Abstract
The purpose of this study was to evaluate the effect of melatonin on oxidative stress occurring in the brain after routine lobectomy neurosurgery procedures. Different concentrations of melatonin (5mg/kg, 15mg/kg and 150mg/kg) were administered 1 hour before lobectomy in a rodent surgical brain injury (SBI) model. The neurological outcomes were assessed at 24 hrs before euthanization for evaluation of brain water content (brain edema) and lipid peroxidation (oxidative stress). The results showed that lower doses (5mg/kg and 15mg/kg) failed to reduce brain edema, but the 15mg/kg dose did lower oxidative stress and improved several neurological parameters. High concentration of melatonin (150mg/kg) significantly increased brain edema and elevated oxidative stress as compared to vehicle-treated group. Furthermore, high-dose melatonin also worsened neurological outcomes compared to other groups. The study suggests that melatonin has dual effects; low-dose melatonin may provide neuroprotective effects against SBI but high dose of melatonin may aggravate some parameters after SBI.
Keywords: Surgical brain injury, melatonin, oxidative stress, brain edema, neuroprotection, lipid peroxidation
Introduction
5-methoxy-N-acetyltryptamine, commonly known as melatonin is a naturally secreted indoleamine from the pineal gland and regulates circadian and seasonal rhythms [1]. It is a well known dietary supplement used for sleep disorders [1]. Melatonin and its metabolites are also known antioxidants that act as direct free radical scavengers [2-5]. Recent experimental studies have shown that melatonin provides neuroprotection against brain injury due to different etiologies such as ischemia [6-15], trauma [16-18], hemorrhage [19], and excitotoxicity [20] as well as in neurodegenerative disorders [21-24] such as Parkinson’s disease and Alzheimer’s disease. Melatonin decreased the brain injury by reducing oxidative stress, preventing the disruption of blood brain barrier (BBB) and attenuating the resulting brain edema in experimental models of cerebrovascular disorders [7,8,13-15].
Blood brain barrier (BBB) disruption and brain edema are also commonly encountered early postoperative complications after routine neurosurgical procedures which can contribute to neurological deficits [25-27]. These events are critical in pathophysiology of surgical brain injury (SBI) [28-31]. Our previous studies have shown that there is also increased oxidative stress in the brain tissue affected by SBI [30]. Free radical toxicity and oxidative stress after brain injury can disrupt the BBB and lead to brain edema [32,33]. Melatonin has been shown to freely cross the BBB and is known to be widely distributed in all tissues allowing it to act on all cells [34]. We used a rodent SBI model to test our hypothesis that melatonin decreases oxidative stress, attenuates post operative complications such as brain edema and improves neurological outcomes after routine neurosurgical procedures.
Materials and Methods
The Animal Care and Use Committee at Loma Linda University School of Medicine approved all the procedures for this study.
Surgical brain injury
Male Sprague Dawley rats weighing between 300 and 350 grams were used for this study. The rodent model of SBI was used as described before [28,29,31]. In brief, following anesthesia (ketamine 100 mg/kg plus xylazine 10mg/kg, intraperitoneal), rats were positioned on a stereotaxic frame and prepared for surgery. The right dorsum of the skull was exposed via a midline incision followed by blunt dissection of skin, connective tissue and periosteal elevation. A micro-drill was used to fashion a square cranial window (5mm edge) such that the left lower corner of the square was at the bregma. The dura was incised and reflected to expose the underlying right frontal lobe. A sharp flat steel blade with the cutting surface measuring 6 mm × 1.5 mm (length × width), was used to make two incisions along sagittal and coronal planes leading away from the bregma to resect an area of brain 2mm lateral in the sagittal plane and 1mm ventral in the coronal plane. The depth of the incisions extended to the base of the skull. Hemostasis was achieved by intraoperative packing and saline irrigation. After confirmation of hemostasis, the drilled bone piece was placed back in position and the skin sutured with 3-0 Ethicon on reverse cutting needle. Vital parameters including temperature (maintained between 35-37° C) and respiration were monitored intraoperatively and postoperatively. All animals received post-operative fluids. Sham surgery included only craniotomy and replacement of the bone flap without any dural incisions. This animal model had zero mortality in any of the groups.
Treatments
Melatonin (5-methoxy-N-acetyltryptamine) was purchased from Sigma Aldrich (St. Louis, United States). The compound was dissolved in 0.9% NS with 10% ethanol to create solutions for dosages for treatment groups receiving 5mg/kg, 15 mg/kg and 150 mg/kg intraperitoneally 1 hr before the surgery. All others groups received equal volume of vehicle.
Brain water content
The brain water content was calculated similar to previous studies by us and others [28-31,35]. The animals were sacrificed under deep anesthesia at 24 hrs after surgery and the brains were immediately divided on ice into frontal ipsilateral (right), frontal contralateral (left), parietal ipsilateral, parietal contralateral, brain stem and cerebellum. These parts were weighed immediately (wet weight) and weighed again after drying in oven at 105° C for 48 hours (dry weight).The percent of water content was calculated as [(wet weight – dry weight) / wet weight] × 100 % as reported previously [28-31,35].
