Abstract
A novel animal-analogue of the human psychomotor vigilance task (PVT) was validated by subjecting rats to 24 h of sleep deprivation (SD) and examining the effect on performance in the rat-PVT (rPVT), and a rat Multiple Sleep latency Test (rMSLT). During a three-phase (separate cohorts) crossover design, vigilance performance in the rPVT was compared to 24 h SD-induced changes in sleepiness assessed by polysomnographic evaluation and the rMSLT. 24 h of SD was produced by brief rotation of activity wheels at regular intervals in which the animals resided throughout the experiment. In the rPVT experiment, exercise controls (EC) experienced the same overall amount of locomotor activity as during SD, but allowed long periods of undisturbed sleep. After 24 h SD response latencies slowed, and lapses increased significantly during rPVT performance when compared to baseline and EC conditions. During the first 3 h of the recovery period following 24 h SD, polysomnographic measures indicated sleepiness. Latency to fall asleep after 24 h SD was assessed six times during the first 3 h after SD. Rats fell asleep significantly faster immediately after SD, than after non-SD baseline sessions. In conclusion, 24 h of SD in rats increased sleepiness, as indicated by polysomnography and the rMSLT, and impaired vigilance as measured by the rPVT. The rPVT closely resembles the human PVT test widely used in human sleep research and will assist investigation of the neurobiological mechanisms that produce vigilance impairments after sleep disruption.
Keywords: Rat, sleep, sleep deprivation, vigilance, sleep latency, psychomotor vigilance task, mean sleep latency test
INTRODUCTION
Sleep disturbance from disease (e.g., insomnia, sleep apnea) and vocational demands (e.g., shift work, doctors, emergency workers) contributes to decreases in work/school efficiency, and is a major contributor to accident rates. Impaired vigilance/attention due to sleep disruption and subsequent sleepiness is thought to be the main cause of sleep loss related accidents (Philip and Akerstedt, 2006).
Developed to test vigilance (AKA, sustained attention), the psychomotor vigilance task (PVT) is widely used in sleep studies to determine the effect of sleep disruption on vigilance. Reliable PVT performance impairments are seen after various types of sleep disruption in humans including: chronic sleep restriction (Belenky et al., 2003, Van Dongen et al, 2003,), sleep deprivation (Adam et al., 2006, Doran et al., 2001, Urrila et al., 2007), sleep disordered breathing (Kribbs et al., 1993, Dinges and Weaver, 2003, Sforza et al, 2004), and insomnia (Czeisler et al., 2005, Varkevisser and Kerkhof, 2005, Raymann and Van Someren, 2007). A recent comparison of the sensitivity of 26 different tests of attention concluded that response latencies in the PVT are the most sensitive measure of impaired attention resulting from sleep disruption (Balkin et al., 2004). Other advantages of the PVT include being highly portable, easy to learn, and quick to perform (Doran et al., 2001).
Despite the widespread use of the PVT in human studies, to our knowledge, there has been no attempt to directly model the PVT in animals. A valid rat-PVT would allow the direct investigation of neurobiological mechanisms underlying sleep-loss induced vigilance impairment. The rat-PVT (rPVT) described herein was designed to closely resemble the human PVT. As in the human PVT, rats are required to monitor a central stimulus location (light) for a brief and unpredictable signal (light flash) and respond with a simple intrinsic behavior (a nose-poke for rats). As in the human PVT, the primary measures of performance in the rPVT are response latency and the number of lapses (failure to respond within a predetermined time).
The effect of 24 h sleep deprivation (SD) on rPVT vigilance performance was compared to the effect of 24 h SD on sleepiness as measured by conventional polysomnographic criteria (Borbely and Neuhaus, 1979, Trachsel et al., 1986, Lancel and Kerkof, 1989, Franken et al., 1991, Lancel et al., 1992) and a newly developed rat-Multiple Sleep latency Test (rMSLT; McKenna et al., 2007). It was predicted that SD would result in both increases in objective measures of sleepiness (sleep latency) and impaired vigilance, in the same way that SD impairs PVT performance in humans (increased lapses and response latencies).
METHODS
Animals
Adult Fischer-Norway rats (Charles River Breeding Laboratories), weighing between 300 and 350 grams were housed under constant temperature (23±1°) and a 12:12 light/dark cycle (lights on at 7am) with food available ad libitum. All animals were treated in accordance with Association for Assessment and Accreditation of Laboratory Animal Care’s policy on care and use of laboratory animals. All experiments conformed to U.S. Department of Veterans Affairs, Harvard University, and U.S. National Institutes of Health guidelines on the ethical use of animals. All measures were taken to minimize the number of animals used and any suffering.
Sleep Deprivation
Rats remained singly-housed throughout the SD experiments in activity wheels custom made by Lafayette Instruments (Lafayette, IN) that were controlled by Lafayette’s ‘Activity Wheel Monitor’ software. These are large, motorized, stainless-steel activity wheels, with a 35.56 cm diameter, and internal wheel width of 10.9 cm. Rats were habituated to the activity wheel environment, including 1 h of activity wheel motion (5 min on: 5 min off) at 11AM for two days before the experiments began. 24 h of SD (11AM-11AM) was produced by the rotational movement of the activity wheel, programmed on a schedule of 3 s on and 12 s off, at a speed of 3m/min. Actual measurements indicated that the duration of wheel movement ranged from 3 s to 5 s, and the wheel was motionless for 11–14 s. Similar parameters have been previously shown to produce greater than 93% wakefulness (Guzman-Marin et al., 2003, Gong et al., 2004). To control for the non-specific effects of the activity wheel (e.g., locomotor activity) an exercise control was used. In the exercise control (EC) condition for the rPVT phase the wheel revolved at 3m/min for 36 min in every three hour period. This ensured that rats experienced the same amount of locomotor activity in both the SD and EC conditions, as well as providing ample opportunity to enter and maintain deep sleep in the EC condition. Rats always performed the rPVT at 11AM each week day regardless of whether they had just experienced sleep deprivation or no sleep deprivation (a ‘baseline’ session). Sleep deprivation conditions and the EC conditions were always separated by at least three full days to allow the rats to recover. SD and EC conditions were performed in a counter-balanced, repeated-measures design.
