Abstract
C. albicans is a diploid organism that exhibits high levels of heterozygosity. Although the precise manner by which this heterozygosity provides advantage for the commensal/pathogenic life styles of C. albicans is not known, heterozygous markers are themselves useful for studying genomic rearrangements, which occur frequently in C. albicans. Treatment of CAI-4 with UV light yielded histidine auxotrophs which could be complemented by HIS4, suggesting that strain CAI-4 is heterozygous for HIS4. These auxotrophs appeared to have undergone mitotic recombination and/or chromosome loss. As expected from a heterozygote, disruption of the functional allele of HIS4 resulted in a his4::hisG-URA3-hisG strain that is auxotrophic for histidine. Sequencing of random clones of the HIS4 ORF from CAI-4 and its precursor SC5314 revealed the presence of 11 SNPs, 7 synonymous and 4 non-synonymous. Site directed mutagenesis indicates that only one of those SNPs, T929G (Gly310Val), is responsible for the non-functionality of the encoded enzyme. HIS4 analysis of five commonly used laboratory strains is reported. This study provides a new, easily measured nutritional marker that can be used in future genetic studies in C. albicans.
Keywords: Candida albicans SC5314, HIS4 heterozygosity, SNPs
1. Introduction
Candida albicans is a normal commensal of the human mucosa, but under some circumstances, including immunosuppression, it can cause superficial or disseminated infections that affect most of the organs in susceptible warm-blood animals. In fact, C. albicans is the most common fungal pathogen of man and the fourth cause of nosocomial infectious diseases in the USA, as well as in other countries where data are available (Calderone, 2002).
Traditionally, C. albicans has been considered to be a diploid or quasi-diploid organism that reproduces mainly by clonal propagation. This concept was recently challenged by the discovery of a sexual locus (MTL-locus) (Hull and Johnson, 1999) as well as by the demonstration of true mating and karyogamy between strains of opposite mating type (Hull et al., 2000; Magee and Magee, 2004), followed by the return of the resulting tetraploid to the diploid state by a process of chromosome loss (Bennett and Johnson, 2003). However, the low frequency of strains homozygous for the MTL locus in nature (Pujol et al., 1993; Lockhart et al., 2002; Legrand et al., 2004), together with the low efficiency of karyogamy between genetically different isolates (Lockhart et al., 2003) and the apparent absence of a true meiotic process (Tzung et al., 2001) suggest that the extent to which sexual recombination contributes to the variability of the organism is rather low (Magee and Magee, 2004). In spite of these limitations, the parasexual cycle may result in new combinations of chromosomes that could provide some genetic variability for C. albicans, since that process can rearrange highly polymorphic alleles observed in clinical isolates (Miller and Johnson, 2006). Therefore, parasexuality may account for the low levels of recombination revealed by several population genetic studies (Gräser et al., 1996; Forche et al., 1999; Xu et al., 1999; Pujol et al., 2003).
Clinical isolates of C. albicans exhibit a substantial degree of natural heterozygosity (Whelan et al., 1980; Whelan and Soll, 1982; Tsang et al., 1999). This implies that for some pairs of alleles one copy is functional whereas the other carries a recessive mutation (e.g., a point mutation). The heterozygosity of C. albicans has been suggested from the observation that clinical isolates of the organism, when treated with moderate doses of UV light, formed auxotrophic progeny in a pattern (limited to one or two traits) that was strain-specific. Evidence was presented indicating that auxotrophs arise by segregation from naturally occurring heterozygotes. It was hypothesized that low doses of UV light likely result in a mitotic crossing over such that both recessive alleles can segregate together resulting in the formation of an auxotrophic phenotype (or any other phenotype) (Whelan and Magee 1981; Whelan and Soll, 1982; Whelan, 1987; Magee, 1990). Interestingly, auxotrophs for different growth factors, including Met, Arg, Ade, Lys, Pro, etc, were isolated, so that each parental strain was heterozygous for some gene in the corresponding biosynthetic pathway. The high degree of heterozygosity has been confirmed and extended by sequencing the C. albicans genome. The diploid sequence of strain SC5314 has 62,534 high confidence polymorphisms, most of them located in intergenic regions. Still 3,579 ORFs contain polymorphisms of which 2,792 alter the translation product (Jones et al., 2004). This may result in differences in the activity of the proteins encoded by two functional alleles. When the protein encoded by one allele is not functional, a single mutational event that inactivates the functional allele results in expression of the recessive phenotype. Under these conditions, uncovering a recessive phenotype is a simple way to detect loss of heterozygosity (LOH). The functional consequences of LOH at intergenic regions are more difficult to determine but include regulatory SNPs in a human genetic disease and in the loss of seed shattering during rice domestication as has been recently reported (De Gobbi et al., 2006; Konishi et al., 2006).
Should heterozygosity be advantageous for C. albicans? It has been suggested that LOH may provide a genetic role similar to sexual recombination (Yesland and Fonzi, 2000), thus resulting in genotypic and, occasionally, phenotypic variability in C. albicans. Indeed, LOH in C. albicans has proved to be crucial for a number of phenomena related to its biology and antifungal susceptibility. For instance, only cells that become homozygous for the MTL locus can mate (Hull et al., 2000; Magee and Magee, 2004). In addition, analysis of a number of clinical isolates or strains induced to fluconazole resistance in vitro has suggested a correlation between homozygosity at the MTL locus and resistance to azoles (Rustad et al., 2002). For instance, LOH at and around the ERG11 locus (which localizes to chromosome 5 and encodes for lanosterol demethylase, the enzyme targeted by fluconazole) has been shown to occur during development of fluconazole-resistance in clinical isolates (White, 1997; Franz et al., 1998). Other studies have indicated that LOH at several loci accompanied the adaptation of C. albicans to fluconazole, although a causal relationship was not apparent (Cowen et al., 2000). More recently, LOH at specific sites of chromosome 5 resulted in the homozygosity of a hyperactive allele of the transcriptional regulator Tac1p and upregulation of the efflux pumps CDR1 and CDR2 in pairs of matched azole-susceptible and azole-resistant strains (Coste et al., 2006).
Interestingly, LOH at several loci occurs at a measurable level during infection and this may be accompanied by changes in karyotypes (Forche et al., 2003; 2004; 2005). Also, multilocus sequence typing (MLST)(Bougnoux et al., 2002; Tavanti et al., 2004) has indicated that following colonization of an individual, the progeny of a C. albicans strain undergo microvariations caused mainly by LOH events (Bougnoux et al., 2006; Odds et al., 2006). In addition, a recent report suggests that recombination in C. albicans may be a more common event than previously thought (Odds et al., 2007). Accordingly, it is possible that at least some of these events influence the virulence and survival of this opportunistic pathogen.
In this scenario, the detection of new, easily scored heterozygous nutritional markers and their characterization in model strains could be extremely useful for the genetic analysis of C. albicans. In particular, LOH caused by either loss of chromosome or gene conversion, as well as the role of recombination genes in these events can be identified. In the present study, we show that in C. albicans SC5314, HIS4 is a heterozygous marker. Further, DNA sequence analysis of the HIS4 ORF has indicated the presence of 11 SNPs, but only one, the T929G transversion, seems relevant to the function of His4p. In addition to sequencing the PCR products, SNP-specific PCR can be also used to readily identify the presence of either allele in other strains.
2. Materials and Methods
2.1. Strains and media
The C. albicans strains used in this study are indicated in Table 1. Prototrophic strain SC5314 (Gillum et al., 1984) has been used in genome sequencing. C. albicans cells were grown routinely at 28°C in YPD (1% yeast extract, 2% Bacto Peptone, 2% glucose). For the isolation of his auxotrophs cells were grown on complete SC and colonies replica-plated onto SC minus histidine.
Table 1.
Strains of C. albicans used in this study.
| Strain | Relevant genotype | Parental | Reference |
|---|---|---|---|
| SC5314 | Wild type | Gillum et al., 1984 Odds et al., 2006 (a gift of W. Fonzi) | |
| CAI-4 | ura3-ΔΔ | SC5314 | Fonzi and Irwin, 1993 (a gift of W. Fonzi) |
| 1001 | Wild type | (ATCC 64385) (a gift of F. Navarro-García) | |
| 1006 | Arg−, Ser−, Lys−, ura3, MpaR | CBS5736*, 834 (STN57) ATCC 32032 DSM3454 IFO 1856 | Van Uden, and Buckley, 1970 Suzuki et al., 1986 Goshorn et al., 1992 Chu et al., 1993 (P. Magee lab) |
| 3153A | Wild type | Evans et al., 1974 (Pasteur Institute) (G. Larriba lab) | |
| 4918 | Wild type | Manning & Mitchell, 1980 (R. Calderone lab) | |
| SN148 | Arg− Leu− His− Ura− | SC5314 | Noble and Johnson, 2005 |
| 7A2A | his4-ΔΔ | CAI-4 | This work |
Different names given to CBS 5736/ATCC 32032
2.2. DNA extraction and analysis
Standard techniques were used for DNA manipulations (Sherman et al., 1972). Genomic DNA was obtained as described (Andaluz et al., 1999). For Southern analysis, genomic DNA was digested with the indicated restriction enzymes, electrophoresed into an agarose gel, transferred to nitrocellulose, and probed with the corresponding DNA fragment at high stringency (6x SSC [1xSSC is 0.15M NaCl plus 0.015M sodium citrate], 5xDenhart’s solution, 0.5% SDS for 12 h at 65°C). The probes used consisted of either PCR products amplified from selected regions of genomic DNA (CZF1, PHR1) using oligonucleotides indicated in Table 2 or the specific disruption cassette (HIS4). They were labeled with 32P using the Random Primed DNA Radiolabel Kit (Roche). CZF1 and PHR1 exhibit restriction fragment length polymorphism (RFLP) in strain SC5314 associated with SspI and BamHI sites, respectively (Xi Chen, personal communication; Chen et al., 2004; Yesland and Fonzi, 2000). Following hybridization, nitrocellulose filters were washed (65°C in 2X SSC, 0.5% SDS and then 0.2X SSC, 0.5% SDS) and analyzed using a Molecular Imager FX (Bio-Rad Laboratories). The Quantity ONE-1D Software (Bio-Rad) was used to determine the intensity of hybridized bands in Southern blots. PCR amplification of the heterozygous marker RBT7 was accomplished using the oligonucleotides indicated in Table 2. Karyotype and MTL locus analysis were carried out as described (Andaluz et al., 2007).
Table 2.