Lipid peroxidation assay
The animals were anesthetized, perfused with ice cold PBS, and brain samples were collected at 24 hours after surgical injury. The level of lipid peroxidation products (malondialdehyde [MDA]) in the ipsilateral (right) frontal lobe were measured using a LPO-586 kit (OxisResearch; Portland, United States) as previously described [19,30]. The brain tissue from right frontal lobe (ipsilateral) was homogenized in 20 mmol / L phosphate buffer (pH 7.4) with 0.5 M butylated hydroxytoluene (Sigma Aldrich Corp, MO, USA) in acetonitrile. The homogenates were centrifuged at 20,800 g for 10 min at 4°C and the supernatants were collected (Centrifuge 5417C/R from Eppendorf AG, 22331 Hamburg, Germany). Protein concentration was measured by DC protein assay (Bio-Rad) and the samples were reacted with a chromogenic reagent at 45 °C for 60 min. After incubation, the samples were centrifuged at 20,800 g for 10 min at 4 °C and supernatants were measured at 586 nm. The level of MDA was calculated as picomoles per milligram protein according to the standard curve.
Neurological evaluation
Neurological status of the animals was evaluated at 24 hrs after surgery by an investigator blinded to the treatment groups using a 18 point scoring system according to previous studies [30]. Briefly, the scoring system consisted of six tests with scores of 1–3 for each test. These six tests included: (i) spontaneous activity; (ii) symmetry in the movement of four limbs; (iii) forepaw outstretching; (iv) climbing; (v) body proprioception; and (vi) response to vibrissae touch. The score given to each rat at the completion of the evaluation was the summation of all six individual test scores. The minimum neurological score was 3 and the maximum was 18. Vibrissae stimulation (paw placement test) was conducted as described previously [36]. Briefly, right and left whiskers were stimulated separately and the ability of left paw to be placed on table was noted. Similarly, right paw was tested, however the contralateral side (left paw) was affected and only those results were reported. Beam walking tests were also performed using 2.4cm diameter × 100 cm length beam and 5 cm diameter × 60 cm length beam. Time spent on beam, distance traveled and speed were calculated.
Data analysis
Data are expressed as mean ± S.E.M. Statistical significance was verified by one-way ANOVA for multiple comparisons with Holm-Sidak test for post-hoc comparison. Overall significance level =0.05 and probability value of p<0.05 was considered statistically significant.
Results
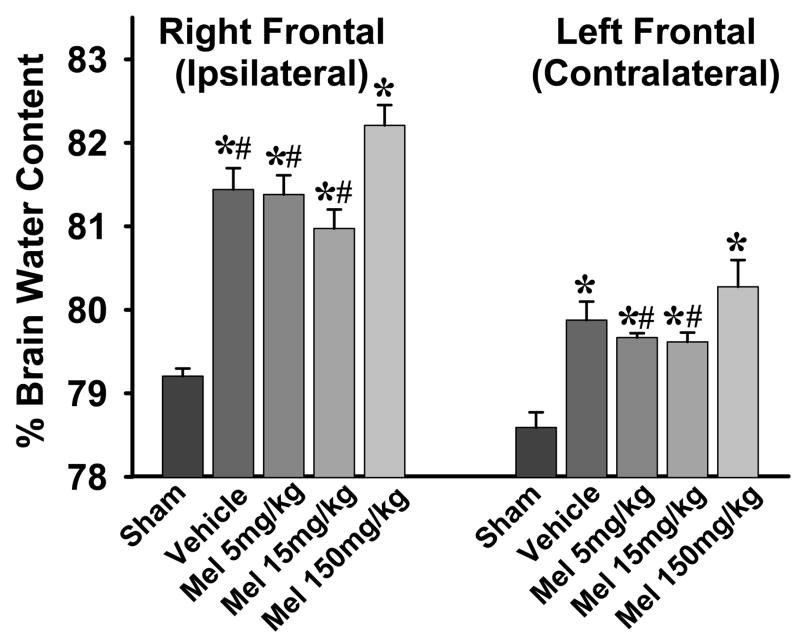
Brain edema was assessed by measuring the brain water content in different areas of the brain at 24 hrs after surgery. There was significantly increased brain water content in the right (ipsilateral) and left (contralateral) frontal lobes in all rats subjected to SBI (with or without melatonin treatment) as compared to their respective sham groups (Fig. 1). The brain water content in right frontal lobe (ipsilateral) was significantly higher than the left frontal lobe (contralateral) in all comparable groups except sham group (p<0.05, not shown in figure). There was no significant difference in the brain water content between the SBI and sham groups in other brain areas (data not shown).
Fig. 1.
Effects of melatonin on brain edema. The figure shows brain water content in right (ipsilateral) and left (contralateral) frontal lobes 24 hrs after surgical injury. Both right and left frontal lobes show increased brain water content compared to respective sham groups, however, the right lobe has much higher water content (by almost 2%). Low-dose melatonin (5mg/kg and 15mg/kg) show a trend towards lowering brain water content, though not statistically significant (p=0.13 with 15mg/kg dose, right frontal lobe). The high-dose melatonin (150mg/kg), however, significantly increases brain water content compared to other groups. (P< 0.05 is considered significant, (*) and (#) indicate significance v/s sham and 150mg/kg melatonin respectively, ANOVA p<0.001). n numbers are as follows: sham = 8; vehicle treated = 8; melatonin 5mg/kg = 8; 15mg/kg = 7; 150mg/kg = 6.
The lower doses of melatonin did not reduce brain edema (p=0.13 for 15mg/kg, right frontal lobe). On the other hand, high-dose melatonin (150mg/kg) significantly increased brain edema in both right and left frontal lobes compared to all other groups (Fig. 1).