rPVT METHODS
Water restriction procedure
In order to motivate the rats to perform the operant rPVT task for water reinforcements, water was unavailable to them for ~ 23 h prior to the daily operant testing sessions during training, baseline performance, and all experimental conditions (i.e. SD and EC exposure). The rPVT rats (N=12), which did not undergo EEG surgery, were introduced to the water restriction schedule over several days by reducing the amount of time each day that water was available in the home cage; within 5 days rats learned to consume their daily amount of water in fewer than 10 min. Rats were weighed weekly to ensure that their body weight did not drop below 90% of their baseline “free water” body weight. After entering the rPVT training protocol, rats received water as a reward for task performance (20 µl/reinforcement). Additional home cage access to free water was available for 5 or 10 min immediately after each rPVT test session. Parametric studies performed in our lab determined that rats receiving > 100 water reinforcements in the rPVT task were satiated by 5 to 7 min of access to free water (satiation was defined as > 2 min without water consumption), whereas 10 min of water access was sufficient to achieve satiation on days without rPVT sessions, or for rats that received fewer than 100 reinforcements in the rPVT. Rats on this water restriction procedure remain active and healthy; for example the rats maintained or gained body weight.
rPVT Apparatus
The rPVT was performed in MED-Associates (Burlington, VT) operant chambers, with infra-red head-detection beams across the front of the water delivery port to record nose-pokes. The target stimulus was a centrally mounted light directly above the water delivery port. A nose-poke into the water delivery port during stimulus illumination (0.5 s), or during the 2.5 s limited-hold period after the stimulus was extinguished, was rewarded with 20 µl of water delivered by automatic dipper. Video cameras allowed observation of the rats during task performance.
rPVT Training
rPVT training (always run at 11AM) initially consisted of two acclimatization sessions (rats in chambers, but no light or dipper activity), followed by two sessions in which water was presented at regular (30 s) intervals and whenever the rat nose-poked the response port in the 30 s between automatic deliveries. By the end of the second such session rats were reliably and continually nose-poking to gain water reinforcement. Thereafter rats were trained to associate nose-poking during stimulus presentation with water reinforcement. Stimulus presentation was initially 30 s, and this was gradually reduced (from 15, to 10, to 5, to 2.5, to 1 and finally to 0.5 s) as each rat individually reached the advancement criterion (>100 reinforced responses and <20 omissions for 3 consecutive sessions) at each stimulus duration. Time-outs for either failing to respond (omission) or a premature response (during the inter-trial interval; ITI) were introduced from the 10 s stimulus duration onward. It took ~5 daily operant (not acclimatization or free-water) sessions for the rats to learn to nose-poke during stimulus presentation in return for water reinforcement. The remaining training time (3 to 5 weeks) was spent adjusting to each new/shorter stimulus duration, and, hence, the total training time to reach criterion levels of performance at the stimulus duration 0.5 s was 4 to 6 weeks. This 4 to 6 week period of training compares favorably to training in other rodent tests of attention. For example, in our hands (Cordova et al, 2006), the 5-Choice Serial Reaction Time task takes between 3 to 5 months of training to reach baseline criterion. Finally, training success rates are very high, in general rPVT training success rates are ~95%. In this particular study all 12 rats originally selected for training successfully reached criterion levels of performance at the target stimulus duration and went on to perform the experiment. Note that, in contrast to rats, humans in PVT experiments learn the PVT essentially immediately because they can act on instructions received from the experimenter, whereas rats require training.
The Rat Psychomotor Vigilance Task
During post-training baseline task performance the ITI varied from 3 to 7 s in 1 s increments in a quasi-random fashion (equal density of intervals throughout session). Responding during the ITI (a ‘premature response’) or failing to respond at all within 3 s (an omission) were treated as errors and resulted in a 10 s ‘time out’ (house light extinguished and absence of trials). At the end of the 10 s time-out the house light was re-illuminated and a new ITI started. The measures of performance in the rPVT were: response latencies, numbers of lapses, omissions, (rewarded/correct) responses, and premature errors. Response latencies were calculated from the onset of the light until the rat nose-poked the water delivery port, excluding lapses and omissions (defined next). Parametric analysis of pilot data revealed that the variability of rat response latencies in the rPVT was much greater than that typical of human responses in the PVT. Hence, lapses were individually defined post hoc as those trials in which response latencies were greater than two-times the average basal response-latency for each rat (including omissions). This definition of lapses did not skew the distribution of the remaining response latencies and provided a sensitive measure of performance. Omissions were defined as a failure to respond within the 3-second window of opportunity, and were used as an indication that rats did simply stop responding. Sessions in which rats made greater than 50 omissions were not included in the final analysis, although large blocks of omissions of this type were very rare. As typical of human PVT studies, omissions were not analyzed independently of lapses. Premature errors were any response during the inter-trial interval. Premature response errors and the overall number of (rewarded/correct) responses were recorded and analyzed as secondary measures of performance. The number of responses per session functioned as both a secondary measure of performance and a, rudimentary, measure of motivation.
rMSLT METHODS
EEG Surgery
A separate group of rats (N=8) were anesthetized (isoflurane anesthesia), and EEG/EMG surgery was performed. For EEG recording, two screw electrodes (Plastics One Inc., Roanoke, VA, USA) were fixed into the skull (dorsal of the temporal cortex). EMG electrodes (Plastics One Inc.) were placed in the nuchal muscles.
