Summary
Background
Mesothelial cells that line the thoracic cavity play an important role in maintaining the local balance between procoagulant and fibrinolytic activity, a role akin to the endothelial cells in blood vessels. The mechanism(s) responsible for increased tissue factor (TF) expression in mesothelial cells in response to injury are at present unclear.
Objective
To investigate whether plasmin or thrombin, two major proteases that may be generated on pleural surface upon injury, induce TF expression in human pleural mesothelial cells (HMC) and elucidate the underlying mechanism(s).
Methods
Confluent monolayers of HMC and human umbilical vein endothelial cells (HUVEC) were exposed to plasmin or thrombin for varying time periods and TF expression was analyzed by measuring its activity in a factor Xa generation assay, TF antigen levels by immunoblot analysis and TF mRNA by Northern blot analysis.
Results
Both plasmin and thrombin treatments increased cell surface TF activity in HMC by 3 to 4-fold. In contrast to thrombin, plasmin-induced TF activity is not dependent on the de novo synthesis of TF. In HUVEC, plasmin had a minimal effect on unperturbed HUVEC whereas it markedly increased TF activity of activated HUVEC. Plasmin treatment neither affected anionic phospholipid levels at the cell surface nor released protein disulfide isomerase, an oxidoreductase protein that was newly described to play a role in TF activation. Plasmin cleaved cell-associated TFPI.
Conclusion
Thrombin upregulates TF activity in HMC through the transcriptional activation of TF whereas plasmin increases TF activity by inactivating the cell-associated TFPI by a limited proteolysis.
Keywords: endothelial cells, mesothelial cells, plasmin, thrombin, tissue factor, tissue factor pathway inhibitor
Introduction
Formation of exudative pleural effusions and intrapleural fibrin deposition are hallmarks of a variety of inflammatory diseases affecting the lung pleura and malignant pleural mesothelioma [1;2]. Intrapleural fibrin deposition that bridges the visceral and parietal pleural surfaces characterizes fibrosing forms of pleuritis, such as those occur in association with complicated forms of parapneumonic effusions. If unchecked by the fibrinolytic system, the fibrinous intrapleural neomatrix leads to loculation that in turn organizes with pleural fibrosis [3]. Lung restriction and symptoms of persistent shortness of breath are common clinical sequellae.
The mesothelial lining of pleura plays a central role in maintaining the hemostatic balance in pleura [4]. Human pleural mesothelial cells may autoregulate fibrin turnover locally by expression of procoagulants and fibrinolysins. Fibrin formation is resulted by the coagulation cascade activation initiated by tissue factor (TF), while the dissolution of fibrin is mediated by tissue plasminogen activator (tPA) and urokinase plasminogen activator (uPA)-dependent plasmin generation. Mesothelial cells express TF and the fibrinolysins, as well as plasminogen activator inhibitor-1 [5;6].
TF expression is typically found at low or undetectable levels in vivo in the mesothelial lining of the normal pleura. Cultured mesothelial cells were shown to express low but measurable levels of tissue factor (TF) [7;8]. TF expression was shown to be upregulated, both in vivo and in vitro, in response to inflammatory mediators or other pathophysiologically relevant stimuli [5–9]. However, our current knowledge is very limited regarding how TF expression is regulated in human pleural mesothelial cells (HMC). Tumor necrosis factor-alpha (TNFα) was shown to increase TF mRNA expression levels in HMC [6]. Serum stimulation of quiescent HMC was found to induce both TF mRNA and TF activity levels by about 3-fold [7]. Interestingly, treating quiescent HMC with plasma resulted in a small but significant decrease in TF expression [7].
Pleural inflammation leads to increased microvascular permeability with formation of exudative effusions that contain plasma constituents. Albumin is the principal protein present in exudative pleural effusions, but coagulation and fibrinolytic proteins are also well represented in pleural effusions [4;10–15]. Although active thrombin or plasmin was difficult to detect in most of the pleural fluids [4], there was a strong indication that they were generated in the pleural space. Coagulation factors necessary to generate thrombin, i.e., TF, FVII, FV, FX and prothrombin, procoagulant activity that is capable of shortening the clotting time upon recalcification of plasma deficient in factor VII, X, VIII, V or prothrombin, and high levels of prothrombin F1.2 fragments suggest that thrombin is generated in vivo in pleural effusions [4;12]. The lack of readily detectable thrombin activity in most pleural fluids was thought to be due to the presence of high levels of antithrombin in pleural fluids [4]. Exudative pleural fluids were also shown to contain high levels of plasminogen and plasminogen activators [4;11;13–15]. The presence of plasmin-derived peptides, plasmin-inhibitor complexes and high levels of fibrin degradation products including D-dimers provide evidence for the generation of plasmin in vivo in pleural effusions [4;11;13;14].
