Abstract
Myelination in the PNS is accompanied by a large induction of the Myelin Protein Zero (Mpz) gene to produce the most abundant component in peripheral myelin. Analyses of knockout mice have shown that the EGR2/Krox20 and SOX10 transcription factors are required for Mpz expression. Our recent work has shown that the dominant EGR2 mutations associated with human peripheral neuropathies cause disruption of EGR2/SOX10 synergy at specific sites, including a conserved enhancer element in the first intron of the Mpz gene. Further investigation of Egr2/Sox10 interactions reveals that activation of the Mpz intron element by Egr2 requires both Sox10-binding sites. In addition, both Egr1 and Egr3 cooperate with Sox10 to activate this element, which indicates that this capacity is conserved among Egr family members. Finally, a conserved composite structure of Egr2/Sox10-binding sites in the genes encoding Mpz, Myelin-associated glycoprotein (Mag), and Myelin Basic Protein (Mbp) genes was used to screen for similar modules in other myelin genes, revealing a potential regulatory element in the periaxin gene. Overall, these results elucidate a working model for developmental regulation of Mpz expression, several facets of which extend to regulation of other peripheral myelin genes.
Keywords: Charcot, Krox20, transcription, myelin, Schwann cells
Introduction
Myelin Protein Zero (Mpz) is the most abundant protein component of peripheral myelin (Greenfield et al., 1973) and is necessary for the formation of compact myelin (Giese et al., 1992). Since the identification of the gene encoding Mpz (Lemke and Axel, 1985), several studies have probed its transcriptional regulation. The large increase of Mpz expression in myelinating Schwann cells depends on axonal signals (Lemke and Chao, 1988) and declines dramatically after nerve injury (Trapp et al., 1988). Moreover, the MPZ gene is commonly mutated in human peripheral neuropathies such as Charcot-Marie-Tooth disease (reviewed in Wrabetz et al., 2004; reviewed in Shy, 2005).
Several studies have shown that Mpz expression in Schwann cells depends on both Egr2/Krox20 and Sox10 (Topilko et al., 1994; Zorick et al., 1999; Peirano et al., 2000; Le et al., 2005). Initial studies of Mpz expression indicated that it was expressed exclusively in myelinating Schwann cells, but subsequent analysis revealed that Mpz is also expressed during embryonic development of Schwann cells from the neural crest, albeit at a much lower basal level (Bhattacharyya et al., 1991; Zhang et al., 1995; Lee et al., 1997). Sox10 has a crucial role in specification of the Schwann cell fate and is required for early embryonic expression of Mpz: indeed Sox10-binding sites have been localized in the Mpz promoter (Peirano et al., 2000; Slutsky et al., 2003). Sox10 and Egr2 are co-expressed in myelinating Schwann cells (Aquino et al., 2006) and recent work has indicated that Sox10 is required for induction of Mpz expression in myelinating Schwann cells (Schreiner et al., 2007).
Although Sox10 has been shown to bind the Mpz promoter, there is substantial evidence to indicate that other regions are involved in regulation of Mpz. Transgenic analysis of the Mpz locus indicates that important control elements reside downstream of the transcription start site (Feltri et al., 1999). Accordingly, we have described a conserved element within the first intron of the Mpz gene (LeBlanc et al., 2006), which contains binding sites for both Egr2 and Sox10. Reporter assays show that this isolated intron element responds to treatments that induce endogenous expression of Mpz in Schwann cells.
The central role of Egr2 and Sox10 in regulating peripheral nerve myelination has been demonstrated by transgenic mouse studies and by the identification of mutations in EGR2 and SOX10 in human peripheral neuropathies. Mutations in SOX10 are associated with a complex syndrome involving peripheral demyelinating neuropathy, central dysmyelinating leukodystrophy, Waardenburg syndrome and Hirschsprung disease (Inoue et al., 2004). Dominant, neuropathy-associated mutations in EGR2 have been identified in all three zinc fingers of the DNA-binding domain (Warner et al., 1998; Bellone et al., 1999; Timmerman et al., 1999; Pareyson et al., 2000; Mikesova et al., 2005), and these mutations generally either impair or prevent DNA-binding (Warner et al., 1999; Musso et al., 2001; Musso et al., 2003). Dominant mutants of EGR2 exert a dominant-negative effect on the activation of some endogenous target genes in Schwann cells by wild type EGR2 (Nagarajan et al., 2001; Arthur-Farraj et al., 2006), with the most striking effects observed on Mpz.
We recently investigated the mechanism of dominant negative effects of Egr2 mutants on Mpz activation. These studies indicate that Egr2-mediated activation of the Mpz intron element, but not the promoter, is sensitive to expression of dominant, neuropathy-associated Egr2 mutants. Binding of Sox10 to the Mpz intron element is required for the dominant-negative effects, which further indicates that the dominant Egr2 mutants interfere with interactions required for Egr2-dependent binding of Sox10 to the conserved intron element of Mpz (LeBlanc et al., 2007). These data highlight the potential importance of direct Egr2/Sox10 interactions in Mpz activation.
Objective
Based on our previous data we wished to further elucidate mechanisms by which Egr2 and Sox10 interact to regulate Mpz expression. Therefore, we used in vivo chromatin Immunoprecipitation (ChIP) assays to investigate the developmental regulation of Egr2 binding in the Mpz locus. In addition, we examined the requirement of Sox10 sites for activation of the Mpz intron element. Finally, a conserved configuration of Egr2/Sox10 sites has been identified in the Mpz, Mbp, Mag, and Connexin 32 genes and a bioinformatic screen was developed to identify where this configuration occurs in peripheral myelin genes.
Methods
Promoter Analysis
Footprinting was carried out by incubating 200 ng of purified, FAM-labeled Mpz intron fragment (from +984–1500 of mouse Mpz relative to transcription start site) in the presence or absence of recombinant Egr2 protein and 500 ng of a non-specific 20 bp oligonucleotide in binding buffer (20% glycerol, 20 mM Tris 7.5, 50 mM KCl, 5 mM MgCl2, 0.01 mM ZnCl2, 1mM DTT, and 0.2 mM PMSF) in a volume of 10 μl. DNAse I (Promega) (1 μl of 1000 U/ml) was added for 1 min at room temperature before adding 90 μl of 0.5% SDS, 100 mM NaCl, and 10 mM EDTA. The resulting fragments were resolved on an ABI Prism 377 Sequencer. Recombinant Egr2 was made by fusing the mouse Egr2 sequence with the 6xHis tag in pET30a (Novagen), and purifying the protein from bacteria using Ni-NTA agarose (Qiagen) according to the manufacturer’s protocol.
Plasmids
Mpz intron-containing (+984–+1749) reporter plasmids have been described previously (LeBlanc et al., 2006; LeBlanc et al., 2007). Site-directed mutagenesis of the Mpz intron reporter was performed to alter individual Sox10 sites to G at positions 4 and 5 on the CA-rich strand, which has been previously reported to abrogate Sox10 binding to DNA (Bondurand et al., 2001). Protein expression constructs were created by cloning the coding regions of Egr1, Egr2, Egr3, and Egr2 180 with an N-terminal HA epitope in the pCite3 vector (Novagen) and the coding region of Sox10 with an N-terminal FLAG epitope as previously described (Kuhlbrodt et al., 1998).
Cell culture conditions and transfection assays
B16/F10 mouse melanocytes were obtained from ATCC. Transfection assays were performed as described (LeBlanc et al., 2006). Fold induction is calculated relative to the luciferase activity of the reporter alone. The average luciferase activity of each sample was normalized to the β-galactosidase activity from the transfected lacZ reporter. Unless otherwise indicated, means and standard deviations of two separate transfections are shown. The S16 rat Schwann cell line were cultured as described (LeBlanc et al., 2005).
Expression analysis
Quantitative RT-PCR was performed by monitoring in real-time the increase in fluorescence of the SYBR-GREEN dye (Morrison et al., 1998) using the TaqMan 7000 Sequence Detection System (Applied Biosystems). Relative amounts of each gene between samples were determined using the Comparative Ct method (Livak and Schmittgen, 2001) and normalized to the relative levels of 18S rRNA.