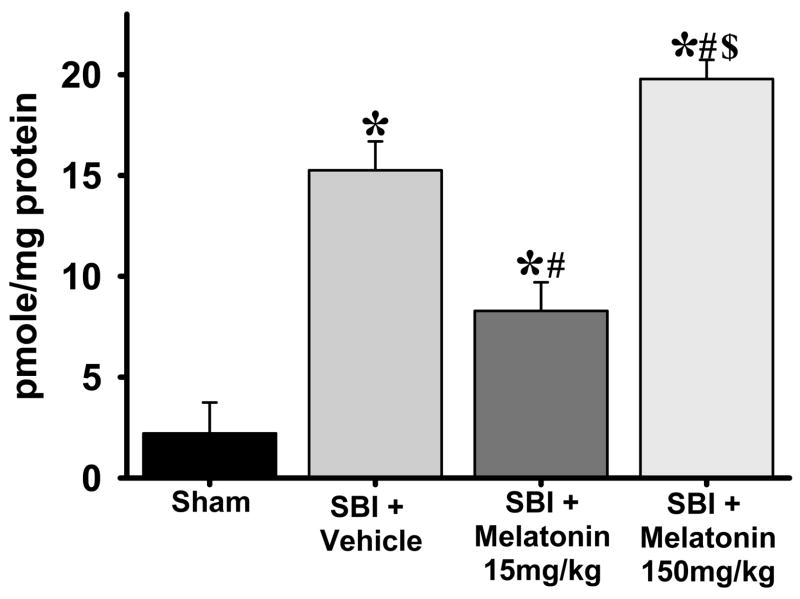
Lipid peroxidation assay (malondialdehyde levels) showed that oxidative stress was dramatically increased (6-fold) in the right frontal lobe at 24 hrs after surgery (vehicle-treated v/s sham group, p<0.05). Low-dose melatonin (15mg/kg) significantly attenuated the oxidative stress compared to vehicle group. This, however, was reversed with the high-dose melatonin (150mg/kg) which showed significantly higher oxidative stress as compared to all other groups (Fig. 2).
Fig. 2.
Effects of melatonin on oxidative stress. The figure shows malondialdehyde levels for lipid peroxidation assay from right (ipsilateral) frontal lobe 24 hrs after surgical injury. All groups subjected to SBI showed increased oxidative stress compared to sham group. Low-dose melatonin (15mg/kg) significantly attenuated the oxidative stress compared to vehicle; however, high-dose melatonin (150mg/kg) significantly increased oxidative stress compared to all other groups. (P< 0.05 is considered significant, (*), (#) and ($) indicates v/s sham and v/s vehicle and v/s 15mg/kg melatonin respectively, ANOVA p<0.001). n numbers are as follows: sham = 4; vehicle treated = 6; melatonin 15mg/kg = 8;150mg/kg = 6.
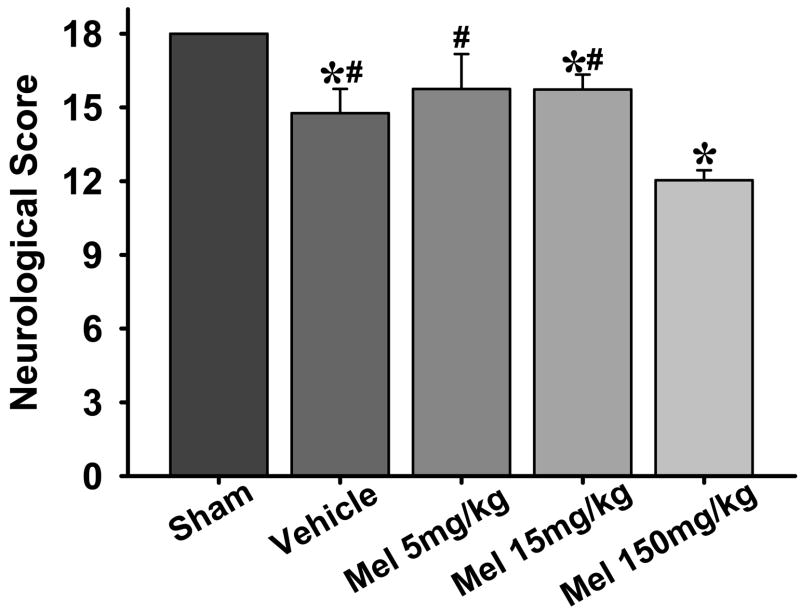
Numerous neurological tests were performed to evaluate the sensorimotor deficits 24 hours after surgery (Figs. 3,4). An 18 point sensorimotor scoring system as used previously [30] showed that all rats subjected to SBI (treated or untreated) had worsened scores as compared to sham group. However, the rats treated with high-dose melatonin (150mg/kg) had further worsening of neurological deficits compared to all other groups (Fig. 3). Low dose melatonin did not show any improvement compared to vehicle treated group.
Fig. 3.
Neurological outcomes. The figures shows an 18 point neurological scoring system which shows that all groups subjected to SBI had significant neurological deficits compared to sham group; however, the groups treated with high-dose melatonin had significantly more neurological deficits as compared to all other groups. (*) and (#) indicates v/s sham and 150mg/kg melatonin respectively for P<0.05. n numbers are as follows: sham = 11; vehicle treated = 15; melatonin 5mg/kg = 8; 15mg/kg = 13; 150mg/kg = 14.