EEG Recording
Rats were attached to EEG/EMG cables one week following surgery, and placed in the activity wheels. A Grass model amplifier system polygraph (15LT Bipolar, Grass Technologies) with four amplifiers (15A54 Quad AC Amplifiers, Grass Technologies) was used for EEG/EMG data collection (GAMMA/SYS Acquisition software, Grass Technologies). Sampling rate was set at 128 Hz. EEG filter settings were set at 0.1 and 100 Hz. Recordings were visually scored, by means of EEG/EMG analysis, in 10 s epochs using the Grass Rodent Sleep Stager program (V4.2, Grass Technologies), and classified into 3 different states: wakefulness, non-REM sleep (NREM), and REM sleep. Raw data was meticulously analyzed, rejecting data where artifact was evident. Average delta power (1–4Hz) in NREM sleep was determined by means of spectral analysis of the EEG recording (Fast Fourier Transform). All measures were compared to the baseline day amounts in the same rats. NREM sleep delta power was represented as average power per hour. Average NREM sleep delta power amounts were normalized to the average of the baseline values for the same rat.
Recovery Period after 24 h SD
Baseline EEG/EMG was recorded for 27 h (11AM-2PM), to provide a 24 h control period and a 3 h recovery period control (11AM-2PM). Five days later, 24 h of SD (11AM-11AM) was produced by the rotational movement of the activity wheel, as described above. EEG/EMG data was then acquired for the first 3 h of the recovery period following 24 h of SD (11AM-2PM). The order of baseline and SD treatment was counterbalanced.
rMSLT
The rMSLT was performed in a third group of rats (N=8). Prior to baseline recordings, rMSLT rats were habituated to 1 h of activity wheel motion for two days (5 min on: 5 min off at 11AM). As well, rats were exposed for 10 min on each of these two days to a gentle sensory stimulation protocol (auditory stimulation without explicit handling) for habituation. The experimental protocols described for EEG recordings above were then conducted, except instead of the rats remained undisturbed during the recovery period following SD, the rMSLT was performed in the same 3 h baseline and recovery periods. The rMSLT included six sleep latency trials. For each trial, the rat was kept awake for 5 min by means of gentle sensory stimulation, and then left undisturbed for 25 min while EEG/EMG data was collected. The trial was then repeated five more times on 30 min intervals, resulting in 3 h of testing. A 10–15 s delay occurred between the end of the gentle sensory stimulation and the beginning of polysomnographic recording (the time it took for the experimenter to exit the testing room and turn on the recording equipment). In order to determine the optimal definition (duration) of sleep onset, separate analyses were performed in which the first incident of sleep lasted either, 10, 20, 30, or 60 sec. For each of the these values sleep onset latency values were defined as the amount of time between the end of gentle sensory stimulation and the first instance of NREM sleep lasting (at least) as long as the duration (in consecutive 10 s epochs), similar to the sleep definition criteria previously reported (McKenna et al, 2007). Again, the order of baseline and SD treatment was counterbalanced.
Statistical Analysis
In the rPVT analysis behavioral measures were averaged within a session to generate four measures from each session (response-latency, number of responses, number of premature errors, and number of lapses) for each rat. In the rMSLT analysis each rat was compared to his own baseline for all polysomnographic measurements. In order to account for inter-individual differences behavioral measures from both the rPVT and rMSLT were analyzed via a mixed-model ANOVA (SPSS, SPSS Inc., Chicago, IL). Any significant analyses in either analysis (rPVT or rMSLT) were further analyzed with post-hoc dependent-means t-tests. All significant p-values, of both the main analyses and the post-hoc analyses, were subjected to modified (ranked) Bonferoni adjustment.
RESULTS
Observation of these rats during the 30 min rPVT baseline and post-SD sessions revealed that the rats did not sleep during task performance, and consistently made a large number of responses (<100) before making any short pauses in their task performance. Two rats were removed from the rPVT analyses due to consistently making greater than 50 omissions after exposure to both SD and EC conditions (leaving N=10).
rPVT
Response Latencies
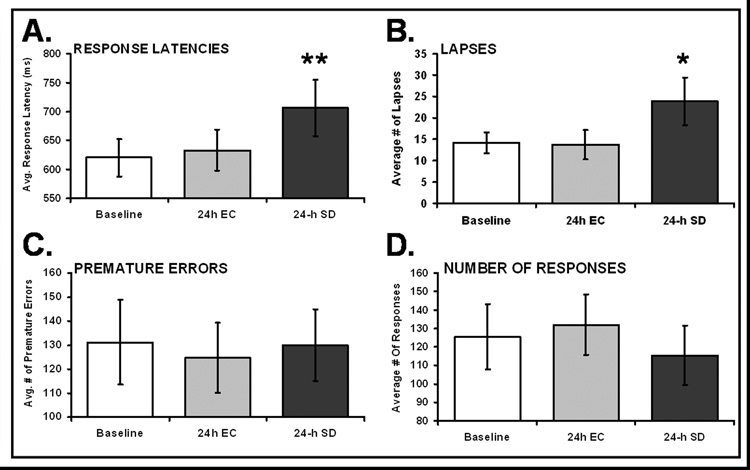
The mixed-model ANOVA reported a significant effect of experimental condition, (F2,9=7.56, p< 0.005); see Figure 1A. Post-Hoc analysis revealed that rats made significantly slower responses after 24 h SD (706.5ms) when compared to 24 h EC (633.4ms) and baseline (620.5ms). (SD & EC: t9=2.55, p<0.05, SD & Baseline: t9=3.47, p<0.005).