Primers used in this study
| 1. Polymorphic loci | |||
| Locus | Oligonucleotide sequence | Size | RFLP-associated restriction site |
| CZF1-F | 5′-CACAATCTGTAGGTTACCTAG-3′ | 382bp | SspI |
| CZF1-R | 5′-TGCTGCTCTGATGGAGACAA-3′ | ||
| PHR1-F | 5′-TAAACGTGACGTCAAGTATT-3′ | 425bp | BamHI |
| PHR1-R | 5′-ACGATCATCTAATGAACCAC-3 | ||
| RBT7-F | 5′-GAGTGAAAAATAAGTGACGAAC-3′ | 1009bp | |
| RBT7-R | 5′-TTAGGATGTACCACACTTCATC-3′ | AluI, MboI | |
| 2. Amplification and sequencing of HIS4 or HIS4 fragments | |||
| Oligonucleotide | Sequence 5’-3’ | Relative position within the ORF (5′ end of each primer) | |
| His41-F | TCCTCCACAAGTGATAAGTT | −71 | |
| His41-R | AGAGAACTTTACCAAGATGC | +40 | |
| His42-F | GAAATATAATGCCAATGAAA | 372 | |
| His42-R | GAATCACTGGAACGTAGTTT | 469 | |
| His43-F | ATTGCCGAAATTAGATAGTA | 876 | |
| His43-R | GTTTAGTGTAGGACCCTTCA | 943 | |
| His44-F | TTTGATGGTGTAAAACTTGA | 1354 | |
| His44-R | TTTTTCTTTTGGTAATTGTG | 1500 | |
| His45-F | ATTTTGTTGCTAGTAATTTG | 1952 | |
| His45-R | CTTTAGCTTGTCTCTCCACT | 2080 | |
| 3. PCR Analysis of the disruption of HIS4 alleles | |||
| Oligonucleotide | Sequence | Size | |
| URA1 | 5’-GGTATAGAAATGCTGGTTGG-3’ | 1,7 Kb | |
| HIS45-R | 5’-CTTTAGCTTGTCTCTCCACT-3’ | ||
| HIS41-F | 5’-TCCTCCACAAGTGATAAGTT-3’ | 2.2 Kb | |
| HIS45-R | 5’- CTTTAGCTTGTCTCTCCACT-3’ | ||
| 4. Site-directed mutagenesis | |||
| Oligonucleotide | Sequence | Change | |
| MUTA769-F | 5’-GGATTGTGACTCTCATGTGATTCAGTTTATGGTGGAGC-3’ | G/C | |
| MUTA769-R | 5’-GCTCCACCATAAACTGAATCACATGAGAGTCACAATCC-3’ | G/C | |
| MUTA929-F | 5’-GATTGGAAAATGCACCTGAAGTGTCCTACACTAAACG-3’ | G/T | |
| MUTA929-R | 5’-CGTTTAGTGTAGGACACTTCAGGTGCATTTTCCAATC-3’ | G/T | |
| 5. RT-PCR | |||
| Oligonucleotide | Sequence | Size | |
| HIS42-F | (see above) | 571 bp | |
| HIS43-R | (see above) | ||
| ACT1-F | 5′-GCTGCTTTAGTTATCGATAACGG-3′ | 276 bp | |
| ACT1-R | 5′- GTGGAGCAACTCTCAATTCATTG-3′ | ||
2.3. Construction of a his4-ΔΔ strain in the CAI-4 background
A his4 null strain was constructed using the URA blaster method (Fonzi and Irwin, 1993). A 3628 bp EcoRI-SacI fragment released from vector pDMHis4, which carries CaHIS4 from strain 1001 (Navarro-Garcia et al., 1998), was subcloned in pGEM-7Z(+) previously cleaved with the same restriction enzymes. This construct was incubated with PstI to release a 1537 bp fragment comprising the central region of the HIS4 ORF, which was substituted with the hisG-URA3-hisG using blunt end ligation. Treatment of this construct with XhoI/SacI releases a 5813 bp fragment, in which the hisG-URA3-hisG module is flanked by 523 and 457 bp of the HIS4 ORF (see Fig. 4). This cassette was used to sequentially disrupt both alleles of HIS4. Accordingly, in the resulting his4-ΔΔ strain, 7A2A, the domain HIS4B of each HIS4 allele (see Results, second paragraph), including bases 856 to 1080, was entirely deleted. Since the encoded protein normally catalyzes the second step of histidine biosynthesis, our his4 null homozygous should accumulate phosphoribosyl-ATP which is the product of the first biosynthetic reaction catalyzed by His1. In addition, since only 523 bp remain on the 5′ side of the HIS4 ORF and HIS4A extends from base 1 to base 855, it is unlikely that His4A is functional. The same is true for His4C, since only the last 457 last bp were left and HIS4C extends from 1080 to base 2517.
Fig. 4.
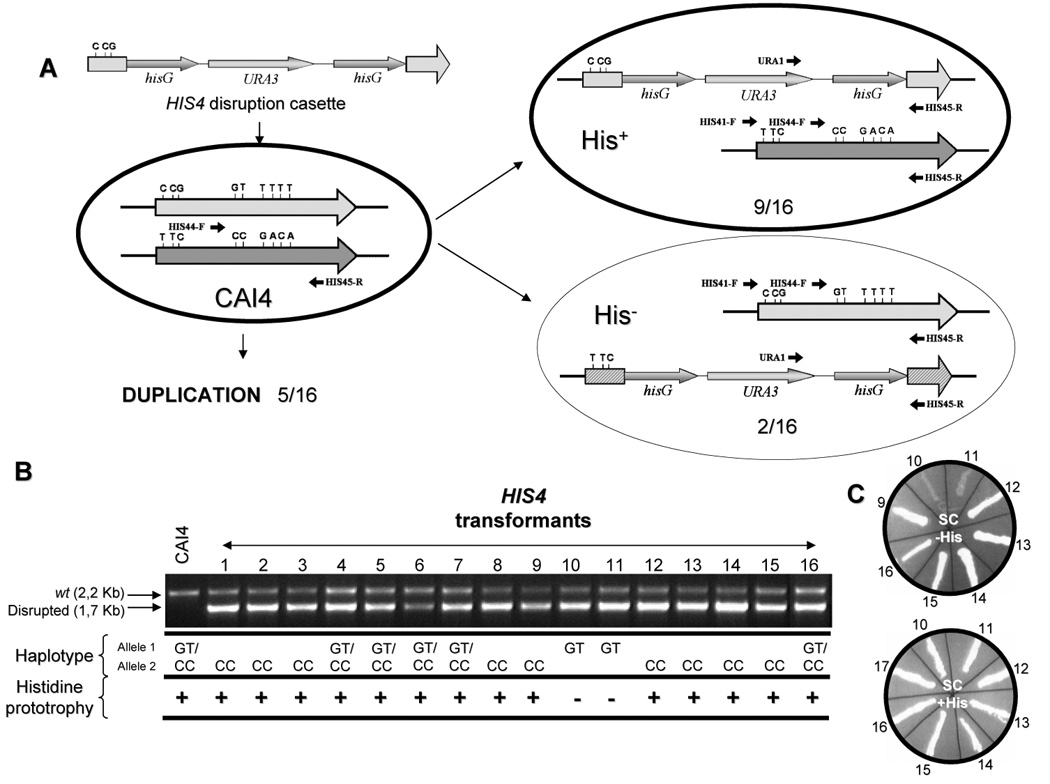
Genetic and functional analysis of the HIS4 alleles in strain CAI-4. A. Diagram showing the possibilities of integration of a disruption cassette prepared from the 1001 allele in CAI-4 cells. Alleles are distinguished with either light (inactive) or dark (active) grey. The presence of three heterologies between the upstream region of the URA blaster and the same region of the active allele is shown. We have indicated three heterozygosities in 5′ region of the disrupted functional allele (*) and labeled that region with an intermediate grey because we have not investigated the bases present in each disruptant (they may belong to either the cassette or the allele, depending on the recombination point). B. PCR of cognate heterozygous clones and sequencing data (for details see the text). For the sake of simplicity only two SNPs are shown but the other supports the expected haplotype. All strains were grown in SC with/without histidine to distinguish between prototrophs (His+) and auxotrophs (His−) (bottom line). C. Behavior of several strains in this assay.
2.4. Cloning and sequencing of HIS4 from CAI-4 and SC5314 strains
HIS4 was amplified by PCR (Expand High Fidelity PCR System; Roche) from genomic DNA of the indicated strains. Primers pairs for PCR included 20-mer oligonucleotides complementary to positions −71 to −51 in relation to the first nucleotide of the ORF and to positions +21 to + 40 of the nucleotide stop codon (Table 2). The amplification products from each strain were purified from an agarose gel yielding a homogeneous product of the expected length (about 2.6 kb), which was then cloned into the vector pGEM-T (Promega). The ligation products were transformed into E. coli and white colonies selected. The presence of the construct was verified by PCR using the same pair of oligonucleotides mentioned above. Clones containing the expected fragments were selected and kept at −80°C. Plasmid DNA from the indicated clones was sequenced using the above mentioned primers plus four additional pairs of 20-mer oligonucleotides (four direct and four reverse) (Table 2). These primers are located in similar relative positions along the ORF to yield reliable sequences and overlapping reaction products that allow assembly of the sequences (see Table 3). Each clone supplied 10 sequences (one per primer) that were analyzed using the SeqMan software (DNAStar, Lasergene) resulting in a single correct sequence. Each set of all consensus sequences from the indicated clones from each strain was compared with those of the SC5314 database using the MegAlign software (DNAStar, Lasergene). When new polymorphisms were found in the selected HIS4 clones, they were verified by sequencing a PCR amplified stretch comprising the polymorphic site using the indicated oligonucleotides and genomic DNA of the corresponding strain as a template.
Table 3.
SNP analysis of the HIS4 ORF
| Strain | SNPs and point mutations* | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 266 | 405 | 496 | 769 | 929 | 1719 | 1770 | 1825 | 1890 | 1908 | 1938 | 1983 | |
| 1001 | C | C | G | G | G | G | T | C | T | T | T | T |
| SC5314 reported | C(S)/T(L) | C/T | G | C | T | G | T | C | T | T | T | T/A |
| CAI-4-1 | C | C | G | C | T | G | T | C | T | T | T | T |
| CAI-4-2 | C | C | G | C | T | G | T | C | T | T | T | T |
| CAI-4-3 | C | C | G | C | T | G | T | C | T | T | T | T |
| CAI-4-4 | C | C | G | C | T | G | T | T(S) | T | T | T | T |
| CAI-4-5 | C | C | G | C | T | G | T | C | G | A | C | A |
| CAI-4-6 | T | T | C | G | G | C | C | C | G | A | C | A |
| CAI-4-7 | T | T | C | G | G | C | C | C | G | A | C | A |
| CAI-4-8 | T | T | C | G | G | C | C | C | G | A | C | A |
| SC5314-1 | T | T | C | G | G | C | C | C | G | A | C | A |
| SC5314-2 | C | C | G | C | T | G | T | C | T | T | T | T |
| SC5314-3 | C | C | G | C | T | G | T | C | T | T | T | T |
| SC5314-4 | C | C | G | C | T | C | C | C | G | A | C | A |
| SC5314-5 | C | C | G | C | G | G | T | C | T | T | T | T |
| SC5314 & CAI-4 Our data | C(S)/T(L) | C/T | G(E)/C(Q) | C(H)/G(D) | T(V)/G(G) | G/C | T/C | T/G | T/A | T/C | T/A | |
Eight clones from CAI-4 (CAI-4-1 to CAI-4-8) and five clones from SC5314 (SC5414-1 to SC5314-5) were sequenced and the haplotypes shown in shadowed cells. Allele 1 is indicated with light grey and allele 2 with dark grey. Portions of each allele in recombinant clones are indicated in the same way. Reported data for strain 1001 and SC5314 strains are indicated at the top. The SNP map of the SC5314 lineage, derived from this work, is shown the bottom line. A point mutation found is italicized.