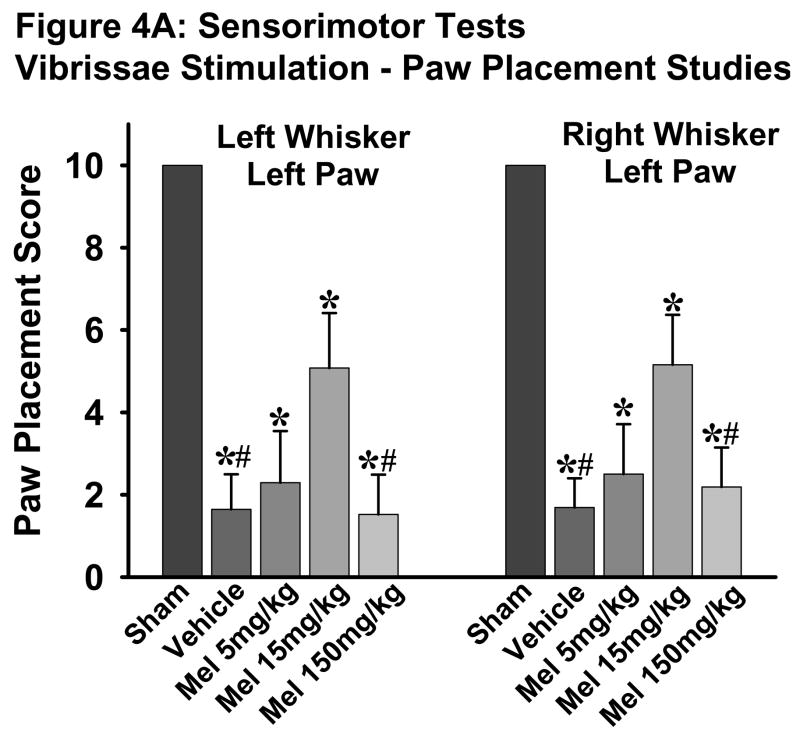
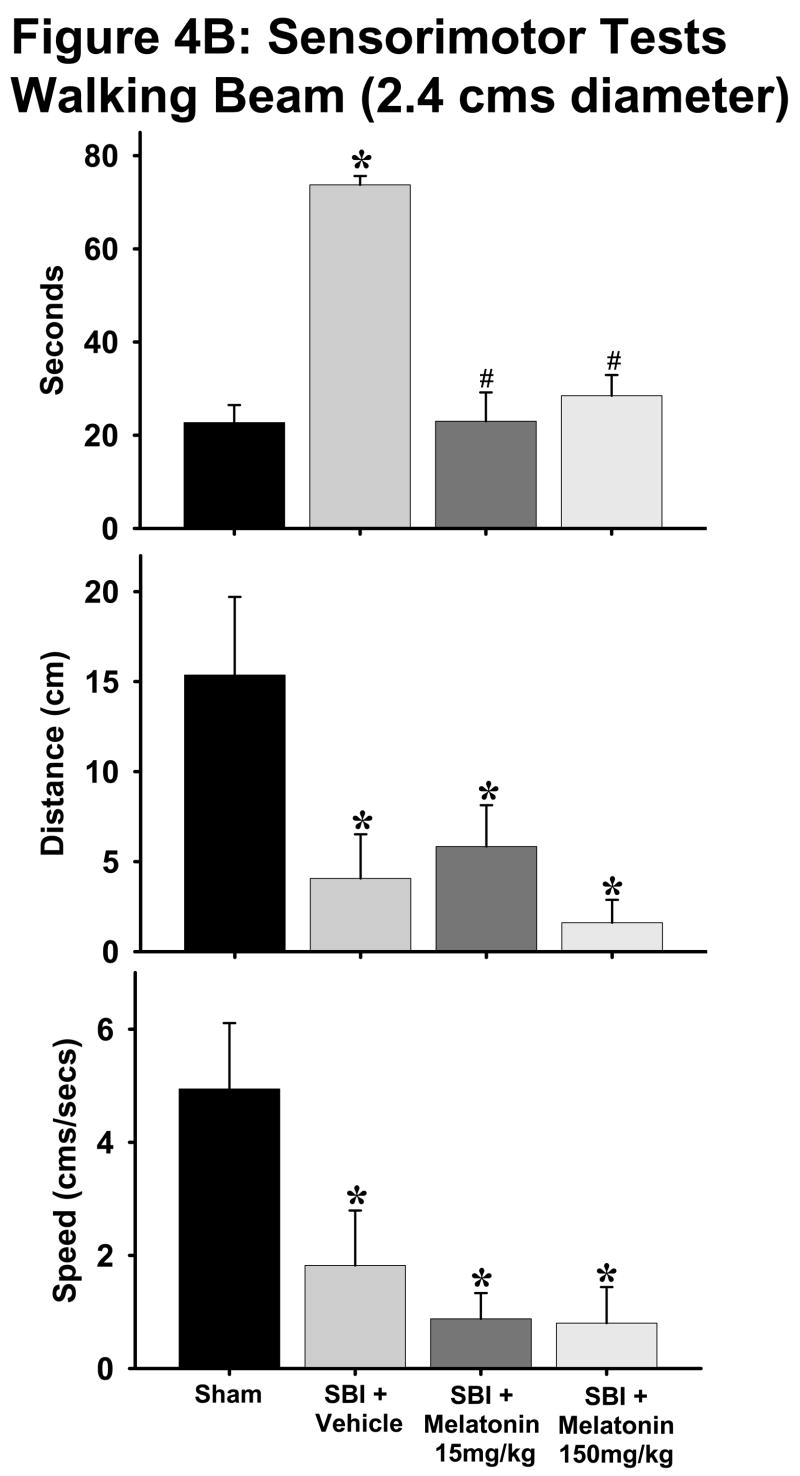
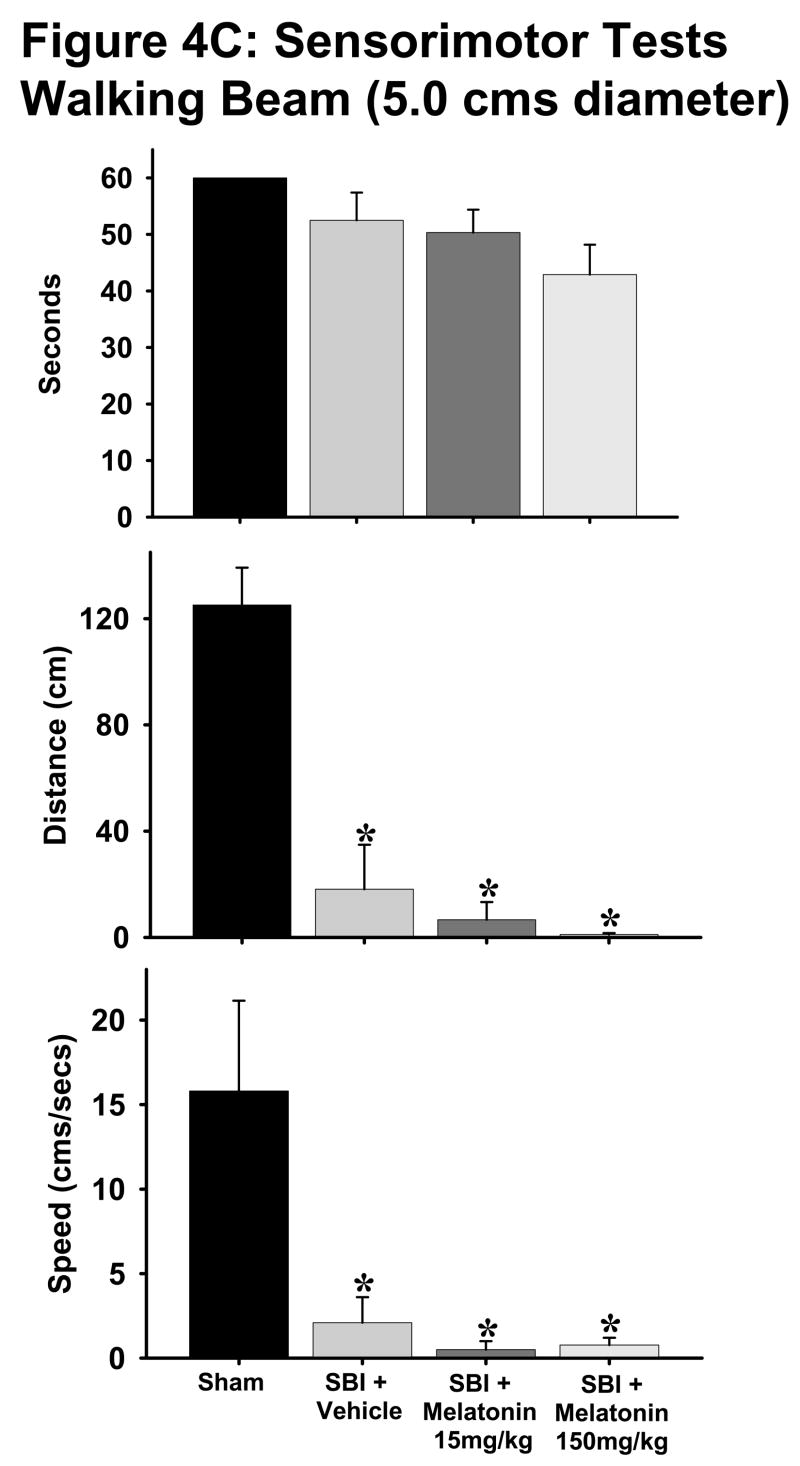
Fig. 4. Sensorimotor tests.
A: Vibrissae stimulation - paw placement test showed worsening scores in all groups subjected to SBI as compared to sham group. The 15mg/kg dose significantly improved the scores compared to vehicle group which were reversed with 150mg/kg dose. (*) and (#) indicates v/s sham and 15mg/kg melatonin respectively for P<0.05. n numbers are as follows: sham = 11; vehicle treated = 15; melatonin 5mg/kg = 8; 15mg/kg = 13; 150mg/kg = 14.
B and C: The beam walking tests on both 2.4cm (Fig. 4B) and 5 cm (Fig. 4C) diameter beams indicated that with the exception of “time spent on beam” parameter for 5cm beam, all vehicle-treated animals showed worsening parameters such as increased time taken, decreased distance traversed and lesser speed on the beams as compared to sham group. Both melatonin groups showed significant improvement in time taken on 2.4cm beam with no significant changes in other parameters. (*) and (#) indicates v/s sham and vehicle respectively. P< 0.05 is considered significant. n numbers are as follows: sham = 11 (2.4 cm beam) and 4 (5cm beam); vehicle treated = 8 (both beams); melatonin 15mg/kg = 6 (both beams); 150mg/kg = 14 (both beams).