Figure 1. Vigilance performance in the rPVT is impaired by 24 h of sleep deprivation (SD).
Panel A. Response latencies are significantly slowed after 24 h of total sleep deprivation (SD), when compared to either baseline (no wheel movement), or 24 h of exercise control (EC), ** = p<0.005. Panel B. The mean number of lapses, including omissions, increased significantly after 24-h SD, when compared to either 24-h EC or 24-h undisturbed baseline. * = p<0.05. Panel C. The number of premature errors (responses during the inter-trial interval) does not change as a function of experimental condition. Panel D. The number of rewarded nose-pokes is somewhat lower after 24 h SD, but this effect was not significant (p=0.08). The data plotted are means ±SEM.
Lapses
Mixed-model ANOVA analysis revealed a significant effect of experimental condition, (F2,9=3.72, p<0.05); see Figure 1B. Post-Hoc analysis revealed that rats made significantly more lapses after 24 h SD (23.9), than after either 24 h EC (15.2) or baseline activity (14.3). (SD & EC: t9=3.45, p<0.01, SD & Baseline: t9=2.01, p<0.05).
Premature Errors
There was no effect of experimental condition for this measure (p=0.66), see Figure 1C.
Number of Responses
The decline in the total number of responses seen after 24 h SD was not statistically significant (p=0.083, see Figure 1D), indicating motivation was not affected by SD or EC sessions. Rats made, on average, 132 responses in an rPVT session after 24 h EC, but made, on average, 115.4 responses after 24 h SD (and 125.4 responses, on average, after 24 h baseline activity).
Polysomnography
Rats were awake for 91.3±2.0% of the 24 h SD period. Conventional polysomnographic measures of sleepiness were evaluated in the 3 h recovery period (11AM-2PM) following 24 h of SD (11AM-11AM). As depicted in Figure 2A, average NREM sleep episode duration was significantly increased, compared to the matched time of day baseline values (from 1 min, 51 s baseline to 4 min, 23 s post-SD; F1,35=78.3, p<0.001). Figure 2B demonstrates a significant increase of normalized NREM sleep delta power following SD (71% elevation post-SD compared to baseline values; F1,35=274.78, p<0.001). The number of awakenings was significantly decreased following SD (Figure 2C; from 10.7 baseline to 6.9 post-SD; F1,35=23.17, p<0.001). A significant increase of the amount of NREM sleep time was also evident, expressed as a percentage of total time per hour (Figure 2D; from 43.5% baseline to 55.5% post-SD; F1,42=13.75, p=0.001). REM sleep percentages (Figure 2E; from 11.8% baseline to 29.0% post-SD; F1,35=103.91, p<0.001) and total sleep time percentages (Figure 2F; from 55.3% baseline to 84.5% post-SD; F1,35=53.88, p<0.001) were also significantly increased. Total REM and NREM sleep amounts for this three hour period (~55%) were slightly decreased compared to our previous study in the Fischer-Norway rats (~60%; McCoy et all, 2007), possibly due to anticipatory activity at this time of day because, for several days prior to the experiment, the rats received habituation training (activity wheel, gentle sensory stimulation) at this same time of day.