2.5. Site-directed mutagenesis
Specific changes in the sequence of HIS4 ORF were introduced using the QuikChangeR II Site Directed Mutagenesis Kit (Stratagene). For that purpose, we released the functional wild type allele of the HIS4 ORF from strain 1001 present in pDMHIS4 (Navarro-Garcia et al., 1998) and subcloned it in vector pGEM-7Z. This construct (pHIS4) was used as a template. Two changes were independently introduced at positions 769 (G-to-C) and 929 (G-to-T) of the nucleotide sequence, by using specific primers that contained the desired alterations in a central position (Table 2). The resulting constructs were sequenced in order to verify the desired changes. A 3628 bp EcoRI-SacI fragment derived from each one of the correct clones was treated with the Klenow polymerase, and the blunt-ended product was subcloned in the SmaI site of vector pRM1 (Pla et al., 1995). The new constructs with changes at positions 769 (pRMH1-769) and (pRMH1-929) each were used to complement the his4-ΔΔ strain 7A2A.
2.6. SNP-specific PCR and RT-PCR
SNP-specific reactions were designed essentially as described by Holmes et al. (2006). For the SNP-specific PCR we used a common forward primer HIS42-F ending at position 370 of the HIS4 ORF (with respect to the A of the initiation codon ATG) and primers specific for either one of two selected SNPs, 769 and 929 (Table 5). For the first SNP (C769G/His257Asp), we used reverse primers specific for allele 1 (CAT 3′, His) or allele 2 (GAT 3′, Asp) which amplify a 419 bp band. PCR reactions were optimized by varying the annealing temperature. When performed at 50° C, the primers proved to discriminate between both alleles (i.e., each primer only amplified its corresponding allele). For the second SNP, T929G/Val310Gly, we used reverse primers specific for allele 1 (GTG 3′, Val) or allele 2 (GGG3′, Gly) which amplify a 579 bp fragment. Following optimization as above, this PCR was specific for the inactive allele (i.e., the CTG primer only amplified allele 1) but not for the active one (allele 2), since the primer GGG3′ amplified both alleles.
Table 5.
SNP-specific PCR primers
| Common Forward primer (HIS42-F): SNP769 | GAAATATAATGCCAATGAAA |
| Allele 1-specific primer | ACCATAAACTGAATCACATG |
| Allele 2-specific primer (ON-F) SNP929 | ACAATAAACTGAATCACATC |
| Allele 1-specific primer (OFF-NF) | TAAACGTTTAGTGTAGGACA |
| Allele 2-specific primer | TAAACGTTTAGTGTAGGACC |
For RT-PCR, RNA samples were extracted with the hot phenol method (Schmitt et al., 1990) and treated with DNAase (Roche). DNA-free RNA samples (5 µg) were used as templates for the production of total cDNA using Random Primer (Invitrogene) and reverse transcriptase Super Script II (RT, RNA-ase H-Reverse transcriptase) (Invitrogen). Controls without transcriptase were also included. Then cDNA was amplified using either HIS4-specific primers His42-F and His43-R or ACT1 specific primers ACT-F and ACT-R (Table 2).
3. Results
3.1. Identification of HIS4 as a heterozygous marker in strain CAI-4
Low level UV light treatment has been used in the past to reveal the presence of heterozygous markers in clinical isolates of C. albicans (Whelan and Magee, 1981). In order to identify auxotrophic heterozygous markers, we investigated the appearance of histidine auxotrophs following treatment of CAI-4 with mild doses of UV light (20 J/m2) that caused a mortality of about 50%. Six out of 2515 survivors were unable to grow in the absence of histidine, indicating a rate of about 3 × 10−3 His auxotrophs per survivor. His+ revertants were not observed among approximately 21000 colonies analyzed from each strain indicating that these His auxotrophs were rather stable.
On the basis of sequence similarity to putative homologues from S. cerevisiae, C. albicans possesses at least seven genes, located on different chromosomes, that catalyze nine well defined steps in the biosynthesis of histidine (Fig. 1): HIS1 (Chr 5), HIS2 (Chr7), HIS3 (Chr 2), HIS4 (Chr4), HIS5 (Chr4), HIS6 (Chr4), and HIS7 (Chr7). Furthermore, the HIS4 gene from S. cerevisiae and, by extrapolation, its homologue from C. albicans (Navarro-Garcia et al., 1998), encodes a three domain protein HIS4A, HIS4B, and HIS4C, each one responsible for a different activity: AMP cyclohydrolase (step 3), phosphoribosyl-ATP pyrophosphatase (step 2), and histidinol dehydrogenase (the two last steps) respectively (Fink, 1964; 1966; Donahue et al., 1982) (Fig. 1).
Fig. 1.
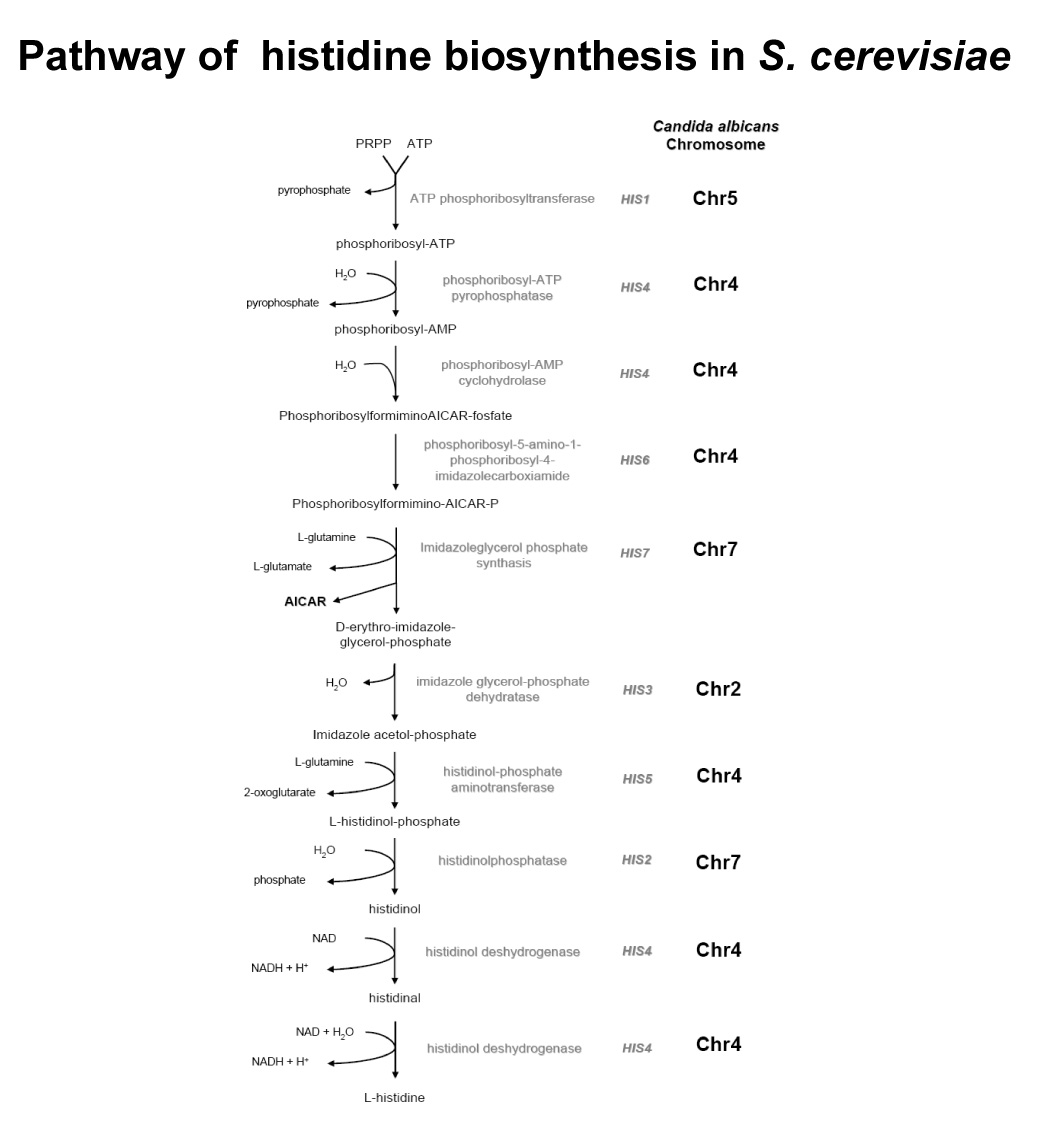
The histidine biosynthetic pathway of Saccharomyces cerevisiae. To the right of each biosynthetic step, the chromosomal location of each C. albicans orthologue is indicated.
In order to detect His auxotrophs altered in the HIS4 ORF, we transformed the six selected His− strains derived from CAI-4 (mentioned above) with plasmid pRMH1, which, in addition to the authentic HIS4 gene from strain 1001 of C. albicans carries the URA3 marker. When colonies from the Uri+ transformants were replica plated onto agar plates lacking His, all grew readily, suggesting that they had been complemented by HIS4. Furthermore, following curing of the plasmid by growing the cells in the presence of 5-FOA as a counter selection agent for Ura+ cells, all survivors were Uri− and His−, an indication that regaining of the His+ phenotype by the mutants was due to transformation and not as a consequence of a reversion caused by mutational event. These results indicated that treatment of CAI-4 with UV light yields His auxotrophs defective in HIS4 and, therefore, strongly suggest that CAI-4 is heterozygous for the HIS4 locus.
3.2. Genetic analysis of the UV-induced His− strains identifies chromosome loss and mitotic recombination as responsible for LOH
In order to investigate the genetic events leading to the generation of the His auxotrophy, we analyzed the copy number of Chr4, where HIS4 is located, in the six UV-induced His auxotrophs (his4.1-his4.6). A comparison of their electrophoretic karyotypes with that of CAI-4 failed to reveal the presence of supernumerary chromosomes (Fig. 2A), suggesting that chromosome breaks had not occurred. All the strains were heterozygous for MTL, except his4.1 which was homozygous for MTLa, suggesting genetic rearrangements in Chr5 (loss of the homologue carrying MTLα, gene conversion, mitotic crossing over, or a large deletion)(Fig. 2B, top row). Next, we analyzed the polymorphic region of RBT7 located in the left arm of Chr4, centromere proximal to HIS4. As shown in Fig. 2B (second row), when genomic DNA from CAI-4 was PCR-amplified and the amplification product cut with AluI, two fragments, one of 394 bp corresponding to the RBT7 allele that lacks an AluI site, and another of 311 bp corresponding to the second allele which carries that site. With the exception of his4.6 which remained heterozygous for the marker, the rest of the strains carry a single allele of RBT7. Analysis of a second heterozygous marker of the left arm closer to the centromere, PHR1, gave the same results (Fig. 2B, third set), raising the possibility that loss of one copy of Chr4 may have occurred with some of the other strains (his4.1–his4.5). Next, we determined the configuration of a second heterozygous marker, CZF1, which is located on the right arm (Sanyal et al., 2004). Again, only the his4.6 strain remained heterozygous for this marker while the others remained homozygous (Fig. 2B, fourth set). However, four of the latter retained the same allele whereas strain his4.3 conserved the other. Some of the several mechanisms that could generate the histidine auxotrophs are shown in Fig. 3.
Fig. 2.