Chromatin immunoprecipitation (ChIP) assays
ChIP analysis of myelinating rat sciatic nerve at postnatal days 5 and 15 was performed as described (Jang et al., 2006; LeBlanc et al., 2006), by mincing pooled sciatic nerves from 8–13 rat pups in 1% formaldehyde for 25 min. Polyclonal antibodies directed against Egr2 (Covance) and Sox10 (Santa Cruz sc-17342) were employed in the immunoprecipitations. All experiments were performed in strict accordance with experimental protocols approved by the University of Wisconsin, School of Veterinary Medicine. Quantitative PCR was carried out on the purified samples in duplicate with primers specific to regions of interest. Percentage recovery relative to input DNA was determined using the Comparative Ct method (Livak and Schmittgen, 2001), and primer sets used for ChIP analysis have been described previously (Jang et al., 2006; LeBlanc et al., 2006). Primers used for ChIP assays in the rat periaxin locus are: GGGCTATTGTGTGAGGGAATGT and CACACAGGAAAAGGTCAGGCATA; and for rat Mag −1.7kb: TGTCTCAGACTGGGAAAGGCA and CACCTCGGGCTTCATGATG.
Coimmunoprecipitation assay
All proteins were translated using an in vitro transcription translation coupled reticulocyte lysate system (Promega). Equal volumes of lysates were mixed and incubated at room temperature for 20 minutes before immunoprecipitating using M2 anti-FLAG-agarose beads (Sigma) as described (LeBlanc et al., 2007) with NaCl adjusted to 75mM. After the final wash, proteins were eluted by boiling for 3 minutes in 2x Laemmli buffer prior to immunoblot analysis using a polyclonal antibody directed against the HA epitope (Roche).
Results
Developmental regulation of Egr2 binding in the Mpz locus
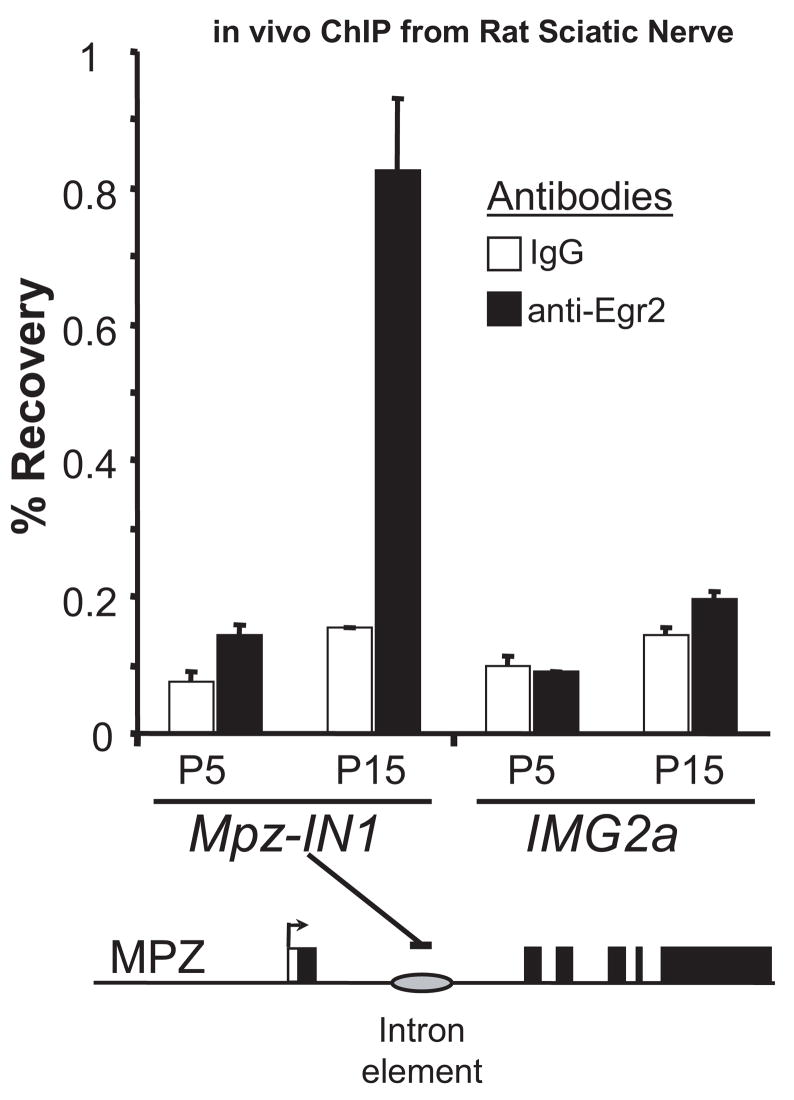
We have shown previously that Egr2 binding to the conserved intron element can be detected using a ChIP assay in the S16 Schwann cell line and in myelinating sciatic nerve from rat pups isolated at postnatal day 10 (Jang et al., 2006; LeBlanc et al., 2006). The specificity of this assay has been tested further by determining if Egr2 occupancy of the Mpz intron element increases in a developmentally regulated manner. Egr2 expression is low at postnatal day 0 in rat sciatic nerve and increases as myelination initiates during the first 7–10 days after birth (Zorick et al., 1996). ChIP assays using sciatic nerves from rat pups at postnatal day 5 (early myelination) or postnatal day 15 (late myelination) revealed little or no detectable binding of Egr2 to the Mpz intron element at postnatal day 5 and significantly higher levels at postnatal day 15 (Fig. 1), relative to a control immunoprecipitation using purified rabbit IgG. In contrast, no binding was observed at a negative control site in a silent immunoglobulin gene. Overall, these data are consistent with developmental regulation of Egr2 binding to the Mpz intron element during the myelination process.
Figure 1. Developmental regulation of Egr2 binding to the Mpz intron element.
Cross-linked chromatin was prepared from pooled sciatic nerves obtained from rat litters at postnatal days 5 and 15 (P5 and P15). Sonicated chromatin was immunoprecipitated with antibodies directed against either Egr2 (filled bars) or purified rabbit IgG (open bars) as a negative control. Purified DNA was then analyzed by quantitative PCR using the indicated primers. Percentage recovery relative to input was determined using quantitative PCR with primers designed for the Mpz intron element. The silent IMG2a (immuglobulin G-2a) gene was used as a negative control.
Regulation of Egr2 binding to conserved sites in the first intron of the Mpz gene
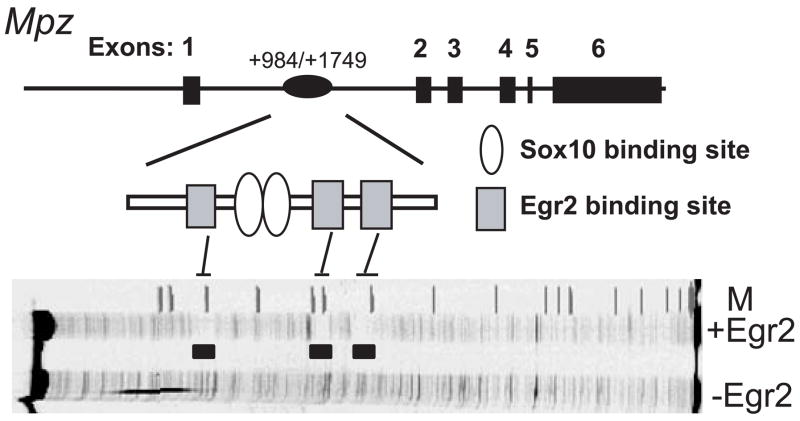
Our initial analysis of the Mpz intron sequence identified three conserved Egr2 binding sites (Fig. 2). To test whether these sites bind Egr2, the mouse Mpz intron element was analyzed by DNase I footprinting. In the presence of recombinant Egr2, strongly protected regions encompass the three predicted sites. Previous analysis of Egr2 binding specificity indicated a strong preference for T in the 4th position of the consensus sequence 5′ GCGTGGGCG 3′ (Swirnoff and Milbrandt, 1995) and each of the three protected sites have a T in position 4. However, mutation of the last two binding sites abrogates induction by Egr2 (LeBlanc et al., 2006), and our recent work indicates a specific requirement for the second site (data not shown), which is located ~50 bp downstream of two Sox10 binding sites. Interestingly, these sites are in an inverted orientation similar to two key Sox10 binding sites in the Mpz promoter (C/C′), to which Sox10 was shown previously to bind cooperatively as a dimer (Peirano et al., 2000; Peirano and Wegner, 2000).