In the present study, we have investigated the effect of thrombin and plasmin on TF expression in HMC. We also compared the effect of these proteases on TF activity in human umbilical vein endothelial cells (HUVEC). The data presented herein show that both thrombin and plasmin enhanced TF activity at the cell surfaces of HMC and HUVEC through distinctly different mechanisms.
Materials and methods
Cell culture
Human pleural mesothelial cells were harvested from pleural effusions of patients suffering from congestive heart failure as described earlier [5]. These materials were obtained under the auspices of an approved protocol from the Human Subjects Institutional Review Board of The University of Texas Health Science Center. HMC were cultured at 37°C and 5 % CO2 in a humidified incubator in DMEM containing high glucose and supplemented with 1% glutamine, 1% penicillin/streptomycin, and 10% heat-inactivated fetal bovine serum (FBS). Primary human umbilical vein endothelial cells (HUVEC) were purchased from Cambrex Bio Science (Walkersville, MD) and cultured to confluency at 37°C and 5% CO2 in EBM-2 basal medium supplemented with growth supplements (Cambrex Bio Science) and 5% fetal bovine serum.
Reagents
Human plasmin, thrombin, factor X, factor V, factor Xa and prothrombin were purchased from either Enzyme Research Laboratories (South Bend, IN) or Haematologic Technologies, Inc. (Essex Junction, VT). Recombinant human factor VIIa (FVIIa) was from Novo Nordisk (Gentofte, Denmark). Monospecific polyclonal antibodies against human TF [16] and TFPI [17] were prepared as described earlier. TF monoclonal antibodies (10H10 and TF 85G9) were kindly provided by Wolfram Ruf (Scripps Research Institute, La Jolla, CA). Polyclonal anti-PDI antibody was obtained from David Essex (University of Texas Health Science Center at San Antonio, San Antonio, TX), and monoclonal anti-PDI (RL90) was from Affinity Bioreagents (Golden, CO). Protease activated receptor agonist peptides, PAR1 (TFLLRN) and PAR2 (SLIGRL) were custom synthesized (Biosynthesis Incorporated, Midland, TX). Recombinant tissue factor pathway inhibitor-2 (TFPI-2) kunitz-type domain 1 (KD1) was a gift from W. Kisiel (University of New Mexico, New Mexico, USA). Active site-inhibited plasmin was prepared by incubating plasmin with a 1000-fold molar excess of D-Phe-L-Phe-L-Arg chloromethyl ketone (PPACK) (Calbiochem, San Diego, CA, USA) for 1 h at 37 °C and then removing the free inhibitor by exhaustive dialysis at 4°C against 10 mM HEPES, 0.15 M NaCl, pH 7.5. Annexin V was kindly provided by Jonathan F. Tait (University of Washington, Seattle, WA).
Treatments
Confluent monolayers of HMC cultured in 24-well plates were washed with serum-free DMEM and deprived of serum for overnight before they were subjected to treatments. The monolayers were treated with control serum-free DMEM (SFM) or SFM containing plasmin (50 nM) or thrombin (5 nM) for varying time periods. For HUVEC, unstimulated confluent monolayers were washed with serum free F12K medium and the monolayers were treated with plasmin or thrombin in serum free EBM-2 medium. Where HUVEC were stimulated to induce TF expression before exposing them to plasmin, the cells were treated with thrombin (5 nM) or TNFα (20 ng/ml) + IL1-β (20 ng/ml) for 5.5 h in serum-free EBM-2 medium following which the cells were washed twice with serum-free F12K medium before plasmin (50 nM) was added to the cells. Following the treatments, the cells were washed with buffer A (10 mM Hepes, 0.15 M NaCl, 4 mM KCl, 11 mM glucose, pH 7.5 buffer) before they were used for measuring TF activity.
Measurement of TF-FVIIa activity on cell surfaces
Confluent monolayers of HMC or HUVEC were incubated with FVIIa (10 nM) in buffer B (buffer A and 1 mg/ml bovine serum albumin) for 5 min at 37°C, followed by the addition containing 5 mM CaCl2 of substrate factor X (175 nM). An aliquot was removed at a specific time point (usually at 10 min) for mesothelial cells and at 1 h for HUVEC into stopping buffer (TBS containing 1 mg/ml BSA and 10 mM EDTA), and FXa in the sample was measured in a chromogenic assay as described earlier [18].
Determination of prothrombin activation on cell surfaces
Monolayers of HMC or HUVEC were incubated with FVa (10 nM) in buffer B followed by the addition of FXa (1 nM) for 5 min at 37°C. Then, prothrombin (1.4 μM) was added to the cells and at the end of the 2-min activation period, an aliquot was removed into the stopping buffer and the thrombin generated was measured in a chromogenic assay using Chromozym TH as described earlier [19].
