Abstract
We report the structure and Young's modulus of switchable films formed by peptide self-assembly at the air–water interface. Peptide surfactant AM1 forms an interfacial film that can be switched, reversibly, from a high- to low-elasticity state, with rapid loss of emulsion and foam stability. Using neutron reflectometry, we find that the AM1 film comprises a thin (approx. 15 Å) layer of ordered peptide in both states, confirming that it is possible to drastically alter the mechanical properties of an interfacial ensemble without significantly altering its concentration or macromolecular organization. We also report the first experimentally determined Young's modulus of a peptide film self-assembled at the air–water interface (E=80 MPa for AM1, switching to E<20 MPa). These findings suggest a fundamental link between E and the macroscopic stability of peptide-containing foam. Finally, we report studies of a designed peptide surfactant, Lac21E, which we find forms a stronger switchable film than AM1 (E=335 MPa switching to E<4 MPa). In contrast to AM1, Lac21E switching is caused by peptide dissociation from the interface (i.e. by self-disassembly). This research confirms that small changes in molecular design can lead to similar macroscopic behaviour via surprisingly different mechanisms.
Keywords: peptide, Young's modulus, interface, film, neutron reflectometry
1. Introduction
Soft interfaces occur at the boundary between air and water, or oil and water, and are ubiquitous in industries ranging from pharmaceuticals and foods to oil and minerals (Lucassen 1981; Debregeas et al. 1998). Such interfaces are often stabilized by chemical or polymer surfactants which, in the case of static emulsions, induce stability to coalescence that can be partly understood within the Derjaguin–Landau–Verwey–Overbeek (DLVO) framework (Israelachvili 1992; Myers 1999). Nevertheless, the dynamic behaviour of surfactant-covered soft interfaces remains poorly understood in many respects and is an area of active research (Fragneto et al. 1995; Chen et al. 2004; Dagastine et al. 2006).
The need for temporary emulsions or foams across a range of areas of practical interest has encouraged a search for switchable surfactants which are able to destabilize such systems in response to a trigger (Rosslee & Abbott 2000; Abbott 2001; Aydogan & Abbott 2001; Dexter et al. 2006; Liu et al. 2006). Several switchable surfactants have been described, including grafted copolymers that undergo a pH-induced structural change (Mathur et al. 1998), azobenzene surfactants triggered by UV irradiation (Shin & Abbott 1999; Shang et al. 2003), ferrocenyl-based surfactants triggered by redox mechanisms (Gallardo et al. 1995, 1996; Aydogan et al. 2002) and an oil-soluble long-chain alkyl amidine surfactant triggered by conversion of the amidine group to a charged amidinium bicarbonate (Liu et al. 2006). These surfactants all switch by mechanisms that change surfactant interfacial affinity (i.e. by reducing or removing the DLVO stabilizing forces). Current systems suffer from slow rates of switching, incomplete removal of material from the interface leading to flocculation but not coalescence, suitability for emulsions but not foams and/or require triggers that are difficult to implement at large scale.
Peptides comprise amino acid residues ordered into an informational chain, constituting part of a protein or else designed de novo, and are increasingly viewed as building blocks for the design of new materials including surfactants (Zhang 2003, 2006; Zhao & Zhang 2004, 2006; Rapaport 2006; Reches & Gazit 2006). Recently, an aqueous-soluble peptide (AM1) has been reported which has switchable surfactant properties (Dexter et al. 2006; Malcolm et al. 2006a). Under conditions where the histidine-containing peptide binds metal ions, AM1 aids the formation of high-quality foams and emulsions, yet these can be efficiently and reversibly destabilized by a simple pH change or by the addition of a chelating agent. The transition between stable macroscopic states is extremely rapid, with coalescence complete in seconds, satisfying a key objective of research into switchable surfactants (Liu et al. 2006). Surprisingly, interfacial tension does not significantly change as a result of the switch (Dexter et al. 2006) suggesting that destabilization is not accompanied by a change in peptide interfacial concentration (i.e. there was no evidence of interfacial depopulation). However, the maximum tensile stress that the interfacial film can transmit changed by more than an order of magnitude and a significant change in interfacial elasticity occurred (from 120 mN m−1 in the strong- or high-elasticity state to less than 30 mN m−1 in the weak- or low-elasticity state). This result suggests that, for AM1, known emulsion and foam stabilization mechanisms might be supplemented with an additional stability-controlling factor that is related to the mechanical properties of the interfacial film.
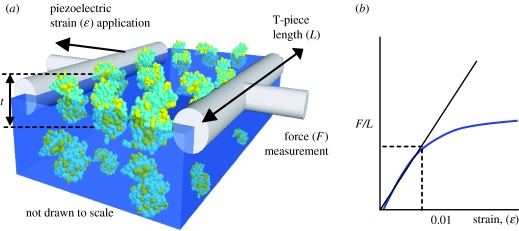
The mechanical properties of protein and peptide films located at fluid–fluid interfaces have been directly measured using the Cambridge interfacial tensiometer (CIT; Jones & Middelberg 2002a–c, 2003; Dexter et al. 2006; Malcolm et al. 2006a,b). The CIT can be thought of as a two-dimensional analogue of conventional volumetric materials stress–strain testing devices. It measures a force–strain curve of an interfacial film connected between two parallel silica fibres (figure 1) from which an interfacial elasticity, which is the product of the Young modulus (E) and the film thickness (t), can be obtained (Jones & Middelberg 2002a–c). In practice, the film thickness t is difficult to experimentally measure for very thin protein and peptide films and can change due to molecular rearrangement in the interfacial plane. These difficulties have, to date, prevented experimental determination of the Young modulus for such films.
