1901–1921. Elsevier; Amsterdam, The Netherlands: 1906. Nobel lecture. [Google Scholar]
- Riddle R.D, Johnson R.L, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. doi:10.1016/0092-8674(93)90626-2 [DOI] [PubMed] [Google Scholar]
- Robertson C.P, Braun M.M, Roelink H. Sonic hedgehog patterning in chick neural plate is antagonized by a Wnt3-like signal. Dev. Dyn. 2004;229:510–519. doi: 10.1002/dvdy.10501. doi:10.1002/dvdy.10501 [DOI] [PubMed] [Google Scholar]
- Roelink H, et al. Floor plate and motor neuron induction by vhh-1, a vertebrate homolog of hedgehog expressed by the notochord. Cell. 1994;76:761–775. doi: 10.1016/0092-8674(94)90514-2. doi:10.1016/0092-8674(94)90514-2 [DOI] [PubMed] [Google Scholar]
- Roelink H, Porter J.A, Chiang C, Tanabe Y, Chang D.T, Beachy P.A, Jessell T.M. Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of Sonic hedgehog autoproteolysis. Cell. 1995;81:445–455. doi: 10.1016/0092-8674(95)90397-6. doi:10.1016/0092-8674(95)90397-6 [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A. Combinatorial Gli gene function in floor plate and neuronal inductions by Sonic hedgehog. Development. 1998;25:2203–2212. doi: 10.1242/dev.125.12.2203. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A. Gli proteins encode context-dependent positive and negative functions: implications for development and disease. Development. 1999;26:3205–3216. doi: 10.1242/dev.126.14.3205. [DOI] [PubMed] [Google Scholar]
- Sandberg M, Kallstrom M, Muhr J. Sox21 promotes the progression of vertebrate neurogenesis. Nat. Neurosci. 2005;8:995–1001. doi: 10.1038/nn1493. doi:10.1038/nn1493 [DOI] [PubMed] [Google Scholar]
- Sasaki H, Hui C, Nakafuku M, Kondoh H. A binding site for Gli proteins is essential for HNF-3beta floor plate enhancer activity in transgenics and can respond to Shh in vitro. Development. 1997;124:1313–1322. doi: 10.1242/dev.124.7.1313. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Nishizaki Y, Hui C, Nakafuku M, Kondoh H. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development. 1999;126:3915–3924. doi: 10.1242/dev.126.17.3915. [DOI] [PubMed] [Google Scholar]
- Scardigli R, Schuurmans C, Gradwohl G, Guillemot F. Crossregulation between Neurogenin2 and pathways specifying neuronal identity in the spinal cord. Neuron. 2001;31:203–217. doi: 10.1016/s0896-6273(01)00358-0. doi:10.1016/S0896-6273(01)00358-0 [DOI] [PubMed] [Google Scholar]
- Scheer N, Groth A, Hans S, Campos-Ortega J.A. An instructive function for Notch in promoting gliogenesis in the zebrafish retina. Development. 2001;128:1099–1107. doi: 10.1242/dev.128.7.1099. [DOI] [PubMed] [Google Scholar]
- Shirasaki R, Pfaff S.L. Transcriptional codes and the control of neuronal identity. Annu. Rev. Neurosci. 2002;25:251–281. doi: 10.1146/annurev.neuro.25.112701.142916. doi:10.1146/annurev.neuro.25.112701.142916 [DOI] [PubMed] [Google Scholar]
- Simmons A.D, Horton S, Abney A.L, Johnson J.E. Neurogenin2 expression in ventral and dorsal spinal neural tube progenitor cells is regulated by distinct enhancers. Dev. Biol. 2001;229:327–339. doi: 10.1006/dbio.2000.9984. doi:10.1006/dbio.2000.9984 [DOI] [PubMed] [Google Scholar]
