Abstract
We previously made the surprising finding that cultures of multipotent precursors can be grown from the dermis of neonatal and adult mammalian skin. These skin-derived precursors (SKPs) display multi-lineage differentiation potential, producing both neural and mesodermal progeny in vitro, and are an apparently novel precursor cell type that is distinct from other known precursors within the skin. In this review, we begin by placing these findings within the context of the rapidly evolving stem cell field. We then describe our recent efforts focused on understanding the developmental biology of SKPs, discussing the idea that SKPs are neural crest-related precursors that (i) migrate into the skin during embryogenesis, (ii) persist within a specific dermal niche, and (iii) play a key role in the normal physiology, and potentially pathology, of the skin. We conclude by highlighting some of the therapeutic implications and unresolved questions raised by these studies.
Keywords: stem cells, dermal papilla, hair follicles, spinal cord injury, cancer, plasticity
1. Introduction
A number of studies published in the past 7 years have challenged the traditional view that stem cells present in somatic tissues are restricted to producing that tissue's cell types. Neural stem cells isolated from the embryonic or adult central nervous system (CNS) have been reported to differentiate into muscle (Galli et al. 2000; Tsai & McKay 2000; Bani-Yaghoub et al. 2004), blood (Bjornson et al. 1999) and endothelial cells (Wurmser et al. 2004), and to contribute to all three primary germ layers when injected into blastocysts (Clarke et al. 2000). Transplanted bone marrow-derived cells have shown evidence of contributing to the liver (Petersen et al. 1999; Theise et al. 2000; Grompe 2003), muscle (LaBarge & Blau 2002; Brazelton et al. 2003; Corbel et al. 2003; Doyonnas et al. 2004; Palermo et al. 2005) and brain (Brazelton et al. 2000; Mezey et al. 2000, 2003; Priller et al. 2001; Cogle et al. 2004), and also to the three primary germ layers following blastocyst injection (Jiang et al. 2002b). These and other studies suggested that ‘tissue-specific’ stem cells can express a wider differentiation potential if exposed to appropriate foreign environments, as occurs in vitro or following heterotopic transplantation. Such findings raise the possibility that tissue-derived stem cells are either more ‘plastic’ than previously thought or that the stem cells present in adult tissues are more similar to embryonic precursors than previously appreciated.
2. Isolation and differentiation of novel multipotent precursors from skin
Skin represents a complex and highly regenerative tissue containing a relatively broad stem cell repertoire, including resident epidermal and mesenchymal stem cells (MSCs), migratory melanocytic stem cells derived from the embryonic neural crest (NC) and blood vessel-associated haematopoietic and endothelial precursors. The idea that skin might provide an accessible, potentially autologous source of neural precursors (NPCs) was first suggested by the finding that Merkel cells, a type of sensory receptor with characteristics of neurons, could be generated within the skin of rodents (Nurse et al. 1984). On the basis of these findings, we hypothesized that skin contained a precursor cell with neurogenic capacity and used methods similar to those used to culture CNS neural stem cells (Reynolds & Weiss 1992; Reynolds et al. 1992) in an attempt to isolate these precursors. When skin cells of neonatal and adult rodents were dissociated to single cells and grown in suspension culture in the presence of the mitogens fibroblast growth factor-2 (FGF2) and epidermal growth factor (EGF), floating spheres of proliferating cells were generated (Toma et al. 2001). These spheres were positive for nestin, an intermediate filament expressed in neural and skeletal muscle precursors. Moreover, differentiation of skin-derived spheres in vitro resulted in the de novo generation of separate subpopulations of cells expressing neuronal, glial, smooth muscle and adipocyte markers (Toma et al. 2001). Importantly, clonally derived colonies were able to produce this same complement of cell types, confirming the multipotency of these cultured precursors from skin.
A direct comparison of these precursors with other types of stem cells and precursors known to be resident in skin indicated that skin-derived precursors (SKPs) were a distinct population (Toma et al. 2001; Fernandes et al. 2004). In particular, they were antigenically distinct from MSCs and, when grown under the same conditions, the MSCs grew adherently while SKPs grew as floating spheres (Toma et al. 2001). SKPs were derived from the dermis and did not apparently produce keratinocytes, distinguishing them from epidermal stem cells (Toma et al. 2001). Finally, SKPs neither express c-kit, a marker of melanocytic stem cells and haematopoietic stem cells, nor other melanocyte stem cell markers such as dct (Fernandes et al. 2004). We also compared SKPs directly to CNS neural stem cells cultured as neurospheres and again found significant differences; while both CNS stem cells and SKPs expressed nestin, SKPs also expressed a number of proteins, including fibronectin, that were not expressed by CNS neural stem cells (Fernandes et al. 2004). In addition, while both CNS stem cells and SKPs could generate one mesodermal cell type, smooth muscle cells, only SKPs were able to generate adipocytes (Toma et al. 2001). Thus, on the basis of these findings, we proposed that SKPs were novel multipotent precursors resident in the mammalian dermis (figure 1).
Figure 1.
Isolation and differentiation of skin-derived precursors. (a) Isolation of SKPs. (i) Fluorescence micrograph of post-natal mouse skin, with nuclei labelled by Hoechst (blue) and epithelial cells of the epidermis and hair follicles labelled with anti-cytokeratin (green). Non-specific cytokeratin staining also illuminates the subcutaneous muscle layer. (ii) Enzymatically and mechanically dissociated mouse skin. (iii) Growth of SKP spheres in suspension cultures when dissociated skin cells are exposed to the mitogens FGF2 and EGF. (b) Differentiation of SKP spheres. Under appropriate conditions, SKP spheres differentiate into neurons that express βIII tubulin, glial cells that express S100β, smooth muscle cells that express smooth muscle SMA and adipocytes containing characteristic intracellular lipid droplets. FGF2, fibroblast growth factor-2; EGF, epidermal growth factor; SMA, smooth muscle actin.
3. SKPs: what, where and why?
The notion of multi-lineage adult stem cell plasticity has been challenged by a number of findings in recent years, including demonstrations of cell fusion by bone marrow-derived cells (Alvarez-Dolado et al. 2003; Vassilopoulos et al. 2003; Wang et al. 2003; Nygren et al. 2004), an inability to repeat earlier reports of plasticity (Morshead et al. 2002; Vallieres & Sawchenko 2003; Wehner et al. 2003) and the production of non-functional cell types (Lu et al. 2004; Neuhuber et al. 2004). These findings raised several fundamental questions regarding the biological properties and in vivo relevance of cultured adult stem cells, which we have attempted to address with regards to SKPs. What is their developmental origin? Are SKPs an endogenous precursor cell? Where are SKPs located in vivo and what are their biological roles?
(a) SKPs exhibit properties of embryonic neural crest precursors
The capacity of SKPs to generate both neural and mesodermal progeny provided our first clue to their potential developmental origin. In particular, one embryonic precursor population with a similar developmental potential is NC stem cells. The embryonic NC is a subpopulation of neuroectodermally derived cells that undergoes an epithelial–mesenchymal transformation and subsequently exits from the dorsal neural tube. These embryonic NC cells consist of a transient population of multipotent and fate-biased precursors that ultimately generate a wide variety of non-CNS cell types. Among the clearly identified derivatives of NC cells are the entire peripheral (sensory, autonomic and enteric) nervous system, catecholaminergic adrenal cells, melanocytes, Merkel cells, and, at the cranial levels, the facial dermis, cartilage, bone, adipocytes and meninges (Halata et al. 1990; Couly et al. 1992; Le Douarin & Kalcheim 1999; Etchevers et al. 2001; Jiang et al. 2002a; Le Douarin & Dupin 2003). The NC also contributes a variety of mesodermal cell types outside the cranial regions, including adipocytes within parasympathetic ganglia of the gut (Le Lievre & Le Douarin 1975), peripheral nerve fibroblasts (Halata et al. 1990; Joseph et al. 2004) and the outflow tract of the heart (Fukiishi & Morriss-Kay 1992; Serbedzija & McMahon 1997; Jiang et al. 2000; Chan et al. 2004). Clonal analysis of embryonic NC cells showed that migrating multipotent NC precursors ultimately reach a variety of different target tissues and, while they were traditionally thought to terminally differentiate within these tissues, recent evidence has raised the possibility that these NC precursors persist in at least some adult tissues such as the gut (Kruger et al. 2002). We therefore examined the possibility that SKPs may be NC-derived precursors that are maintained into adulthood within the dermis of the skin.
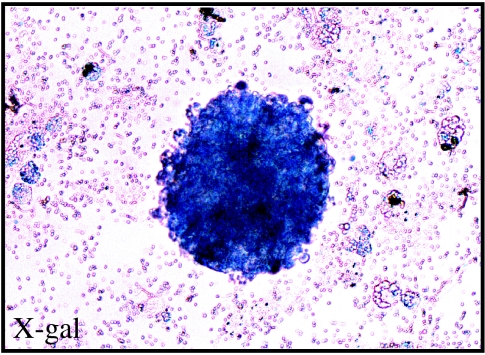
These studies revealed four lines of evidence supporting this possibility (Fernandes et al. 2004). First, a side-by-side comparison of gene expression in SKPs and NC-containing embryonic neural tube revealed that SKPs express a variety of NC-associated transcription factors including Slug, Snail, Twist, Pax3 and Sox9. Moreover, SKPs also expressed SHOX2 and Dermo-1, the transcription factors associated with the mesenchymal capability of cranial NC cells. In contrast, few of these transcription factors were expressed by CNS neural stem cells. Second, phenotypic analysis of the neural cells differentiated from SKPs revealed that they were largely peripheral nervous system cell types including Schwann cells and catecholaminergic neurons, cells that are derived from the NC during normal development. Third, when genetically tagged SKPs were transplanted into NC migratory pathways in the embryonic chick, they were able to specifically follow the host NC migratory streams, ultimately ending up in peripheral sensory and autonomic ganglia, peripheral nerves and the skin. Fourth, when SKPs were derived from the facial skin of Wnt1-cre;R26R compound transgenic mice, which selectively and indelibly express β-galactosidase in peripheral NC derivatives, SKP spheres were β-galactosidase positive (Fernandes et al. 2004; figure 2).
Figure 2.
SKPs from the facial skin of Wnt1-cre;R26R compound transgenic mice. Spheres generated from the facial skin of Wnt1-cre;R26R transgenic mice, which indelibly express β-galactosidase in neural crest derivatives, react positively with the β-galactosidase substrate X-gal.
Together these data strongly support the hypothesis that SKPs are NC-related precursors in the skin that retain multipotency into adulthood. These data also indicate that SKPs generated from facial skin derive from the NC embryonically. Do SKPs generated from trunk skin share this embryonic origin? Unfortunately, attempts to use the same lineage tracing approach to answer this question were confounded by the partial penetrance of the Wnt1-cre;R26R β-galactosidase transgene in back skin (Fernandes et al. 2004). In particular, while only a fraction of SKPs isolated from the back skin of these mice were β-galactosidase positive, many known NC progeny, including some melanocytes and Schwann cells, were also not β-galactosidase positive in the back skin (Fernandes et al. 2004), making such studies inconclusive. Thus, it is formally possible that trunk-derived SKPs have all of the properties of NC precursors, but derive from a different, as-yet-uncharacterized population of NC-like precursors in the embryo, and/or that they derive from NC stem cells that do not express Wnt1.