Figure 2. EEG measures indicated sleepiness during undisturbed recovery (Post-SD, 11AM-2PM) following sleep deprivation (SD, 11AM-11AM)).
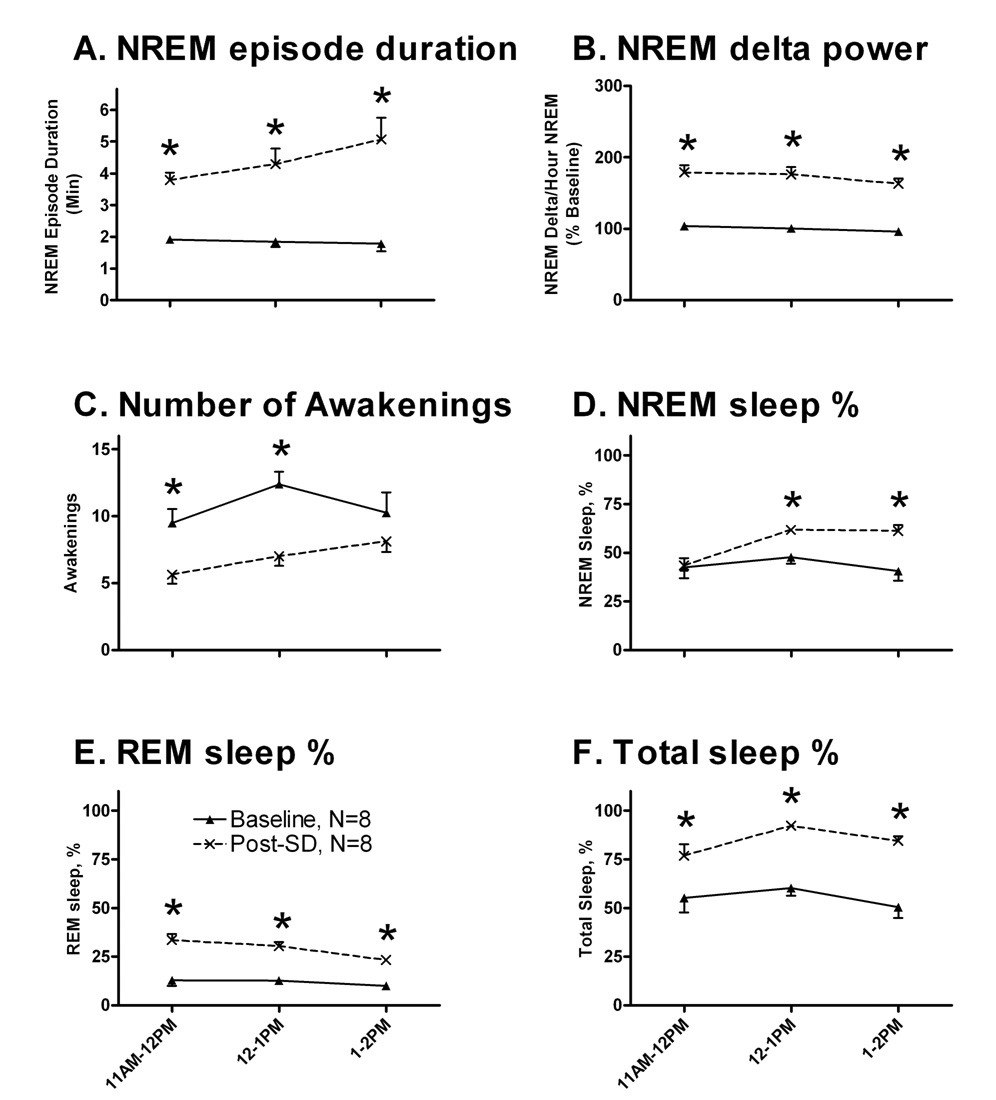
NREM sleep episode duration (Panel A), and normalized NREM delta values (Panel B) were elevated in the recovery period. A significant decrease in the number of awakenings per hour was noted (Panel C). Panels D, E, and F demonstrate significant increases of the hourly amounts of NREM, REM, and total sleep time, expressed as percentages of total time per hour. Baseline data are represented by closed triangles, and post-SD values by crosses. The data plotted are means ±SEM. Each animal was used as its own control. Asterisks indicate significant pairwise post-hoc comparisons between baseline and post-SD values, Bonferroni corrected.
rMSLT
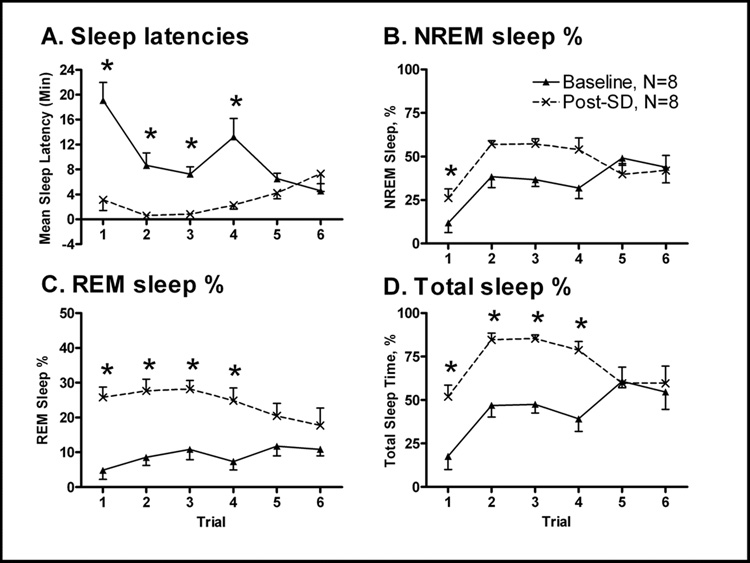
Baseline sleep latency values were first acquired, and then compared to the rMSLT, performed during the 3h (11AM-2PM) following 24h SD (11AM-11AM). No significant differences were noted between each of the four sleep onset durations (10, 20, 30 or 60 sec.), when comparing values following baseline 11AM-11AM (F3,161=0.03, P=0.993), or post-SD 11AM-11AM (F3,161=0.00, P=1.000). Therefore, varying the definition of sleep onset did not significantly influence sleep latency measures. Consequentially, subsequent analysis was performed adopting the definition of 30 sec as the criteria of sleep onset, as this definition was used by previous groups (Veasey et al., 2004a, b). Rats that experienced 24 h of SD had a significantly decreased average sleep latency when compared to the average baseline sleep latency values (Figure 3A; 9 min, 51 s baseline vs. 3 min, 2 s post-SD; F1,77=45.56, p<0.001). The interaction analysis was also significant (F5,77=6.99, p<0.001) and post-hoc analyses revealed a significant difference in sleep latencies between baseline and post-SD conditions during trials #1–4 (Trial #1: t7=3.81, p=0.012, #2: t7=3.94, p=0.03, #3: t7=4.92, p=0.028, #4: t7=3.37, p=0.036). Significant increases of NREM (Figure 3B; from 35.3% baseline to 45.9% post-SD; F1,84=9.71, P=0.003), REM (Figure 3C; from 9.0% baseline to 24.0% post-SD; F1,77=71.49, P<0.001), and total sleep (Figure 3D; from 44.5% baseline to 70.0% post-SD; F1,77=41.93, P<0.001) amounts were demonstrated, where values were represented as percentage of total time per hour.
Figure 3. The rMSLT (Post-SD, 11AM-2PM) revealed sleep deprivation (SD) effects on sleepiness.
Sleep latencies were significantly decreased following 24h of SD (Panel A; 11AM-11AM). Sleep rebound was evident during the period of the rMSLT trials when the animal was left undisturbed. NREM sleep % (Panel B), REM sleep % (Panel C), and total sleep % amounts per hour (Panel 3D) were all elevated following SD, represented as percentages of total time per hour. Baseline data are represented by closed triangles, and post-SD values by crosses. The data plotted are means ±SEM. Each animal was used as its own control. Asterisks indicate significant pairwise post-hoc comparisons between baseline and post-SD values, Bonferroni corrected.