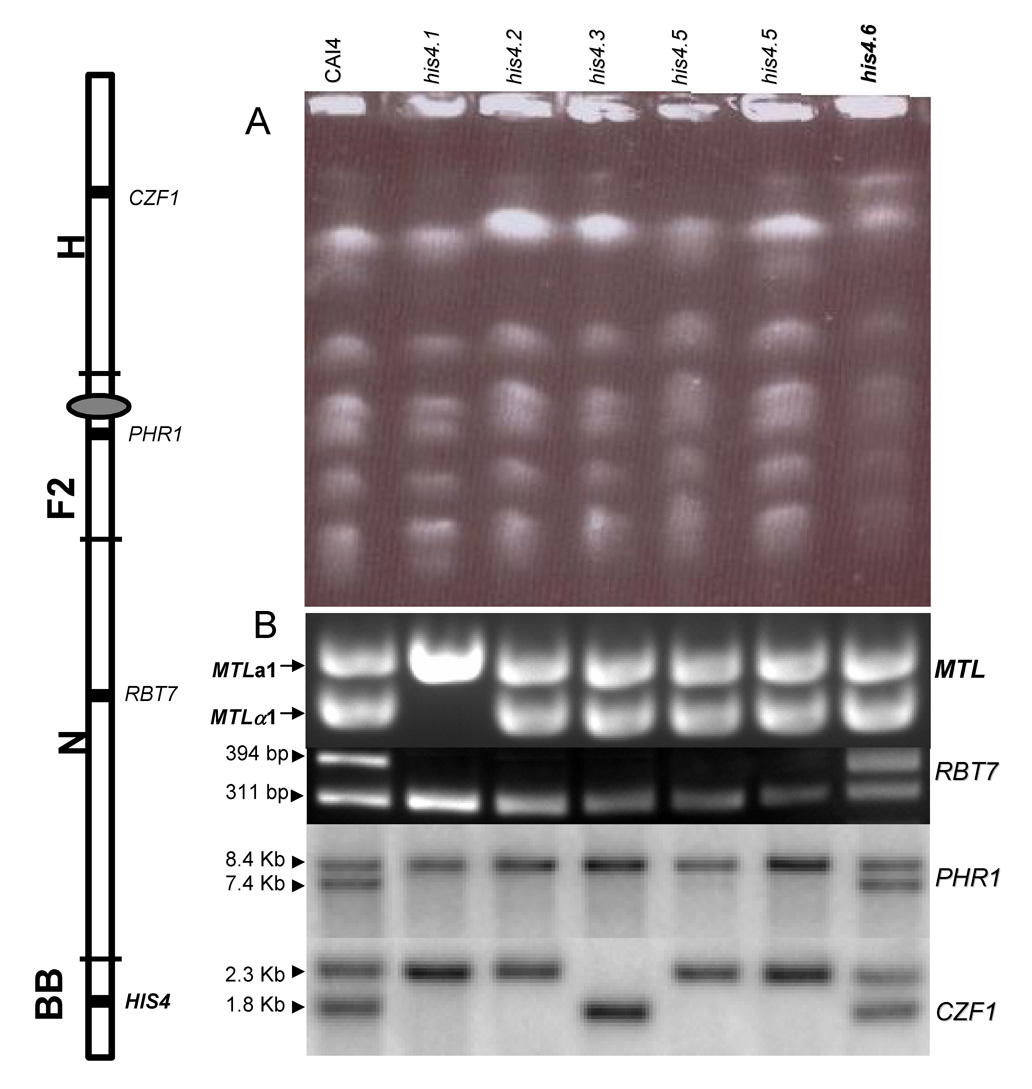
Genetic characterization of CAI-4 and six histidine auxotrophs (his4.1 to his4.6) obtained by UV treatment. A. Electrokaryotypes. B. Analysis of the heterozygosity of the indicated loci (MTL, RBT7, CZF1, and PHR1). See text for details. Left: A map of Chr4, indicating the location of the several loci (Candida Genome Database), SfiI fragments (Chu et al., 1993) and centromere (elipse) (Sanyal et al., 2004).
Fig. 3.
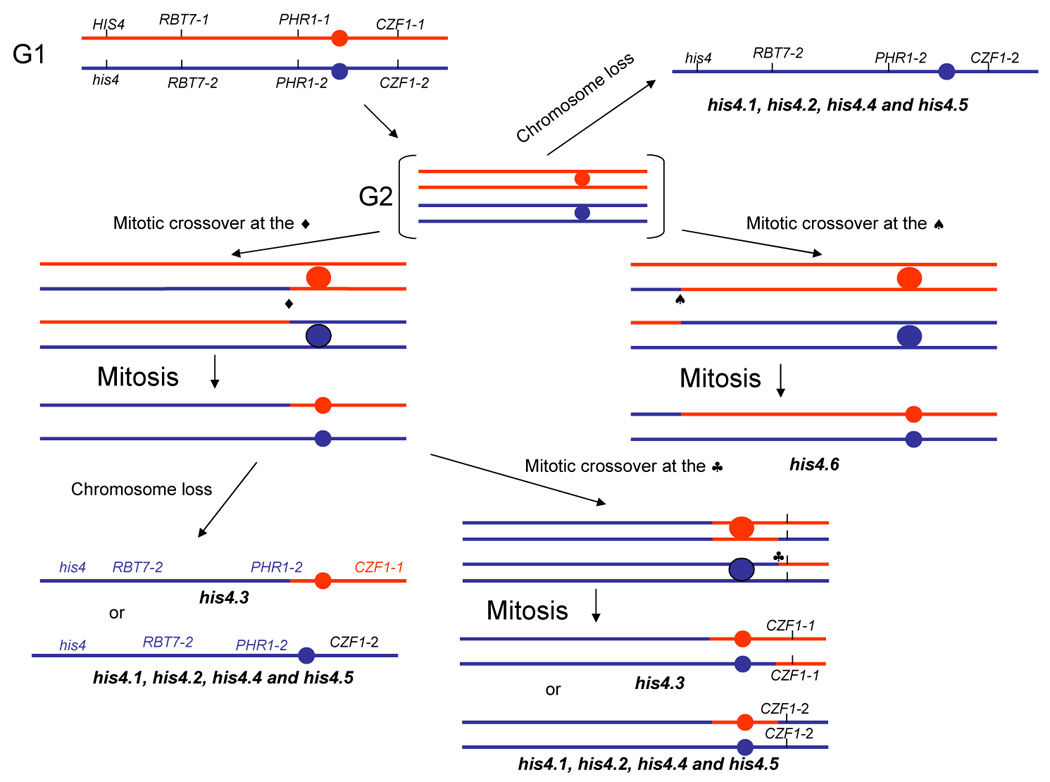
A diagram showing several possible mechanisms leading to the generation of histidine auxotrophs following UV light treatment. Filled circles indicate centromeres. ♠, ♦ and ♣ indicate alternative recombination sites. Sites ♦ and ♣ are very close to the centromere; therefore, a double crossover at these sites (lower right), although potentially possible, is highly unlikely.
3.3. SNP analysis of the HIS4 ORF distinguishes two alleles in both CAI-4 and its parental SC5314 strain
The experiments shown in Section 3.1 strongly suggest that HIS4 is heterozygous in strain CAI-4. In order to test that hypothesis, HIS4 was amplified by PCR from genomic DNA of CAI-4, the PCR product ligated into a plasmid, and eight HIS4 independent clones (CAI-4-1 to CAI-4-8) were sequenced. As expected from the size of the HIS4 gene reported in the Candida Genome Database (http://www.candidagenome.org/), each sequence contained an ORF of 2517 bp that encodes a protein of 838 amino acids. The sequences were then compared with those reported for CaHIS4, one allele of strain 1001, and both alleles of strain SC5314. For strain SC5314, three SNPs were reported to occur at positions 266 (T/C), 405 (T/C) and 1983 (T/A) (Jones et al., 2004), but only the T→C change at position 266 results in a change in an amino acid (Leu to Ser) that presumably has no effect on the activity of the protein. Surprisingly, in CAI-4 we found, in addition to those described for SC5314, eight new polymorphisms that define two alleles: allele 1, C266C405G496C769T929G1719T1770T1890T1908T1938T1983 and allele 2, T266T405C496G769G929C1719C1770G1890A1908C1938A1983 (Table 3). For the sake of simplicity, we will refer to these alleles as C769T929 or haplotype CT (allele 1), and G769G929 or haplotype GG (allele 2). As shown later, these pairs of bases remain linked in all clones analyzed from CAI-4-1 and its parent SC5314. We also found a possible recombinant clone (CAI-4-5) in which an intragenic crossing over presumably could have occurred between positions 1770 and 1890, resulting in the sequence of allele 1 until the base 1770 and the sequence of allele 2 after position 1889 (see Discussion). Also, we found a single base change C-to-T in clone CAI-4-4 at position 1825, resulting in a shift from Pro-to-Ser (CAI-4-4).
Since CAI-4 is derived from strain SC5314, it appeared possible that the eight additional SNPs in strain CAI-4 arose during its construction from SC5314. Furthermore, according to the Candida Genome Database, both HIS4 alleles of strain SC5314 are identical. These observations prompted us to sequence these regions in SC5314. For that purpose, the polymorphisms were amplified by PCR and the amplified product sequenced. Surprisingly, we found the same polymorphisms described above in both strains (see below). In addition, sequencing of several clones derived from SC5314 revealed that the association between the polymorphic bases in each allele was identical to those found in CAI-4 (Table 3). Also, we found a suggestion of recombination, including a crossing over between bases 929 and 1719 in clone 4, and an apparent homozygosity at SNP929 in clone 5; the latter could be due to either a point mutation or gene conversion comprising a short stretch of DNA around that position, although other interpretations of these results are possible (see Discussion). Accordingly, both alleles were already present in the same form in the parental strain SC5314, an indication that SNPs included in the HIS4 ORF are generally inherited as blocks (haplotypes).
3.4. Functional analysis of both alleles in the SC5314 lineage
It is clear that the HIS4 allele sequenced from strain 1001 is active (Table 3, first line). Not only was the clone able to complement a his4-34 mutation from S. cerevisiae (at least activities His4A and His4B activities, which were inactivated by the mutation) (Navarro-Garcia et al., 1998) (see also below), but also complemented the His auxotrophs obtained by UV irradiation (see above). With regard to CAI-4, at least one HIS4 allele is active because this strain is His+ but our experiments with UV exposure suggest that the other allele is non-functional. However, it is not clear which allele is functional since allele 1 is similar to the 1001 allele, but differs at positions 769 (G instead C, which results in His instead Asp at position 257 of His4p) and 929 (T instead G, which results in Val instead Gly at position 310 of His4p), and allele 2, that like strain 1001 carries G769 and G929 (Asp at position 257 and Gly at position 310 of the protein), but carries the alternative base in the 9 residual SNPs.