(b) SKPs are endogenous precursors that arise in skin during embryogenesis and persist into adulthood
A key question that has emerged in the stem cell field over the past several years is to what extent cultured precursor cells reflect the properties of the endogenous cells from which they are derived (Fernandes et al. 2004). This issue has arisen as a consequence of studies showing (i) that biased progenitors can dedifferentiate in culture to become multipotent (Kondo & Raff 2000), (ii) that cells with similarities to embryonic stem cells can be derived upon long-term culture of, for example, bone marrow cells (Jiang et al. 2002b,c), and (iii) in the most remarkable example, that nuclei isolated from even terminally differentiated cells like olfactory receptor neurons can be used to clonally generate an entire mouse (Eggan et al. 2004; Li et al. 2004). Thus, while it has always been thought that embryonic precursor cells like NC stem cells exhibit broader potential in culture than in their more restrictive in vivo environments, these new studies raise the possibility that much more profound alterations can occur as a consequence of long-term culture.
Is the multipotential nature of SKPs a consequence of such culture-induced alterations? Two lines of evidence argue against this possibility. In one series of studies, we asked whether skin contained a precursor cell capable of generating neurons, a SKP-derived cell type never found in skin, without any passaging and expansion as spheres. In these experiments, skin cells that were plated under conditions used to analyse the neurogenic potential of primary embryonic NC stem cells (Stemple & Anderson 1992; Shah et al. 1996; Morrison et al. 1999) were capable of generating cells with the morphological and antigenic characteristics of differentiated peripheral catecholaminergic neurons (Fernandes et al. 2004). Thus, skin contains a NPC cell that normally does not generate catecholaminergic neurons in vivo, but that can do so when placed in the appropriate environmental conditions in culture. In a second series of studies, we cultured skin cells under conditions where single cells gave rise to single, clonal spheres. These primary clones had all of the characteristics of SKPs, and when differentiated were multipotent. Thus, SKPs that had never been passaged had the same properties as SKPs that had been expanded in culture (Fernandes et al. 2004). Moreover, this clonal sphere assay allowed us to determine when the endogenous SKPs first arrive in skin. These studies indicated that mouse SKPs could first be detected in the back skin at embryonic day 15 (E15), that their numbers are then relatively high during late embryogenesis, decrease to lower levels at birth and then are maintained into adulthood (Fernandes et al. 2004). Together, these studies support the idea that SKPs represent an endogenous embryonic NC-derived precursor cell that persists into adulthood, and whose full potential is normally restricted by its in vivo environment but is revealed in culture.
(c) One niche for SKPs is the dermal papillae of hair and whisker follicles
If SKPs are restricted by their skin environment from generating, for example, neurons, then what is their in vivo environment? Four lines of evidence indicate that one niche for SKPs is the dermal papillae of hair and whisker follicles (Fernandes et al. 2004). First, in situ hybridization for transcription factors expressed by SKPs (as well as embryonic NC stem cells) such as Slug, Snail and Twist revealed that the most robust expression of these markers in adult mouse skin occurred in dermal papillae of follicles during the anagen growth phase. Thus, follicle papillae cells express SKP markers. Second, SKPs express markers that are enriched in follicle dermal papillae, including nexin, Wnt5a and versican. Third, when dermal papillae are dissected from whisker follicles and cultured under SKP conditions, they generate floating spheres of cells with the characteristics of SKPs. Finally, lineage tracing with the Wnt1-Cre;R26R mice revealed that whisker follicle dermal papillae are entirely NC derived, and that even dermal papillae of the back skin hair follicles contain NC-derived cells. Thus, these findings support the idea that follicle dermal papillae are a niche for the endogenous NC-derived SKPs. However, this is probably not the only niche for SKPs in skin, since SKPs can be routinely derived from human foreskin (Toma et al. 2005; described below), a tissue that contains no hair follicles.
Interestingly, follicular dermal cells, including dermal papillae cells, have long been thought to be multipotent precursors that potentially play an important role in normal skin turnover and in wound healing (Jahoda & Reynolds 2001; Gharzi et al. 2003). In this regard, cultured follicular dermal papilla cells have been shown to generate mesodermal cell types, including adipocytes and osteoblasts (Jahoda et al. 2003), and to even contribute to the haematopoietic system (Lako et al. 2002). They are also known to be the key regulators of hair follicle initiation and the follicle growth cycle, something that is discussed below. Like SKPs, follicle papillary cells proliferate in response to FGF2 and have been described as growing in clusters (Jahoda & Oliver 1984). Finally, follicle dermal papillae first condense at about E15 in mouse back skin, at the same time-point when SKPs can first be isolated, and the cells that form these embryonic papillae are positive for both TrkC and the p75 neurotrophin receptor (Botchkarev et al. 1998, 1999), markers of embryonic NC precursors (Stemple & Anderson 1992; Luo et al. 2003). Thus, the convergence of findings for follicular papillary cells and SKPs strongly support the idea that NC-derived SKPs comprise at least a fraction of the cells within the follicle dermal papillae.
4. Prospects for therapeutic applications of SKPs
One of the most exciting aspects of the ongoing work on SKPs is the prospect that they will provide an accessible, multipotent human precursor cell for a variety of purposes. While the most obvious of these is their potential use as an accessible source of cells for transplantation, human SKPs could also provide accessible human precursors for discovery research, something discussed in more detail below. As a requisite to such studies, however, it was essential to derive methods for isolating and expanding human SKPs, and to demonstrate that, like the rodent SKPs, they represented an accessible, multipotent precursor cell population.
(a) Human SKPs are similar to rodent SKPs
The initial work indicating that isolation of human SKPs was feasible was performed using ‘scalp tags’ from the placement of the stereotaxic neurosurgical apparatus on patients ranging from 40 to 70 years of age; small clusters of nestin-positive, fibronectin-positive cells could be derived from these very small scalp biopsies (Toma et al. 2001), suggesting that human skin also contained a SKP-like cell. More recent work using other human skin sources has now verified that human SKPs can be isolated and expanded, and are very similar to their rodent counterparts in terms of their potential. In our own work, we have focused upon human foreskins from 0–2-year-old males, and using a protocol very similar to that used for the rodent cells, we can routinely isolate and expand human SKPs. These human SKPs express markers typical of rodent SKPs, including NC transcription factors, and can differentiate into both neural and mesodermal progeny. Importantly, clonal analysis demonstrated that a single human cell can give rise to cells of both embryonic lineages. In addition, analysis of expanded human SKPs demonstrated that they maintain their differentiative abilities for at least 1 year in culture, and that these long-term passaged human SKPs maintained a normal karyotype, at least as analysed by G-banding. With regard to their embryonic origin, it is difficult to definitively ascertain whether human SKPs represent NC-derived precursors that are laid down in skin during embryogenesis and persist post-natally, as we believe to be the case for rodent SKPs. However, a number of lines of evidence indicate that they may well be. First, human SKPs express transcription factors expressed by NC stem cells. Second, human foreskin contains a population of NPCs that can differentiate into neurons without any isolation, passaging or expansion. Third, human SKPs have a potential similar to NC precursors, generating mesodermal progeny such as adipocytes, and neural cell types that are peripheral in nature, such as Schwann cells and catecholaminergic neurons (Toma et al. 2005).
Two recent papers have provided independent evidence in support of the existence of SKP-like NPCs in human skin (Belicchi et al. 2004; Joannides et al. 2004). In one study, the dermis of skin biopsy samples from 41 to 77-year-old healthy adults were grown into SKP-like spheres using FGF2 and EGF, and then the isolated spheres were adherently expanded using a serum-based protocol. These expanded adherent cultures retained the ability to generate spheres expressing the NPC markers nestin and musashi when placed in SKP-like serum-free conditions, and clonal analysis revealed a neural/mesodermal bipotentiality. Spheres generated following serum-based expansion were able to produce neurons upon differentiation, and neurogenesis was enhanced in the presence of hippocampal astrocyte-conditioned medium (Joannides et al. 2004), as previously reported for CNS NPCs (Song et al. 2002).
In a second study, skin samples were obtained from 14-week aborted foetuses and from 12 to 65-year-old healthy volunteers. In this study, skin cells were first fluorescent activated cell sorted for the human AC133(+) fraction, which has previously been shown to contain the sphere-forming cells from multiple stem cell populations, including human neural stem cells (Uchida et al. 2000). Consistent with this notion, no sphere-forming activity was found in the AC133-negative population. Single AC133(+) cells grew into spheres that could be clonally passaged, expressed nestin, and could differentiate into neuronal cells. Following transplantation into the lateral ventricles of neonatal immune-compromised SCID mice, cells from the AC133(+)-derived spheres displayed robust migration and some evidence of neuronal and glial differentiation (Belicchi et al. 2004). Thus, cells with the properties of SKPs have been isolated from human skin of differing ages and origins, supporting the notion that SKPs provide an accessible, autologous human stem cell population.
(b) Functionality of SKP-derived neural and mesodermal cells
For any precursor cell population to be used therapeutically, it is essential that rigorous functional assays be performed to definitively establish that they actually generate bona fide differentiated cell types. This is particularly true as there are experimental limitations to many of the assays that are commonly used. For example, some groups have found that bone marrow-derived cells can, under some conditions, undergo a rapid apparent neuronal transdifferentiation, but recent studies indicate that such ‘neurons’ are actually non-functional (Lu et al. 2004; Neuhuber et al. 2004). In addition, the apparent transition of bone marrow to neural, cardiomyocyte and hepatic lineages in vivo may be largely, if not completely, due to cell fusion (Alvarez-Dolado et al. 2003; Vassilopoulos et al. 2003; Wang et al. 2003; Nygren et al. 2004). Thus, it is essential to ensure the functionality of cell types generated from stem cells via a variety of assays with complementary strengths. In this regard, our approach has been to first pursue culture studies, looking for (i) appropriate morphological changes, (ii) selective induction and expression of cell type-specific genes, and (iii) stability of differentiated cellular phenotypes, and to then use both ex vivo and in vivo transplantation to assay functionality. We are currently pursuing such an approach to ascertain the validity and functionality of SKP-derived neural and mesodermal cell types, and will discuss our neural work in more detail here.