The vibrissae stimulation studies showed significantly lower scores in all animals subjected to SBI (treated and untreated) as compared to sham group when tested for following paradigms: left whisker left paw and right whisker left paw. The low dose melatonin (15mg/kg) significantly improved these scores compared to the vehicle-treated group, which were significantly reversed by the high-dose melatonin (150mg/kg) treatment (Fig. 4A, p<0.05).
With the exception of ‘time spent on beam’ parameter for 5cm diameter beam, all rats subjected to SBI showed significant deficits compared to sham animals on both 2.4cm and 5cm diameter beams for all parameters tested (Figs. 4B, C). Both low and high doses of melatonin significantly lowered the time spent on 2.4cm diameter beam to almost sham group levels (Fig. 4B, p<0.05). Melatonin treatment did not have any effect on any other parameters tested on the walking beams.
Discussion
The present study evaluates the effects of melatonin, a powerful antioxidant, for the first time in SBI after neurosurgical operation. Patients undergoing neurosurgical procedures usually have to be monitored closely which translates into longer hospital stays. Medicolegal implications of SBI and practice of defensive medicine further burdens the healthcare system in the amount of billions of dollars each year. Presently, there are no standard therapeutic modalities that are used before, during or after neurosurgical operations to alleviate the post-operative complications such as brain edema. A recent study indicates that melatonin exerts anti-edema effects by downregulation of vascular endothelial growth factor (VEGF) and aquaporin-4 (water transport channel) expression [13]. Previous studies have shown that melatonin is neuroprotective against different types of brain injuries ranging from ischemic to traumatic and hemorrhagic to excitotoxic [6–20]. Furthermore, melatonin is also considered to be beneficial in neurodegenerative disorders such as Parkinson’s disease and Alzheimer’s disease [21–24]. Melatonin also activates antioxidant enzymes such as superoxide dismutase, catalase, and gluthathione peroxidase [37]. Studies using other models have suggested that melatonin has anti-apoptotic, anti-inflammatory, anti-excitotoxicity actions in addition to activating neuroprotective signaling pathways [9,10,38].
In the present study, melatonin (15mg/kg) significantly improved sensorimotor neurological outcomes. This effect of melatonin seems related to its potent antioxidant properties. Low dose of melatonin (15mg/kg) significantly reduced the oxidative stress in the brain region affected by SBI, which is consistent with our previous observations that oxidative stress occurs in the affected brain tissue due to SBI [30]. This neuroprotective effect of melatonin on oxidative stress was however not translated into brain edema reduction. Low dose melatonin (15mg/kg) fails to significantly lower the localized brain edema which occurs in the susceptible brain regions after surgery (predominantly right frontal lobe). The lack of effect of melatonin in attenuating brain edema in our study may be attributed to the type of brain injury itself. The direct surgical injury to brain tissues which leads to dramatic edematous changes and oxidative stress is localized only to the brain tissue surrounding site of resection. Surgical brain injury-induced edema may be more severe than brain edema resulted from cerebral ischemia.
Surprisingly, high dose of melatonin (150mg/kg) showed contrasting effects. High dose melatonin worsened brain edema as compared to all other groups. Melatonin is generally thought to be a very safe drug with practically no toxicity [3,4]. However, there is limited information about effects of high-dose melatonin. One study which used high concentration of melatonin (100mg/kg) comparable to the dose used in the present study reported a lack of antioxidant effects of melatonin at that particular dose [39] in spinal injury. In the present study, too, high-dose melatonin (150mg/kg) did not lower oxidative stress. On the contrary, it significantly worsened the oxidative stress following SBI. This dose of melatonin also worsened neurological outcomes (Fig. 3). In some of sensorimotor tests high dose melatonin actually reversed the benefits of low dose melatonin (Fig. 4A). It could be argued that the sedative effects [40] of high dose melatonin likely affected the neurological outcomes especially at 24 hrs after surgery. Future experiments evaluating later time points will address this issue. However, in light of deleterious effects of high dose melatonin on brain edema as well as oxidative stress, it is likely that the worsened neurological scores are a direct outcome of possible toxic effects of melatonin and/or its metabolites when the indole is given at high doses. There is a need to further study the safety of high dose melatonin which is widely perceived as safe, is readily available and inexpensive.
In conclusion, the present study suggests dual effects of melatonin; low dose melatonin reduces oxidative stress after SBI and may exert neuroprotective effects, whereas high-dose melatonin is deleterious by paradoxically increasing oxidative stress, worsening brain edema and deteriorating neurological outcomes.
Acknowledgments
This study was partially supported by grants from NIH NS45694, HD43120, and NS43338 to JHZ.