In order to test our hypothesis that only one allele is functional, we first constructed a heterozygote HIS4/his4 using the URA blaster method and the CAI-4 parental strain as a recipient (Fig. 4). Following transformation, cells were plated on complete minimal medium without uridine. This medium contains histidine in order to allow growth of putative His auxotrophs. Analysis of 31 transformants by Southern blot (not shown) and PCR using the appropriate primers (HIS41-F and HIS45-R −2.2 Kb- for wild type ORF and HIS45-R and URA1 −1.7 kb- for the hisG-URA3-hisG disrupted ORF, Fig. 4A) indicated that in 16 of them the cassette integrated correctly, since they showed both the wild type and the disrupted alleles (Fig. 4B). Next, the region comprising nucleotides 1352 to 2059 of the wild type allele remaining in each heterozygote was amplified using oligonucleotides HIS44-F and HIS45-R that delimit a region of 726 bp containing six SNPs and sequenced (Table 2, Fig. 4A). As shown in Fig. 4B only two transformants retained the haplotype corresponding to allele 1 (i.e., both carry a disruption of allele 2), whereas most of them (9) retained the haplotype corresponding to allele 2, exhibiting a disruption of allele 1 (for the sake of simplicity only two SNPs are presented, but the other four support the expected haplotype). This bias could be explained by the fact that the disruption cassette used was the one derived from strain 1001, whose 5′-flanking region is identical to allele 1 and differs in three positions (Fig. 4A, see also Table 3) from the same region of allele 2. Five heterozygous disruptants still carried both HIS4 alleles (in addition to the disrupted one), indicating that one of the original alleles had duplicated prior to disruption (probably as a consequence of the duplication of the corresponding homologue, leading to Chr4 triploidy) and then one of the two copies had been targeted by the disruption cassette. These heterozygotes could be easily distinguished in the PCR analysis because the bands corresponding to the wild type allele exhibited a relative intensity significantly higher (Fig. 4B, lanes 4, 5, 6, 7, and 16) as compared with their counterparts from a normal heterozygote. Finally, we analyzed the ability of the 16 heterozygous disruptants to grow in the absence of histidine. Interestingly, both heterozygotes carrying only allele 1 were His auxotrophs (lanes 10 and 11), whereas those carrying allele 2 remained His prototrophs (Fig. 4B and 4C). Two of these heterozygous disruptants which carry only the active (strain 2C, lane 1) or the inactive (strain 4C, lane 10) alleles respectively, were used in further studies. These results clearly indicate that allele 1 is inactive and confirm the results derived from the UV irradiation analysis that suggested that CAI-4 is heterozygous for HIS4.
3.5. Identification of the SNP responsible for the inactivation of allele 1
Why is allele 2 active and allele 1 inactive? It should be noted that most of the single base changes between both alleles are synonymous, but four of them have a non-conserved change in protein sequence: C266T, G496C, C769G, and T929G, which result in the Ser89Leu, Glu166Gln, His257Asp, and Val310Gly polymorphisms respectively. On the other hand, as mentioned above, the active allele from strain 1001 is identical to the inactive allele 1 except for the bases at positions 769 and 929, where the alternative base is found, suggesting that at least one of these two changes is key to the function of the allele. Because the non-conserved change in the protein would appear to drastically affect activity, we first constructed a His257Asp mutation in the strain 1001 allele using site directed mutagenesis. However, the resulting allele complemented a null his4-ΔΔ strain to the same extent as the original His257 indicating that the presence of Asp257 in allele 1 is not the cause of the inactivation of His4. In contrast, modification of the other SNP, G929T/Gly310Val, prevented the resulting allele from complementing the his4-ΔΔ strain. Therefore, Asp257 is not crucial for the activity of His4p, whereas the Gly310Val leads to its inactivation.
3.6. Analysis of the heterozygosity of HIS4 in other strains
The presence of a functional and a non-functional allele of HIS4 in the SC5314 lineage prompted us to determine whether this phenomenon could be extended to other clinical isolates that have been used frequently in different laboratories (see Table 1). For that purpose, we PCR amplified and sequenced a region 1109 bp between bases 372 and 1500 that contains three non-synonymous SNPs (496, 769, and 929) in strain SC5314 using the oligonucleotides His42-F and His44-R. As shown in Table 4, the polymorphism was found in strain 3153A, whereas strains 1001, 1006, and 4918, have Gly codons in both alleles. The polymorphism G496T was conserved in all but strain 4918. Strain 1006 was particularly polymorphic in the region analyzed, showing 7 new polymorphisms, whereas strain 4918 did not exhibit a single polymorphism in that region. Surprisingly, strain 4918 (isolated by Ardell Proctor in 1975 from blood of a patient suffering systemic candidiasis at Duke Hospital, see reference in Table 1) seemed to be related to strain 1006 (whose parental CBS5736 is a strain of Dutch origin, Table 1) since it retained one of the two bases from four (positions 726, 729, 798, 893) out of the seven 1006 specific SNPs, and in all of these, the base was different from the one found in the rest of the strains.
Table 4.
SNPs analysis of the HIS4 ORF from five C. albicans clinical isolates commonly used in different laboratories
 |
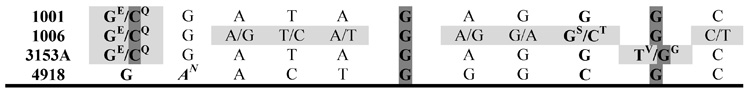 |
The region comprised between bases 370 and 1479 was amplified using oligonucleotides His42-F and His44-R and the amplified product sequenced. SNPs (X/Y) are within light grey cells. Non-synonymous SNPs are indicated in bold with the amino acid corresponding to each base indicated as a superscript index and the bases corresponding to the active allele in SC5314 enclosed in dark grey boxes. Single base changes are italicized.
If the SNP929 Val310Gly polymorphism alone accounts for the HIS4/his4 heterozygosity, then strains SC5314 and 3153A but not the other three strains should produce these auxotrophs following treatment with UV light. This prediction proved to be true for all the strains except 3153A, which was unable to generate His auxotrophs. A closer look at this strain suggests that is trisomic for Chr4, as indicated by the different intensity of both alleles from PHR1 and RBT7, such that one allele of each gene yielded an intensity about twice that of the other (Fig. 5). We also investigated CZF1, but in contrast to strain SC5314 (see above), strain 3153A was homozygous for this marker (Fig. 5A). The same conclusion was reached by comparing the amount of each base of the SNP929 in the sequence of the amplified product. As shown in Fig 5B, the G/T ratio was consistently 2:1 for strain 3153A, and 1:1 for strain SC5314, further confirming the presence of three alleles, two active and one inactive, in the former, and two alleles, one active and one inactive, in the second. This explains the absence of His auxotrophs in strain 3153A. Accordingly, the generation of His auxotrophs following treatment with UV light implies the presence of a single inactive and a single active allele of HIS4. Strains unable to produce a His auxotroph have two HIS4 active alleles regardless of the presence of an inactive one.
Fig. 5.
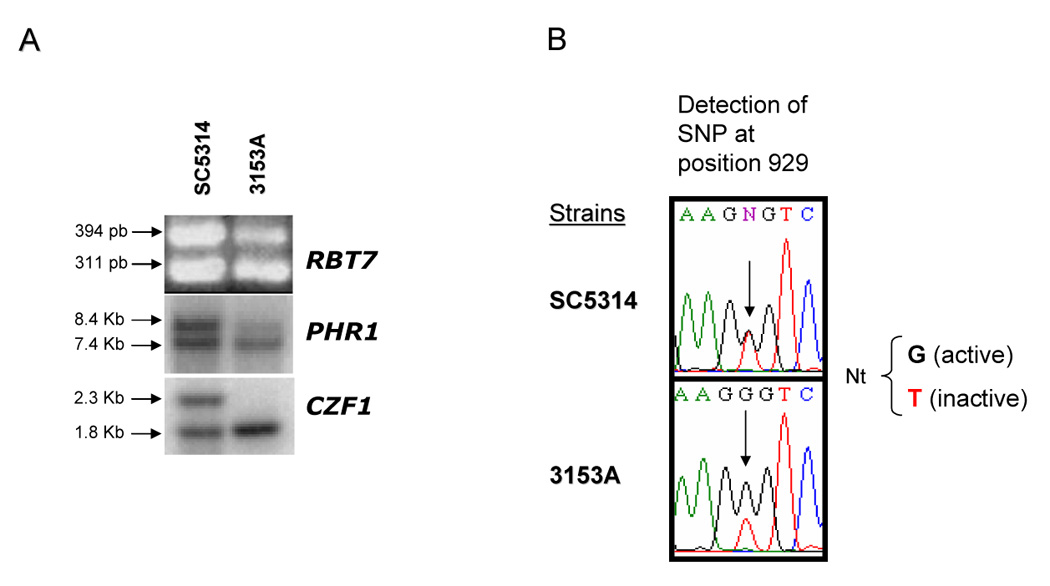
Ploidy analysis of Chr4 of strain 3153A deduced from sequencing of the HIS4 ORF and RFLP of three polymorphic markers (RBT7, CZF1, and PHR1) in strain. A. RFLP analysis of the RBT7 locus (top), and Southern blot analysis of PHR1 (middle) and CZF1 (bottom). B. Detail of a trace of the HIS4 sequence in the region surrounding SNP929. Four additional determinations yielded the same result. For details see legend of Fig. 2 and text.
In order to carry out an easy and rapid assay to determine the presence of one or the other allele in clinical isolates, we designed allele-specific PCRs (Table 5; see Materials and Methods). For that purpose, we first used SNP929. Importantly, the specific primer for allele 1 (non functional, OFF-NF) showed specificity (i.e., annealed with allele 1 but not with allele 2) when tested under previously optimized conditions. However, the allele 2-specific primer (active) did not show the expected specificity, since it annealed with both alleles under any of the conditions tested. Accordingly, this PCR reaction may be potentially used to detect the inactive allele in any strain. As shown in Fig. 6A, the inactive allele was identified in strains 3153A, SC5314, CAI-4, the HIS4 heterozygous His auxotroph 4C, and the six UV-induced His auxotrophs. It also was present in the CAI-4 derived strain SN148 (Noble and Johnson, 2005) (not shown). By contrast, it was absent in strains 1001, 1006, 4918, and the HIS4 heterozygous His prototroph 2C. In addition, to identifying unambiguously the presence of the active allele, we made use of SNP C769G that was always linked to SNP929 in such a way that the inactive allele was represented by the haplotype C769-T929 and the active one by the haplotype G769-G929. When SNP C769G-specific PCR reactions were carried out under optimized conditions, the specific primer for allele 1 only amplified DNA from its own allele and failed to amplify DNA from allele 2, and vice versa. As shown in Fig. 6A, where only the results of the SNP769-specific PCR for the second allele (functional, ON-F) are shown, this allele was present in the His+ homozygous strains 1001, 1006, 4918, the heterozygous counterparts 3153A, SC5314, and CAI-4, and the HIS4 heterozygous His prototroph 2C, and absent in all the His auxotrophs. The results obtained with the SNP769-specific PCR for the inactive allele (not shown) fully agree with those mentioned above for the SNP929-specific PCR. Accordingly, SNP-specific PCR matched the DNA sequence analysis and therefore this approach can be used routinely to analyze the configuration of the HIS4 locus in any C. albicans strain.
Fig. 6.