As mentioned previously, SKPs apparently generate peripheral neural progeny, consistent with a NC origin. Schwann cells are a peripheral glial cell type of therapeutic interest, owing to their ability to myelinate axons and to provide a conducive growth and regeneration environment for CNS axons (Richardson et al. 1980, 1984; David & Aguayo 1981; Xu 1995b, 1997, 1999; Pearse et al. 2004). To ask whether SKPs generate bona fide Schwann cells, we performed a series of culture and in vivo studies, all of which strongly support the conclusion that SKP-derived Schwann cells are ‘real’ (McKenzie et al. 2006). First, SKPs responded to extrinsic cues that have been shown to enhance the differentiation and the proliferation of Schwann cells from NC stem cells to produce cultures highly enriched in cells with the morphological and biochemical phenotype of Schwann cells; they generated parallel arrays of bipolar cells that expressed the Schwann cell markers S100β, glial fibrillary acidic protein (GFAP), myelin basic protein (MBP), protein 0 (P0), peripheral myelin protein-22 (PMP22) and p75NTR (Fernandes et al. 2004). Importantly, they do not express the proteins characteristic of neurons or other mesodermal cell types. Second, when these SKP-derived Schwann cells were cultured in the presence of peripheral neural explants, they associated with axons, divided and expressed myelin proteins, while at the same time undergoing a morphological differentiation consistent with myelination. Third, when SKPs were transplanted into either the peripheral nerve or the CNS of shiverer mutant mice, which are genetically deficient in a key myelin protein, MBP (Rosenbluth 1980a,b), they myelinated axons, as shown by electron microscopy. Significantly, this myelination was also seen with human SKPs. Thus, by all criteria, including production of functional myelin, SKPs generate bona fide Schwann cells (McKenzie et al. 2006).
What about SKP-derived neurons? Differentiation of SKPs under neurogenic conditions has resulted in the production of cells that fulfil most, but not yet all, criteria for neuronal differentiation (Fernandes 2006). In culture, differentiating rodent SKPs undergo an appropriate sequence and time-course of changes, progressing from proliferating cells that express the NPC marker nestin to non-proliferating cells that adopt complex neuron-like morphologies and express pan-neuronal genes (βIII tubulin, neurofilaments, GAP-43 and MAP2). These SKP-derived neurons are probably peripheral in nature, since they express peripherin and proteins characteristic of a catecholaminergic phenotype, and almost all of them are positive for p75NTR, a hallmark of peripheral neurons. These putative neurons do not express any non-neuronal protein that we have examined. These SKP-derived neurons further mature when they are transplanted into slice cultures derived from the hippocampus, as they express and maintain expression of the catecholaminergic neurotransmitter enzyme, tyrosine hydroxylase, for at least five weeks, and begin to express neuronal voltage-gated calcium channels. Interestingly, the neurons differentiated from SKPs maintain their peripheral phenotype, even in this CNS environment. However, the gold standard for demonstrating functional neuronal differentiation is appropriate electrophysiological characteristics, including the generation of depolarization-induced action potentials, something that we are starting to assay now. In general, reaching this standard using multipotent stem cells has proven to be difficult, requiring long and somewhat complex differentiation protocols and the presence of astrocyte-associated factors. Nonetheless, this is a necessary demonstration that will provide the ultimate proof that a given stem cell-derived cell is a bona fide neuron.
(c) Potential clinical applications for human SKPs
We are currently exploring several potential therapeutically relevant uses for SKPs, including autologous cell-replacement strategies, the effects of genetic alterations on stem cell function and differentiation and the potential involvement of transformed SKP-like precursors in tumours of NC-derived tissues.
(i) Cell-replacement strategies
The ability to differentiate SKPs into functional cell types has potential therapeutic implications for autologous cell-replacement strategies. As described above, a particularly promising avenue is cell therapy using SKP-derived glial cells, as we have generated considerable data indicating that SKPs can be used to produce functional myelinating Schwann cells which have potent growth-promoting effects on injured CNS neurons, and have extensively been used to promote regeneration of CNS axons in animal spinal cord injury models (Richardson et al. 1980; Paino et al. 1994; Xu 1995a,b, 1997, 1999; Guest et al. 1997; Menei et al. 1998; Oudega et al. 2001; Bunge & Pearse 2003; Pearse et al. 2004; Fouad et al. 2005). Moreover, the ability of Schwann cells to myelinate may make them useful replacement cells for oligodendrocytes that degenerate following spinal cord injury (Crowe et al. 1997; Shuman et al. 1997; Li et al. 1999; Casha et al. 2001; Warden et al. 2001; Beattie et al. 2002) or as a consequence of multiple sclerosis (Brierley et al. 2001; Halfpenny et al. 2002; Bachelin et al. 2005).
Besides neural cell types, we have also found that SKP cultures can be directed to generate skeletogenic derivatives normally associated with cranial NC cells. Specifically, SKPs reproducibly generate characteristic nodules that express chondrocyte oligomeric matrix protein and type II collagen, synthesize chondrocytic proteoglycans and ultimately produce mineralized calcium deposits associated with osteoblast activity (K. Fernandes, J. Biernaskie, J.-F. Lavoie and F. Miller 2002, unpublished data). Thus, SKPs may be useful as a source of certain mesenchymal derivatives.
(ii) Mechanisms of disease development in genetic disorders
In addition to transplantation-based therapies, the availability of accessible, autologous NPCs also opens novel doors to exploring the mechanisms and potential treatments of genetically based neural disorders. SKPs derived from humans having neurodegenerative conditions may serve as useful tools for investigating the effects of specific genetic alterations on the essential properties of NPCs (i.e. survival, proliferation and differentiation) or, alternatively, on the characteristics and functions of cells differentiated from mutant NPCs. Such studies on the cell biological properties of genetically compromised precursors, neurons or glial cells could provide novel information on the mechanisms of neurodegeneration. A similar approach has recently proved useful in defining the role of gut-derived NC stem cells in Hirschprung's disease (Iwashita et al. 2003).
(iii) Involvement of SKP-like cells in tumours of neural crest-derived tissues
One particularly exciting idea emerges from recent work documenting putative ‘cancer stem cells’ in paediatric neural tumours (Singh et al. 2003, 2004a,b). Such studies raise the possibility that transformed SKP-like ‘neural crest cancer stem cells’ may be important for the genesis of peripheral tumours of the NC. If this were the case, then cultures of transformed SKP-like cells isolated from biopsies could be expanded in vitro for drug screening and optimization of anti-cancer treatments. Conveniently, in at least some instances, tumorigenic and non-tumorigenic precursors could even be isolated from the same individuals, providing an ideal control tissue. In this regard, one candidate disorder is neuroblastoma (NB), which is the most common children's tumour and the most common extracranial paediatric solid tumour. NB affects migrating early NC precursors of the sympathoadrenal lineage and results in tumours whose primary sites are the adrenal glands and paraspinal sympathetic ganglia, with metastases to bone, lymph nodes, liver and even skin (Kushner 2004; Henry et al. 2005). A second candidate is neurofibromatosis type 1 (NF1; Lakkis & Tennekoon 2000; De Schepper et al. 2005). NF1 is a highly prevalent (1 : 3500) autosomal dominant disorder that results from mutations in the gene encoding neurofibromin. Clinical symptoms of NF1 are primarily observed in NC-derived cells and tissues, and include nerve sheath tumours, melanocytic hamartomas, Café au Lait spots, freckling in sun-protected areas and bone lesions. The presence of Schwann cell, fibroblastic and melanocytic tumour types in NF1 is particularly intriguing in light of the phenotypic instability between these NC-derived cells (Rizvi et al. 2002; Dupin et al. 2003; Real et al. 2005). A third class of disorders that may contain transformed SKP-like cells are tumours of the melanocyte NC lineage. The common dermal dendritic melanocytic proliferations contain embryonic-like spindled and dendritic melanocytes, and the classifications include dermal melanocytic hamartomas, several variants of benign blue naevi, malignant melanocytic tumours (malignant blue naevi) as well as borderline melanocytic tumours that appear to be of mixed neuroectodermal origin (Zembowicz & Mihm 2004).
5. Implications and unresolved questions
(a) Relationship between SKPs and other skin stem cells
Our findings that SKPs are a multipotent NC-related precursor that is laid down in the dermis during embryogenesis and persists into adulthood in a follicle dermal papilla niche raise a number of intriguing questions concerning possible physiological roles for SKPs.
One potential role for SKPs is in the regulation of the hair and whisker follicle growth cycle, given their niche in the follicle dermal papillae. The dermal cells that reside in these papillae are essential for the hair follicle growth cycle and have therefore been studied extensively. Interestingly, follicle dermal papillae are known to contain a resident precursor cell population: cultured papillary cells can differentiate into adipocytes and osteoblasts (Jahoda et al. 2003), and can even contribute to a small degree to the haematopoietic system in reconstitution experiments (Lako et al. 2002). In vivo, the cells within the follicle dermal papillae undergo marked changes during the hair cycle, increasing in number during anagen stages of hair follicle growth and decreasing during catagen stages of follicle decline. These changes may be partially due to the exchange of cells between the papilla and the nearby follicular connective tissue sheath (Tobin 2003a,b). The papilla cells are essential for hair follicle formation initially during embryogenesis and then on an ongoing basis in mature animals. During the normal hair cycle, potent papilla-derived signals such as Noggin and perhaps Wnts instruct the downward migration of epidermal stem cells residing within the hair follicle bulge, which then differentiate into new hair follicle cells and consequently generate a new hair shaft (Lavker et al. 2003). As a reflection of this essential regulatory role, transplanted dermal papillae are capable of inducing de novo hair follicle formation within the interfollicular epidermis (Reynolds & Jahoda 1992) and play a role in the conversion of adult corneal epithelium to follicular epithelium in response to embryonic dermis (Pearton et al. 2005). Our recent findings, therefore, raise a number of key questions in this regard. First, what proportion of the cells within the follicle dermal papillae are SKPs? Second, are SKPs the same cells as the previously characterized precursor cell population resident in this niche? Finally, are the precursor cells within the follicle papillae the same cells that regulate the hair follicle growth cycle?
SKPs may also provide a resident adult stem cell source for NC-derived cell types in the skin. In the face, the entire dermis is comprised of NC-derived progeny (Le Lievre & Le Douarin 1975; Nakamura 1982), while even in the thoracic regions, the dermis contains many different NC-derived cell types, including Schwann cells, Merkel cells and melanocytes. Intriguingly, Merkel cells are now known to be NC derived (Szeder et al. 2003), and previous work has demonstrated that the dermis contains a precursor cell capable of giving rise to Merkel cells in vivo (Nurse et al. 1984). Could SKPs be this resident dermal NC precursor cell? Given the multipotent nature of SKPs, they might also contribute to the mesodermal components of the dermis, including dermal fibroblasts, either during normal physiological turnover or during wound healing. In this regard, cells of the follicle dermal papillae and sheath have previously been proposed to participate in wound healing in the skin (Jahoda & Reynolds 2001).