Figure 1.
Principle of the CIT. (a) Proteins self-assemble into a mechanically strong but non-switchable film at the air–water interface, allowing the stress–strain behaviour of the film to be experimentally determined using the CIT (Jones & Middelberg 2002a). The film in this schematic comprises lysozyme (PDB ID 1LYZ) arrayed at the air–water interface (Lu et al. 1998; Malcolm et al. 2006b) to give a film connected between two T-piece fibres that have been modified to promote protein binding (Jones & Middelberg 2002a). Hydrophobic residues (yellow) are distributed over the protein surface. (b) The force (F) measured by the CIT is normalized by T-piece length (L) to give a curve of interfacial stress (F/L) versus strain (ϵ). Interfacial elasticity (E×t), which is the product of Young's modulus (E) and film thickness (t), is calculated as the gradient of the linear region (ϵ<1%) of the interfacial stress–strain curve.
The detailed structure of the AM1 peptide interfacial film and hence the basis for rapid switchability have not previously been known, and the absence of data on film thickness has prevented calculation of the Young modulus. We have hypothesized that precise ordering of the peptide units at the interface leads to the separate control of peptide interface and peptide–peptide bonding in a way that is not possible with complex protein molecules and that this might be the basis for rapid switching of film strength. In the present study, we carried out neutron reflectivity studies of the AM1 interfacial film to directly determine film thickness and also to investigate how the individual peptide molecules are organized at the interface. Neutron reflectometry is a direct method for probing the amount of material adsorbed at a soft interface (Lu et al. 1996, 1998), while selective deuteration allows the reflectivity profile of a nanostructure to be altered without changing its chemical composition (Fragneto et al. 1995; Strzalka et al. 2004). We confirmed that the AM1 surface coverage is approximately the same in both high- and low-elasticity states and showed that in both cases the film comprises a thin layer of ordered peptides. We also report here a designed peptide sequence, Lac21E (Fairman et al. 1996), which we show can be reversibly switched by pH change to give rapid and large changes in mechanical strength and also interfacial tension. Neutron reflectometry confirmed that, unlike AM1, the Lac21E film switches via interfacial depopulation (i.e. by self-disassembly). The results suggest that switchable peptide surfactants can be designed to allow independent control of phase destabilization through two different mechanisms—the known depopulation mechanism used by switchable chemical and polymer surfactants and a new mechanism related to the Young modulus of the interfacial film.
2. Experimental procedures
Lac21E was custom synthesized by GenScript Corporation (Piscataway, NJ) or GL Biochem (Shanghai). Lac21E incorporating deuterated valine and leucine (dLac21E, d8-valine, d10-leucine) and AM1 incorporating deuterated valine and leucine (dAM1, d8-valine, d10-leucine) were custom synthesized by AnaSpec (San Jose, CA). Purity in each case was more than 95% by RP-HPLC. Peptide content was determined by quantitative amino acid analysis (Australian Proteome Analysis Facility, Sydney). Sodium 4-(2-hydroxyethyl)-1-piperazine ethanesulphonate (HEPES) was from Spectrum Chemicals (Gardena, CA) and was chosen for its capacity to buffer at both pH 3 and 7, as well as its low-binding affinity for metal ions. All other reagents were analytical grade.
2.1 Peptide film mechanical properties
Lac21E (5 μM, 6.5 ml) in HEPES buffer at pH 3.0 or 7.0 was filled into a polytetrafluoroethylene bath forming part of the CIT. The film was aged for 60 min before being subjected to initial tension–compression cycles to 5 and 300% strain. To switch a preformed interface, a small volume of 1 N HCl or NaOH, sufficient to adjust the pH to the desired final value, was added to the bath by pipetting beneath the T-pieces, and the film was allowed to age a further 120 min before the tension–compression cycles were repeated.
2.2 Interfacial tension
Air bubbles (approx. 8 μl) were formed via a U-shaped needle in a quartz Hellma cuvette (8 ml) containing a solution of Lac21E (5 μM) in 25 mM HEPES at pH 3.0 or 7.4 under magnetic stirring. The bubble shape was monitored automatically over 10 min to follow changes in the interfacial tension (DSA-10, Krüss, Hamburg, Germany). For switching experiments, a small aliquot of NaOH or HCl was added to the cuvette to switch the pH rapidly between 3 and 7.4. Control experiments on buffer in the absence of peptide showed a stable interfacial tension close to 73 mN m−1.
2.3 Foam switching
Lac21E (100 nmol) was dissolved in 1.0 ml of 10 mM HEPES (pH 3.0) and transferred into a custom-made foam preparation apparatus. A foam of approximately 6.5 cm height was formed by passing air (7 ml) through the peptide solution via a sintered glass disc. The foam was allowed to stand for 5 min during which time slight foam coarsening, but insignificant alteration in foam height, was noted. An aliquot (8 μl) of NaOH (1.0 N) was added to the top of the foam, causing foam collapse within 60 s. A new foam of approximately 6.5 cm height was prepared from the neutralized peptide solution (pH 7.0) by the passage of air as explained above. The foam was unstable and collapsed within 60 s.