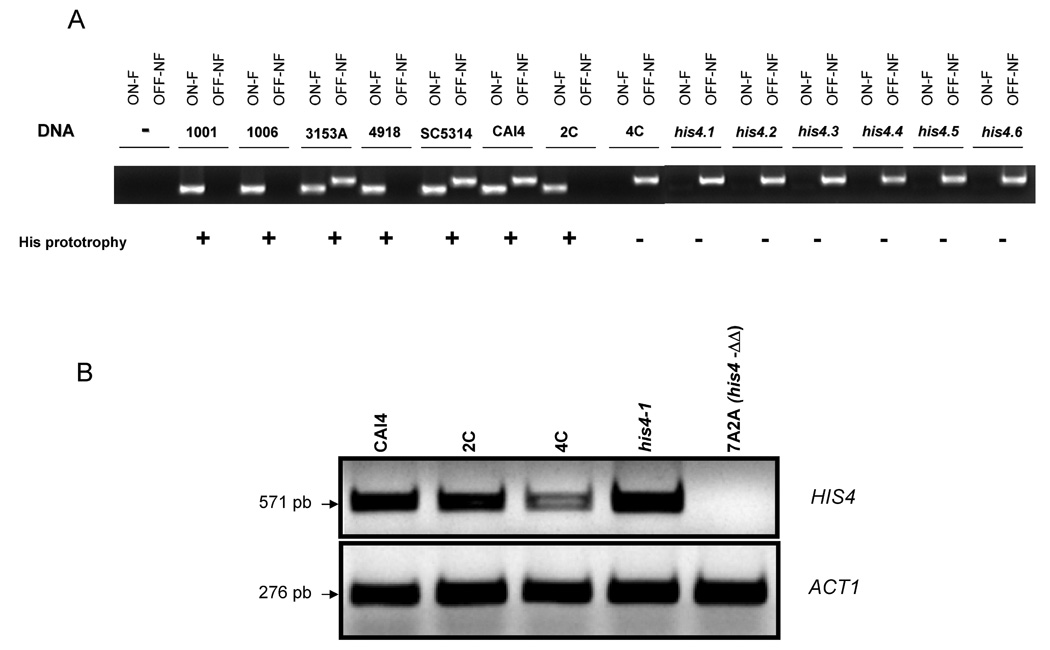
A. Detection of both HIS4 alleles by SNP-specific PCR in the indicated strains. The common primer is indicated in Table 5. For PCR, 50 ng of genomic DNA was used. The specific primer is indicated in each case. ON-F denotes that the primer was specific for the SNP769 of the functional allele and OFF-NF that it was specific for the SNP929 of the non-functional counterpart. For details, see the text. A control without DNA failed to show amplification B. RT-PCR. The indicated strains were grown at 30°C in synthetic medium containing histidine. RNA was extracted as indicated in Material and Methods and used as a template for cDNA synthesis, which was amplified using either HIS4 or ACT1 specific primers as indicated in Table 2. For details see the text.
3.7. Both HIS4 alleles, active and inactive, can be expressed
Finally, in order to determine whether both alleles were expressed in the HIS4 heterozygous isolates, we performed RT-PCR. As shown in Fig. 6B, specific HIS4 mRNA was expressed in CAI-4 (lane 1) as well as in both heterozygous disruptant strains 2C and 4C. Heterozygote 2C (active allele, lane 2) yielded higher levels of the message than its counterpart 4C (inactive allele, lane 3), even though ACT1 mRNA levels were similar in both strains, suggesting preferential expression of the active allele. However, since the method used is not quantitative and each allele is in a different genetically manipulated clone, caution is needed with this interpretation. Strain his4.1, which only carries the inactive allele, also expressed the HIS4 message. By contrast, no HIS4 mRNA was detected in the null homozygous 7A2A (lane 5), whereas the expression level of ACT1 was similar to the rest of the strains, indicating that the RT-PCR was specific for HIS4. These results rule out the possibility that the inactive allele cannot be expressed because of alterations in the promoter region when coexisting with the active one in SC5314 or CAI-4. However, no extrapolations can be made to the expression level of each allele in either its corresponding heterozygote or wild type. This issue is beyond the scope of the present study.
4. Discussion
In the present study, we have investigated the presence of a nutritional heterozygous marker in strain CAI-4 of C. albicans, by analyzing the generation of auxotrophic clones following UV treatment. Among the survivors, we have found histidine auxotrophs at a rather high rate (3 × 10−3). These auxotrophs could be complemented with HIS4, indicating that strain CAI-4 is heterozygous for this gene. Histidine auxotrophs of C. albicans have been isolated before using UV light, either alone (Whelan and Magee, 1981) or combined with HNO2 (Kakar et al., 1983), as well as with nitrosoguanidine (Altboum et al., 1990). In the first case, his4 (A642) and his5 (A5155) mutants were obtained (it should be noticed that the nomenclature used for these complementation groups is unrelated to the currently used gene names), and in the second, the activity affected was His4C. A his1-ΔΔ mutant (RM1000) has also been isolated by sequential deletion of both HIS1 alleles (Negredo et al., 1997). However, no further studies were conducted with those mutants.
Our allelic analysis at the molecular level has demonstrated that strain CAI-4 as well as its parent (SC5314) carries two HIS4 alleles, but only one is functional, due to a SNP at position 929 (G929T; Gly310Val). The active allele has a G:C pair (Gly310), whereas the other, the inactive allele has a T:A pair (Val310), so that a G→T transversion in the former leads to both elimination of the SNP929 and inactivation of the gene-product. In agreement with this observation, six independent UV-induced His auxothrophs carry exclusively Val310. Generation of these strains likely involves mitotic recombination between homologues followed by segregation of the non-functional alleles into the same cell, loss of one copy of Chr4, or a combination of both (see Fig. 3).
Changes of Gly residues to Val or Asp in some oncogenic variants of ras oncogenes (Reddy et al., 1982; Santos et al., 1983) prevent hydrolysis of GTP which remains locked in the active site and leads to a permanent signaling, which results in malignant transformation (Pai et al., 1990). Gly residues play also a crucial role in ATP hydrolysis of a number of proteins including the HSV-1 helicase (Graves-Woodward et al., 1997) and the retina-specific human ATP binding cassette transporter (Suárez et al., 2002), since its mutation to Val or Ala respectively abolished or severely decreased the ATP-ase activity of the respective enzymes. It should be noted that the change Gly310Val that inactivates His4p occurs in the His4B domain, which is a phosphoribosyl ATP-pyrophosphatase which converts phosphorybosyl-ATP into phosphorybosyl AMP, the second step in the biosynthesis of histidine. It is possible that the Gly310 residue interacts with ATP and promotes its hydrolysis, whereas its substitution by Val locks the ATP-phosphorybosyl-HisB complex in an inactive complex. Regardless of other considerations, the demonstration of the HIS4 heterozygosity in the reference strain SC5314 is noteworthy, since it provides a new, easily scored nutritional marker that can be used in future genetic studies.
In addition to SNP929, ten SNPs, seven of which are synonymous, were found in the HIS4 ORF of the SC5314 lineage. Whereas the amino acid sequence differences between alleles caused by non-synonymous SNPs likely affect function, as revealed by our analysis of SNP929, the role of the silent counterparts is far from evident. Still, their abundance suggests that they may confer some selective advantage. It has been shown recently that haplotypes of the human catechol-O-methyltransferase modulate protein expression by altering mRNA secondary structure even though the associated SNPs are synonymous (Nackley et al., 2006). Also, a silent SNP in the MDR transporter changes specificity by altering translation kinetics and, as a consequence, protein folding (Kimchi-Sarfaty et al., 2007). These types of regulatory mechanisms could be especially appropriate for an organism that like C. albicans exhibits a high SNPs density in its genome.
Although the 11 SNPs define two rather well conserved haplotypes one per allele, hints of mitotic recombination within the HIS4 ORF were found at a significant frequency. Sequencing of 8 HIS4 clones from CAI-4 revealed the occurrence of a new allele (haplotype) that could have arisen by intragenic recombination between the other two. In addition, at least another candidate to be a recombinant allele, and possibly two, were detected during sequencing of five HIS4 clones derived from SC5314. Our initial thought is that the detection of hybrid clones in cultures of strains CAI-4 and SC5314 supports the notion that mitotic recombination is quite common in C. albicans, as previously reported (Enloe et al., 2000; Lephart and Magee, 2006; Odds et al., 2007). One explanation for their apparent high frequency in our samples is that the HIS4 is located only 24 kb from the telomere. It has been reported that the large nearly homozygous regions of the C. albicans genome are near telomeres, suggesting that the frequency of homologous recombination is higher in those regions (Jones et al., 2004). However, the fact that we have observed identical association between the polymorphic bases in strains CAI-4 and SC5314 argues against such a high frequency of recombination and suggests that it could be an artifact. For instance, it could be attributed to the late effect of an early recombination event in the culture. It is also possible that our results are biased by the low number of clones sequenced. Still, another possibility is that the putative recombinant clones are PCR artifacts. Since C. albicans is diploid, both alleles are present in the DNA used as a template. Whenever the synthesis of a DNA strand is terminated before it reaches the binding site for the second primer, the DNA can bind to the complementary strand in the next PCR cycle, which can be the one originating from the other allele, resulting in hybrid PCR products containing sequences from both original alleles. Of course, if this is the case, the proposed mechanism has to occur at a high frequency in vitro. Otherwise, even if it takes place early during PCR, the number of accurately replicated DNA molecules would considerably outnumber those of the recombinant artifact. In the absence of a Luria-Delbruck fluctuation experiment (or one of the modifications of it, Lephart and Magee, 2006) that determines the actual recombination rate (events/cell/generation) in a particular region of the genome, these possibilities remain open to discussion.
Interestingly, upon transformation of CAI-4 with the HIS4 disruption cassette, the non-functional allele was targeted with an approximate five-fold preference over the functional counterpart. This result may be attributed to the presence of three heterozygosities in the 5′ region of the hisG-URA3-hisG module when compared to the corresponding region of the functional allele, whereas the same 5′ region was identical to the non-functional allele. This observation is not surprising, since Yesland and Fonzi (2000) found that a PHR1 disruption cassette showed a three- to four-fold preference for integration in one of the two alleles (the homologous allele), and this was shown to be due to heterologies in the 5′ and 3′ flanking regions of the disruption cassette (Yesland and Fonzi, 2000). Allele specific targeting may be quite common in C. albicans in view of the high number of polymorphisms that seem to significantly exceed that found during the sequencing project of the C. albicans genome. An additional possibility for the preferential integration of the disruption cassette in the non-functional allele could reflect the ability of this allele to be transcribed more efficiently than the active one. Yesland and Fonzi (2000) have suggested that it would not be surprising to find allelic variations in transcription, and this has been substantiated for the SAP2 (Staib et al., 2002) and ADE2 (Tsang et al., 1999) genes. In addition, in C. albicans, transcriptional activity increases recombination with exogenous DNA (Srikantha et al., 1995). Obviously, the answer to this question needs detailed studies on the differential expression of both HIS4 alleles in the parental strain SC5314. In addition to allele-specific targeting, we have found that with a high frequency (nearly one third), one allele, either the active or the inactive, duplicates before the integration of the cassette, most probably as a consequence of the duplication of the whole chromosome. Then, the disruption cassette integrates in one of the resulting copies. Given the allele-specific gene targeting effect, we are tempted to propose that the homologue carrying the non-functional allele is the one that undergoes duplication. Again, additional studies are needed to evaluate this possibility.