In addition to questions regarding their potential physiological role, the elucidation of a novel precursor cell in skin also leads to questions about their interactions with other resident precursor cell populations. For example, MSCs are widespread throughout a variety of tissues (Young et al. 1995), including the dermal layer of the skin, where they are considered the source of the dermal connective tissue. Since MSCs themselves are embryonically derived from either the NC (cranially) or the mesoderm (sub-cranially), further work will be required to clarify the relationship between MSCs and SKPs. One possibility is that SKPs can differentiate in the dermis into a more mesodermally restricted precursor cell with the characteristics of MSCs. A second possibility is that these two populations of cells are independently generated within the dermis, with the MSCs contributing to mesodermal cell types, and SKPs representing a more multipotent precursor that also generates other NC-derived progeny found in the dermis.
Similar issues arise regarding SKPs and other known NC cells resident in the hair follicle. A number of NC-derived cell types are present within the hair follicle bulge and sub-bulge regions, including melanocyte stem cells (Nishimura et al. 2002), recently described epidermal NC stem cells (eNCSCs; Sieber-Blum et al. 2004), and non-innervated Merkel-like cells of unknown function (Narisawa et al. 1994). Melanocyte stem cells are clearly labelled in the bulge/sub-bulge area in dct–lacZ transgenic mice, and these generate transit-amplifying cells that differentiate into mature melanocytes in the hair follicle matrix immediately above the dermal papilla (Nishimura et al. 2002). Little is known regarding the functions of eNCSCs and non-innervated Merkel-like cells, though it has been hypothesized that they may serve a key regulatory function within this critical epidermal stem cell niche (Christiano 2004). Although SKPs and bulge NC-derived cells are present in different layers of the skin, it is possible that they are developmentally related and/or functionally interact, particularly given the regular emigration of cells from the bulge into the lower follicle and from the dermal papillae into the overlying connective tissue sheath (Taylor et al. 2000; Lavker et al. 2003; Tobin 2003a,b; Blanpain et al. 2004; Tumbar et al. 2004). It is even possible that under some conditions SKPs might give rise to more restricted NC precursors within the follicle such as melanocyte stem cells and/or might exchange with the eNCSCs in the bulge region. It also remains to be established whether any of these populations of NC precursors are related to recently described follicular cells that express a nestin–GFP transgene and that are reported to have the ability to differentiate into blood vessels, neurons and myelinating Schwann cells (Li et al. 2003; Amoh et al. 2004, 2005a–c).
(b) Potential origins of SKPs
Our recent data indicate that the first cells with SKP-forming ability arrive in murine back skin at E15, and that their numbers are high during late embryogenesis, decline somewhat into the early post-natal period, and then again in the adult (Fernandes et al. 2004). Given their probable NC origin, the most parsimonious explanation for this time-course is that the relevant NC precursors migrate into skin at E14/E15. In this regard, NC cells could arrive in the skin via a number of migratory routes. In mice, a first wave of trunk NC migration occurs at approximately E9–9.5, when crest cells migrate ventromedially from the neural tube (Serbedzija et al. 1990, 1994; Wilson et al. 2004). These cells generate diverse peripheral nervous system derivatives, including the peripheral nerves that eventually innervate targets that include the skin. Interestingly, multipotent NC stem cells (NCSCs) are present in the rodent peripheral sciatic nerve at E15 (Morrison et al. 1999), the time-point when (i) mouse back skin is first innervated (Peters et al. 2002), (ii) SKPs are first detected in mouse back skin (Fernandes et al. 2004), and (iii) the follicle dermal papillae first form from a population of cells expressing TrkC and p75NTR (Botchkarev et al. 1998, 1999), both of which are expressed on NC precursors (Stemple & Anderson 1992; Luo et al. 2003). Within the peripheral nerve, these NCSCs then apparently disappear by birth (Morrison et al. 1999), something that has been attributed to their terminal differentiation within the nerve itself. However, we propose that a subpopulation of these nerve NCSCs migrate into the skin, participate in the formation of the follicle dermal papillae, and then are maintained as SKPs into adulthood. Indeed, some evidence for nerve-derived NC cells populating the skin has been described in quail–chick chimera experiments (Halata et al. 1990). One prediction of this model is that other peripheral tissues might also contain SKP-like cells that are laid down during embryogenesis when they are first innervated.
Two other NC pathways could contribute NC precursors to skin. First, a second wave of trunk NC migration begins at approximately E10.5 (Serbedzija et al. 1990, 1994; Wilson et al. 2004), when crest cells delaminating from the neural tube shift into a dorsolateral migration pathway, taking them through the developing dermis, from where they ultimately migrate directly into the epidermis of the skin. Cells migrating along this pathway are predominantly of the melanocyte lineage (Wakamatsu et al. 1998). This migration reaches the dermis well before E15, and thus is unlikely to represent the predominant route for SKPs. Another potential source of NC-derived cells in skin is NC boundary cap cells. Boundary cap cells are NC cells that cluster at the sites of spinal and cranial nerve root entry/exit points into the CNS, i.e. the dorsal root entry zone and the ventral motor exit points. Embryonically, boundary cap cells help to regulate the passage of cells and axonal projections between the spinal roots and the CNS (Golding & Cohen 1997; Vermeren et al. 2003), but following the end of NC cell migration from the neural tube, they subsequently migrate distally down peripheral nerves, generating Schwann cells within the spinal roots and nociceptive neurons and satellite cells within the sensory ganglia (Maro et al. 2004; Hjerling-Leffler et al. 2005). Owing to the lack of specific boundary cap markers, it remains unclear how far distally these cells migrate, and whether they enter peripheral target tissues. However, it is possible that boundary cap cells correspond to previously identified cell populations that migrate along peripheral nerves to colonize sensory ganglia and skin (Sharma et al. 1995) and various peripheral target tissues (ventrally emigrating neural tube cells; Ali et al. 1999, 2003a,b; Sohal 1999a,b, 2002) such as trigeminal ganglia, facial cartilage, the gut, heart and vestibular system.
While we have focused here upon potential NC sources for SKPs, we have only formally shown that cranial SKPs are NC derived. Is it possible that non-cranial (thoracic) SKPs express NC-like characteristics without actually being embryologically derived from the NC? In this respect, it is worth noting the following points. First, there are a number of cell types and tissues that are produced developmentally by both the NC and the mesoderm, depending upon their location. For example, bone, cartilage and the entire dermis are produced by NC-derived mesenchymal cells in the craniofacial area (Le Lievre & Le Douarin 1975) and by mesoderm-derived mesenchymal cells in most of the rest of the body. Consistent with the notion that cells of different embryonic lineages can express similar genetic programmes, a recent study has shown that whisker follicle dermal papilla cells, which are NC derived (Fernandes et al. 2004), behave virtually indistinguishably from bone marrow-derived MSCs in their ability to expand and differentiate into bone, cartilage, fat and muscle (Hoogduijn et al. 2006). Thus, even though back skin dermal papilla express considerable NC-like properties, as further illustrated in a recent gene profiling study (Rendl et al. 2005), it is possible that they are derived from the mesodermal lineage.
Second, consider the examples of the vertebrate neurogenic placodes, particularly the olfactory, trigeminal, otic and epibranchial placodes (Graham & Begbie 2000). Developmentally, the neurogenic placodes are identified as focal regions of thickened cranial ectoderm located outside the neural plate (which is the source of the CNS and NC). These placodes give rise to a large percentage of cranial sensory neurons (including olfactory sensory neurons, trigeminal neurons and vestibular neurons), as well as a number of more specialized cell types (such as glial cells, olfactory epithelium, neuroendocrine cells and sensory hair cells). Thus, during normal development, neurons and glial cells can be generated from areas besides the CNS and NC. Can neurogenic placodes give rise to SKP-like cells? We have previously asked this question focusing upon the olfactory placode-derived olfactory epithelium. Using SKP culture conditions, we have isolated a population of cells from the P14 olfactory epithelium that (i) grows and can be expanded as spheres in response to FGF2 and EGF, (ii) expresses a marker profile similar to SKPs, including expression of nestin and fibronectin, and (iii) differentiates into neurons, glia and smooth muscle cells (J.G. Toma, A. Gloster, and F.D. Miller 1994, unpublished data; figure 3). Similar results have been obtained by others with the human olfactory epithelium (Zhang et al. 2004; Murrell et al. 2005). Thus, placodally derived structures contain a SKP-like cell, raising the possibility that SKPs themselves could potentially derive from an embryonic source other than the NC.
Figure 3.
Characterization and differentiation of precursors isolated from the rodent olfactory epithelium. (a) Immunocytochemical comparison of spheres grown from embryonic rodent cortex, post-natal rodent olfactory epithelium and post-natal rodent skin. Note that olfactory epithelium-derived spheres have an immunocytochemical phenotype intermediate to cortical and skin-derived spheres. For example, while only very rare cells from SKP spheres were positive for the p75 neurotrophin receptor (p75NTR) and many cells from CNS cortical neurospheres were positive, a significant fraction of cells from the olfactory epithelium also expressed this receptor. (b) Cells differentiating for 1 day from olfactory epithelium-derived spheres co-express nestin and fibronectin, similar to SKPs. (c) After 2–3 weeks of differentiation, olfactory epithelium-derived spheres generate neurons that express βIII tubulin, MAP2 and NFM, smooth muscle cells that express SMA, and glial cells that express CNPase and GFAP. MAP2, microtubule-associated protein-2; NFM, medium neurofilaments; SMA, smooth muscle actin; CNPase, cyclic nucleotide phosphohydrolase; GFAP, glial fibrillary acidic protein.
6. Conclusions
Regardless of embryonic origin, the finding that SKP-like cells reside in the adult olfactory epithelium, together with the possibility that SKPs migrate into peripheral tissues via embryonic peripheral nerves, raises the possibility that SKP-like cells may be present in many peripheral tissues. Further support for this idea derives from a recent report where SKP culture conditions were used to identify a multipotent cell from human dental pulp, a NC-derived tissue (Miura et al. 2003). Whether the SKP-like cells that have been isolated from these different tissues are similar in terms of their potency and/or origin is still an open question, but these findings do raise the provocative possibility that many adult tissues contain multipotent NC-related precursors that were laid down in those tissues during embryogenesis, and that have persisted into the adult.
Acknowledgments
The authors would like to thank Anne Aumont and Mahnaz Akhavan for their excellent technical assistance, and members of the laboratories of Dr Freda Miller and Dr David Kaplan for their constructive discussions. This work was supported by research grants from the Canadian Stem Cell Network and the Canadian Institutes of Health Research (CIHR). K.J.L.F. was supported by fellowships from CIHR/Neurotrauma and the Toronto Hospital for Sick Children Restracomp programme. F.D.M. is the Canada Research Chair in Developmental Neurobiology.
Footnotes
One contribution of 14 to a Theme Issue ‘Stem cells and brain repair’.