2.4 Neutron reflectivity
Neutron reflection experiments were performed using the SURF reflectometer, a time-of-flight instrument at Rutherford Appleton Laboratory (ISIS, Oxfordshire, UK; wavelength 0.55–6.8 Å corresponding to a momentum transfer between 0.048 and 0.6 Å−1 at an incident angle of 1.5°). Peptide solution (5 μM in 25 mM HEPES buffer, pH 3.0 or 7.4, in the presence of 100 μM ZnSO4 or 100 μM EDTA) was added to a Teflon trough and aged for 1.5–6 h before measurement. Repeated experiments showed that there was no reflection signal difference for aged samples. Together with selective deuteration of peptides, contrast variation was carried out by using a HEPES buffer with four different D2O/H2O ratios (8.1, 39.1, 70 and 100% (v/v) D2O). Reflectivity data were calibrated to an absolute scale using pure D2O standard and are presented in the electronic supplementary material. The average reflectivity between 0.27 and 0.6 Å−1 was taken as background and subtracted from each reflectivity curve. Peptide molecular volume was calculated from the partial specific volume of amino acids (Jacrot & Zaccai 1981) taking into account the contribution of N-terminal peptide acetylation and C-terminal amidation. Scattering length was calculated from the chemical composition of peptides (Jacrot & Zaccai 1981). Exchange of labile peptide hydrogens with solvent was considered to be complete before reflectivity measurement. A model of two sub-layers was used to represent the peptide film at the interface, with each sub-layer described by a Gaussian (Lu et al. 1996). The solvent profile at the interface was described by an improved solvent distribution function (Penfold & Thomas 2002). Assuming that a peptide in protonated and deuterated form has identical chemical structure under different D2O/H2O ratios, the detailed structure of a peptide film was obtained by simultaneously fitting the data at different contrast variations. Goodness of fit is shown in the electronic supplementary material.
3. Results and discussion
Although peptide adsorption at interfaces has been extensively researched (Middelberg et al. 2000; Jones & Middelberg 2002b; Lu et al. 2003, 2004; Sjogren & Ulvenlund 2004), only AM1 (Dexter et al. 2006) has, to date, been reported to act as a switchable peptide surfactant. The surprising finding that AM1 could switch the stability of a foam or emulsion, in seconds and without a significant change in interfacial tension, stimulated us to characterize the interface using neutron reflectometry. To the best of our knowledge, neutron reflectometry has been used only once before to probe the adsorption of small well-defined peptide sequences at the air–water interface (Lu et al. 2003). In that study, a 14-residue non-switchable β-hairpin peptide adsorbed at the air–water interface gave an area per molecule of 210 Å2 and a peptide Gaussian layer thickness of approximately 10 Å. Deuteration was not used in that study to obtain high-resolution information on amino acid location, the layer was not switchable, and its mechanical properties were not determined.
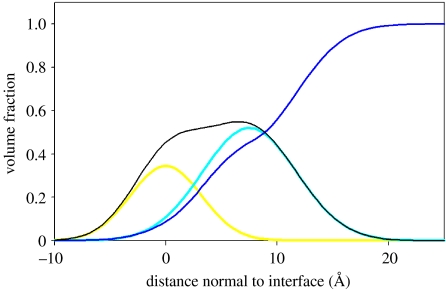
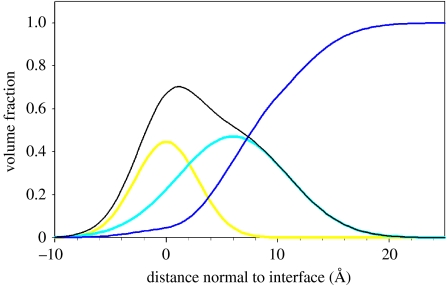
Figure 2 shows neutron reflectometry results for peptide AM1 self-assembled at the air–water interface, under conditions shown previously to create a mechanically strong film (interfacial elasticity, E×t of 120 mN m−1 and maximum interfacial tensile stress of 6.9 mN m−1) which is able to stabilize an emulsion or foam (Dexter et al. 2006). All valine and leucine residues in the 21-residue AM1 sequence were deuterated, allowing contrast matching to reveal different molecular features (Fragneto et al. 1995; Strzalka et al. 2004). Data were described using a two-sub-layer hydrophilic–hydrophobic Gaussian model, with an allowance for roughness of the water surface (Lu & Thomas 1998; Penfold & Thomas 2002). An area per molecule of 380±15 Å2, a volume fraction of 55±5% and sub-layer thicknesses of 9±2 Å for the hydrophobic component, and 12 Å for the hydrophilic component, were determined. The data indicate a spatial separation of the hydrophobic and hydrophilic amino acids in the peptide sequence. Deuterated leucine and valine residues partitioned to the air phase, such that the hydrophobic and hydrophilic layers were separated by 7.5 Å or slightly less than the full diameter of an α-helix (approx. 10 Å). These results suggest that AM1 folds into an α-helix during interfacial self-assembly (figure 3), creating a thin ordered layer at the air–water interface. The formation of an ordered film at the soft interface occurs even though the peptide has a random coil conformation in bulk solution at the concentration used, as assessed by circular dichroism (data not shown). The interface imparts a dominant ordering effect that allows individual peptides to interact in such a way that a thin, but strong, interfacial film is formed.
Figure 2.
Structure of the strong film formed from the switchable peptide surfactant AM1 at the air–water interface, determined by neutron reflectometry of deuterated peptide. The overall peptide distribution (black line) comprises hydrophobic (yellow line) and hydrophilic (cyan line) distributions separated by approximately 7.5 Å. Surface roughness is represented by a distribution of water density (blue line). The film was formed by self-assembly from a solution of 5 μM protonated or partially deuterated AM1 in 25 mM HEPES buffer at pH 7.4 in the presence of 100 μM ZnSO4.
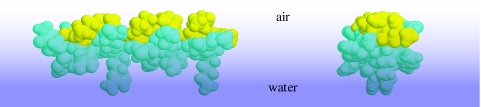
Figure 3.