We have found SNP929 (G929T) in two clinical isolates. One of them, SC5314 (and its derivative CAI-4), yielded His auxotrophs upon treatment with UV light. The other, strain 3153A, behaved anomalously in that it carried the SNP but did not produce a His auxotroph. This seems to be due to the fact that the strain used is trisomic for Chr4 and has two active and one inactive HIS4 alleles. The other three isolates analyzed lack the SNP, and that event was correlated with the lack of His auxotrophs. Why do some C. albicans strains have a SNP929 while others do not? Recently, allelic differences in CDR2, which encodes the drug efflux pump protein Cdr2p, have been shown to result in different abilities to transport azoles (Holmes et al., 2006). However, both CDR2 alleles were equally frequent among 69 clinical isolates, and two azole-resistant isolates had not lost the allele that confers greater azole sensitivity. It was argued that possession of two alleles with different activities may be advantageous, although the reason seems to be other than the azole resistance and could be related to the adaptation of C. albicans to the different environmental conditions it encounters in the human host (Holmes et al., 2006).
Could a similar reasoning apply to the HIS4 heterozygosity detected in the present study? Although the strains with the SNP929 account for 40% of the total strains analyzed, their low numbers raise the possibility that it represents a random mutation which is either neutral or has not yet been selected against. Clearly, a survey of a higher number of clinical isolates from both blood and mucosa from several individual is needed. Although other mutations may also render HIS4 inactive, the critical role played by SNP929 raises the possibility that it acts as a switch that regulates the synthesis of histidine in response to the environmental conditions. For instance, we have shown here that both alleles can be potentially expressed in strain SC5314. This may lead to the synthesis of both active and inactive His4p, and therefore to a competition between the His4B components, which, in turn, could lead to a lower level of the reaction product phosphorybosyl-AMP. It should be emphasized that the proposed survey is not intended for phylogenetic and epidemiological studies, where the MLST approach that investigates several different, well chosen and validated target genes is certainly superior (Bougnoux et al., 2002; Tavanti et al., 2004; Bougnoux et al., 2006; Odds et al., 2006; 2007). However, it could provide some hints for the frequent occurrence of heterozygosity in C. albicans.
Acknowledgements
This study was supported by a Public Health Service grant, NIH-NIAID 1 R01 AI51949 to R.C and G.L. and grants PI042599 from Fondo de Investigaciones Sanitarias and 2PR03A044 from Junta de Extremadura to E.A. We thank Jesús Pla and Federico Navarro-Garcia for the HIS4 clone. We also thank to R. Cueva and T. Ciudad of the Larriba lab, and P.T. Magee (University of Minnesota) for their helpful suggestions, and Belén Hermosa for her technical support. JGR is the recipient of a fellowship from the NIH-NIAID 1 R01 AI51949 grant. Belén Hermosa was partially supported by grant 2PR03A044.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altboum Z, Gottlieb S, Lebens GA, Polacheck I, Segal E. Isolation of the Candida albicans histidinol dehydrogenase (HIS4) gene and characterization of a histidine auxothroph. J. Bacteriol. 1990;171:3898–3904. doi: 10.1128/jb.172.7.3898-3904.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andaluz E, Gomez-Raja J, Hermosa B, Ciudad T, Rustchenko E, Calderone R, Larriba G. Loss and fragmentation of chromosome 5 are major events linked to the adaptation of rad52-ΔΔ strains of Candida albicans to sorbose. Fungal Genet. Biol. 2007;44:789–798. doi: 10.1016/j.fgb.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andaluz E, Calderone R, Reyes G, Larriba G. Phenotypic analysis and virulence of Candida albicans LIG4 mutants. Infect Immun. 2001;69:137–147. doi: 10.1128/IAI.69.01.137-147.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andaluz E, Ciudad A, Rubio Coque J, Calderone R, Larriba G. Cell cycle regulation of a DNA ligase-encoding gene (CaLIG4) from Candida albicans. Yeast. 1999;15:1199–1210. doi: 10.1002/(SICI)1097-0061(19990915)15:12<1199::AID-YEA447>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Bennett RJ, Johnson AD. Completion of a parasexual cycle in Candida albicans by induced chromosome loss in tetraploid strains. EMBO J. 2003;22:2505–2515. doi: 10.1093/emboj/cdg235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougnoux M-E, Diogo D, François N, Sendid B, Veirmeire S, Colombel JF, Bouchier C, Van Kruiningen H, dÉnfert C, Poulain D. Multilocus sequence typing reveals intrafamilial transmission and microevolutions of Candida albicans isolates from the human digestive tract. J Clin Microbiol. 2006;44:1810–1820. doi: 10.1128/JCM.44.5.1810-1820.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougnoux M-E, Morand S, d'Enfert C. Usefulness of multilocus sequence typing for characterization of clinical isolates of Candida albicans. J. Clin. Microbiol. 2002;41:1290–1297. doi: 10.1128/JCM.40.4.1290-1297.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone R. Candida and Candidiasis. Washington: ASM press; 2002. [Google Scholar]
- Chen X, Magee BB, Dawson D, Magee PT, Kumamoto CA. Chromosome 1 trisomy compromises the virulence of Candida albicans. Mol. Microbiol. 2004;51:551–565. doi: 10.1046/j.1365-2958.2003.03852.x. [DOI] [PubMed] [Google Scholar]
- Chu WS, Magee BB, Magee PT. Construction of an SfiI macrorestriction map of the Candida albicans genome. J. Bacteriol. 1993;175:6637–6651. doi: 10.1128/jb.175.20.6637-6651.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciudad T, Andaluz E, Steinberg-Neifach O, Lue NF, Lue NF, Gow NAR, Calderone RA, Larriba G. Homologous recombination in Candida albicans: role of CaRad52p in DNA repair, integration of linear DNA fragments and telomere length. Mol. Microbiol. 2004;53:1177–1194. doi: 10.1111/j.1365-2958.2004.04197.x. [DOI] [PubMed] [Google Scholar]
- Coste AT, Tumer V, Isher F, Morschlauser J, Forche A, Selmecki A, et al. A mutation in Tac1, a transcription factor regulating CDR1 and CDR2, is coupled with loss of heterozygosity at chromosome 5 to mediate antifungal resistance in Candida albicans. Genetics. 2006;172:2139–2156. doi: 10.1534/genetics.105.054767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen LE, Sanglard D, Calabrese D, Sirjusingh C, Anderson JB, Kohn L. Evolution of drug resistance in experimental populations in C. albicans. J. Bacteriol. 2000;182:1515–1522. doi: 10.1128/jb.182.6.1515-1522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gobbi M, Viprakasit V, Hughes JR, Fisher C, Bucle VJ, et al. A regulatory SNP causes a human genetic disease by creating a new transcriptional promoter. Science. 2006;312:1215–1217. doi: 10.1126/science.1126431. [DOI] [PubMed] [Google Scholar]
- Donahue TF, Farabaugh FJ, Fink GR. The nucleotide sequence of the HIS4 region of yeast. Gene. 1982;18:47–59. doi: 10.1016/0378-1119(82)90055-5. [DOI] [PubMed] [Google Scholar]
- Enloe B, Diamond A, Mitchell AP. A single-transformation gene function test in diploid Candida albicans. J. Bacteriol. 2000;182:5730–5736. doi: 10.1128/jb.182.20.5730-5736.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans EG, Odds FC, Richardson MD, Holland KT. The effect of growth medium of filament production in Candida albicans. Sabourandia. 1974;12:112–119. doi: 10.1080/00362177485380151. [DOI] [PubMed] [Google Scholar]
- Fink GR. Gene-enzyme relations in histidine biosynthesis in yeast. Science. 1964;146:525–527. doi: 10.1126/science.146.3643.525. [DOI] [PubMed] [Google Scholar]
- Fink GR. A cluster of genes controlling three enzymes in histidine biosynthesis in Saccharomyces cerevisiae. Genetics. 1966;53:445–459. doi: 10.1093/genetics/53.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzi WA, Irwin MY. Isogenic stain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forche A, Magee PT, Magee BB, May G. Genome-wide single nucleotide polymorphism map for Candida albicans. Eukaryot. Cell. 2004;3:705–714. doi: 10.1128/EC.3.3.705-714.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forche A, May G, Magee PT. Demonstration of loss of heterozygosity by single-nucleotide polymorphism microarray analysis and alterations in strain morphology in Candida albicans strains during infection. Eukaryot. Cell. 2005;4:156–165. doi: 10.1128/EC.4.1.156-165.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forche A, May G, Beckerman J, Kauffman S, Becker J, Magee PT. A system for studying genetic changes in Candida albicans during infection. Fungal Genet. Biol. 2003;39:38–50. doi: 10.1016/s1087-1845(02)00585-6. [DOI] [PubMed] [Google Scholar]
- Forche A, Schönian G, Gräser Y, Vilgalys R, Mitchell TG. Genetic structure of typical and atypical populations of Candida albicans from Africa. Fungal Genet. Biol. 1999;28:107–125. doi: 10.1006/fgbi.1999.1164. [DOI] [PubMed] [Google Scholar]
- Franz R, Kelly DC, Lamb DC, Kelly DE, Ruhnke M, Morschhauser J. Multiple molecular mechanisms contribute to stepwise development of fluconazole resistance in clinical Candida albicans strains. Antimicrob. Agents Chemother. 1998;42:3065–3072. doi: 10.1128/aac.42.12.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyF mutations. Mol. Gen. Genetics. 1984;198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- Goshorn AK, Scherer S. Genetic analysis of prototrophic natural variants of Candida albicans. Genetics. 1989;123:667–673. doi: 10.1093/genetics/123.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräser Y, Volovsek M, Arrington J, Schönian G, Presber W, Mitchell TG, Vilgalys R. Molecular markers reveal that population structure of the human pathogen Candida albicans exhibits both clonality and recombination. Proc. Natl. Acad. Sci. USA. 1996;93:12473–12477. doi: 10.1073/pnas.93.22.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves-Woodward KL, Gottlieb J, Challberg MD, Weller SK. Biochemical analysis of mutations in the HSV-1 helicase-primase that alter ATP-hydrolysis, DNA unwinding, and coupling between hydrolysis and unwinding. J. Biol. Chem. 1997;272:4623–4630. doi: 10.1074/jbc.272.7.4623. [DOI] [PubMed] [Google Scholar]
- Holmes AR, Tssao S, Ong S-W, Niimi K, Monk BC, Niimi M, Kaneko A, Holland BR, Schmid J, Cannon RD. Heterozygosity and functional allelic variation in the Candida albicans efflux pump genes CDR1 and CDR2. Mol. Microbiol. 2006;62:170–186. doi: 10.1111/j.1365-2958.2006.05357.x. [DOI] [PubMed] [Google Scholar]
- Hull CM, Johnson AD. Identification of a mating type-like locus in the asexual pathogenic yeast Candida albicans. Science. 1999;285:1271–1275. doi: 10.1126/science.285.5431.1271. [DOI] [PubMed] [Google Scholar]