Figure 2. Conserved configuration of Egr2/Sox10-binding sites in the first intron of the Mpz gene.
The six exons of Mpz and the location of a conserved intron element containing Egr2 and Sox10 sites (gray squares and open ovals, respetively). DNase I footprinting analysis of a fragment (+984–+1500) of the mouse Mpz intron in the presence of recombinant Egr2 protein reveals three footprinted regions that correspond to the previously identified conserved sites (filled areas) (LeBlanc et al., 2006). M=marker lane
Activation of the Mpz intron element by Egr2 depends upon Sox10 binding to adjacent sites
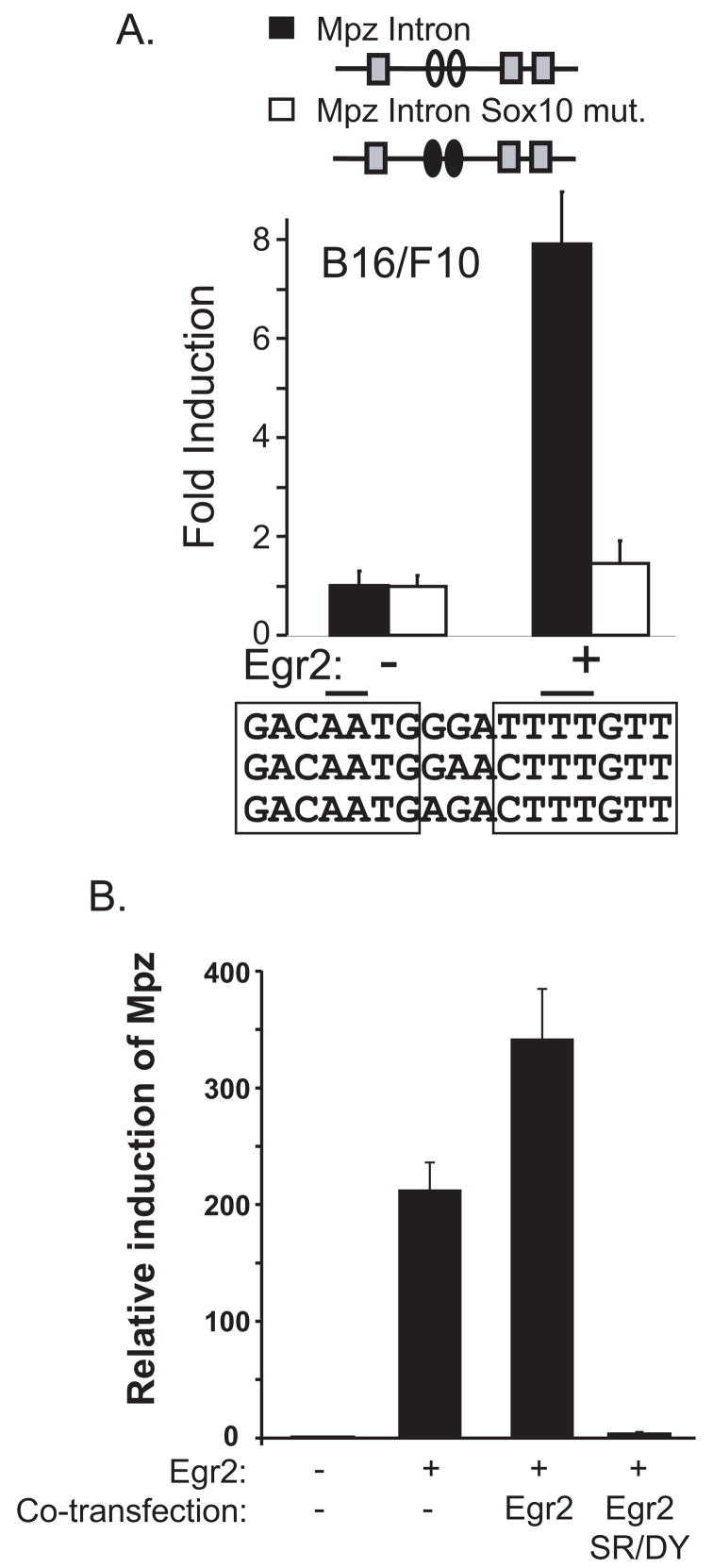
Transfection assays in primary rat Schwann cells show that mutation of Sox10 sites severely diminished the capacity of Egr2 to activate through the intron element (LeBlanc et al., 2007). To independently test the role of endogenous Sox10 in activation through the intron element, transfection assays were performed in the B16/F10 melanocyte cell line. This cell line expresses Sox10 (Kamaraju et al., 2002) but lacks Egr2 expression and has been used previously in studies of the Mpz promoter (Slutsky et al., 2003). As shown in Figure 3A, a reporter containing the wild type intron element fused to a minimal promoter is strongly activated by Egr2 expression in the melanocyte cell line. Mutation of the Sox10 sites abolishes this activation, which indicates that endogenous levels of Sox10 contribute significantly to activation of the intron element by Egr2. Overall, these data indicate that Egr2 activation of the Mpz intron element in Sox10-expressing cells (Schwann cells and melanocytes) depends largely on interaction with Sox10 that binds to the adjacent sites.
Figure 3. Egr2 activation of the Mpz intron element depends on Sox10 binding sites.
A) The Mpz intron reporters (wild type and Sox10 mut) were cotransfected in B16/F10 cells with an expression plasmid for Egr2 (30 ng). The putative Sox10- and Egr2-binding sites are indicated by ovals and squares, respectively. The filled ovals in the diagram represent the intron element that contains mutant Sox10-binding sites. The sequence alignment shows conservation of the Sox10-binding sites in mouse, rat and human Mpz loci, with mutated bases indicated by lines over the sequence. Fold induction is calculated relative to the activity of each reporter alone. Means and standard deviations of six replicate assays are shown. The fold induction of the Mpz intron reporter is significantly more than the fold induction of the Mpz intron Sox10 mut reporter (P<0.0001).
B) B16/F10 cells were transfected with expression constructs for wild type Egr2 (450 ng) and, where indicated, an equal amount of either wild-type or dominant mutant (S382R, D383Y) Egr2 (450 ng) expression plasmid. At 48 hours after transfection, RNA was prepared and the relative expression of Mpz was determined by quantitative PCR relative to a transfection control. A CMV-lacZ construct was co-transfected and quantitation of lacZ mRNA levels revealed that the transfection efficiency differed less than 2-fold among the samples. These data are representative of three independent experiments. Expression of the dominant Egr2 mutant alone did not significantly increase Mpz expression (data not shown).
The B16/F10 cell line expresses Sox10 so we also examined activation of the endogenous Mpz gene in the B16/F10 melanocyte cell line to determine if it behaves similarly to activation of the endogenous Mpz gene by Egr2 in primary Schwann cells (Nagarajan et al., 2001; Arthur-Farraj et al., 2006). These studies had shown that endogenous Mpz in Schwann cells is sensitive to a dominant Egr2 mutant (LeBlanc et al., 2007). Transfection of a plasmid that expresses Egr2 gives rise to >100-fold increase in Mpz RNA in B16/F10 cells (Fig. 3B). Co-expression of the neuropathy-associated Egr2 mutant (S382R, D383Y) reduces Mpz expression to almost background levels. In these respects, the responses of the endogenous Mpz gene in the B16/F10 melanocyte cell line to expression of wild type and mutant Egr2 are similar to previous observations in primary Schwann cells (Nagarajan et al., 2001; Arthur-Farraj et al., 2006). Given the relative ease of transfection of these cells, we used the B16/F10 cell line for further studies of Egr2/Sox10 interaction in Mpz expression.
Both Sox10 sites are required for Egr2 activation of the Mpz intron element
The inverted arrangement of the Sox10 sites in the conserved intron element suggested cooperative binding of a Sox10 dimer, as shown previously for the C/C′ sites in the Mpz promoter (Peirano et al., 2000; Peirano and Wegner, 2000). To test the requirement of each site, they were mutated individually and the responses of these reporters to Egr2 activation were compared with the double-site mutation used previously. As shown in Figure 4, mutation of either site individualy significantly diminished activation by Egr2 in the Sox10-expressing B16/F10 melanocyte cell line, although the level of activation was still above the background of the double-site mutant. These data indicate that binding of a Sox10 dimer is required for efficient activation of the Mpz intron element by Egr2.