Possible helix structure of AM1 at the air–water interface showing the relationship to the distributions in figure 2. Deuterated hydrophobic residues (yellow) show clear separation from the hydrophilic residues (blue), unlike proteins (e.g. figure 1). Right-handed α-helical conformations of N- and C-terminally capped peptides were generated using PepBuild (www.imtech.res.in/bvs/pepbuild/). Visual representations were generated using Protein Explorer (http://www.umass.edu/microbio/chime/pe_beta/pe/protexpl/frntdoor.htm).
The mechanical properties of the AM1 thin film have been quantitatively determined by direct stress–strain measurement, in situ and in the plane of the interface, using the CIT (figure 1; Dexter et al. 2006). Previous studies using the CIT (Jones & Middelberg 2002a–c, 2003; Dexter et al. 2006; Malcolm et al. 2006a) do not report an experimental Young's modulus for interfacial films, as direct measures of film thickness were not available. In this study, neutron reflectometry provides information on interfacial structure and thickness. From figure 2, thickness can be estimated as t=15.0 Å by taking the peak width at half maximum height while recognizing that the distribution is non-symmetric. This result, coupled with the measured interfacial modulus of 120 mN m−1, allows a Young modulus of E=80 MPa to be calculated. This quantitative result is consistent with estimates for other biological materials such as peptide fibres (1–20 MPa; Leon et al. 1998), collagen fibres in buffer (200–500 MPa; van der Rijt et al. 2006) and the wall of the yeast cell (100–130 MPa; Smith et al. 2000a,b). To the best of our knowledge, this result represents the first in situ experimental determination of the Young modulus of a biological material self-assembled at a soft interface and has been performed on a molecularly thin film.
We next addressed the question of whether the characteristics of the interfacial film changed substantially when the array was in the ‘off’ state (i.e. when a mechanically weak film exists at the interface). While restructuring is known for peptides following self-assembly at an interface, the extent and nature of interfacial ordering or disordering is highly dependent on the sequence of amino acids in the peptide, the nature of the interface (hard or soft) and the physico-chemical conditions of self-assembly (Degrado & Lear 1985; Holt et al. 2002; Lu et al. 2004; Henderson et al. 2005; Rapaport 2006). Therefore, we repeated the neutron reflectometry study for an AM1 film assembled at the air–water interface in the off state (in the presence of the chelating agent EDTA) and found a very slight but insignificant decrease in molecular density (molecular area increased to 390 Å2, volume fraction decreased to 54% and film thickness decreased to 14.6 Å). In the off state, the film has an interfacial elasticity of less than 30 mN m−1 and a maximum interfacial tensile stress of 0.5 mN m−1 (Dexter et al. 2006), giving a Young modulus of less than 20 MPa. Neutron reflectometry of deuterated peptide confirmed the structure of the interfacial film which was almost identical to that assembled in the ‘on’ or high-elasticity state (data not shown); in both states the film comprised a thin layer of peptide having clear separation of the hydrophobic and hydrophilic regions. For AM1 it can be concluded that a change in the in-plane interactions, rather than interfacial depopulation or rearrangement, leads to the transition from high- to low Young's modulus. This finding supports our proposal that, for AM1, it is the existence of a mechanically strong film at the interface, rather than the density or thickness of the peptide film, that is important for foam stabilization.
It appears that the air–water interface imparts a dominant ordering effect on AM1, which has the classic 4,3 hydrophobic repeat characteristic of coiled coils (Cohen & Parry 1990; Kohn & Hodges 1998). Crick (1952, 1953) showed that some coiled coil architectures adopt a ‘knobs-in-holes’ geometry whereby the hydrophobic side chains from one helix pack into holes between side chains on an associated helix, thus minimizing the contact of hydrophobic residues with water. Other non-hydrophobic residues in the sequence drive inter-peptide interactions, for example by ion pairs (Cohen & Parry 1990; Kohn & Hodges 1998). At the air–water interface the hydrophobic driver for self-assembly is retained. However, the interface imposes less steric constraint and so the conformational entropy (Lee et al. 2000) of the hydrophobic side chains is higher than when packed in a constrained hole. Consequently, the hydrophobic driver of AM1 self-assembly may stabilize peptide ordering independently of whether or not adjacent peptides interact with each other, for example through ion-pair interactions (Cohen & Parry 1990; Kohn & Hodges 1998) or, in the case of AM1, switchable metal ion coordination (Ruben et al. 2004; Dexter et al. 2006). This interfacial uncoupling of the energies of interfacial ordering and peptide–peptide interaction makes it possible to have AM1 interfacial films that are similarly populated, but which display very different mechanical properties.