DISCUSSION
The rat-psychomotor vigilance task (rPVT) was developed to closely resemble the human psychomotor vigilance task (PVT) widely used in human sleep studies. Rats that experienced 24 h SD just prior to performing the rPVT demonstrated a behavioral impairment analogous to that of sleep deprived humans; response latencies slowed, and lapses increased. Moreover, rats were sleepy during the first 3 h after 24 h SD as demonstrated by both the latencies to sleep onset, and by polysomnographic measures of sleepiness. Thus, we conclude that the sleepiness produced by 24 h of SD resulted in vigilance impairments analogous with that seen in sleep deprived humans performing the human PVT. Importantly however, the rats did not fall asleep during the rPVT sessions indicating that 24 h SD produced sleepiness and vigilance impairments, but did not produce profound sleep that could grossly interfere with operant task performance.
These rPVT results are consistent with a previous study examining the effect of 4, 7 & 10 h total sleep deprivation on 5-choice serial reaction time (5CSRT) performance in rats (Cordova et al., 2006). In both tasks response latencies, and lapses (‘omissions’ in the 5CSRT), increased significantly, and the number of premature errors did not change as a result of increased sleepiness. Advantages of the rPVT compared to the 5CSRT include the comparability of rPVT findings to human PVT findings and the fact that the rPVT is easier for rats to learn and thus can train to criterion much more quickly.
The impairment in rPVT performance after 24 h SD in rats as reported in this study resembles the deterioration in PVT performance seen in clinical or experimental populations of sleepy humans. For example, several studies have shown that subjects with sleep apnea show a profound behavioral impairment on the PVT that is related to the frequency of arousals (Sforza et al., 2004, Kim et al., 2007). Indeed, in unpublished observations we found significant rPVT impairments after 24 h of experimental sleep fragmentation in a rat model of sleep apnea. In experimental settings, humans start to show impairments in the PVT after ~20 h of experimental SD. Specifically, during up to 80 h of total sleep deprivation human PVT performance begins to deteriorate (reaction times slow, and numbers of errors increase) after approximately 20 h of deprivation, and thereafter worsens rapidly (Adam et al., 2006, Doran et al., 2001, Urrila et al., 2007). Similarly, human subjects that experience varying degrees of chronic sleep restriction (i.e., 3, 5 or 7 h of time in bed for up to 14 days) show duration dependent PVT deficits (Belenky et al., 2003, Van Dongen et al., 2003). Hence, it will be of interest to see in the future if rats show a similar duration-dependent effect in rPVT performance during several days of chronic sleep restriction.
The rPVT and the human PVT provide comparable measures of performance. Herein, response latencies and lapses were the most sensitive measures for the detection of sleep loss induced impairments in vigilance, similar to the human literature (Adam et al., 2006, Doran et al., 2001, Urrila et al., 2007, Balkin et al., 2004). The criterion for lapses in the present study was individually determined for each rat as two-times their pre-experimental baseline response-latency performance. Although this differs from the human PVT, setting the lapse criterion at two-times the baseline was necessary because of the high variability of response latencies between rats in the rPVT, which was largely due to different starting positions when each rat initiated a nose poke. In the rPVT lapses increased significantly after 24 h-SD, in the same way that lapses increased in sleep human subjects after SD (Adam et al., 2006, Doran et al., 2001, Durmer and Dinges 2005, Urrila et al., 2007), and after other forms of sleep disruption (Dinges and Weaver 2003, Czeisler et al., 2005, Van Dongen et al., 2003). Furthermore, although there was a small numerical difference in lapses between the control and experimental differences in the rPVT, the difference was nonetheless meaningful. It is also worth noting that the numerical difference between lapses made in control and experimental conditions in human PVT studies is equally small (for example see Van Dongen et al., 2003, Czeisler et al 2005).
Unlike humans who are intrinsically motivated to perform the PVT, rats are motivated to perform the rPVT by restricting their access to water. Short periods of total sleep deprivation have been shown to increase feeding, and presumably hunger, in rats (Everson et al., 1989), and a pattern of short sleep duration is associated with obesity in man (Hasler et al., 2004). In contrast, 2 to 5 days of selective REM deprivation reduced operant responding for food reward, although the findings suggested a decline in reward and/or attention mechanism rather than a decrease in hunger (Hanlon et al., 2005). This notwithstanding, to our knowledge, no publications exist on the interaction between SD and thirst in rats. However, recent work in our lab suggest that SD-induced changes in thirst motivation are not a potential confound. Specifically, we found that 24h of SD in the activity wheels did not alter water consumption in water restricted rats (N=8; baseline= 13.0 ± 1.2ml/day, 24h SD = 12.8 ± 1.5 ml/day; unpublished observations). Similarly, endocrinological stress responses due to the activity wheels are unlikely to underlie the changes seen in rPVT performance after 24h SD as our measures of plasma corticosterone (CORT) indicate that SD in the wheel does NOT elevate CORT (both controls and SD rats have < 7 ng/ml CORT levels after 24 h SD; unpublished observations). Thus, by itself, 24 h SD in an activity wheel does not produce an endocrinological stress response, and therefore stress is unlikely to be responsible for the deterioration in vigilance performance observed.