Figure 4. Mutagenesis of Sox10-binding sites disrupts synergistic activation of the Mpz intron element by Egr2/Sox10.
The two Sox10 sites in the Mpz intron element reporter were mutated by site-directed mutagenesis. The Mpz intron reporters (wild type, Sox10 mut, 5′ Sox10 mut, and 3′ Sox10 mut) were cotransfected in B16/F10 cells with either 15 ng or 30 ng of the expression plasmid for Egr2. The putative Sox10- and Egr2-binding sites are indicated with ovals and squares, respectively. The filled ovals in the diagram represent mutated Sox10 sites. Fold-induction is calculated relative to the activity of each reporter alone. Means and standard deviations of four replicates are shown. Inductions of the mutant reporters (Sox10 mut, 5′ Sox10 mut, 3′ Sox10 mut) were significantly less than the wild-type reporter (P<0.0001).
Identification of evolutionarily conserved SOX/EGR2 binding modules in myelin genes
The structure of the Mpz intron sequences includes an inverted dimer of Sox10 sites ~50 bp upstream of a critical Egr2 binding site (LeBlanc et al., 2006). Although this spacing is larger than expected for cooperating proteins, previous analyses of other myelin genes have identified other cases of this arrangement of Sox10/Egr2 sites (Table 1). To determine whether the Egr2/Sox10 binding-site configuration has predictive value in identifying regulatory elements in other myelin genes, a database screen was devised to identify this binding site configuration in genomic sequence. Examination of inverted dimeric Sox10 binding sites in myelin genes (Table 1) reveals a common spacing of 3–4 bp between sites (Peirano et al., 2000; Peirano and Wegner, 2000; Bondurand et al., 2001; Ghislain et al., 2003; Denarier et al., 2005). These features cannot be modeled accurately using the SOX-binding profile available in the TRANSFAC database (M00410), which only includes a monomeric binding site. Therefore, to construct a better model of Sox10-binding sites in myelin genes, the inverted dimeric sites were used to construct two weight matrices, with a spacing of either 3 or 4bp between two SOX sites (Supplementary Fig. 1).
Table 1.
Conserved Composite Elements in Myelin-associated Genes
| Gene | Sox10 binding sites | Spacing | Egr2 binding site |
|---|---|---|---|
| Mpz intron | GACAATGggaTTTTGTT | 50 bp | GTGTGGGCT* |
| MBP Mod4 (−9 kb) | ACCATGGgcctTTTTGTT | 80 bp | GTGTGGGCC* |
| Mag intron | AACAGGActcTTTTGTA | 50 bp | GAGAGGGCG |
| Connexin 32 promoter | CACAAAGgtccCATTGTA | −50 bp | GGGTGGGCG |
| Krox20 NCE | AACAGAGccCTTTGTG | 70 bp | GCGTGGGTT |
| Periaxin intron | AGCAGACtggcCATTGTG | −21 bp | GGGTGGGCA |
| Mag −1.7 kb | TTCAATGaacCATTGTC | 64 bp | GTGGGGGTG |
Mouse Connexin 32 (GJB1) sequences are analogous to the sites in the human promoter (described in Bondurand et al., 2001). Mouse Myelin Basic Protein (MBP) sequences are derived from M13, M14 (Sox10) and M20 motifs (Denarier et al., 2005). Mouse Krox20 sequences are derived from the Neural Crest Enhancer (Ghislain et al., 2003). The negative spacing numbers for Connexin 32 and Periaxin indicate that Egr2 sites are upstream of the inverted Sox10 sites, relative to the direction of transcription. Asterisks indicate that Egr2 sites as written are on opposite strand from the Sox10 sites. The Periaxin intron and Mag −1.7 kb sites are novel occurrences of the module (corresponding to first and last entries in Table 2) that were tested in the ChIP assays in Figure 5.
There is considerable degeneracy in the sequences recognized by Egr2 and Sox10, so we chose to screen specific myelin gene loci rather than employing a genome-wide screen. The genomic sequences around four myelin genes (Prx, Mpz, Mag, and Mbp) in human, mouse and rat were collected from the latest genome builds in the UCSC genome browser (Supplementary Table 1). The program TBA (Blanchette et al., 2004) was used to make a multiple sequence alignment using human, mouse and rat sequences from the same locus. Using the constructed SOX dimer binding profile and the EGR2-binding profile from the TRANSFAC database (M00246, M00807 and M00982), SOX and EGR2-binding sites in four myelin gene loci were identified using the program PATSER (Stormo et al., 1982). Both strands of the genomic sequence were searched in this analysis. Based on the multiple sequence alignments generated by TBA, only binding sites conserved between human, mouse and rat were considered in the search for myelin regulatory modules, which are defined as a combination of SOX dimer sites and at least one EGR2 site, with the distance between the SOX site and the EGR2 site <200 bp. This screen identified several previously described sites (Table 2), including sites in the MBP upstream enhancer region, and Mpz and Mag intron regions (Denarier et al., 2005; Jang et al., 2006; LeBlanc et al., 2007).
Table 2.
Evolutionarily conserved SOX/EGR2 modules found in the four myelin genes.
| Gene | 1st SOX position | SOX score | Spacing(bp) | EGR position | EGR score | Distance to SOX site |
|---|---|---|---|---|---|---|
| Mag | −2137 | 8.35 | 3 | −2073 | 5.16 | 64 |
| −168 | 13.2 | 4 | −140 | 6.92 | 28 | |
| * | 1516 | 11.44 | 3 | 1552 | 4.14 | 36 |
| Mbp* | −8744 | 8.52 | 4 | −8947 | 6.77 | 203 |
| −209 | 9.38 | 3 | −22 | 7.61 | 187 | |
| Mpz | −4220 | 11.53 | 3 | −4239 | 4.48 | 19 |
| * | 1220 | 12.97 | 3 | 1289 | 5.13 | 69 |
| Prx | 4536 | 5.49 | 4 | 4504 | 4.14 | 21 |
Sites marked with an asterisk have been identified previously (Denarier et al., 2005; Jang et al., 2006; LeBlanc et al., 2007). Identification of the Periaxin (Prx) sequence requires relaxing the stringency of Sox10 dimer-site selection. Positions are indicated relative to transcription start sites in UCSC genome browser (genome.ucsc.edu) (July 2007, mm9 assembly).