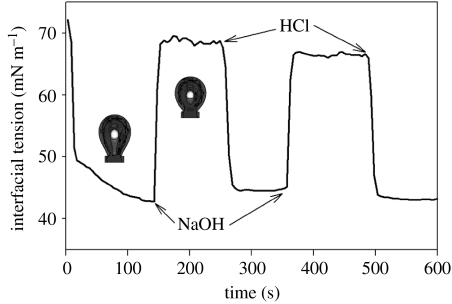
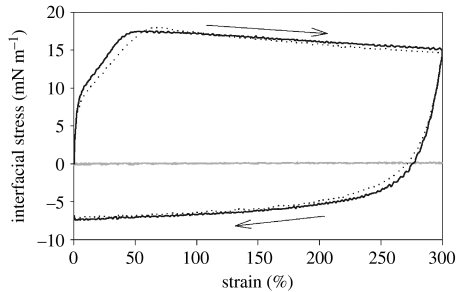
We next asked whether changes in the amino acid sequence of the peptide would alter the mechanism of film switching. Could we develop or discover a peptide which assembles a concentrated and precisely ordered film, similar to AM1, but which switches by changing the interfacial peptide concentration? The peptide Lac21E (Ac-MEELADS LEELARQ VEELESA-NH2) contains a high content of carboxylate residues and is closely related to the AM1 parent sequence Lac21 (Fairman et al. 1996). Figure 4 shows rapid switching of interfacial tension with Lac21E as a function of pH. At freshly formed interface, with a bulk solution pH of 3, the peptide adsorbed to give an interfacial tension of 43 mN m−1 at 140 s in a stirred cell. Addition of NaOH to adjust the pH to 7.4 (arrow) gave a rapid increase in interfacial tension to 69 mN m−1 as the peptide desorbed from the interface. Reacidification (arrow) lowered the interfacial tension to 45 mN m−1 as the peptide readsorbed. A second cycle of switching gave similar results. We probed the strength of the interfacial film under both conditions using the CIT (figure 5). An interfacial film assembled from Lac21E at pH 3 showed an interfacial elasticity of 430 mN m−1 and a maximum interfacial stress of 17.5 mN m−1. On addition of sufficient NaOH to adjust the bulk solution to pH 7.4, followed by re-equilibration of the interface, the mechanical strength of the interface was lost (interfacial elasticity 5 mN m−1 and maximum interfacial stress 0.3 mN m−1). Subsequent addition of HCl to return the solution pH to 3 restored the original mechanical properties (interfacial elasticity 380 mN m−1 and maximum interfacial stress 18.1 mN m−1). Figure 6 shows differences in foam stability at different pH values and confirms that a more stable foam is achieved under conditions that lead to a strong interfacial film as reported previously for AM1 (Malcolm et al. 2006a).
Figure 4.
Rapid switching of interfacial tension with a pH trigger using peptide surfactant Lac21E. A bubble formed in a solution of Lac21E at pH 3 reached a low interfacial tension, which was rapidly increased on addition of NaOH to increase the pH to 7.4, but could be decreased by addition of HCl to restore the pH to 3 (arrows). The cycle could be repeated giving similar results. Inset: bubble images at pH 7.4 (top) and pH 3 (bottom).
Figure 5.
Switching the mechanical properties of a Lac21E film by pH change. Lac21E self-assembly at pH 3 (solid line) gives a strong film. Addition of NaOH to adjust the pH to 7.0 (grey line) dissipated the film, while reacidification to pH 3 (dotted line) restored it.
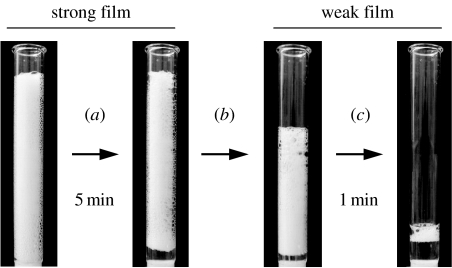
Figure 6.
Rapid modulation of the stability of a Lac21E-stabilized foam by changes in pH. A foam prepared from a Lac21E solution at pH 3.0 (a) was stable on standing for 5 min. After (b), adjustment of the pH to 7.0, the foam collapsed completely within 1 min (not shown) and a new foam was prepared which (c) collapsed completely in 1 min.
We directly determined the structure of the interfacial Lac21E film, using neutron reflectometry of deuterated Lac21E, as already done for AM1. The interfacial area per molecule of 1400±200 Å2 was significantly higher at neutral pH than at acid pH (330±10 Å2). The corresponding volume fractions at the interface were 17 and 70%, respectively. The Lac21E reversible switch operates by the dissociation of peptide from the interfacial film, in a very different way to the mechanism for AM1, and is consistent with the peptides being highly charged at neutral pH. In this case, loss of the strong state is accompanied by a decrease in interfacial molecular density; the peptides transfer back into the bulk aqueous solution in a process of self-disassembly. Figure 7 shows the interfacial structure at pH 3, where the strong film is formed. The result closely resembles the structure of the AM1 film (figure 2) suggesting that Lac21E, like AM1, is precisely organized into an interfacial layer of interacting α-helical peptides. In this case, the layer thickness is estimated as 12.8 Å, giving an initial Young's modulus of E=335 MPa, switching to E<4 MPa. This high modulus in the populated ‘on’ state is stronger than that of the AM1 film (80 MPa), most probably as a consequence of sequence differences and the higher peptide concentration at the interface (70% volume fraction for Lac21E compared with 55% for AM1). The Lac21E modulus is comparable to that reported for collagen fibres (200–500 MPa; van der Rijt et al. 2006), which comprise a dense nanostructure of interacting helices. These results suggest an upper limit for the Young modulus of hydrated biomaterials comprising interacting peptide or protein helices and a significant dependence of modulus on peptide concentration.
Figure 7.
Structure of the Lac21E film at the air–water interface determined by neutron reflectometry of deuterated peptide. The overall peptide volume distribution (black line) comprises hydrophobic (yellow line) and hydrophilic (cyan line) distributions; surface roughness is represented by a distribution of water density (blue line). The film was formed under conditions where the interface is strongly populated (5 μM deuterated Lac21E in 25 mM HEPES buffer at pH 3).