Radiolabeling of proteins
FVIIa, TF mAB and annexin V were labeled with 125I using IODOGEN (Pierce Biotechnology, Rockford, IL)-coated polypropylene tubes and Na125I (Perkin Elmer Life Sciences, Wellesley, MA) according to the manufacturer’s technical bulletin and as described previously [20]. Briefly, 100 μg of protein was incubated with Na125I (1 mCi) in a 10 μg of IODOGEN-coated tube on ice for 20–30 min, and the reaction was stopped by the addition of 1% potassium iodide. The sample was then dialyzed for 18–20 h in HEPES buffer (10 mM HEPES, 150 mM NaCl, pH 7.4) at 4°C with 3 changes (2 liters each time) to remove the free iodide.
Radioligand binding studies
Monolayers of HMC or HUVEC, unperturbed or stimulated with plasmin ± other agonists, were chilled on ice for 10 min before the radiolabeled ligand was added to the cells. The cells were incubated with the radiolabeled ligand ± unlabeled competitors at 4°C for 2 h. Then, the supernatant was removed, and the cells were washed four times with ice-cold buffer B to remove the unbound radiolabeled protein. The surface-bound radiolabeled ligand was eluted by treating the cells with 0.1 M glycine (pH 2.3) for 3 min at the room temperature. The surface eluted radioactivity was counted in a γ-counter.
Northern blot analysis
Total RNA was extracted with Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Total RNA (10 μg) was subjected to gel electrophoresis in 1.1% agarose/6% formaldehyde gels and transferred onto the nitrocellulose membrane by a capillary blot method. Northern blots analysis with 32P-labeled TF cDNA was performed as described earlier [18].
Immunoblot analysis
Following specific treatments, the overlying media were removed and the cells were washed with SFM before lysing them in 100 μl (24-well plate) of SDS-PAGE buffer. Cell lysates (10 to 20 μl) were subjected to non-reduced SDS–PAGE on 12% polyacrylamide gel, transferred onto PVDF membrane by electroblotting and probed with specific antibodies using standard procedures. Densitometric measurement of TF band in TF immunoblots were used to quantify relative TF antigen levels in cell extracts.
Immunofluorescence confocal microscopy
Cells cultured on glass coverslips were incubated with control vehicle, plasmin or thrombin for 30 min or 6 h at 37°C. Then the cells were fixed with 4% paraf ormaldehyde, permeabilized with 0.05% Triton X-100 and processed for immunostaining for TF as described earlier [21]. Nuclei were stained with DAPI. The immunostained cells were viewed using a Axio Observer. Z1 microscope at 63X magnification (oil) plan-apo chromate lens. Images were acquired of a field of view at 1-μm Z-axis increments using LSM 510 Meta confocal system (Zeiss, Germany). The laser setting wavelengths were, 369 ±10 nm excitation and 450 ± 30 nm emission for DAPI and 488 ±10 nm excitation and 525 ± 10 nm emission for Oregon-Green. The images were processed using LSM software Zen 2007 (Zeiss).
Data collection and statistical analysis
All experiments were repeated 3 to 6 times. Data shown in the figures represent mean ± SEM or a representative experiment. Increase in TF activity and antigen levels overtime in plasmin and thrombin treated cells were compared with vehicle-treated controls using a repeated measures one-way ANOVA followed by Dunnet’s post-hoc test. Statistical significance of differences in TF activity between the treatment and the control or between the two treatments was analyzed using paired t-test or nonparametric Mann-Whitney test. Unless otherwise stated, P<0.05 was considered statistically significant difference for all data analyses.