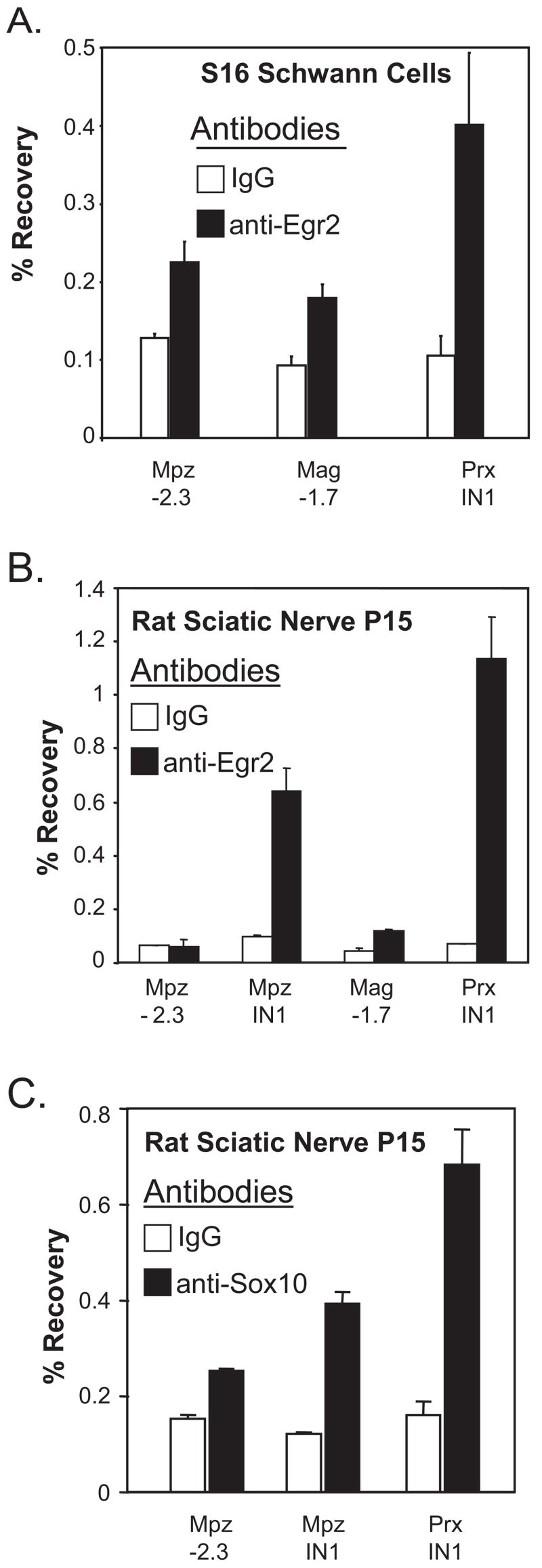
In addition, several potential sites of Egr2/Sox10 interaction have been uncovered in this screen. As a preliminary analysis of these sites, ChIP assays were performed to test binding of Egr2 to two of the novel candidate sites in both the S16 Schwann cell line (Hai et al., 2002), and in freshly dissected sciatic nerves of rat pups (postnatal day 15). The site in the periaxin gene (located within the first intron, ~4.5 kb downstream of the transcription start site) was chosen because its expression depends on Egr2 (Parkinson et al., 2003) and is developmentally regulated in a manner similar to the Mpz gene (Gillespie et al., 1994). In addition, the Mag upstream site was chosen because our previous work has detected binding of Egr2 to the intron-associated site at +1552 (Jang et al., 2006; LeBlanc et al., 2007). The −2073 site in the mouse Mag gene is homologous to the corresponding site at −1.7 kb in the rat Mag locus. These assays reveal significant binding of Egr2 to the Periaxin site compared to the negative control immunoprecipitations in the S16 cell line and in myelinating sciatic nerves (Fig. 5). In contrast, binding of Egr2 to the Mag −1.7 kb site is only marginally above background in these assays. As a further test of the Periaxin site, an in vivo ChIP assay for Sox10 revealed significant binding above background (Fig. 5C).
Figure 5. Binding of Egr2 to a conserved module in the periaxin gene.
A) Cross-linked chromatin was prepared from the S16 rat Schwann cell line. Sonicated chromatin was immunoprecipitated with antibodies directed against either Egr2 (filled bars) or purified rabbit IgG (open bars) as a negative control. Purified DNA was then analyzed by quantitative PCR using primers designed for the Mpz upstream region (−2.3 kb) as a negative control. Additional primer sets were used to detect binding of Egr2 at −1.7 kb upstream of the Mag gene and 4.5 kb downstream of the periaxin transcription start site. Percentage recovery relative to input was determined using quantitative PCR. These data are representative of two independent ChIP experiments.
B) Similar ChIP assays were performed using pooled sciatic nerves obtained from a rat litter at postnatal day 15. An additional primer set in the Mpz intron element (IN1) was used as a positive control. These data are representative of assays performed with two independent litters of rat pups.
C) A ChIP assay for Sox10 binding to the indicated sites in pooled rat sciatic nerve (postnatal day 15) was performed as described above.
Do other Egr family members activate in a Sox10-dependent manner?
Egr2 is closely related to several other members of the EGR family. Egr1 is expressed in embryonic Schwann cell development but is silenced in myelinating Schwann cells and is confined to nonmyelinating Schwann cells (Topilko et al., 1997). In addition, Egr3 has been shown recently to be expressed in nonmyelinating Schwann cells (Gao et al., 2007). Some Egr family members are expressed in nonmyelinating Schwann cells, which has led to the hypothesis that high levels of Mpz expression in Egr2-expressing myelinating Schwann cells might result from the unique ability of Egr2 to interact with Sox10. To test this, we expressed Egr1 and Egr3 in the transfection assays in B16/F10 cells (Fig. 6A). These assays revealed that Egr1 and Egr3 can activate expression through the Mpz intron element, but that activation depends on the integrity of the Sox10 binding sites.
Figure 6. Several Egr family members interact with Sox10.
A) The Mpz intron reporters (wild type and Sox10 mut) were cotransfected in B16/F10 cells with expression plasmids for Egr2, Egr3 (either 25ng or 100ng), and Egr1 (either 50ng or 200ng). The putative Sox10- and Egr2-binding sites are indicated by ovals and squares, respectively. The filled ovals in the diagram represent mutated Sox10 sites. Fold-induction is calculated relative to the activity of each reporter alone. Means and standard deviations of two replicate assays are shown. The fold inductions of the Mpz intron reporter by Egr1, Egr2, and Egr3 are statistically significant compared to that of the Mpz intron Sox10 mut reporter (P<0.01 in each case).<
B) In vitro translated Egr1, Egr2, Egr3 and Egr2 180, each containing an N-terminal HA epitope, were individually mixed and incubated with FLAG-Sox10. Sox10 was immunoprecipitated (IP) using an anti-FLAG affinity resin. After washing, binding of the Egr family members was detected using an anti-HA antibody. The input lanes represent 10% of the amount of lysate used in the anti-FLAG IP. This experiment is representative of two independent assays.
Previous work indicates that Sox10 interacts with many DNA-binding domains, including that of Egr2 (Wissmuller et al., 2006), and this binding is further stabilized by the N-terminus of Egr2 (LeBlanc et al., 2007). Similar coimmunoprecipitation assays reveal that Egr1 and Egr3 also interact physically with Flag-tagged Sox10 in vitro (Fig. 6B), which is consistent with their Sox10-dependent activation of the Mpz intron element. Control assays indicate much weaker binding of Egr2 that lacks an N-terminus (Δ180) and that binding of full length Egr2 depends on addition of Flag-Sox10 (data not shown).
Conclusions
Egr2 binding to the Mpz intron element, detected by in vivo ChIP assays, is developmentally regulated.
Activation of the Mpz intron element requires the adjacent dimeric Sox10-binding site.
There are several occurrences of a conserved composite element consisting of Egr2 and Sox10-binding sites in myelin genes, and a composite matrix that incorporates these features has predictive value.
Other Egr family members (Egr1 and Egr3) are capable of Sox10-dependent activation of the Mpz intron element.
Discussion
Our recent studies have highlighted a configuration of Egr2 and Sox10-binding sites in a conserved region of the first intron of the Mpz gene that responds to many of the signals that induce expression of the endogenous Mpz gene in Schwann cells(LeBlanc et al., 2006; LeBlanc et al., 2007). Compared to the Mpz promoter, this element is also sensitive to the effects of neuropathy-associated, dominant EGR2 mutants that downregulate Mpz expression (Nagarajan et al., 2001; Arthur-Farraj et al., 2006). The dominant mutants of EGR2 appear to exert dominant-negative effects on Mpz by interfering with cooperative binding of Egr2 and Sox10 to the intron element (LeBlanc et al., 2007). Together with previous studies of the Mpz promoter (Peirano et al., 2000; Slutsky et al., 2003), we propose that Egr2/Sox10 binding at the intron element cooperates with Sox10 binding in the proximal promoter region to more effectively recruit transcription complexes to the gene (Fig. 7).