Neutron reflectometry coupled with deuteration of selected amino acids has provided a unique insight into the detailed structure of switchable interfacial films. Both AM1 and Lac21E create thin but remarkably strong films with neutron reflectivity suggesting, but not proving, ordered folding of the peptides into interacting α-helices. The molecular areas in populated states are similar for both peptides (18 Å2 per residue for AM1 and 16 Å2 for Lac21E) and are consistent with earlier reports for non-switchable β-hairpin peptides at the air–water interface (15 Å2/residue, Lu et al. 2003) and for peptides adsorbed at solid interfaces (15–17 Å2, Lu et al. 2004; Strzalka et al. 2004). This interfacial ordering occurs despite the fact that the peptide surfactant has random-coil characteristics in bulk solution as assessed by circular dichroism (data not shown).
4. Conclusions
Neutron reflectometry has provided detailed information on the organization of interfacial films that self-assemble from aqueous peptide solutions to give structures that can be reversibly switched between an ‘on’ state having high elasticity and an ‘off’ state where the film is weaker. For peptide AM1, the interfacial film structure and concentration are almost the same in both states, and the reflectivity profile is consistent with a thin but ordered layer of interacting α-helices. Experimental determination of the AM1 film thickness (approx. 15 Å) allowed the Young modulus of the interfacial film to be calculated (80 MPa in the ‘on’ state and less than 20 MPa in the ‘off’ state). To the best of our knowledge, this result, obtained for a molecularly thin film, represents the first experimental determination of the Young modulus of a biological film self-assembled at a soft interface. Previous results have shown that AM1 stabilizes emulsions and foams in the high-elasticity state, but not when the film is weak. This result, coupled with the current finding that the interfacial structure does not significantly change when switched, suggests a fundamental link between the Young modulus of the interfacial film and the macroscopic stability of foam, and possibly emulsions, formed using peptide surfactants. We also studied a second designed peptide, Lac21E, which also switches between ‘on’ and ‘off’ states. Unlike AM1, the reduction in Young's modulus for Lac21E, from 335 MPa to less than 4 MPa, following a pH trigger, is caused by very substantial transfer of peptide from the interfacial film back into the aqueous bulk (i.e. by self-disassembly). Nevertheless, neutron reflectometry using deuterated peptide confirmed that the structure of the Lac21E strong film is very similar to that formed by AM1. The extent of foam destabilization following the pH trigger is also similar.
Lac21E and AM1 allow the surfactant effect to be switched off by either depopulation of the interface (Lac21E) or by disconnection of the peptides within the interfacial film (AM1). This complementarity of mechanism in closely related peptides potentially allows independent specification of both interfacial strength and interfacial tension in basic soft-matter studies and suggests that conventional DLVO stabilization mechanisms do not fully describe the properties of peptide-covered soft interfaces. These molecular tools therefore have the potential to enable new fundamental insights in emulsions and foams, by drawing on the richness of the amino acid toolkit to design unique interfacial structures. Knowledge gained by their scientific study may motivate a search for new bio-inspired switchable surfactants and a more complete understanding of the mechanisms governing foam and emulsion stability.
Acknowledgments
This work was supported by grants from the Australian Research Council (FF0348465 to A.P.J.M.), the Research Councils UK (to Rutherford Appleton Laboratories) and through an Australian Research Council Federation Fellowship to A.P.J.M. The authors thank Mr Andrew Malcolm for assistance in preparing figure 1.
Supplementary Material
Raw data from neutron reflecometry showing model fit
References
- Abbott N.L. New horizons for surfactant science in chemical engineering. AIChE J. 2001;47:2634–2639. doi: 10.1002/aic.690471202. [DOI] [Google Scholar]
- Aydogan N, Abbott N.L. Comparison of the surface activity and bulk aggregation of ferrocenyl surfactants with cationic and anionic headgroups. Langmuir. 2001;17:5703–5706. doi: 10.1021/la010178e. [DOI] [Google Scholar]
- Aydogan N, Rosslee C.A, Abbott N.L. Reassessment of the surface activity of ferrocenyldimethylammonium surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2002;201:101–109. doi: 10.1016/S0927-7757(01)00823-8. [DOI] [Google Scholar]
- Chen N.H, Kuhl T, Tadmor R, Lin Q, Israelachvili J. Large deformations during the coalescence of fluid interfaces. Phys. Rev. Lett. 2004;92:024 501. doi: 10.1103/PhysRevLett.92.024501. [DOI] [PubMed] [Google Scholar]
- Cohen C, Parry D.A.D. α-Helical coiled coils and bundles: how to design an α-helical protein. Proteins. 1990;7:1–15. doi: 10.1002/prot.340070102. [DOI] [PubMed] [Google Scholar]