An increase in sleepiness following 24 h SD was evident with both conventional polysomnographic measures (post-SD EEG/EMG analysis), and the rMSLT. Thus, these findings support the hypothesis that the decrements in vigilance performance observed were the result of increased sleepiness and not other non-specific factors. Measurement of such polysomnographic measures as increased average NREM sleep episode duration, average NREM sleep delta power, and NREM sleep amounts, as well as decreased number of awakenings, have been proposed to reflect an elevation of the homeostatic sleep drive, indicating sleepiness (Borbely and Neuhaus 1979, Franken et al., 1991, Trachsel et al., 1986, Lancel and Kerkof 1989, Lancel et al., 1992, McKenna et al., 2007). Previously, the rMLST has been used to directly measure increased sleepiness following 6 and 24 h of sleep fragmentation (McKenna et al., 2007), just as human clinical studies have measured sleepiness by means of the Multiple Sleep Latency Test (Carskadon and Dement 1979, Reynolds et al., 1982, Carskadon et al., 1986, Roehrs and Roth 1992). In human studies, if the average latency to sleep across the day during nap periods is significantly shorter than that observed in the general clinical population, the patient is determined to be sleepy. Although MSLT procedures for rodents have been published (Shiromani et al., 1991, Blanco-Centurion et al., 2006) our rMSLT procedure is based on, and complements, that developed by Veasey et al. (2004a, b) in the mouse. The rMSLT protocol used here minimized the sleep loss associated with the testing procedure (rats are kept awake for only 5 min of each 30 min trial) and required only 3 h of the investigator’s time to collect the rMSLT data.
The rPVT is preferred over other tests of sustained attention used in rats because it is an animal-analogue of the human PVT task and will thus better enable direct comparisons between species. The rPVT will readily lend itself to investigations of other types of sleep disruption in rats (e.g., sleep fragmentation, chronic sleep restriction), and importantly, will allow investigation of the neurobiological mechanisms underlying sleepiness and the vigilance impairments associated with sleep disruption.
ACKNOWLEDGEMENTS
We thank Lauren Kane, Stephanie Cummings, Jeremy Beech, and Courtney Foster for technical assistance. This research was supported by the Department of Veterans Affairs Medical Research Service Award to RES, NHLBI - P50 HL060292 (RES & RWM), NIMH - R37 MH039683 (RWM), NHLBI - T32 HL07901 (MAC & JTM), NIMH - F32 MH070156 (JTM).
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- Abrantes-Pais F, Friedman JK, Lovallo WR, Ross ED. Psychological or physiological: Why are tetraplegic patients content? Neurology. 2007;69:261–267. doi: 10.1212/01.wnl.0000262763.66023.be. [DOI] [PubMed] [Google Scholar]
- Adam M, Retey JV, Khatami R, Landolt HP. Age-related changes in the time course of vigilant attention during 40 hours without sleep in man. Sleep. 2006;29:55–57. doi: 10.1093/sleep/29.1.55. [DOI] [PubMed] [Google Scholar]
- Balkin TJ, Bliese PD, Belenky G, et al. Comparative utility of instruments for monitoring sleepiness-related performance decrements in the operational environment. J. Sleep Res. 2004;13:219–227. doi: 10.1111/j.1365-2869.2004.00407.x. [DOI] [PubMed] [Google Scholar]
- Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP, Russo MB, Balkin TJ. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- Blanco-Centurion C, Xu M, Murillo-Rodriguez E, Gerashchenko D, Shiromani AM, Salin-Pascual RJ, Hof PR, Shiromani PJ. Adenosine and sleep homeostasis in the basal forebrain. J. Neurosci. 2006;26:8092–8100. doi: 10.1523/JNEUROSCI.2181-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbely AA, Neuhaus HU. Sleep-deprivation: effects on sleep and EEG in the rat. J Comp Physiol. 1979;133:71–87. [Google Scholar]
- Carskadon MA, Dement WC. Effects of total sleep loss on sleep tendency. Percept Mot Skills. 1979;48:495–506. doi: 10.2466/pms.1979.48.2.495. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Dement WC, Mitler MM, Roth T, Westbrook PR, Keenan S. Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness. Sleep. 1986;9:519–524. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- Cordova CA, Said BO, McCarley RW, Baxter MG, Chiba AA, Strecker RE. Sleep deprivation in rats produces attentional impairments on a 5-choice serial reaction time task. Sleep. 2006;29:69–76. [PMC free article] [PubMed] [Google Scholar]
- Czeisler CA, Walsh JK, Roth T, et al. U.S. Modafinil in Shift Work Sleep Disorder Study Group. Modafinil for excessive sleepiness associated with shift-work sleep disorder. N Engl J Med. 2005;353:476–486. doi: 10.1056/NEJMoa041292. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Weaver TE. Effects of modafinil on sustained attention performance and quality of life in OSA patients with residual sleepiness while being treated with nCPAP. Sleep Med. 2003;4:393–402. doi: 10.1016/s1389-9457(03)00108-4. [DOI] [PubMed] [Google Scholar]
- Doran SM, Van Dongen HP, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Arch Ital Biol. 2001;139:253–267. [PubMed] [Google Scholar]
- Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Sem Neurol. 2005;25:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Everson CA, Bergmann BM, Recjtschaffen A. Sleep deprivation in the rat III: Total sleep deprivation. Sleep. 1989;12:13–21. doi: 10.1093/sleep/12.1.13. [DOI] [PubMed] [Google Scholar]