References
- 1.Harderland R, Pandi-Perumal S, Cardinali D. Melatonin. The International Journal of Biochem Cell Biol. 2006;28:313–316. doi: 10.1016/j.biocel.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 2.Cheung RT, Tipoe GL, Tam S, Ma ES, Zou LY, Chan PS. Preclinical evaluation of pharmacokinetics and safety of melatonin in propylene glycol for intravenous administration. J Pineal Res. 2006;41:337–343. doi: 10.1111/j.1600-079X.2006.00372.x. [DOI] [PubMed] [Google Scholar]
- 3.Cheung RT. The utility of melatonin in reducing cerebral damage resulting from ischemia and reperfusion. J Pineal Res. 2003;34:153–160. doi: 10.1034/j.1600-079x.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- 4.Reiter RJ, Tan DX, Terron MP, Flores LJ, Czarnocki Melatonin and its metabolites: new findings regarding their production and their radical scavenging actions. Acta Biochim Pol. 2007;54:1–9. [PubMed] [Google Scholar]
- 5.Tan DX, Manchester LC, Terron MP, et al. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res. 2007;42:28–42. doi: 10.1111/j.1600-079X.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 6.Reiter RJ, Tan DX, Leon J, Kilic U, Kilic E. When melatonin gets on your nerves: its beneficial actions in experimental models of stroke. Exp Biol Med (Maywood) 2005;230:104–17. doi: 10.1177/153537020523000205. [DOI] [PubMed] [Google Scholar]
- 7.Chen HY, Chen TY, Lee MY, et al. Melatonin decreases neurovascular oxidative/nitrosative damage and protects against early increases in the blood-brain barrier permeability after transient focal cerebral ischemia in mice. J Pineal Res. 2006;41:175–182. doi: 10.1111/j.1600-079X.2006.00351.x. [DOI] [PubMed] [Google Scholar]
- 8.Chen TY, Lee MY, Chen HY, et al. Melatonin attenuates the postischemic increase in blood-brain barrier permeability and decreases hemorrhagic transformation of tissue-plasminogen activator therapy following ischemic stroke in mice. J Pineal Res. 2006;40:242–250. doi: 10.1111/j.1600-079X.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 9.Kilic U, Kilic E, Reiter RJ, Bassetti CL, Hermann DM. Signal transduction pathways involved in melatonin-induced neuroprotection after focal cerebral ischemia in mice. J Pineal Res. 2005;38:67–71. doi: 10.1111/j.1600-079X.2004.00178.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee MY, Kuan YH, Chen HY, et al. Intravenous administration of melatonin reduces the intracerebral cellular inflammatory response following transient focal cerebral ischemia in rats. J Pineal Res. 2007;42:297–309. doi: 10.1111/j.1600-079X.2007.00420.x. [DOI] [PubMed] [Google Scholar]
- 11.Pei Z, Fung PC, Cheung RT. Melatonin reduces nitric oxide level during ischemia but not blood-brain barrier breakdown during reperfusion in a rat middle cerebral artery occlusion stroke model. J Pineal Res. 2003;34:110–118. doi: 10.1034/j.1600-079x.2003.00014.x. [DOI] [PubMed] [Google Scholar]
- 12.Pei Z, Pang SF, Cheung RT. Pretreatment with melatonin reduces volume of cerebral infarction in a rat middle cerebral artery occlusion stroke model. J Pineal Res. 2002;32:168–172. doi: 10.1034/j.1600-079x.2002.1o847.x. [DOI] [PubMed] [Google Scholar]
- 13.Kaur C, Sivakumar V, Zhang Y, Ling EA. Hypoxia-induced astrocytic reaction and increased vascular permeability in the rat cerebellum. Glia. 2006;54:826–839. doi: 10.1002/glia.20420. [DOI] [PubMed] [Google Scholar]
- 14.Kondoh T, Uneyama H, Nishino H, Torii K. Melatonin reduces cerebral edema formation caused by transient forebrain ischemia in rats. Life Sci. 2002;72:583–590. doi: 10.1016/s0024-3205(02)02256-7. [DOI] [PubMed] [Google Scholar]
- 15.Torii K, Uneyama H, Nishino H, Kondoh T. Melatonin suppresses cerebral edema caused by middle cerebral artery occlusion/reperfusion in rats assessed by magnetic resonance imaging. J Pineal Res. 2004;36:18–24. doi: 10.1046/j.1600-079x.2003.00097.x. [DOI] [PubMed] [Google Scholar]
- 16.Maldonado MD, Murillo-Cabezas F, Terron MP, et al. The potential of melatonin in reducing morbidity-mortality after craniocerebral trauma. J Pineal Res. 2007;42:1–11. doi: 10.1111/j.1600-079X.2006.00376.x. [DOI] [PubMed] [Google Scholar]
- 17.Beni SM, Kohen R, Reiter RJ, Tan DX, Shohami E. Melatonin-induced neuroprotection after closed head injury is associated with increased brain antioxidants and attenuated late-phase activation of NF-kappaB and AP-1. FASEB J. 2004;18:149–151. doi: 10.1096/fj.03-0323fje. [DOI] [PubMed] [Google Scholar]
- 18.Ozdemir D, Uysal N, Gonenc S, et al. Effect of melatonin on brain oxidative damage induced by traumatic brain injury in immature rats. Physiol Res. 2005;54:631–637. [PubMed] [Google Scholar]