- Crick F.H.C. Is α-keratin a coiled coil? Nature. 1952;170:882–883. doi: 10.1038/170882b0. [DOI] [PubMed] [Google Scholar]
- Crick F.H.C. The packing of α-helices—simple coiled-coils. Acta Crystallogr. 1953;6:689–697. doi: 10.1107/S0365110X53001964. [DOI] [Google Scholar]
- Dagastine R.R, Manica R, Carnie S.L, Chan D.Y.C, Stevens G.W, Grieser F. Dynamic forces between two deformable oil droplets in water. Science. 2006;313:210–213. doi: 10.1126/science.1125527. [DOI] [PubMed] [Google Scholar]
- Debregeas G, de Gennes P.G, Brochard-Wyart F. The life and death of “bare” viscous bubbles. Science. 1998;279:1704–1707. doi: 10.1126/science.279.5357.1704. [DOI] [PubMed] [Google Scholar]
- Degrado W.F, Lear J.D. Induction of peptide conformation at apolar/water interfaces. 1. A study with model peptides of defined hydrophobic periodicity. J. Am. Chem. Soc. 1985;107:7684–7689. doi: 10.1021/ja00311a076. [DOI] [Google Scholar]
- Dexter A.F, Malcolm A.S, Middelberg A.P.J. Reversible active switching of the mechanical properties of a peptide film at a fluid–fluid interface. Nat. Mater. 2006;5:502–506. doi: 10.1038/nmat1653. [DOI] [PubMed] [Google Scholar]
- Fairman R, Chao H.G, Lavoie T.B, Villafranca J.J, Matsueda G.R, Novotny J. Design of heterotetrameric coiled coils: evidence for increased stabilization by Glu(−)–Lys(+) ion pair interactions. Biochemistry. 1996;35:2824–2829. doi: 10.1021/bi952784c. [DOI] [PubMed] [Google Scholar]
- Fragneto G, Thomas R.K, Rennie A.R, Penfold J. Neutron reflection study of bovine beta-casein adsorbed on OTS self-assembled monolayers. Science. 1995;267:657–660. doi: 10.1126/science.7839141. [DOI] [PubMed] [Google Scholar]
- Gallardo B.S, Hwa M.J, Abbott N.L. In-situ and reversible control of the surface-activity of ferrocenyl surfactants in aqueous-solutions. Langmuir. 1995;11:4209–4212. doi: 10.1021/la00011a008. [DOI] [Google Scholar]
- Gallardo B.S, Metcalfe K.L, Abbott N.L. Ferrocenyl surfactants at the surface of water: principles for active control of interfacial properties. Langmuir. 1996;12:4116–4124. doi: 10.1021/la960199m. [DOI] [Google Scholar]
- Henderson M, Perriman A, Robson-Marsden H, White J. Protein–poly(silicic) acid interactions at the air/solution interface. J. Phys. Chem. B. 2005;109:20 878–20 886. doi: 10.1021/jp051908k. [DOI] [PubMed] [Google Scholar]
- Holt S.A, Henderson M.J, White J.W. Thermal denaturation of interfacial protein layers. Aust. J. Chem. 2002;55:449–459. doi: 10.1071/CH02100. [DOI] [Google Scholar]
- Israelachvili J.N. Academic Press; London, UK: 1992. Intermolecular and surface forces. [Google Scholar]
- Jacrot B, Zaccai G. Determination of molecular weight by neutron scattering. Biopolymers. 1981;20:2413–2426. doi: 10.1002/bip.1981.360201110. [DOI] [Google Scholar]
- Jones D.B, Middelberg A.P.J. Direct determination of the mechanical properties of an interfacially adsorbed protein film. Chem. Eng. Sci. 2002a;57:1711–1722. doi: 10.1016/S0009-2509(02)00057-X. [DOI] [Google Scholar]
- Jones D.B, Middelberg A.P.J. Mechanical properties of interfacially adsorbed peptide networks. Langmuir. 2002b;18:10 357–10 362. doi: 10.1021/la0262203. [DOI] [Google Scholar]
- Jones D.B, Middelberg A.P.J. Micromechanical testing of interfacial protein networks demonstrates ensemble behaviour characteristic of a nanostructured biomaterial. Langmuir. 2002c;18:5585–5591. doi: 10.1021/la020090g. [DOI] [Google Scholar]
- Jones D.B, Middelberg A.P.J. Interfacial protein networks and their impact on droplet breakup. AIChE J. 2003;49:1533–1541. doi: 10.1002/aic.690490617. [DOI] [Google Scholar]
- Kohn W.D, Hodges R.S. De novo design of α-helical coiled coils and bundles: models for the development of protein-design principles. Trends Biotechnol. 1998;16:379–389. doi: 10.1016/S0167-7799(98)01212-8. [DOI] [Google Scholar]
- Lee A.L, Kinnear S.A, Wand A.J. Redistribution and loss of side chain entropy upon formation of a calmodulin–peptide complex. Nat. Struct. Biol. 2000;7:72–77. doi: 10.1038/71280. [DOI] [PubMed] [Google Scholar]
- Leon E, Verma N, Zhang S, Lauffenburger D, Kamm R. Mechanical properties of a self-assembling oligopeptide matrix. J. Biomater. Sci. Polym. Ed. 1998;9:297–312. doi: 10.1163/156856298x00668. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jessop P.G, Cunningham M, Eckert C.A, Liotta C.L. Switchable surfactants. Science. 2006;313:958–960. doi: 10.1126/science.1128142. [DOI] [PubMed] [Google Scholar]
- Lu J, Thomas R. Neutron reflection from wet interfaces. J. Chem. Soc. Faraday Trans. 1998;94:995–1018. doi: 10.1039/a707853f. [DOI] [Google Scholar]
- Lu J.R, Lee E.M, Thomas R.K. The analysis and interpretation of neutron and X-ray specular reflection. Acta Crystallogr. A. 1996;52:11–41. doi: 10.1107/S0108767395011202. [DOI] [Google Scholar]
- Lu J.R, Perumal S, Powers E.T, Kelly J.W, Webster J.R.P, Penfold J. Adsorption of beta-hairpin peptides on the surface of water: a neutron reflection study. J. Am. Chem. Soc. 2003;125:3751–3757. doi: 10.1021/ja0292290. [DOI] [PubMed] [Google Scholar]