References
- Ali A.A, Ali M.M, Dai D, Sohal G.S. Ventrally emigrating neural tube cells differentiate into vascular smooth muscle cells. Gen. Pharmacol. 1999;33:401–405. doi: 10.1016/s0306-3623(99)00034-8. doi:10.1016/S0306-3623(99)00034-8 [DOI] [PubMed] [Google Scholar]
- Ali M.M, Farooqui F.A, Sohal G.S. Ventrally emigrating neural tube cells contribute to the normal development of heart and great vessels. Vascul. Pharmacol. 2003a;40:133–140. doi: 10.1016/s1537-1891(03)00003-x. doi:10.1016/S1537-1891(03)00003-X [DOI] [PubMed] [Google Scholar]
- Ali M.M, Jayabalan S, Machnicki M, Sohal G.S. Ventrally emigrating neural tube cells migrate into the developing vestibulocochlear nerve and otic vesicle. Int. J. Dev. Neurosci. 2003b;21:199–208. doi: 10.1016/s0736-5748(03)00036-4. [DOI] [PubMed] [Google Scholar]
- Alvarez-Dolado M, Pardal R, Garcia-Verdugo J.M, Fike J.R, Lee H.O, Pfeffer K, Lois C, Morrison S.J, Alvarez-Buylla A. Fusion of bone marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. doi:10.1038/nature02069 [DOI] [PubMed] [Google Scholar]
- Amoh Y, Li L, Yang M, Moossa A.R, Katsuoka K, Penman S, Hoffman R.M. Nascent blood vessels in the skin arise from nestin-expressing hair-follicle cells. Proc. Natl Acad. Sci. USA. 2004;101:13 291–13 295. doi: 10.1073/pnas.0405250101. doi:10.1073/pnas.0405250101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoh Y, Li L, Campillo R, Kawahara K, Katsuoka K, Penman S, Hoffman R.M. Implanted hair follicle stem cells form Schwann cells that support repair of severed peripheral nerves. Proc. Natl Acad. Sci. USA. 2005a;102:17 734–17 738. doi: 10.1073/pnas.0508440102. doi:10.1073/pnas.0508440102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoh Y, Li L, Katsuoka K, Penman S, Hoffman R.M. Multipotent nestin-positive, keratin-negative hair-follicle bulge stem cells can form neurons. Proc. Natl Acad. Sci. USA. 2005b;102:5530–5534. doi: 10.1073/pnas.0501263102. doi:10.1073/pnas.0501263102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoh Y, Li L, Yang M, Jiang P, Moossa A.R, Katsuoka K, Hoffman R.M. Hair follicle-derived blood vessels vascularize tumors in skin and are inhibited by Doxorubicin. Cancer Res. 2005c;65:2337–2343. doi: 10.1158/0008-5472.CAN-04-3857. doi:10.1158/0008-5472.CAN-04-3857 [DOI] [PubMed] [Google Scholar]
- Bachelin C, et al. Efficient myelin repair of the macaque spinal cord by autologous grafting of Schwann cells. Brain. 2005;128:540–549. doi: 10.1093/brain/awh406. doi:10.1093/brain/awh406 [DOI] [PubMed] [Google Scholar]
- Bani-Yaghoub M, Kendall S.E, Moore D.P, Bellum S, Cowling R.A, Nikopoulos G.N, Kubu C.J, Vary C, Verdi J.M. Insulin acts as a myogenic differentiation signal for neural stem cells with multilineage differentiation potential. Development. 2004;131:4287–4298. doi: 10.1242/dev.01295. doi:10.1242/dev.01295 [DOI] [PubMed] [Google Scholar]
- Beattie M.S, Hermann G.E, Rogers R.C, Bresnahan J.C. Cell death in models of spinal cord injury. Prog. Brain Res. 2002;137:37–47. doi: 10.1016/s0079-6123(02)37006-7. [DOI] [PubMed] [Google Scholar]
- Belicchi M, et al. Human skin-derived stem cells migrate throughout forebrain and differentiate into astrocytes after injection into adult mouse brain. J. Neurosci. Res. 2004;77:475–486. doi: 10.1002/jnr.20151. doi:10.1002/jnr.20151 [DOI] [PubMed] [Google Scholar]
- Bjornson C.R, Rietze R.L, Reynolds B.A, Magli M.C, Vescovi A.L. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999;283:534–537. doi: 10.1126/science.283.5401.534. doi:10.1126/science.283.5401.534 [DOI] [PubMed] [Google Scholar]
- Blanpain C, Lowry W.E, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. doi:10.1016/j.cell.2004.08.012 [DOI] [PubMed] [Google Scholar]
- Botchkarev V.A, Botchkarev N.V, Albers K.M, van der Veen C, Lewin G.R, Paus R. Neurotrophin-3 involvement in the regulation of hair follicle morphogenesis. J. Invest. Dermatol. 1998;111:279–285. doi: 10.1046/j.1523-1747.1998.00277.x. doi:10.1046/j.1523-1747.1998.00277.x [DOI] [PubMed] [Google Scholar]
- Botchkareva N.V, Botchkarev V.A, Chen L.H, Lindner G, Paus R. A role for p75 neurotrophin receptor in the control of hair follicle morphogenesis. Dev. Biol. 1999;216:135–153. doi: 10.1006/dbio.1999.9464. doi:10.1006/dbio.1999.9464 [DOI] [PubMed] [Google Scholar]
- Brazelton T.R, Rossi F.M, Keshet G.I, Blau H.M. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. doi:10.1126/science.290.5497.1775 [DOI] [PubMed] [Google Scholar]
- Brazelton T.R, Nystrom M, Blau H.M. Significant differences among skeletal muscles in the incorporation of bone marrow-derived cells. Dev. Biol. 2003;262:64–74. doi: 10.1016/s0012-1606(03)00357-9. doi:10.1016/S0012-1606(03)00357-9 [DOI] [PubMed] [Google Scholar]
- Brierley C.M, Crang A.J, Iwashita Y, Gilson J.M, Scolding N.J, Compston D.A, Blakemore W.F. Remyelination of demyelinated CNS axons by transplanted human Schwann cells: the deleterious effect of contaminating fibroblasts. Cell Transplant. 2001;10:305–315. [PubMed] [Google Scholar]
- Bunge M.B, Pearse D.D. Transplantation strategies to promote repair of the injured spinal cord. J. Rehabil. Res. Dev. 2003;40:55–62. doi: 10.1682/jrrd.2003.08.0055. [DOI] [PubMed] [Google Scholar]
- Casha S, Yu W.R, Fehlings M.G. Oligodendroglial apoptosis occurs along degenerating axons and is associated with FAS and p75 expression following spinal cord injury. Neuroscience. 2001;103:203–218. doi: 10.1016/s0306-4522(00)00538-8. doi:10.1016/S0306-4522(00)00538-8 [DOI] [PubMed] [Google Scholar]
- Chan W.Y, Cheung C.S, Yung K.M, Copp A.J. Cardiac neural crest of the mouse embryo: axial level of origin, migratory pathway and cell autonomy of the splotch (Sp2H) mutant effect. Development. 2004;131:3367–3379. doi: 10.1242/dev.01197. doi:10.1242/dev.01197 [DOI] [PubMed] [Google Scholar]
- Christiano A.M. Epithelial stem cells: stepping out of their niche. Cell. 2004;118:530–532. doi: 10.1016/j.cell.2004.08.024. doi:10.1016/j.cell.2004.08.024 [DOI] [PubMed] [Google Scholar]
- Clarke D.L, Johansson C.B, Wilbertz J, Veress B, Nilsson E, Karlstrom H, Lendahl U, Frisen J. Generalized potential of adult neural stem cells. Science. 2000;288:1660–1663. doi: 10.1126/science.288.5471.1660. doi:10.1126/science.288.5471.1660 [DOI] [PubMed] [Google Scholar]
- Cogle C.R, Yachnis A.T, Laywell E.D, Zander D.S, Wingard J.R, Steindler D.A, Scott E.W. Bone marrow transdifferentiation in brain after transplantation: a retrospective study. Lancet. 2004;363:1432–1437. doi: 10.1016/S0140-6736(04)16102-3. doi:10.1016/S0140-6736(04)16102-3 [DOI] [PubMed] [Google Scholar]
- Corbel S.Y, Lee A, Yi L, Duenas J, Brazelton T.R, Blau H.M, Rossi F.M. Contribution of hematopoietic stem cells to skeletal muscle. Nat. Med. 2003;9:1528–1532. doi: 10.1038/nm959. doi:10.1038/nm959 [DOI] [PubMed] [Google Scholar]
- Couly G.F, Coltey P.M, Le Douarin N.M. The developmental fate of the cephalic mesoderm in quail-chick chimeras. Development. 1992;114:1–15. doi: 10.1242/dev.114.1.1. [DOI] [PubMed] [Google Scholar]
- Crowe M.J, Bresnahan J.C, Shuman S.L, Masters J.N, Beattie M.S. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat. Med. 1997;3:73–76. doi: 10.1038/nm0197-73. doi:10.1038/nm0197-73 [DOI] [PubMed] [Google Scholar]
- David S, Aguayo A.J. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. doi:10.1126/science.6171034 [DOI] [PubMed] [Google Scholar]
- De Schepper S, Boucneau J, Lambert J, Messiaen L, Naeyaert J.M. Pigment cell-related manifestations in neurofibromatosis type 1: an overview. Pigment Cell Res. 2005;18:13–24. doi: 10.1111/j.1600-0749.2004.00206.x. doi:10.1111/j.1600-0749.2004.00206.x [DOI] [PubMed] [Google Scholar]
- Doyonnas R, LaBarge M.A, Sacco A, Charlton C, Blau H.M. Hematopoietic contribution to skeletal muscle regeneration by myelomonocytic precursors. Proc. Natl Acad. Sci. USA. 2004;101:13 507–13 512. doi: 10.1073/pnas.0405361101. doi:10.1073/pnas.0405361101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupin E, Real C, Glavieux-Pardanaud C, Vaigot P, Le Douarin N.M. Reversal of developmental restrictions in neural crest lineages: transition from Schwann cells to glial-melanocytic precursors in vitro. Proc. Natl Acad. Sci. USA. 2003;100:5229–5233. doi: 10.1073/pnas.0831229100. doi:10.1073/pnas.0831229100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggan K, Baldwin K, Tackett M, Osborne J, Gogos J, Chess A, Axel R, Jaenisch R. Mice cloned from olfactory sensory neurons. Nature. 2004;428:44–49. doi: 10.1038/nature02375. doi:10.1038/nature02375 [DOI] [PubMed] [Google Scholar]
- Etchevers H.C, Vincent C, Le Douarin N.M, Couly G.F. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development. 2001;128:1059–1068. doi: 10.1242/dev.128.7.1059. [DOI] [PubMed] [Google Scholar]
- Fernandes K.J, et al. A dermal niche for multipotent adult skin-derived precursor cells. Nat. Cell Biol. 2004;6:1082–1093. doi: 10.1038/ncb1181. doi:10.1038/ncb1181 [DOI] [PubMed] [Google Scholar]