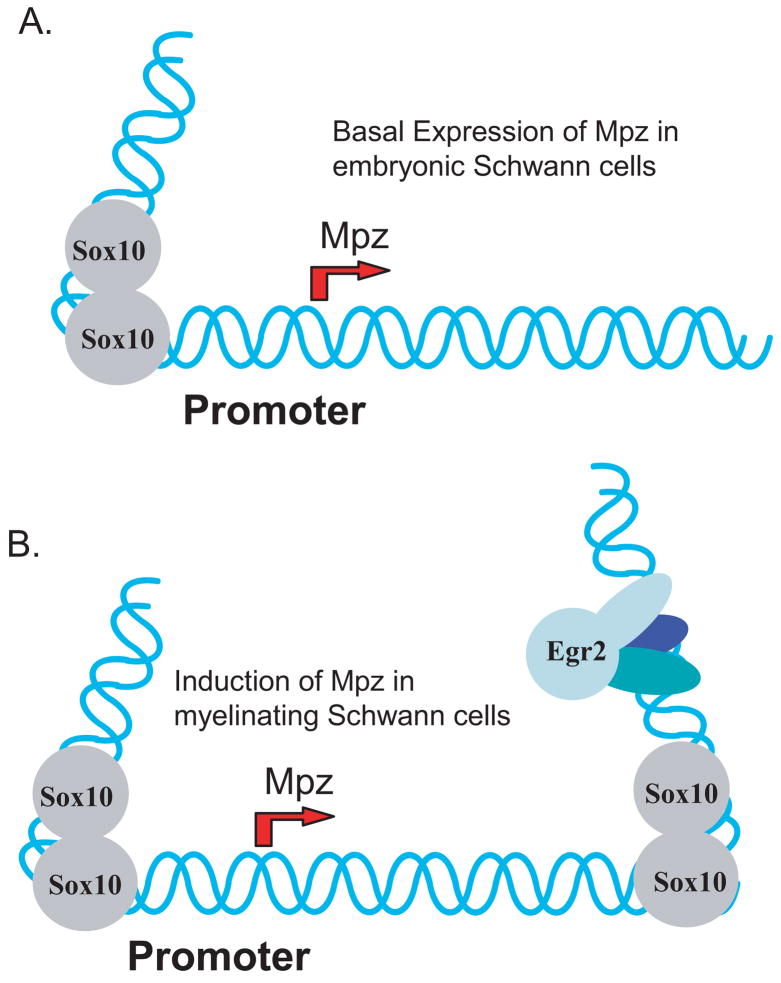
Figure 7. Model for activation of the Mpz gene by Egr2/Sox10.
This model depicts a potential mechanism for the induction of Mpz. A) Previous work (Peirano et al., 2000; Peirano and Wegner, 2000) indicates that Sox10 is responsible for the low basal level of Mpz expression in embryonic Schwann cells. B) Our work indicates that the large induction of Mpz expression that accompanies Egr2 induction in myelinating Schwann cells can be at least partially attributed to Egr2-dependent binding of Sox10 to an enhancer in the first intron of the Mpz gene. In turn, Sox10 is largely required for the large induction of Mpz expression by Egr2, perhaps by serving an architectural function because of its ability to bend DNA. It is proposed that similar configurations of Sox10/Egr2 sites are involved in induction of other myelin genes.
In vitro binding assays show that efficient binding of Sox10 to the Mpz intron element depends on binding of Egr2 to the adjacent sites (LeBlanc et al., 2006; LeBlanc et al., 2007). Similarly, transfection experiments indicate that full activation of the Mpz intron element by Egr2 in Sox10-expressing cells (Schwann cells and melanocytes) depends largely on recruitment of Sox10 to its binding sites. Elegant structure/function studies of Sox10 binding to the Mpz promoter show that Sox10 binds to several sites as a monomer, but that it binds cooperatively with higher affinity to two inverted sites with a defined spacing between the sites (Peirano et al., 2000; Peirano and Wegner, 2000). A similar inverted pair of Sox10-binding sites occurs in the intron element and our data indicate the importance of recruiting a Sox10 dimer because both Sox10-binding sites are required for maximal activation by Egr2. The mechanism of transcriptional activation by Sox10 has not been elucidated, but it is possible that Sox10 and Egr2 interact to cooperatively recruit cofactor(s) that are crucial for transcriptional activation. Alternatively, Sox10 introduces substantial bends in DNA (Peirano and Wegner, 2000), which might facilitate communication of the promoter with the intron-associated activating sequences and profoundly alter chromatin architecture of the Mpz locus.
Although the structural basis of the Egr2/Sox10 interaction has not been defined it is expected that the previously described interaction between Egr2 and Sox10 DNA-binding domains (Wißmüller et al., 2006) are necessary but probably not sufficient for stable interaction (LeBlanc et al., 2007). The assays described here indicate that the capacity for interaction with Sox10 to activate the Mpz intron element is not restricted to Egr2, but is also exhibited by Egr1 and Egr3 and, therefore, is probably mediated by a domain that is shared by these family members. Therefore, the role of Egr2 as a master regulator of myelination might not result from unique properties of the Egr2 protein itself but might reflect the large induction of Egr2/Krox20 in myelinating Schwann cells. Knockin analysis of Egr proteins has not been performed, but a recent analysis shows that insertion of Sox8 coding sequences in the Sox10 locus rescues several of the deleterious components of the Sox10-knockout phenotype (Kellerer et al., 2006). Therefore, the cooperative functions described here might reflect a general capacity of Egr family members and E class Sox proteins (Sox8, Sox9, and Sox10) to interact in various developmental contexts.
Based on sequence patterns derived from the Egr2/Sox10 binding sites in the Mag, Mbp, Connexin 32, and Mpz genes, we identified several novel occurrences of a conserved configuration of Egr2/Sox10 binding sites in four myelin genes, including a site within the periaxin gene that tests positive in an Egr2 ChIP assay. The importance of these sequences to periaxin regulation has not been established, but it is likely that elements downstream of the promoter are involved because constructs containing up to 12 kb of upstream sequence do not respond to either Egr2 expression or cAMP induction (Parkinson et al., 2003). If the intron-associated site is functional, it is possible that intron-associated enhancers are a common theme in myelin gene regulation because binding of Egr2 and Sox10 to conserved sites has also been detected in introns of the Mag and Mpz genes (Jang et al., 2006; LeBlanc et al., 2007). Another outcome of these studies is that few of the Egr2 sites in these composite modules are high affinity sites that correspond strictly to the consensus sequence for Egr binding 5′ GCGTGGGCG 3′ (Swirnoff and Milbrandt, 1995). It is tempting to speculate that somewhat weaker Egr2 sites have evolved to allow finer regulation of Egr2 binding and consequent myelin gene induction. It is also possible that Egr2 binding at these weaker sites is stabilized by interactions with a flanking Sox10 dimer.
Previous work on periaxin regulation indicates that Egr2 acts as an amplifier of pre-existing myelin gene expression because there is a reduced, but significant, level of periaxin induction even in the absence of Egr2/Krox20 (also Mpz and Mag) (Parkinson et al., 2003). Moreover, expression of periaxin temporally precedes Egr2 induction in developing Schwann cells, which indicates that there is an Egr2-independent mode of periaxin induction. The ability of Egr1 and Egr3 to activate in a Sox10-dependent manner might explain these observations because Egr1 is expressed in embryonic Schwann cells and is also co-expressed with Egr2 at postnatal day 1 (Topilko et al., 1997). Therefore, it is possible that Egr1 might cooperate with Sox10 to initiate periaxin (and Mpz) induction, which is then further enhanced by high expression of Egr2 in myelinating Schwann cells. Interestingly, peripheral nerves of a double knockout of the Egr1/Egr3 genes have thinner myelin, which is associated with lower levels of the p75 nerve growth factor receptor (Gao et al., 2007).
Overall, combining our results with previously published data suggest a model for the regulation of Myelin Protein Zero in which binding of Sox10 to multiple sites in the Mpz promoter region is responsible for the basal expression of Mpz in embryonic Schwann cells, although other factors may have a role. Furthermore, we conclude that interaction between Egr2 and Sox10 is important for facilitated binding of Sox10 (LeBlanc et al., 2007) to novel sites, including the conserved intron element of the Mpz gene. Therefore, formation of an Egr2/Sox10 complex would achieve Schwann cell-specific regulation by targeting sites that are not occupied in other tissues that express Sox10 (e.g. melanocytes).
Supplementary Material
Acknowledgments
We thank Stephen Johnson for assistance with ChIP analysis, Richard Quarles for providing the S16 cell line, Vivian Fu and David Jarrard for assistance with footprinting, and Lawrence Wrabetz for providing clones of the Mpz locus. We also thank Scott LeBlanc for helpful discussion. This work was supported by a grant from the National Institutes of Health (HD41590) to JS, and a core grant to the Waisman Center from the National Institute of Child Health and Human Development (P30 HD03352). GM was supported by a training grant from the National Institutes of Health (T32 GM08688).