- Lu J.R, Perumal S, Hopkinson I, Webster J.R.P, Penfold J, Hwang W, Zhang S.G. Interfacial nano-structuring of designed peptides regulated by solution pH. J. Am. Chem. Soc. 2004;126:8940–8947. doi: 10.1021/ja049477r. [DOI] [PubMed] [Google Scholar]
- Lu J.R, Su T.J, Thomas R.K, Penfold J, Webster J. Structural conformation of lysozyme layers at the air/water interface studied by neutron reflection. J. Chem. Soc. Faraday Trans. 1998;94:3279–3287. doi: 10.1039/a805731a. [DOI] [Google Scholar]
- Lucassen J. Dynamic properties of free liquid films and foams. In: Lucassen-Reynders E.H, editor. Anionic surfactants: physical chemistry of surfactant action. Surfactant science series. vol. 11. Marcel Dekker, Inc; New York, NY: 1981. pp. 217–265. [Google Scholar]
- Malcolm A.S, Dexter A.F, Middelberg A.P.J. Foaming properties of a peptide designed to form stimuli–responsive interfacial films. Soft Matter. 2006a;2:1057–1066. doi: 10.1039/b609960b. [DOI] [PubMed] [Google Scholar]
- Malcolm A.S, Dexter A.F, Middelberg A.P.J. Mechanical properties of interfacial films formed by lysozyme self-assembly at the air–water interface. Langmuir. 2006b;22:8897–8905. doi: 10.1021/la060565u. [DOI] [PubMed] [Google Scholar]
- Mathur A.M, Drescher B, Scranton A.B, Klier J. Polymeric emulsifiers based on reversible formation of hydrophobic units. Nature. 1998;392:367–370. doi: 10.1038/32856. [DOI] [Google Scholar]
- Middelberg A.P.J, Radke C.J, Blanch H.W. Peptide interfacial adsorption is kinetically limited by the thermodynamic stability of self association. Proc. Natl Acad. Sci. USA. 2000;97:5054–5059. doi: 10.1073/pnas.080042597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers D. Wiley-VCH; New York, NY: 1999. Surfaces, interfaces, and colloids: principles and applications. [Google Scholar]
- Penfold J, Thomas R. Solvent distribution in non-ionic surfactant monolayers. Phys. Chem. Chem. Phys. 2002;4:2648–2652. doi: 10.1039/b108698g. [DOI] [Google Scholar]
- Rapaport H. Ordered peptide assemblies at interfaces. Supramol. Chem. 2006;18:445–454. doi: 10.1080/10610270600665905. [DOI] [Google Scholar]
- Reches M, Gazit E. Controlled patterning of aligned self-assembled peptide nanotubes. Nat. Nanotechnol. 2006;1:195–200. doi: 10.1038/nnano.2006.139. [DOI] [PubMed] [Google Scholar]
- Rosslee C, Abbott N.L. Active control of interfacial properties. Curr. Opin. Colloid Interf. Sci. 2000;5:81–87. doi: 10.1016/S1359-0294(00)00035-2. [DOI] [Google Scholar]
- Ruben M, Rojo J, Romero-Salguero F.J, Uppadine L.H, Lehn J.M. Grid-type metal ion architectures: functional metallosupramolecular arrays. Angew. Chem. Int. Ed. 2004;43:3644–3662. doi: 10.1002/anie.200300636. [DOI] [PubMed] [Google Scholar]
- Shang T.G, Smith K.A, Hatton T.A. Photoresponsive surfactants exhibiting unusually large, reversible surface tension changes under varying illumination conditions. Langmuir. 2003;19:10 764–10 773. doi: 10.1021/la0350958. [DOI] [Google Scholar]
- Shin J.Y, Abbott N.L. Using light to control dynamic surface tensions of aqueous solutions of water soluble surfactants. Langmuir. 1999;15:4404–4410. doi: 10.1021/la981477f. [DOI] [Google Scholar]
- Sjogren H, Ulvenlund S. Effects of pH, ionic strength, calcium, and molecular mass on the arrangement of hydrophobic peptide helices at the air–water interface. J. Phys. Chem. B. 2004;108:20 219–20 227. doi: 10.1021/jp047858l. [DOI] [Google Scholar]
- Smith A.E, Moxham K.E, Middelberg A.P.J. Wall material properties of yeast cells. Part II. Analysis. Chem. Eng. Sci. 2000a;55:2043–2053. doi: 10.1016/S0009-2509(99)00501-1. [DOI] [Google Scholar]
- Smith A.E, Zhang Z.B, Thomas C.R, Moxham K.E, Middelberg A.P.J. The mechanical properties of Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA. 2000b;97:9871–9874. doi: 10.1073/pnas.97.18.9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strzalka J, Gibney B.R, Satija S, Blasie J.K. Specular neutron reflectivity and the structure of artificial protein maquettes vectorially oriented at interfaces. Phys. Rev. E. 2004;70:061 905. doi: 10.1103/PhysRevE.70.061905. [DOI] [PubMed] [Google Scholar]
- van der Rijt J.A.J, van der Werf K.O, Bennink M.L, Dijkstra P.J, Feijen J. Micromechanical testing of individual collagen fibrils. Macromol. Biosci. 2006;6:697–702. doi: 10.1002/mabi.200600063. [DOI] [PubMed] [Google Scholar]
- Zhang S.G. Fabrication of novel biomaterials through molecular self-assembly. Nat. Biotechnol. 2003;21:1171–1178. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- Zhang S. Another brick in the wall. Nat. Nanotechnol. 2006;1:169–170. doi: 10.1038/nnano.2006.154. [DOI] [PubMed] [Google Scholar]
- Zhao X.J, Zhang S.G. Fabrication of molecular materials using peptide construction motifs. Trends Biotechnol. 2004;22:470–476. doi: 10.1016/j.tibtech.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Zhao X, Zhang S. Molecular designer self-assembling peptides. Chem. Soc. Rev. 2006;35:1105–1110. doi: 10.1039/b511336a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Raw data from neutron reflecometry showing model fit