- Fernandes, K. J., Kobayashi, N. R., Gallagher, C. J., Barnabe´-Heider, F., Aumont, A., Kaplan, D. R. & Miller, F. D. 2006. Analysis of the neurogenic potential of multipotent skin-derived precursors. Exp. Neurol.201, 32–48. [DOI] [PubMed]
- Fouad K, Schnell L, Bunge M.B, Schwab M.E, Liebscher T, Pearse D.D. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J. Neurosci. 2005;25:1169–1178. doi: 10.1523/JNEUROSCI.3562-04.2005. doi:10.1523/JNEUROSCI.3562-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukiishi Y, Morriss-Kay G.M. Migration of cranial neural crest cells to the pharyngeal arches and heart in rat embryos. Cell Tissue Res. 1992;268:1–8. doi: 10.1007/BF00338048. doi:10.1007/BF00338048 [DOI] [PubMed] [Google Scholar]
- Galli R, et al. Skeletal myogenic potential of human and mouse neural stem cells. Nat. Neurosci. 2000;3:986–991. doi: 10.1038/79924. doi:10.1038/79924 [DOI] [PubMed] [Google Scholar]
- Gharzi A, Reynolds A.J, Jahoda C.A. Plasticity of hair follicle dermal cells in wound healing and induction. Exp. Dermatol. 2003;12:126–136. doi: 10.1034/j.1600-0625.2003.00106.x. doi:10.1034/j.1600-0625.2003.00106.x [DOI] [PubMed] [Google Scholar]
- Golding J.P, Cohen J. Border controls at the mammalian spinal cord: late-surviving neural crest boundary cap cells at dorsal root entry sites may regulate sensory afferent ingrowth and entry zone morphogenesis. Mol. Cell Neurosci. 1997;9:381–396. doi: 10.1006/mcne.1997.0647. doi:10.1006/mcne.1997.0647 [DOI] [PubMed] [Google Scholar]
- Graham A, Begbie J. Neurogenic placodes: a common front. Trends Neurosci. 2000;23:313–316. doi: 10.1016/s0166-2236(00)01606-4. doi:10.1016/S0166-2236(00)01606-4 [DOI] [PubMed] [Google Scholar]
- Grompe M. The role of bone marrow stem cells in liver regeneration. Semin. Liver Dis. 2003;23:363–372. doi: 10.1055/s-2004-815560. doi:10.1055/s-2004-815560 [DOI] [PubMed] [Google Scholar]
- Guest J.D, Rao A, Olson L, Bunge M.B, Bunge R.P. The ability of human Schwann cell grafts to promote regeneration in the transected nude rat spinal cord. Exp. Neurol. 1997;148:502–522. doi: 10.1006/exnr.1997.6693. doi:10.1006/exnr.1997.6693 [DOI] [PubMed] [Google Scholar]
- Halata Z, Grim M, Christ B. Origin of spinal cord meninges, sheaths of peripheral nerves, and cutaneous receptors including Merkel cells. An experimental and ultrastructural study with avian chimeras. Anat. Embryol. (Berl) 1990;182:529–537. doi: 10.1007/BF00186459. [DOI] [PubMed] [Google Scholar]
- Halfpenny C, Benn T, Scolding N. Cell transplantation, myelin repair, and multiple sclerosis. Lancet Neurol. 2002;1:31–40. doi: 10.1016/s1474-4422(02)00004-2. doi:10.1016/S1474-4422(02)00004-2 [DOI] [PubMed] [Google Scholar]
- Henry M.C, Tashjian D.B, Breuer C.K. Neuroblastoma update. Curr. Opin. Oncol. 2005;17:19–23. doi: 10.1097/01.cco.0000147901.12325.90. doi:10.1097/01.cco.0000147901.12325.90 [DOI] [PubMed] [Google Scholar]
- Hjerling-Leffler J, Marmigere F, Heglind M, Cederberg A, Koltzenburg M, Enerback S, Ernfors P. The boundary cap: a source of neural crest stem cells that generate multiple sensory neuron subtypes. Development. 2005;132:2623–2632. doi: 10.1242/dev.01852. doi:10.1242/dev.01852 [DOI] [PubMed] [Google Scholar]
- Hoogduijn M.J, Gorjup E, Genever P.G. Comparative characterization of hair follicle dermal stem cells and bone marrow mesenchymal stem cells. Stem Cells Dev. 2006;15:49–60. doi: 10.1089/scd.2006.15.49. doi:10.1089/scd.2006.15.49 [DOI] [PubMed] [Google Scholar]
- Iwashita T, Kruger G.M, Pardal R, Kiel M.J, Morrison S.J. Hirschsprung disease is linked to defects in neural crest stem cell function. Science. 2003;301:972–976. doi: 10.1126/science.1085649. doi:10.1126/science.1085649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahoda C.A, Oliver R.F. Vibrissa dermal papilla cell aggregative behaviour in vivo and in vitro. J. Embryol. Exp. Morphol. 1984;79:211–224. [PubMed] [Google Scholar]
- Jahoda C.A, Reynolds A.J. Hair follicle dermal sheath cells: unsung participants in wound healing. Lancet. 2001;358:1445–1448. doi: 10.1016/S0140-6736(01)06532-1. doi:10.1016/S0140-6736(01)06532-1 [DOI] [PubMed] [Google Scholar]
- Jahoda C.A, Whitehouse J, Reynolds A.J, Hole N. Hair follicle dermal cells differentiate into adipogenic and osteogenic lineages. Exp. Dermatol. 2003;12:849–859. doi: 10.1111/j.0906-6705.2003.00161.x. doi:10.1111/j.0906-6705.2003.00161.x [DOI] [PubMed] [Google Scholar]
- Jiang X, Rowitch D.H, Soriano P, McMahon A.P, Sucov H.M. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Jiang X, Iseki S, Maxson R.E, Sucov H.M, Morriss-Kay G.M. Tissue origins and interactions in the mammalian skull vault. Dev. Biol. 2002a;241:106–116. doi: 10.1006/dbio.2001.0487. doi:10.1006/dbio.2001.0487 [DOI] [PubMed] [Google Scholar]
- Jiang Y, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002b;418:41–49. doi: 10.1038/nature00870. doi:10.1038/nature00870 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Vaessen B, Lenvik T, Blackstad M, Reyes M, Verfaillie C.M. Multipotent pro-genitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp. Hematol. 2002c;30:896–904. doi: 10.1016/s0301-472x(02)00869-x. doi:10.1016/S0301-472X(02)00869-X [DOI] [PubMed] [Google Scholar]
- Joannides A, Gaughwin P, Schwiening C, Majed H, Sterling J, Compston A, Chandran S. Efficient generation of neural precursors from adult human skin: astrocytes promote neurogenesis from skin-derived stem cells. Lancet. 2004;364:172–178. doi: 10.1016/S0140-6736(04)16630-0. doi:10.1016/S0140-6736(04)16630-0 [DOI] [PubMed] [Google Scholar]
- Joseph N.M, et al. Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells. Development. 2004;131:5599–5612. doi: 10.1242/dev.01429. doi:10.1242/dev.01429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. doi:10.1126/science.289.5485.1754 [DOI] [PubMed] [Google Scholar]
- Kruger G.M, Mosher J.T, Bixby S, Joseph N, Iwashita T, Morrison S.J. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential and factor responsiveness. Neuron. 2002;35:657–669. doi: 10.1016/s0896-6273(02)00827-9. doi:10.1016/S0896-6273(02)00827-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner B.H. Neuroblastoma: a disease requiring a multitude of imaging studies. J. Nucl. Med. 2004;45:1172–1188. [PubMed] [Google Scholar]
- LaBarge M.A, Blau H.M. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111:589–601. doi: 10.1016/s0092-8674(02)01078-4. doi:10.1016/S0092-8674(02)01078-4 [DOI] [PubMed] [Google Scholar]
- Lakkis M.M, Tennekoon G.I. Neurofibromatosis Type 1. General overview. J. Neurosci. Res. 2000;62:755–763. doi: 10.1002/1097-4547(20001215)62:6<755::AID-JNR1>3.0.CO;2-W. doi:10.1002/1097-4547(20001215)62:6<755::AID-JNR1>3.0.CO;2-W [DOI] [PubMed] [Google Scholar]
- Lako M, Armstrong L, Cairns P.M, Harris S, Hole N, Jahoda C.A. Hair follicle dermal cells repopulate the mouse haematopoietic system. J. Cell Sci. 2002;115:3967–3974. doi: 10.1242/jcs.00060. doi:10.1242/jcs.00060 [DOI] [PubMed] [Google Scholar]
- Lavker R.M, et al. Hair follicle stem cells. J. Investig. Dermatol. Symp. Proc. 2003;8:28–38. doi: 10.1046/j.1523-1747.2003.12169.x. doi:10.1046/j.1523-1747.2003.12169.x [DOI] [PubMed] [Google Scholar]
- Le Douarin N.M, Dupin E. Multipotentiality of the neural crest. Curr. Opin. Genet. Dev. 2003;13:529–536. doi: 10.1016/j.gde.2003.08.002. doi:10.1016/j.gde.2003.08.002 [DOI] [PubMed] [Google Scholar]
- Le Douarin N.M, Kalcheim C. 2nd edn. Cambridge University Press; Cambridge, UK: 1999. The neural crest. [Google Scholar]
- Le Lievre C.S, Le Douarin N.M. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J. Embryol. Exp. Morphol. 1975;34:125–154. [PubMed] [Google Scholar]
- Li G.L, Farooque M, Holtz A, Olsson Y. Apoptosis of oligodendrocytes occurs for long distances away from the primary injury after compression trauma to rat spinal cord. Acta Neuropathol. (Berl) 1999;98:473–480. doi: 10.1007/s004010051112. doi:10.1007/s004010051112 [DOI] [PubMed] [Google Scholar]
- Li L, Mignone J, Yang M, Matic M, Penman S, Enikolopov G, Hoffman R.M. Nestin expression in hair follicle sheath progenitor cells. Proc. Natl Acad. Sci. USA. 2003;100:9958–9961. doi: 10.1073/pnas.1733025100. doi:10.1073/pnas.1733025100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ishii T, Feinstein P, Mombaerts P. Odorant receptor gene choice is reset by nuclear transfer from mouse olfactory sensory neurons. Nature. 2004;428:393–399. doi: 10.1038/nature02433. doi:10.1038/nature02433 [DOI] [PubMed] [Google Scholar]
- Lu P, Blesch A, Tuszynski M.H. Induction of bone marrow stromal cells to neurons: differentiation, transdifferentiation, or artifact? J. Neurosci. Res. 2004;77:174–191. doi: 10.1002/jnr.20148. doi:10.1002/jnr.20148 [DOI] [PubMed] [Google Scholar]
- Luo R, Gao J, Wehrle-Haller B, Henion P.D. Molecular identification of distinct neurogenic and melanogenic neural crest sublineages. Development. 2003;130:321–330. doi: 10.1242/dev.00213. doi:10.1242/dev.00213 [DOI] [PubMed] [Google Scholar]
- Maro G.S, Vermeren M, Voiculescu O, Melton L, Cohen J, Charnay P, Topilko P. Neural crest boundary cap cells constitute a source of neuronal and glial cells of the PNS. Nat. Neurosci. 2004;7:930–938. doi: 10.1038/nn1299. doi:10.1038/nn1299 [DOI] [PubMed] [Google Scholar]