- Franken P, Tobler I, Borbély AA. Sleep homeostasis in the rat: simulation of the time course of EEG slow-wave activity. Neurosci Lett. 1991;130:141–144. doi: 10.1016/0304-3940(91)90382-4. Erratum in: Neurosci Lett 1991 Nov 11;132:279. [DOI] [PubMed] [Google Scholar]
- Goghari VM, Rehm K, Carter CS, MacDonald AW. Regionally specific cortical thinning gray matter abnormalities in the healthy relatives of schizophrenia patients. Cerebral Cortex. 2007;17:415–424. doi: 10.1093/cercor/bhj158. [DOI] [PubMed] [Google Scholar]
- Gong H, McGinty D, Guzman-Marin R, Chew KT, Stewart D, Szymusiak R. Activation of c-fos in GABAergic neurons in the preoptic area during sleep and in response to sleep deprivation. J Physiol. 2004;556:935–946. doi: 10.1113/jphysiol.2003.056622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán-Marín R, Suntsova N, Stewart DR, Gong H, Szymusiak R, McGinty D. Sleep deprivation reduces proliferation of cells in the dentate gyrus of the hippocampus in rats. J Physiol. 2003;549:563–571. doi: 10.1113/jphysiol.2003.041665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon EC, Andrzejewski ME, Harder BK, Kelley AE, Benca RM. The effect of REM sleep deprivation on motivation for food reward. Beh Brain Res. 2005;163:58–69. doi: 10.1016/j.bbr.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Hasler G, Buysse DJ, Klaghofer R, Gamma A, Ajdacic V, Eich D, et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27:661–666. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- Kim H, Dinges DF, Young T. Sleep-disordered breathing and psychomotor vigilance in a community-based sample. Sleep. 2007;30:1309–1316. doi: 10.1093/sleep/30.10.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kribbs NB, Pack AI, Kline LR, et al. Effects of one night without nasal CPAP treatment on sleep and sleepiness in patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:1162–1168. doi: 10.1164/ajrccm/147.5.1162. [DOI] [PubMed] [Google Scholar]
- Lancel M, Kerkhof GA. Effects of repeated sleep deprivation in the dark- or light-period on sleep in rats. Physiol Behav. 1989;45:289–297. doi: 10.1016/0031-9384(89)90130-3. [DOI] [PubMed] [Google Scholar]
- Lancel M, van Riezen H, Glatt A. Enhanced slow-wave activity within NREM sleep in the cortical and subcortical EEG of the cat after sleep deprivation. Sleep. 1992;15:102–118. doi: 10.1093/sleep/15.2.102. [DOI] [PubMed] [Google Scholar]
- McCoy JG, Tartar JL, Bebis AC, Ward CP, McKenna JT, Baxter MG, McGaughy J, McCarley RW, Strecker RE. Experimental sleep fragmentation impairs attentional set-shifting in rats. Sleep. 2007;30:52–60. doi: 10.1093/sleep/30.1.52. [DOI] [PubMed] [Google Scholar]
- McKenna JT, Tartar JL, Ward CP, Thakkar MM, Cordeira JW, McCarley RW, Strecker RE. Sleep fragmentation elevates behavioral, electrographic and neurochemical measures of sleepiness. Neuroscience. 2007;146:1462–1473. doi: 10.1016/j.neuroscience.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip P, Akerstedt T. Transport and industrial safety, how are they affected by sleepiness and sleep restriction? Sleep Med Rev. 2006;10:347–356. doi: 10.1016/j.smrv.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Raymann RJ, Van Someren EJ. Time-on-task impairment of psychomotor vigilance is affected by mild skin warming and changes with aging and insomnia. Sleep. 2007;1(30):96–103. doi: 10.1093/sleep/30.1.96. [DOI] [PubMed] [Google Scholar]
- Reynolds CF, 3rd, Coble PA, Kupfer DJ, Holzer BC. Application of the multiple sleep latency test in disorders of excessive sleepiness. Electroencephalogr Clin Neurophysiol. 1982;53:443–452. doi: 10.1016/0013-4694(82)90009-8. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Roth T. Multiple Sleep Latency Test: technical aspects and normal values. J Clin Neurophysiol. 1992;9:63–67. [PubMed] [Google Scholar]
- Shiromani PJ, Velazquez-Moctezuma J, Overstreet D, Shalauta M, Lucero S, Floyd C. Effects of sleep deprivation on sleepiness and increased REM sleep in rats selectively bred for cholinergic hyperactivity. Sleep. 1991;14:116–120. [PubMed] [Google Scholar]
- Sforza E, Haba-Rubio J, de BF, Rochat T, Ibanez V. Performance vigilance task and sleepiness in patients with sleep-disordered breathing. Eur Respir J. 2004;24:279–285. doi: 10.1183/09031936.04.00091903. [DOI] [PubMed] [Google Scholar]
- Trachsel L, Tobler I, Borbély AA. Sleep regulation in rats: effects of sleep deprivation, light, and circadian phase. Am J Physiol. 1986;251:R1037–R1044. doi: 10.1152/ajpregu.1986.251.6.R1037. [DOI] [PubMed] [Google Scholar]
- Urrila AS, Stenuit P, Huhdankoski O, Kerkhofs M, Porkka-Heiskanen T. Psychomotor vigilance task performance during total sleep deprivation in young and postmenopausal women. Behav Brain Res. 2007;180:42–47. doi: 10.1016/j.bbr.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- Varkevisser M, Kerkhof GA. Chronic insomnia and performance in a 24-h constant routine study. J Sleep Res. 2005;14:49–59. doi: 10.1111/j.1365-2869.2004.00414.x. [DOI] [PubMed] [Google Scholar]
- Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, Gow A. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep. 2004a;27:194–201. doi: 10.1093/sleep/27.2.194. [DOI] [PubMed] [Google Scholar]
- Veasey SC, Yeou-Jey H, Thayer P, Fenik P. Murine multiple sleep latency test: phenotyping sleep propensity in mice. Sleep. 2004b;27:388–393. doi: 10.1093/sleep/27.3.388. [DOI] [PubMed] [Google Scholar]