- 19.Ayer RE, Sugawara T, Chen W, Tong W, Zhang JH. Melatonin decreases mortality following severe subarachnoid hemorrhage. J Pineal Res. 2008;44:197–204. doi: 10.1111/j.1600-079X.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee SH, Chun W, Kong PJ, et al. Sustained activation of Akt by melatonin contributes to the protection against kainic acid-induced neuronal death in hippocampus. J Pineal Res. 2006;40:79–85. doi: 10.1111/j.1600-079X.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- 21.Mayo JC, Sainz RM, Tan DX, Antolín I, Rodríguez C, Reiter RJ. Melatonin and Parkinson’s disease. Endocrine. 2005;27:169–78. doi: 10.1385/ENDO:27:2:169. [DOI] [PubMed] [Google Scholar]
- 22.Alvira D, Tajes M, Verdaguer E, Acuna-Castroviejo D, et al. Inhibition of the cdk5/p25 fragment formation may explain the antiapoptotic effects of melatonin in an experimental model of Parkinson’s disease. J Pineal Res. 2006;40:251–258. doi: 10.1111/j.1600-079X.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang JZ, Wang ZF. Role of melatonin in Alzheimer-like neurodegeneration. Acta Pharmacol Sin. 2006;27:41–49. doi: 10.1111/j.1745-7254.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- 24.Srinivasan V, Pandi-Perumal SR, Maestroni GJ, Esquifino AI, Hardeland R, Cardinali DP. Role of melatonin in neurodegenerative diseases. Neurotox Res. 2005;7:293–318. doi: 10.1007/BF03033887. [DOI] [PubMed] [Google Scholar]
- 25.Fasano VA, Penna G. [Postoperative complications in neurosurgery] Minerva Anestesiol. 1992;58:15–21. [PubMed] [Google Scholar]
- 26.Manninen PH, Raman SK, Boyle K, el-Beheiry H. Early postoperative complications following neurosurgical procedures. Can J Anaesth. 1999;46:7–14. doi: 10.1007/BF03012507. [DOI] [PubMed] [Google Scholar]
- 27.Tommasino C. Postoperative cerebral edema. Physiopathology of the edema and medical therapy. Minerva Anestesiol. 1992;58:35–42. [PubMed] [Google Scholar]
- 28.Jadhav V, Matchett G, Hsu FP, Zhang JH. Inhibition of Src tyrosine kinase and effect on outcomes in a new in vivo model of surgically induced brain injury. J Neurosurg. 2007;106:680–686. doi: 10.3171/jns.2007.106.4.680. [DOI] [PubMed] [Google Scholar]
- 29.Jadhav V, Solaroglu I, Obenaus A, Zhang JH. Neuroprotection against surgically induced brain injury. Surg Neurol. 2007;67:15–20. doi: 10.1016/j.surneu.2006.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo W, Bravo T, Jadhav V, Titova E, Zhang JH, Tang J. NADPH oxidase inhibition improves neurological outcomes in surgically-induced brain injury. Neurosci Lett. 2007;414:228–232. doi: 10.1016/j.neulet.2006.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamaguchi M, Jadhav V, Obenaus A, Colohan A, Zhang JH. Matrix metalloproteinase inhibition attenuates brain edema in an in vivo model of surgically-induced brain injury. Neurosurgery. 2007;61:1067–1075. doi: 10.1227/01.neu.0000303203.07866.18. [DOI] [PubMed] [Google Scholar]
- 32.Del Zoppo GJ. Stroke and neurovascular protection. N Engl J Med. 2006;354:553–555. doi: 10.1056/NEJMp058312. [DOI] [PubMed] [Google Scholar]
- 33.Gursory-Ozdemir Y, Can A, Dalkara T. Reperfusion-induced oxidative/nitrative injury to neurovascular unit after focal cerebral ischemia. Stroke. 2004;35:1449–1453. doi: 10.1161/01.STR.0000126044.83777.f4. [DOI] [PubMed] [Google Scholar]
- 34.Costa EJ, Lopes RH, Lamy-Freund MT. Permeability of pure lipid bilayers to melatonin. J Pineal Res. 1995;19:123–126. doi: 10.1111/j.1600-079x.1995.tb00180.x. [DOI] [PubMed] [Google Scholar]
- 35.Xi G, Hua Y, Keep RF, Younger JG, Hoff JT. Brain edema after intracerebral hemorrhage: the effects of systemic complement depletion. Acta Neurochir Suppl. 2002;81:253–256. doi: 10.1007/978-3-7091-6738-0_66. [DOI] [PubMed] [Google Scholar]
- 36.Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33:2478–2484. doi: 10.1161/01.str.0000032302.91894.0f. [DOI] [PubMed] [Google Scholar]
- 37.Tomas-Zapico C, Coto-Montes A. A proposed mechanism to explain the stimulatory effect of melatonin on antioxidative enzymes. J Pineal Res. 2005;39:99–104. doi: 10.1111/j.1600-079X.2005.00248.x. [DOI] [PubMed] [Google Scholar]
- 38.Yon JH, Carter LB, Reiter RJ, Jevtovic-Todorovic V. Melatonin reduces the severity of anesthesia-induced apoptotic neurodegeneration in the developing rat brain. Neurobiol Dis. 2006;21:522–530. doi: 10.1016/j.nbd.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Gul S, Celik SE, Kalayci M, Tasyurekli M, Cokar N, Bilge T. Dose-dependent neuroprotective effects of melatonin on experimental spinal cord injury in rats. Surg Neurol. 2005;64:355–361. doi: 10.1016/j.surneu.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 40.Sack RL, Hughes RJ, Edgar DM, Lewy AJ. Sleep-promoting effects of melatonin: at what dose, in whom, under what conditions, and by what mechanisms? Sleep. 1997;20:908–915. doi: 10.1093/sleep/20.10.908. [DOI] [PubMed] [Google Scholar]