- Smith E, Hargrave M, Yamada T, Begley C.G, Little M.H. Coexpression of SCL and GATA3 in the V2 interneurons of the developing mouse spinal cord. Dev. Dyn. 2002;224:231–237. doi: 10.1002/dvdy.10093. doi:10.1002/dvdy.10093 [DOI] [PubMed] [Google Scholar]
- Sommer L, Ma Q, Anderson D.J. neurogenins, a novel family of atonal-related bHLH transcription factors, are putative mammalian neuronal determination genes that reveal progenitor cell heterogeneity in the developing CNS and PNS. Mol. Cell Neurosci. 1996;8:221–241. doi: 10.1006/mcne.1996.0060. doi:10.1006/mcne.1996.0060 [DOI] [PubMed] [Google Scholar]
- Stamataki D, Ulloa F, Tsoni S.V, Mynett A, Briscoe J. A gradient of Gli activity mediates graded Sonic hedgehog signaling in the neural tube. Genes Dev. 2005;19:626–641. doi: 10.1101/gad.325905. doi:10.1101/gad.325905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit A, Berliner A.J, Papanayotou C, Sirulnik A, Stern C.D. Initiation of neural induction by FGF signalling before gastrulation. Nature. 2000;406:74–78. doi: 10.1038/35017617. doi:10.1038/35017617 [DOI] [PubMed] [Google Scholar]
- Takebayashi H, Nabeshima Y, Yoshida S, Chisaka O, Ikenaka K. The basic helix-loop-helix factor Olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr. Biol. 2002a;12:1157–1163. doi: 10.1016/s0960-9822(02)00926-0. doi:10.1016/S0960-9822(02)00926-0 [DOI] [PubMed] [Google Scholar]
- Takebayashi H, Ohtsuki T, Uchida T, Kawamoto S, Okubo K, Ikenaka K, Takeichi M, Chisaka O, Nabeshima Y. Non-overlapping expression of Olig3 and Olig2 in the embryonic neural tube. Mech. Dev. 2002b;113:169–174. doi: 10.1016/s0925-4773(02)00021-7. doi:10.1016/S0925-4773(02)00021-7 [DOI] [PubMed] [Google Scholar]
- Uchikawa M, Ishida Y, Takemoto T, Kamachi Y, Kondoh H. Functional analysis of chicken Sox2 enhancers highlights an array of diverse regulatory elements that are conserved in mammals. Dev. Cell. 2003;4:509–519. doi: 10.1016/s1534-5807(03)00088-1. doi:10.1016/S1534-5807(03)00088-1 [DOI] [PubMed] [Google Scholar]
- Vallstedt A, Muhr J, Pattyn A, Pierani A, Mendelsohn M, Sander M, Jessell T.M, Ericson J. Different levels of repressor activity assign redundant and specific roles to Nkx6 genes in motor neuron and interneuron specification. Neuron. 2001;31:743–755. doi: 10.1016/s0896-6273(01)00412-3. doi:10.1016/S0896-6273(01)00412-3 [DOI] [PubMed] [Google Scholar]
- Vallstedt A, Klos J.M, Ericson J. Multiple dorsoventral origins of oligodendrocyte generation in the spinal cord and hindbrain. Neuron. 2005;45:55–67. doi: 10.1016/j.neuron.2004.12.026. doi:10.1016/j.neuron.2004.12.026 [DOI] [PubMed] [Google Scholar]
- van Straaten H.W, Hekking J.W, Thors F, Wiertz-Hoessels E.L, Drukker J. Induction of an additional floor plate in the neural tube. Acta Morphol. Neerl. Scand. 1985;23:91–97. [PubMed] [Google Scholar]
- Wakamatsu Y, Maynard T.M, Weston J.A. Fate determination of neural crest cells by NOTCH-mediated lateral inhibition and asymmetrical cell division during gangliogenesis. Development. 2000;127:2811–2821. doi: 10.1242/dev.127.13.2811. [DOI] [PubMed] [Google Scholar]
- Wang B, Fallon J.F, Beachy P.A. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423–434. doi: 10.1016/s0092-8674(00)80678-9. doi:10.1016/S0092-8674(00)80678-9 [DOI] [PubMed] [Google Scholar]