- McKenzie I.A, Biernaskie J, Toma J.G, Midha R, Miller F.D. Skin-derived precursors generate myelinating Schwann cells for the injured and dysmyelinated nervous system. J. Neurosci. 2006;26:6651–6660. doi: 10.1523/JNEUROSCI.1007-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menei P, Montero-Menei C, Whittemore S.R, Bunge R.P, Bunge M.B. Schwann cells genetically modified to secrete human BDNF promote enhanced axonal regrowth across transected adult rat spinal cord. Eur. J. Neurosci. 1998;10:607–621. doi: 10.1046/j.1460-9568.1998.00071.x. doi:10.1046/j.1460-9568.1998.00071.x [DOI] [PubMed] [Google Scholar]
- Mezey E, Chandross K.J, Harta G, Maki R.A, McKercher S.R. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. doi:10.1126/science.290.5497.1779 [DOI] [PubMed] [Google Scholar]
- Mezey E, Key S, Vogelsang G, Szalayova I, Lange G.D, Crain B. Transplanted bone marrow generates new neurons in human brains. Proc. Natl Acad. Sci. USA. 2003;100:1364–1369. doi: 10.1073/pnas.0336479100. doi:10.1073/pnas.0336479100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M, Gronthos S, Zhao M, Lu B, Fisher L.W, Robey P.G, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc. Natl Acad. Sci. USA. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. doi:10.1073/pnas.0937635100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S.J, White P.M, Zock C, Anderson D.J. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999;96:737–749. doi: 10.1016/s0092-8674(00)80583-8. doi:10.1016/S0092-8674(00)80583-8 [DOI] [PubMed] [Google Scholar]
- Morshead C.M, Benveniste P, Iscove N.N, van der Kooy D. Hematopoietic competence is a rare property of neural stem cells that may depend on genetic and epigenetic alterations. Nat. Med. 2002;8:268–273. doi: 10.1038/nm0302-268. doi:10.1038/nm0302-268 [DOI] [PubMed] [Google Scholar]
- Murrell W, et al. Multipotent stem cells from adult olfactory mucosa. Dev. Dyn. 2005;233:496–515. doi: 10.1002/dvdy.20360. doi:10.1002/dvdy.20360 [DOI] [PubMed] [Google Scholar]
- Nakamura H. Mesenchymal derivatives from the neural crest. Arch. Histol. Jpn. 1982;45:127–138. doi: 10.1679/aohc.45.127. [DOI] [PubMed] [Google Scholar]
- Narisawa Y, Hashimoto K, Kohda H. Merkel cells of the terminal hair follicle of the adult human scalp. J. Invest. Dermatol. 1994;102:506–510. doi: 10.1111/1523-1747.ep12373164. doi:10.1111/1523-1747.ep12373164 [DOI] [PubMed] [Google Scholar]
- Neuhuber B, Gallo G, Howard L, Kostura L, Mackay A, Fischer I. Reevaluation of in vitro differentiation protocols for bone marrow stromal cells: disruption of actin cytoskeleton induces rapid morphological changes and mimics neuronal phenotype. J. Neurosci. Res. 2004;77:192–204. doi: 10.1002/jnr.20147. doi:10.1002/jnr.20147 [DOI] [PubMed] [Google Scholar]
- Nishimura E.K, et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416:854–860. doi: 10.1038/416854a. doi:10.1038/416854a [DOI] [PubMed] [Google Scholar]
- Nurse C.A, Macintyre L, Diamond J. Reinnervation of the rat touch dome restores the Merkel cell population reduced after denervation. Neuroscience. 1984;13:563–571. doi: 10.1016/0306-4522(84)90249-5. doi:10.1016/0306-4522(84)90249-5 [DOI] [PubMed] [Google Scholar]
- Nygren J.M, Jovinge S, Breitbach M, Sawen P, Roll W, Hescheler J, Taneera J, Fleischmann B.K, Jacobsen S.E. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat. Med. 2004;10:494–501. doi: 10.1038/nm1040. doi:10.1038/nm1040 [DOI] [PubMed] [Google Scholar]
- Oudega M, Gautier S.E, Chapon P, Fragoso M, Bates M.L, Parel J.M, Bunge M.B. Axonal regeneration into Schwann cell grafts within resorbable poly (a-hydroxyacid) guidance channels in the adult rat spinal cord. Biomaterials. 2001;22:1125–1136. doi: 10.1016/s0142-9612(00)00346-x. doi:10.1016/S0142-9612(00)00346-X [DOI] [PubMed] [Google Scholar]
- Paino C.L, Fernandez-Valle C, Bates M.L, Bunge M.B. Regrowth of axons in lesioned adult rat spinal cord: promotion by implants of cultured Schwann cells. J. Neurocytol. 1994;23:433–452. doi: 10.1007/BF01207115. doi:10.1007/BF01207115 [DOI] [PubMed] [Google Scholar]
- Palermo A.T, Labarge M.A, Doyonnas R, Pomerantz J, Blau H.M. Bone marrow contribution to skeletal muscle: a physiological response to stress. Dev. Biol. 2005;279:336–344. doi: 10.1016/j.ydbio.2004.12.024. doi:10.1016/j.ydbio.2004.12.024 [DOI] [PubMed] [Google Scholar]
- Pearse D.D, Pereira F.C, Marcillo A.E, Bates M.L, Berrocal Y.A, Filbin M.T, Bunge M.B. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat. Med. 2004;10:610–616. doi: 10.1038/nm1056. doi:10.1038/nm1056 [DOI] [PubMed] [Google Scholar]
- Pearton D.J, Yang Y, Dhouailly D. Transdifferentiation of corneal epithelium into epidermis occurs by means of a multistep process triggered by dermal developmental signals. Proc. Natl Acad. Sci. USA. 2005;102:3714–3719. doi: 10.1073/pnas.0500344102. doi:10.1073/pnas.0500344102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters E.M, Botchkarev V.A, Muller-Rover S, Moll I, Rice F.L, Paus R. Developmental timing of hair follicle and dorsal skin innervation in mice. J. Comp. Neurol. 2002;448:28–52. doi: 10.1002/cne.10212. doi:10.1002/cne.10212 [DOI] [PubMed] [Google Scholar]
- Petersen B.E, Bowen W.C, Patrene K.D, Mars W.M, Sullivan A.K, Murase N, Boggs S.S, Greenberger J.S, Goff J.P. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. doi:10.1126/science.284.5417.1168 [DOI] [PubMed] [Google Scholar]
- Priller J, Persons D.A, Klett F.F, Kempermann G, Kreutzberg G.W, Dirnagl U. Neogenesis of cerebellar Purkinje neurons from gene-marked bone marrow cells in vivo. J. Cell Biol. 2001;155:733–738. doi: 10.1083/jcb.200105103. doi:10.1083/jcb.200105103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Real C, Glavieux-Pardanaud C, Vaigot P, Le-Douarin N, Dupin E. The instability of the neural crest phenotypes: Schwann cells can differentiate into myofibroblasts. Int. J. Dev. Biol. 2005;49:151–159. doi: 10.1387/ijdb.041940cr. doi:10.1387/ijdb.041940cr [DOI] [PubMed] [Google Scholar]
- Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 2005;3:1910–1924. doi: 10.1371/journal.pbio.0030331. doi:10.1371/journal.pbio.0030331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A.J, Jahoda C.A. Cultured dermal papilla cells induce follicle formation and hair growth by transdifferentiation of an adult epidermis. Development. 1992;115:587–593. doi: 10.1242/dev.115.2.587. [DOI] [PubMed] [Google Scholar]
- Reynolds B.A, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. doi:10.1126/science.1553558 [DOI] [PubMed] [Google Scholar]
- Reynolds B.A, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J. Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson P.M, McGuinness U.M, Aguayo A.J. Axons from CNS neurons regenerate into PNS grafts. Nature. 1980;284:264–265. doi: 10.1038/284264a0. doi:10.1038/284264a0 [DOI] [PubMed] [Google Scholar]
- Richardson P.M, Issa V.M, Aguayo A.J. Regeneration of long spinal axons in the rat. J. Neurocytol. 1984;13:165–182. doi: 10.1007/BF01148324. doi:10.1007/BF01148324 [DOI] [PubMed] [Google Scholar]
- Rizvi T.A, Huang Y, Sidani A, Atit R, Largaespada D.A, Boissy R.E, Ratner N. A novel cytokine pathway suppresses glial cell melanogenesis after injury to adult nerve. J. Neurosci. 2002;22:9831–9840. doi: 10.1523/JNEUROSCI.22-22-09831.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth J. Central myelin in the mouse mutant shiverer. J. Comp. Neurol. 1980a;194:639–648. doi: 10.1002/cne.901940310. doi:10.1002/cne.901940310 [DOI] [PubMed] [Google Scholar]
- Rosenbluth J. Peripheral myelin in the mouse mutant Shiverer. J. Comp. Neurol. 1980b;193:729–739. doi: 10.1002/cne.901930310. doi:10.1002/cne.901930310 [DOI] [PubMed] [Google Scholar]
- Serbedzija G.N, McMahon A.P. Analysis of neural crest cell migration in Splotch mice using a neural crest-specific LacZ reporter. Dev. Biol. 1997;185:139–147. doi: 10.1006/dbio.1997.8551. doi:10.1006/dbio.1997.8551 [DOI] [PubMed] [Google Scholar]
- Serbedzija G.N, Fraser S.E, Bronner-Fraser M. Pathways of trunk neural crest cell migration in the mouse embryo as revealed by vital dye labeling. Development. 1990;108:605–612. doi: 10.1242/dev.108.4.605. [DOI] [PubMed] [Google Scholar]
- Serbedzija G.N, Bronner-Fraser M, Fraser S.E. Developmental potential of trunk neural crest cells in the mouse. Development. 1994;120:1709–1718. doi: 10.1242/dev.120.7.1709. [DOI] [PubMed] [Google Scholar]
- Shah N.M, Groves A.K, Anderson D.J. Alternative neural crest cell fates are instructively promoted by TGFbeta superfamily members. Cell. 1996;85:331–343. doi: 10.1016/s0092-8674(00)81112-5. doi:10.1016/S0092-8674(00)81112-5 [DOI] [PubMed] [Google Scholar]
- Sharma K, Korade Z, Frank E. Late-migrating neuroepithelial cells from the spinal cord differentiate into sensory ganglion cells and melanocytes. Neuron. 1995;14:143–152. doi: 10.1016/0896-6273(95)90248-1. doi:10.1016/0896-6273(95)90248-1 [DOI] [PubMed] [Google Scholar]