- Hull CM, Raisner RM, Johnson AD. Evidence for mating of the asexual yeast Candida albicans. Science. 2000;289:307–310. doi: 10.1126/science.289.5477.307. [DOI] [PubMed] [Google Scholar]
- Jones T, Federspiel NA, Chibana H, Dungan, et al. The diploid genome sequence of Candida albicans. Proc. Natl. Acad. Sci. USA. 2004;101:7329–7334. doi: 10.1073/pnas.0401648101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakar SN, Partridge RM, Magee PT. A genetic analysis of Candida albicans: isolation of a wide variety of auxotrophs and demonstration of linkage and complementation. Genetics. 1983;104:241–255. doi: 10.1093/genetics/104.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, Gottesman MM. A "silent" polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315:525–528. doi: 10.1126/science.1135308. [DOI] [PubMed] [Google Scholar]
- Konishi S, Izawa T, Lin SY, Ebana K, Fukuta Y, Sasaki T, Yano M. An SNP caused loss of seed shattering during rice domestication. Science. 2006;312:1392–1396. doi: 10.1126/science.1126410. [DOI] [PubMed] [Google Scholar]
- Legrand M, Lephart P, Forche A, Magee PT, Magee BB. Homozygosity at the MTL locus in clinical strains of Candida albicans is correlated with karyotypic rearrangements. Mol. Microbiol. 2004;52:1451–1462. doi: 10.1111/j.1365-2958.2004.04068.x. [DOI] [PubMed] [Google Scholar]
- Lephart PR, Magee PT. Effect of the major repeat sequence on mitotic recombination in Candida albicans. Genetics. 2006;174:1737–1744. doi: 10.1534/genetics.106.063271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart SR, Daniels KJ, Zhao R, Wessels D, Soll DR. Cell biology of mating in Candida albicans. Eukaryot Cell. 2003;2:49–61. doi: 10.1128/EC.2.1.49-61.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart SR, Pujol C, Daniels KJ, Miller MG, Johnson AD, Pfaller MA, Soll DR. In Candida albicans, white-opaque switchers are homozygous for mating type. Genetics. 2002;162:737–745. doi: 10.1093/genetics/162.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee BB, Magee PT. Through a glass opaquely: the biological significance of mating in Candida albicans. Curr. Opin. Microbiol. 2004;7:661–665. doi: 10.1016/j.mib.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Magee BB, Magee PT. Induction of mating in Candida albicans by construction of MTLa and MTLalpha strains. Science. 2000;289:310–313. doi: 10.1126/science.289.5477.310. [DOI] [PubMed] [Google Scholar]
- Magee PT. The use of genetic analysis in the study of the biology of Candida albicans. In: Kirsch DR, Kelly R, Kurtz MB, editors. The genetics of Candida. Boca Raton: CRC Press; 1990. pp. 187–196. [Google Scholar]
- Manning M, Mitchell TG. Strain variation and morphogenesis of yeast- and mycelial-phase Candida albicans in low-sulfate, synthetic medium. J. Bacteriol. 1980;142:714–719. doi: 10.1128/jb.142.2.714-719.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Johnson AD. Mating and parasexual genetics in Candida albicans. In: Heitman J, Filler SG, Edwards JE, Mitchell AP, editors. Molecular principles of fungal pathogenesis. Washington, DC: ASM Press; 2006. [Google Scholar]
- Nackley AG, Shabalina SA, Tchivileve IE, Satterfield K, Korchynslyi O, Makarov SS, Maixner W, Diatchenko L. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 2006;314:1930–1933. doi: 10.1126/science.1131262. [DOI] [PubMed] [Google Scholar]
- Navarro-Garcia F, Perez-Diaz RM, Negredo AI, Pla J, Nombela C. Cloning and sequence of a 3.835 kbp DNA fragment containing the HIS4 gene and a fragment of a PEX5-like gene from Candida albicans. Yeast. 1998;14:1147–1157. doi: 10.1002/(SICI)1097-0061(19980915)14:12<1147::AID-YEA297>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Negredo A, Monteoliva L, Gil C, Pla J, Nombela C. Cloning, analysis and one step disruption of the ARG5,6 gene of Candida albicans. Microbiology. 1997;143:297–302. doi: 10.1099/00221287-143-2-297. [DOI] [PubMed] [Google Scholar]
- Noble SM, Johnson AD. Strains and strategies for large-scale deletions studies of the diploid human fungal pathogen Candida albicans. Eucaryot. Cell. 2005;4:298–309. doi: 10.1128/EC.4.2.298-309.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds FC, Bougnoux ME, Shaw DJ, Bain JM, Davidson AD, Diogo D, Jacobsen MD, Lecomte M, Li SY, Tavanti A, Maiden MC, Gow NAR, d'Enfert C. Molecular phylogenetics of Candida albicans. Eukaryot. Cell. 2007;6:1041–1052. doi: 10.1128/EC.00041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds FC, Davidson AD, Jacobsen MD, Tavanti A, Whyte JA, Kibbler CC, Ellis DH, Maiden MCJ, Shaw DJ, Gow NAR. Candida albicans strain maintenance, replacement, and microvariation, demonstrated by multilocus sequence typing. J. Clin. Microbiol. 2006;44:3647–3658. doi: 10.1128/JCM.00934-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai EF, Krengel U, Petsko GA, Goody RS, Kabsch W, Wittinghofer A. Refined crystal structure of the triphosphate conformation of H-ras p21 at 1.35 A resolution: implications for the mechanism of GTP hydrolysis. EMBO J. 1990;9:2351–2359. doi: 10.1002/j.1460-2075.1990.tb07409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pla J, Perez-Diaz RM, Navarro-Garcia F, Sanchez M, Nombela C. Cloning of the Candida albicans HIS1 gene by direct complementation of a C. albicans histidine auxotroph using an improved double-ARS shutle vector. Gene. 1995;165:115–120. doi: 10.1016/0378-1119(95)00492-o. [DOI] [PubMed] [Google Scholar]
- Pujol C, Messer SA, Pfaller M, Soll DR. Drug resistance is not directly affected by mating type locus zygosity in Candida albicans. Antimicrob. Agents Chemother. 2003;47:1207–1212. doi: 10.1128/AAC.47.4.1207-1212.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol C, Reynes J, Renaud F, Raymond M, Tibayrenc M, Ayala FJ, Janbon F, Mallié M, Bastide J. The yeast Candida albicans has a clonal mode of reproduction in a population of infected human immunodeficiency virus-positive patients. Proc. Natl. Acad. Sci. USA. 1993;90:9456–9459. doi: 10.1073/pnas.90.20.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy EP, Reynolds RK, Santos E, Barbacid M. A point mutation is responsible for the acquisition of transforming properties by the T24 human bladder carcinoma oncogne. Nature. 1982;300:149–152. doi: 10.1038/300149a0. [DOI] [PubMed] [Google Scholar]
- Rustad TR, Stevens DA, Pfaller MA, White TC. Homozygosity at the Candida albicans MTL locus associated with azole resistance. Microbiology. 2002;148:1061–1072. doi: 10.1099/00221287-148-4-1061. [DOI] [PubMed] [Google Scholar]
- Santos E, Reddy EP, Pulciani S, Feldman RJ, Barbacid M. Spontaneous activation of a human proto-oncogene. Proc. Natl. Acad. Sci. USA. 1983;80:4679–4683. doi: 10.1073/pnas.80.15.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal K, Baum M, Carbon J. Centromeric DNA sequences in the pathogenic yeast Candida albicans are all different and unique. Proc. Natl. Acad. Sci. USA. 2004;101:11374–11379. doi: 10.1073/pnas.0404318101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt ME, Brown TA, Trumpower BL. A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res. 1990;18:3091–3092. doi: 10.1093/nar/18.10.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F, Fink GR, Lawrence CW. Methods in yeast genetics. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- Srikantha T, Morrow B, Schroppel K, Soll DR. The frequency of integrative transformation at phase-specific genes of Candida albicans correlates with their transcriptional state. Mol. Gen. Genet. 1995;246:342–352. doi: 10.1007/BF00288607. [DOI] [PubMed] [Google Scholar]
- Staib P, Krestmar M, Nichterlein T, Hof H, Morschhäuser J. Host versus in vitro signals and intrastrain allelic differences in the expression of a Candida albicans virulence gene. Mol. Microbiol. 2002;44:1351–1366. doi: 10.1046/j.1365-2958.2002.02967.x. [DOI] [PubMed] [Google Scholar]
- Suárez T, Biswas SB, Biswas EE. Biochemical defect in retina-specific human ATP-binding cassette transporter nucleotide binding domain 1 mutants associated with macular degeneration. J. Biol. Chem. 2002;277:21759–21767. doi: 10.1074/jbc.M202053200. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Rogers AL, Magee PT. Inter- and intra-species crosses between Candida albicans and Candida guillermondii. Yeast. 1986;2:53–58. doi: 10.1002/yea.320020104. [DOI] [PubMed] [Google Scholar]
- Tavanti A, Gow NAR, Maiden MCJ, Odds FC, Shaw DJ. Genetic evidence for recombination in Candida albicans based in haplotype analysis. Fungal Genet. Biol. 2004;41:553–562. doi: 10.1016/j.fgb.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Tsang PWK, Cao B, Siu PYL, Wang J. Loss of heterozygosity, by mitotic gene conversion and crossing over, causes strain-specific adenine mutants in constitutive diploid Candida albicans. Microbiology. 1999;145:1623–1629. doi: 10.1099/13500872-145-7-1623. [DOI] [PubMed] [Google Scholar]
- Tzung KW, Williams RM, Scherer S, Federspiel N, Jones T, Hansen N, Bivolarevic V, Huizar L, Komp C, Surzycki R, Tamse R, Davis RW, Agabian N. Genomic evidence for a complete sexual cycle in Candida albicans. Proc. Natl. Acad. Sci. USA. 2001;98:3249–3253. doi: 10.1073/pnas.061628798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Uden N, Buckley H. In: Genus 2. Candida Berkhout. Lodder J, editor. The Yeast, North-Holland Publishing Co; 1970. pp. 893–1087. [Google Scholar]
- Whelan WL, Magee PT. Natural heterozygosity in Candida albicans. J. Bacteriol. 1981;145:896–903. doi: 10.1128/jb.145.2.896-903.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan WL. The genetics of medically important fungi. Crit. Rev. Microbiol. 1987;14:99–170. doi: 10.3109/10408418709104437. [DOI] [PubMed] [Google Scholar]
- Whelan WL, Soll DR. Mitotic recombination in Candida albicans: recessive lethal alleles linked to a gene required for methionine biosynthesis. Mol. Gen. Genetics. 1982;187:477–485. doi: 10.1007/BF00332632. [DOI] [PubMed] [Google Scholar]
- Whelan WL, Partridge RM, Magee PT. Heterozygosity and segregation in Candida albicans. Mol. Gen. Genet. 1980;180:107–113. doi: 10.1007/BF00267358. [DOI] [PubMed] [Google Scholar]
- White TC. The presence of an R467K amino acid substitution and loss of allelic variation correlate with an azole-resistant lanosterol 14alpha demethylase in Candida albicans. Antimicrob. Agents Chemother. 1997;41:1488–1494. doi: 10.1128/aac.41.7.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Mitchell TG, Vilgalys R. PCR-restriction fragment length polymorphism (RFLP) analyses reveal both extensive clonality and local genetic differences in Candida albicans. Mol. Ecol. 1999;8:59–73. doi: 10.1046/j.1365-294x.1999.00523.x. [DOI] [PubMed] [Google Scholar]
- Yesland K, Fonzi WA. Allele-specific gene targeting in Candida albicans results from heterology between alleles. Microbiology. 2000;146:2097–2104. doi: 10.1099/00221287-146-9-2097. [DOI] [PubMed] [Google Scholar]