- Watterson R.L, Goodheart C.R, Lindberg G. The influence of adjacent structures upon the shape of the neural tube and neural plate of chick embryos. Anat. Rec. 1955;122:539–559. doi: 10.1002/ar.1091220405. doi:10.1002/ar.1091220405 [DOI] [PubMed] [Google Scholar]
- Wettstein D.A, Turner D.L, Kintner C. The Xenopus homolog of Drosophila suppressor of hairless mediates Notch signaling during primary neurogenesis. Development. 1997;124:693–702. doi: 10.1242/dev.124.3.693. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter J.A, Jessell T.M. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. doi:10.1016/S0092-8674(02)00835-8 [DOI] [PubMed] [Google Scholar]
- Wijgerde M, McMahon J.A, Rule M, McMahon A.P. A direct requirement for hedgehog signaling for normal specification of all ventral progenitor domains in the presumptive mammalian spinal cord. Genes Dev. 2002;16:2849–2864. doi: 10.1101/gad.1025702. doi:10.1101/gad.1025702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L, Maden M. The mechanisms of dorsoventral patterning in the vertebrate neural tube. Dev. Biol. 2005;282:1–13. doi: 10.1016/j.ydbio.2005.02.027. doi:10.1016/j.ydbio.2005.02.027 [DOI] [PubMed] [Google Scholar]
- Wilson S.I, Graziano E, Harland R, Jessell T.M, Edlund T. An early requirement for FGF signalling in the acquisition of neural cell fate in the chick embryo. Curr. Biol. 2000;10:421–429. doi: 10.1016/s0960-9822(00)00431-0. doi:10.1016/S0960-9822(00)00431-0 [DOI] [PubMed] [Google Scholar]
- Wilson S.I, Rydstrom A, Trimborn T, Willert K, Nusse R, Jessell T.M, Edlund T. The status of Wnt signalling regulates neural and epidermal fates in the chick embryo. Nature. 2001;411:325–330. doi: 10.1038/35077115. doi:10.1038/35077115 [DOI] [PubMed] [Google Scholar]
- Wilson L, Gale E, Chambers D, Maden M. Retinoic acid and the control of dorsoventral patterning in the avian spinal cord. Dev. Biol. 2004;269:433–446. doi: 10.1016/j.ydbio.2004.01.034. doi:10.1016/j.ydbio.2004.01.034 [DOI] [PubMed] [Google Scholar]
- Yamada T, Placzek M, Tanaka H, Dodd J, Jessell T.M. Control of cell pattern in the developing nervous system: polarizing activity of the floor plate and notochord. Cell. 1991;64:635–647. doi: 10.1016/0092-8674(91)90247-v. doi:10.1016/0092-8674(91)90247-V [DOI] [PubMed] [Google Scholar]
- Zecca M, Basler K, Struhl G. Sequential organizing activities of engrailed, hedgehog and decapentaplegic in the Drosophila wing. Development. 1995;121:2265–2278. doi: 10.1242/dev.121.8.2265. [DOI] [PubMed] [Google Scholar]
- Zhang X.M, Ramalho-Santos M, McMahon A.P. Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R symmetry by the mouse node. Cell. 2001;106:781–792. doi:10.1016/S0092-8674(01)00385-3 [PubMed] [Google Scholar]
- Zhou Q, Anderson D.J. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109:61–73. doi: 10.1016/s0092-8674(02)00677-3. doi:10.1016/S0092-8674(02)00677-3 [DOI] [PubMed] [Google Scholar]
- Zhou Q, Wang S, Anderson D.J. Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron. 2000;25:331–343. doi: 10.1016/s0896-6273(00)80898-3. doi:10.1016/S0896-6273(00)80898-3 [DOI] [PubMed] [Google Scholar]
- Zhou Q, Choi G, Anderson D.J. The bHLH transcription factor Olig2 promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron. 2001;31:791–807. doi: 10.1016/s0896-6273(01)00414-7. doi:10.1016/S0896-6273(01)00414-7 [DOI] [PubMed] [Google Scholar]