Footnotes
Statement of Interest: None
References
- Aquino JB, Hjerling-Leffler J, Koltzenburg M, Edlund T, Villar MJ, Ernfors P. In vitro and in vivo differentiation of boundary cap neural crest stem cells into mature Schwann cells. Exp Neurol. 2006;198:438–449. doi: 10.1016/j.expneurol.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Arthur-Farraj P, Mirsky R, Parkinson DB, Jessen KR. A double point mutation in the DNA-binding region of Egr2 switches its function from inhibition to induction of proliferation: A potential contribution to the development of congenital hypomyelinating neuropathy. Neurobiol Dis. 2006;24:159–169. doi: 10.1016/j.nbd.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Bellone E, Di Maria E, Soriani S, Varese A, Doria LL, Ajmar F, Mandich P. A novel mutation (D305V) in the early growth response 2 gene is associated with severe Charcot-Marie-Tooth type 1 disease. Hum Mutat. 1999;14:353–354. doi: 10.1002/(SICI)1098-1004(199910)14:4<353::AID-HUMU17>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya A, Frank E, Ratner N, Brackenbury R. P0 is an early marker of the Schwann cell lineage in chickens. Neuron. 1991;7:831–844. doi: 10.1016/0896-6273(91)90285-8. [DOI] [PubMed] [Google Scholar]
- Blanchette M, Kent WJ, Riemer C, Elnitski L, Smit AF, Roskin KM, Baertsch R, Rosenbloom K, Clawson H, Green ED, Haussler D, Miller W. Aligning multiple genomic sequences with the threaded blockset aligner. Genome Res. 2004;14:708–715. doi: 10.1101/gr.1933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondurand N, Girard M, Pingault V, Lemort N, Dubourg O, Goossens M. Human Connexin 32, a gap junction protein altered in the X-linked form of Charcot-Marie-Tooth disease, is directly regulated by the transcription factor SOX10. Hum Mol Genet. 2001;10:2783–2795. doi: 10.1093/hmg/10.24.2783. [DOI] [PubMed] [Google Scholar]
- Denarier E, Forghani R, Farhadi HF, Dib S, Dionne N, Friedman HC, Lepage P, Hudson TJ, Drouin R, Peterson A. Functional organization of a Schwann cell enhancer. J Neurosci. 2005;25:11210–11217. doi: 10.1523/JNEUROSCI.2596-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltri ML, D’Antonio M, Quattrini A, Numerato R, Arona M, Previtali S, Chiu SY, Messing A, Wrabetz L. A novel P0 glycoprotein transgene activates expression of lacZ in myelin-forming Schwann cells. Eur J Neurosci. 1999;11:1577–1586. doi: 10.1046/j.1460-9568.1999.00568.x. [DOI] [PubMed] [Google Scholar]
- Gao X, Daugherty RL, Tourtellotte WG. Regulation of low affinity neurotrophin receptor (p75(NTR)) by early growth response (Egr) transcriptional regulators. Mol Cell Neurosci. 2007;36:501–514. doi: 10.1016/j.mcn.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghislain J, Desmarquet-Trin-Dinh C, Gilardi-Hebenstreit P, Charnay P, Frain M. Neural crest patterning: autoregulatory and crest-specific elements co-operate for Krox20 transcriptional control. Development. 2003;130:941–953. doi: 10.1242/dev.00318. [DOI] [PubMed] [Google Scholar]
- Giese KP, Martini R, Lemke G, Soriano P, Schachner M. Mouse P0 gene disruption leads to hypomyelination, abnormal expression of recognition molecules, and degeneration of myelin and axons. Cell. 1992;71:565–576. doi: 10.1016/0092-8674(92)90591-y. [DOI] [PubMed] [Google Scholar]
- Gillespie CS, Sherman DL, Blair GE, Brophy PJ. Periaxin, a novel protein of myelinating Schwann cells with a possible role in axonal ensheathment. Neuron. 1994;12:497–508. doi: 10.1016/0896-6273(94)90208-9. [DOI] [PubMed] [Google Scholar]
- Greenfield S, Brostoff S, Eylar EH, Morell P. Protein composition of myelin of the peripheral nervous system. J Neurochem. 1973;20:1207–1216. doi: 10.1111/j.1471-4159.1973.tb00089.x. [DOI] [PubMed] [Google Scholar]
- Hai M, Muja N, DeVries GH, Quarles RH, Patel PI. Comparative analysis of Schwann cell lines as model systems for myelin gene transcription studies. J Neurosci Res. 2002;69:497–508. doi: 10.1002/jnr.10327. [DOI] [PubMed] [Google Scholar]
- Inoue K, Khajavi M, Ohyama T, Hirabayashi S, Wilson J, Reggin JD, Mancias P, Butler IJ, Wilkinson MF, Wegner M, Lupski JR. Molecular mechanism for distinct neurological phenotypes conveyed by allelic truncating mutations. Nat Genet. 2004;36:361–369. doi: 10.1038/ng1322. [DOI] [PubMed] [Google Scholar]
- Jang SW, LeBlanc SE, Roopra A, Wrabetz L, Svaren J. In vivo detection of Egr2 binding to target genes during peripheral nerve myelination. Journal of Neurochemistry. 2006;98:1678–1687. doi: 10.1111/j.1471-4159.2006.04069.x. [DOI] [PubMed] [Google Scholar]
- Kamaraju AK, Bertolotto C, Chebath J, Revel M. Pax3 down-regulation and shut-off of melanogenesis in melanoma B16/F10.9 by interleukin-6 receptor signaling. J Biol Chem. 2002;277:15132–15141. doi: 10.1074/jbc.M200004200. [DOI] [PubMed] [Google Scholar]
- Kellerer S, Schreiner S, Stolt CC, Scholz S, Bosl MR, Wegner M. Replacement of the Sox10 transcription factor by Sox8 reveals incomplete functional equivalence. Development. 2006;133:2875–2886. doi: 10.1242/dev.02477. [DOI] [PubMed] [Google Scholar]
- Kuhlbrodt K, Herbarth B, Sock E, Hermans-Borgmeyer I, Wegner M. Sox10, a novel transcriptional modulator in glial cells. J Neurosci. 1998;18:237–250. doi: 10.1523/JNEUROSCI.18-01-00237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le N, Nagarajan R, Wang JY, Araki T, Schmidt RE, Milbrandt J. Analysis of congenital hypomyelinating Egr2Lo/Lo nerves identifies Sox2 as an inhibitor of Schwann cell differentiation and myelination. Proc Natl Acad Sci U S A. 2005;102:2596–2601. doi: 10.1073/pnas.0407836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc SE, Jang SW, Ward RM, Wrabetz L, Svaren J. Direct Regulation of Myelin Protein Zero Expression by the Egr2 Transactivator. J Biol Chem. 2006;281:5453–5460. doi: 10.1074/jbc.M512159200. [DOI] [PubMed] [Google Scholar]
- LeBlanc SE, Srinivasan R, Ferri C, Mager GM, Gillian-Daniel AL, Wrabetz L, Svaren J. Regulation of cholesterol/lipid biosynthetic genes by Egr2/Krox20 during peripheral nerve myelination. J Neurochem. 2005;93:737–748. doi: 10.1111/j.1471-4159.2005.03056.x. [DOI] [PubMed] [Google Scholar]
- LeBlanc SE, Ward RM, Svaren J. Neuropathy-associated Egr2 mutants disrupt cooperative activation of myelin protein zero by Egr2 and Sox10. Mol Cell Biol. 2007;27:3521–3529. doi: 10.1128/MCB.01689-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Brennan A, Blanchard A, Zoidl G, Dong Z, Tabernero A, Zoidl C, Dent MA, Jessen KR, Mirsky R. P0 is constitutively expressed in the rat neural crest and embryonic nerves and is negatively and positively regulated by axons to generate non-myelin-forming and myelin-forming Schwann cells, respectively. Mol Cell Neurosci. 1997;8:336–350. doi: 10.1006/mcne.1996.0589. [DOI] [PubMed] [Google Scholar]
- Lemke G, Axel R. Isolation and sequence of a cDNA encoding the major structural protein of peripheral myelin. Cell. 1985;40:501–508. doi: 10.1016/0092-8674(85)90198-9. [DOI] [PubMed] [Google Scholar]