- Shuman S.L, Bresnahan J.C, Beattie M.S. Apoptosis of microglia and oligodendrocytes after spinal cord contusion in rats. J. Neurosci. Res. 1997;50:798–808. doi: 10.1002/(SICI)1097-4547(19971201)50:5<798::AID-JNR16>3.0.CO;2-Y. doi:10.1002/(SICI)1097-4547(19971201)50:5<798::AID-JNR16>3.0.CO;2-Y [DOI] [PubMed] [Google Scholar]
- Sieber-Blum M, Grim M, Hu Y.F, Szeder V. Pluripotent neural crest stem cells in the adult hair follicle. Dev. Dyn. 2004;231:258–269. doi: 10.1002/dvdy.20129. doi:10.1002/dvdy.20129 [DOI] [PubMed] [Google Scholar]
- Singh S.K, Clarke I.D, Terasaki M, Bonn V.E, Hawkins C, Squire J, Dirks P.B. Identification of a cancer stem cell in human brain tumours. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- Singh S.K, Clarke I.D, Hide T, Dirks P.B. Cancer stem cells in nervous system tumours. Oncogene. 2004a;23:7267–7273. doi: 10.1038/sj.onc.1207946. doi:10.1038/sj.onc.1207946 [DOI] [PubMed] [Google Scholar]
- Singh S.K, Hawkins C, Clarke I.D, Squire J.A, Bayani J, Hide T, Henkelman R.M, Cusimano M.D, Dirks P.B. Identification of human brain tumour initiating cells. Nature. 2004b;432:396–401. doi: 10.1038/nature03128. doi:10.1038/nature03128 [DOI] [PubMed] [Google Scholar]
- Sohal G.S, Ali M.M, Ali A.A, Dai D. Ventrally emigrating neural tube cells contribute to the formation of Meckel's and quadrate cartilage. Dev. Dyn. 1999a;216:37–44. doi: 10.1002/(SICI)1097-0177(199909)216:1<37::AID-DVDY6>3.0.CO;2-P. doi:10.1002/(SICI)1097-0177(199909)216:1<37::AID-DVDY6>3.0.CO;2-P [DOI] [PubMed] [Google Scholar]
- Sohal G.S, Ali M.M, Ali A.A, Dai D. Ventrally emigrating neural tube cells differentiate into heart muscle. Biochem. Biophys. Res. Commun. 1999b;254:601–604. doi: 10.1006/bbrc.1998.0109. doi:10.1006/bbrc.1998.0109 [DOI] [PubMed] [Google Scholar]
- Sohal G.S, Ali M.M, Farooqui F.A. A second source of precursor cells for the developing enteric nervous system and interstitial cells of Cajal. Int. J. Dev. Neurosci. 2002;20:619–626. doi: 10.1016/s0736-5748(02)00103-x. doi:10.1016/S0736-5748(02)00103-X [DOI] [PubMed] [Google Scholar]
- Song H, Stevens C.F, Gage F.H. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. doi:10.1038/417039a [DOI] [PubMed] [Google Scholar]
- Stemple D.L, Anderson D.J. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell. 1992;71:973–985. doi: 10.1016/0092-8674(92)90393-q. doi:10.1016/0092-8674(92)90393-Q [DOI] [PubMed] [Google Scholar]
- Szeder V, Grim M, Halata Z, Sieber-Blum M. Neural crest origin of mammalian Merkel cells. Dev. Biol. 2003;253:258–263. doi: 10.1016/s0012-1606(02)00015-5. doi:10.1016/S0012-1606(02)00015-5 [DOI] [PubMed] [Google Scholar]
- Taylor G, Lehrer M.S, Jensen P.J, Sun T.T, Lavker R.M. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–461. doi: 10.1016/s0092-8674(00)00050-7. doi:10.1016/S0092-8674(00)00050-7 [DOI] [PubMed] [Google Scholar]
- Theise N.D, Badve S, Saxena R, Henegariu O, Sell S, Crawford J.M, Krause D.S. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000;31:235–240. doi: 10.1002/hep.510310135. doi:10.1002/hep.510310135 [DOI] [PubMed] [Google Scholar]
- Tobin D.J, Gunin A, Magerl M, Handijski B, Paus R. Plasticity and cytokinetic dynamics of the hair follicle mesenchyme: implications for hair growth control. J. Invest. Dermatol. 2003a;120:895–904. doi: 10.1046/j.1523-1747.2003.12237.x. doi:10.1046/j.1523-1747.2003.12237.x [DOI] [PubMed] [Google Scholar]
- Tobin D.J, Gunin A, Magerl M, Paus R. Plasticity and cytokinetic dynamics of the hair follicle mesenchyme during the hair growth cycle: implications for growth control and hair follicle transformations. J. Invest. Dermatol. Symp. Proc. 2003b;8:80–86. doi: 10.1046/j.1523-1747.2003.12177.x. doi:10.1046/j.1523-1747.2003.12177.x [DOI] [PubMed] [Google Scholar]
- Toma J.G, Akhavan M, Fernandes K.J, Barnabe-Heider F, Sadikot A, Kaplan D.R, Miller F.D. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat. Cell Biol. 2001;3:778–784. doi: 10.1038/ncb0901-778. doi:10.1038/ncb0901-778 [DOI] [PubMed] [Google Scholar]
- Toma J.G, McKenzie I.A, Bagli D, Miller F.D. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727–737. doi: 10.1634/stemcells.2004-0134. doi:10.1634/stemcells.2004-0134 [DOI] [PubMed] [Google Scholar]
- Tsai R.Y, McKay R.D. Cell contact regulates fate choice by cortical stem cells. J. Neurosci. 2000;20:3725–3735. doi: 10.1523/JNEUROSCI.20-10-03725.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry W.E, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. doi:10.1126/science.1092436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Buck D.W, He D, Reitsma M.J, Masek M, Phan T.V, Tsukamoto A.S, Gage F.H, Weissman I.L. Direct isolation of human central nervous system stem cells. Proc. Natl Acad. Sci. USA. 2000;97:14 720–14 725. doi: 10.1073/pnas.97.26.14720. doi:10.1073/pnas.97.26.14720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallieres L, Sawchenko P.E. Bone marrow-derived cells that populate the adult mouse brain preserve their hematopoietic identity. J. Neurosci. 2003;23:5197–5207. doi: 10.1523/JNEUROSCI.23-12-05197.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilopoulos G, Wang P.R, Russell D.W. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901–904. doi: 10.1038/nature01539. doi:10.1038/nature01539 [DOI] [PubMed] [Google Scholar]
- Vermeren M, Maro G.S, Bron R, McGonnell I.M, Charnay P, Topilko P, Cohen J. Integrity of developing spinal motor columns is regulated by neural crest derivatives at motor exit points. Neuron. 2003;37:403–415. doi: 10.1016/s0896-6273(02)01188-1. doi:10.1016/S0896-6273(02)01188-1 [DOI] [PubMed] [Google Scholar]
- Wakamatsu Y, Mochii M, Vogel K.S, Weston J.A. Avian neural crest-derived neurogenic precursors undergo apoptosis on the lateral migration pathway. Development. 1998;125:4205–4213. doi: 10.1242/dev.125.21.4205. [DOI] [PubMed] [Google Scholar]
- Wang X, et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. doi:10.1038/nature01531 [DOI] [PubMed] [Google Scholar]
- Warden P, Bamber N.I, Li H, Esposito A, Ahmad K.A, Hsu C.Y, Xu X.M. Delayed glial cell death following wallerian degeneration in white matter tracts after spinal cord dorsal column cordotomy in adult rats. Exp. Neurol. 2001;168:213–224. doi: 10.1006/exnr.2000.7622. doi:10.1006/exnr.2000.7622 [DOI] [PubMed] [Google Scholar]
- Wehner T, et al. Bone marrow-derived cells expressing green fluorescent protein under the control of the glial fibrillary acidic protein promoter do not differentiate into astrocytes in vitro and in vivo. J. Neurosci. 2003;23:5004–5011. doi: 10.1523/JNEUROSCI.23-12-05004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson Y.M, Richards K.L, Ford-Perriss M.L, Panthier J.J, Murphy M. Neural crest cell lineage segregation in the mouse neural tube. Development. 2004;131:6153–6162. doi: 10.1242/dev.01533. doi:10.1242/dev.01533 [DOI] [PubMed] [Google Scholar]
- Wurmser A.E, Nakashima K, Summers R.G, Toni N, D'Amour K.A, Lie D.C, Gage F.H. Cell fusion-independent differentiation of neural stem cells to the endothelial lineage. Nature. 2004;430:350–356. doi: 10.1038/nature02604. doi:10.1038/nature02604 [DOI] [PubMed] [Google Scholar]
- Xu X.M, Guenard V, Kleitman N, Aebischer P, Bunge M.B. A combination of BDNF and NT-3 promotes supraspinal axonal regeneration into Schwann cell grafts in adult rat thoracic spinal cord. Exp. Neurol. 1995a;134:261–272. doi: 10.1006/exnr.1995.1056. doi:10.1006/exnr.1995.1056 [DOI] [PubMed] [Google Scholar]
- Xu X.M, Guenard V, Kleitman N, Bunge M.B. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J. Comp. Neurol. 1995b;351:145–160. doi: 10.1002/cne.903510113. doi:10.1002/cne.903510113 [DOI] [PubMed] [Google Scholar]
- Xu X.M, Chen A, Guenard V, Kleitman N, Bunge M.B. Bridging Schwann cell transplants promote axonal regeneration from both the rostral and caudal stumps of transected adult rat spinal cord. J. Neurocytol. 1997;26:1–16. doi: 10.1023/a:1018557923309. doi:10.1023/A:1018557923309 [DOI] [PubMed] [Google Scholar]
- Xu X.M, Zhang S.X, Li H, Aebischer P, Bunge M.B. Regrowth of axons into the distal spinal cord through a Schwann-cell-seeded mini-channel implanted into hemisected adult rat spinal cord. Eur. J. Neurosci. 1999;11:1723–1740. doi: 10.1046/j.1460-9568.1999.00591.x. doi:10.1046/j.1460-9568.1999.00591.x [DOI] [PubMed] [Google Scholar]
- Young H.E, Mancini M.L, Wright R.P, Smith J.C, Black A.C, Jr, Reagan C.R, Lucas P.A. Mesenchymal stem cells reside within the connective tissues of many organs. Dev. Dyn. 1995;202:137–144. doi: 10.1002/aja.1002020205. [DOI] [PubMed] [Google Scholar]
- Zembowicz A, Mihm M.C. Dermal dendritic melanocytic proliferations: an update. Histopathology. 2004;45:433–451. doi: 10.1111/j.1365-2559.2004.01975.x. doi:10.1111/j.1365-2559.2004.01975.x [DOI] [PubMed] [Google Scholar]
- Zhang X, Klueber K.M, Guo Z, Lu C, Roisen F.J. Adult human olfactory neural progenitors cultured in defined medium. Exp. Neurol. 2004;186:112–123. doi: 10.1016/j.expneurol.2003.10.022. doi:10.1016/j.expneurol.2003.10.022 [DOI] [PubMed] [Google Scholar]