- Lemke G, Chao M. Axons regulate Schwann cell expression of the major myelin and NGF receptor genes. Development. 1988;102:499–504. doi: 10.1242/dev.102.3.499. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mikesova E, Huhne K, Rautenstrauss B, Mazanec R, Barankova L, Vyhnalek M, Horacek O, Seeman P. Novel EGR2 mutation R359Q is associated with CMT type 1 and progressive scoliosis. Neuromuscul Disord. 2005;15:764–767. doi: 10.1016/j.nmd.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Morrison TB, Weis JJ, Wittwer CT. Quantification of low-copy transcripts by continuous SYBR Green I monitoring during amplification. Biotechniques. 1998;24:954–962. [PubMed] [Google Scholar]
- Musso M, Balestra P, Bellone E, Cassandrini D, Di Maria E, Doria LL, Grandis M, Mancardi GL, Schenone A, Levi G, Ajmar F, Mandich P. The D355V mutation decreases EGR2 binding to an element within the Cx32 promoter. Neurobiol Dis. 2001;8:700–706. doi: 10.1006/nbdi.2001.0397. [DOI] [PubMed] [Google Scholar]
- Musso M, Balestra P, Taroni F, Bellone E, Mandich P. Different consequences of EGR2 mutants on the transactivation of human Cx32 promoter. Neurobiol Dis. 2003;12:89–95. doi: 10.1016/s0969-9961(02)00018-9. [DOI] [PubMed] [Google Scholar]
- Nagarajan R, Svaren J, Le N, Araki T, Watson M, Milbrandt J. EGR2 mutations in inherited neuropathies dominant-negatively inhibit myelin gene expression. Neuron. 2001;30:355–368. doi: 10.1016/s0896-6273(01)00282-3. [DOI] [PubMed] [Google Scholar]
- Pareyson D, Taroni F, Botti S, Morbin M, Baratta S, Lauria G, Ciano C, Sghirlanzoni A. Cranial nerve involvement in CMT disease type 1 due to early growth response 2 gene mutation. Neurology. 2000;54:1696–1698. doi: 10.1212/wnl.54.8.1696. [DOI] [PubMed] [Google Scholar]
- Parkinson DB, Dickinson S, Bhaskaran A, Kinsella MT, Brophy PJ, Sherman DL, Sharghi-Namini S, Duran Alonso MB, Mirsky R, Jessen KR. Regulation of the myelin gene periaxin provides evidence for Krox-20-independent myelin-related signalling in Schwann cells. Mol Cell Neurosci. 2003;23:13–27. doi: 10.1016/s1044-7431(03)00024-1. [DOI] [PubMed] [Google Scholar]
- Peirano RI, Goerich DE, Riethmacher D, Wegner M. Protein zero gene expression is regulated by the glial transcription factor Sox10. Mol Cell Biol. 2000;20:3198–3209. doi: 10.1128/mcb.20.9.3198-3209.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirano RI, Wegner M. The glial transcription factor Sox10 binds to DNA both as monomer and dimer with different functional consequences. Nucleic Acids Res. 2000;28:3047–3055. doi: 10.1093/nar/28.16.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner S, Cossais F, Fischer K, Scholz S, Bosl MR, Holtmann B, Sendtner M, Wegner M. Hypomorphic Sox10 alleles reveal novel protein functions and unravel developmental differences in glial lineages. Development. 2007;134:3271–3281. doi: 10.1242/dev.003350. [DOI] [PubMed] [Google Scholar]
- Shy ME. HMSN Related to MPZ (P0) Mutations. In: Dyck PJ, Thomas PK, editors. Peripheral Neuropathy. W.B. Saunders; 2005. pp. 1681–1706. [Google Scholar]
- Slutsky SG, Kamaraju AK, Levy AM, Chebath J, Revel M. Activation of myelin genes during transdifferentiation from melanoma to glial cell phenotype. J Biol Chem. 2003;278:8960–8968. doi: 10.1074/jbc.m210569200. [DOI] [PubMed] [Google Scholar]
- Stormo GD, Schneider TD, Gold L, Ehrenfeucht A. Use of the ‘Perceptron’ algorithm to distinguish translational initiation sites in E. coli. Nucleic Acids Res. 1982;10:2997–3011. doi: 10.1093/nar/10.9.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirnoff AH, Milbrandt J. DNA-binding specificity of NGFI-A and related zinc finger transcription factors. Mol Cell Biol. 1995;15:2275–2287. doi: 10.1128/mcb.15.4.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman V, De Jonghe P, Ceuterick C, De Vriendt E, Lofgren A, Nelis E, Warner LE, Lupski JR, Martin JJ, Van Broeckhoven C. Novel missense mutation in the early growth response 2 gene associated with Dejerine-Sottas syndrome phenotype. Neurology. 1999;52:1827–1832. doi: 10.1212/wnl.52.9.1827. [DOI] [PubMed] [Google Scholar]
- Topilko P, Levi G, Merlo G, Mantero S, Desmarquet C, Mancardi G, Charnay P. Differential regulation of the zinc finger genes Krox-20 and Krox-24 (Egr-1) suggests antagonistic roles in Schwann cells. J Neurosci Res. 1997;50:702–712. doi: 10.1002/(SICI)1097-4547(19971201)50:5<702::AID-JNR7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Topilko P, Schneider-Maunoury S, Levi G, Baron-Van Evercooren A, Chennoufi AB, Seitanidou T, Babinet C, Charnay P. Krox-20 controls myelination in the peripheral nervous system. Nature. 1994;371:796–799. doi: 10.1038/371796a0. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Hauer P, Lemke G. Axonal regulation of myelin protein mRNA levels in actively myelinating Schwann cells. J Neurosci. 1988;8:3515–3521. doi: 10.1523/JNEUROSCI.08-09-03515.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner LE, Mancias P, Butler IJ, McDonald CM, Keppen L, Koob KG, Lupski JR. Mutations in the early growth response 2 (EGR2) gene are associated with hereditary myelinopathies. Nat Genet. 1998;18:382–384. doi: 10.1038/ng0498-382. [DOI] [PubMed] [Google Scholar]
- Warner LE, Svaren J, Milbrandt J, Lupski JR. Functional consequences of mutations in the early growth response 2 gene (EGR2) correlate with severity of human myelinopathies. Hum Mol Genet. 1999;8:1245–1251. doi: 10.1093/hmg/8.7.1245. [DOI] [PubMed] [Google Scholar]
- Wissmuller S, Kosian T, Wolf M, Finzsch M, Wegner M. The high-mobility-group domain of Sox proteins interacts with DNA-binding domains of many transcription factors. Nucleic Acids Res. 2006;34:1735–1744. doi: 10.1093/nar/gkl105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wißmüller S, Kosian T, Wolf M, Finzsch M, Wegner M. The high-mobility-group domain of Sox proteins interacts with DNA-binding domains of many transcription factors. Nucleic Acids Res. 2006;34:1735–1744. doi: 10.1093/nar/gkl105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrabetz L, Feltri ML, Kleopa KA, Scherer SS. Inherited Neuropathies: Clinical, Genetic and Biological Features. In: Lazzarini RA, editor. Myelin Biology and Disorders. Elsevier Academic Press; 2004. pp. 905–951. [Google Scholar]
- Zhang SM, Marsh R, Ratner N, Brackenbury R. Myelin glycoprotein P0 is expressed at early stages of chicken and rat embryogenesis. J Neurosci Res. 1995;40:241–250. doi: 10.1002/jnr.490400213. [DOI] [PubMed] [Google Scholar]
- Zorick TS, Syroid DE, Arroyo E, Scherer SS, Lemke G. The Transcription Factors SCIP and Krox-20 Mark Distinct Stages and Cell Fates in Schwann Cell Differentiation. Mol Cell Neurosci. 1996;8:129–145. doi: 10.1006/mcne.1996.0052. [DOI] [PubMed] [Google Scholar]
- Zorick TS, Syroid DE, Brown A, Gridley T, Lemke G. Krox-20 controls SCIP expression, cell cycle exit and susceptibility to apoptosis in developing myelinating Schwann cells. Development. 1999;126:1397–1406. doi: 10.1242/dev.126.7.1397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.