Results
Plasmin and thrombin enhance TF activity in HMC
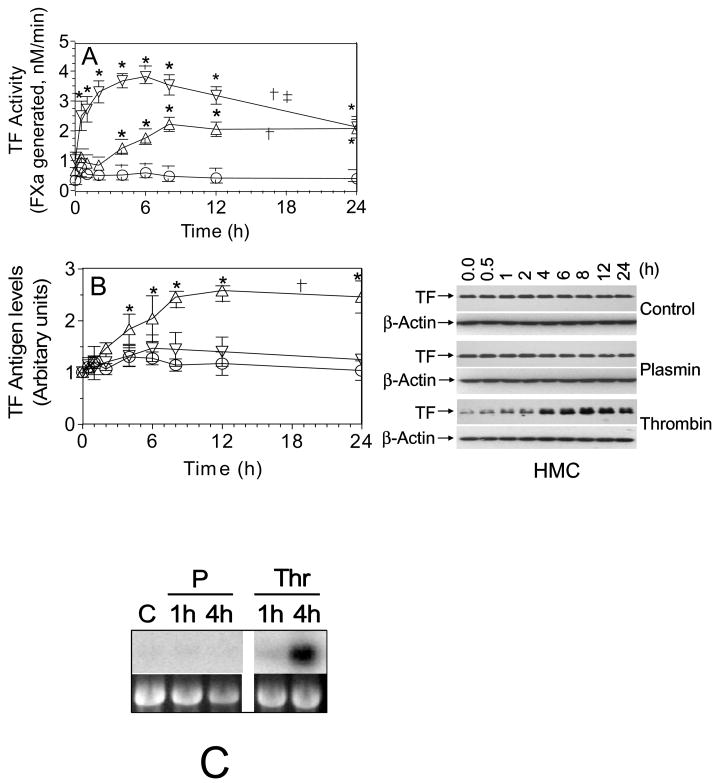
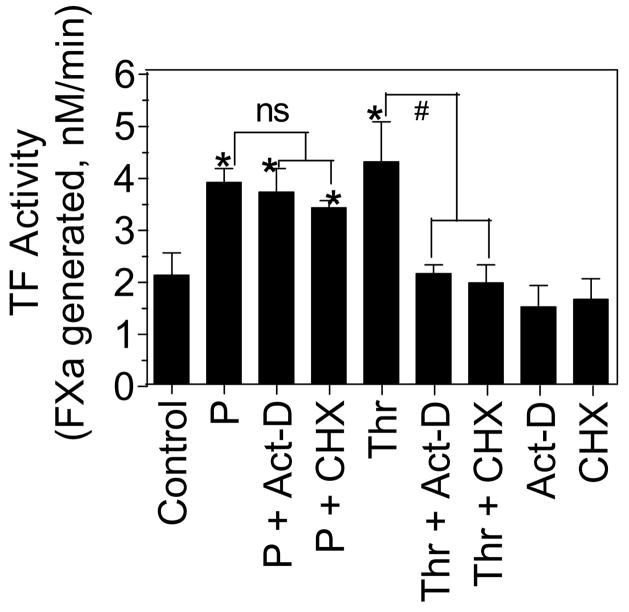
Quiescent monolayers of HMC were exposed to plasmin (50 nM) and thrombin (5 nM) for varying time periods (0.5 h – 24 h) at 37°C and the cell surface TF activity was measured as described in the methods. As noted earlier [7;8], unstimulated HMC in cell culture expressed low levels of TF constitutively. Both plasmin and thrombin treatments markedly increased the cell surface TF activity of HMC (Fig. 1A). In thrombin-stimulated cells, TF activity increased slowly, after a lag period of 2 h, in a time-dependent manner, reaching the maximum level at 8 h. By contrast, plasmin treatment increased TF activity markedly within 30 min. Measurement of TF antigen levels by immunoblot analysis showed a time-dependent increase in TF antigen levels in thrombin-treated HMC but not in plasmin-treated HMC (Fig. 1B). Similarly, Northern blot analysis revealed increased TF mRNA levels in HMC treated with thrombin but not with plasmin (Fig 1C). These data suggest that, as observed with endothelial cells [22;23], thrombin upregulates TF activity in HMC by inducing de novo synthesis of TF. However, plasmin-increased TF activity appeared to be independent of de novo synthesis of TF. To confirm that the plasmin-induced increase in TF activity is independent of de novo synthesis of TF, HMC were treated with the mRNA synthesis inhibitor, actinomycin-D (Act-D) or protein synthesis inhibitor, cycloheximide (CHX) for 1 h prior to the addition of plasmin or thrombin. Pretreatment of HMC with Act-D or CHX failed to inhibit plasmin-induced TF activity whereas they completely attenuated thrombin-induced TF activity (Fig. 2).
Fig. 1.
Upregulation of TF coagulant activity on human mesothelial cells exposed to plasmin or thrombin. Quiescent HMC monolayers were exposed to control vehicle (○), plasmin, 50 nM (▽) or thrombin, 5 nM (△) for varying time periods. At the end of the treatment period, plasmin or thrombin was removed and the cells were washed twice in buffer A and the cell surface TF activity (A) and TF antigen (B) were measured. Values are shown as mean ± SEM (n=3 to 6 experiments). The right panel shown in Fig 1B was a representative TF immunoblot. The data were analyzed by repeated measures one-way ANOVA followed by Dunnet’s post-hoc test. In panel A, † denotes TF activity over time in plasmin- and thrombin-treated cells significantly differs from that of control vehicle-treated cells (p < 0.01). ‡ denotes TF activity overtime in plasmin-treated cells significantly differs from that of thrombin-treated cells (p < 0.001). In panel B, † denotes TF antigen over time in thrombin-treated cells significantly differs from that of control vehicle- or plasmin-treated cells (p < 0.01). TF antigen over time in plasmin-treated cells does not significantly differ from that of control vehicle- treated cells. * represents the value significantly differs from the value noted at zero time (p < 0.05). (C) Effect of plasmin and thrombin on HMC TF mRNA expression. Quiescent HMC were treated with serum free medium alone for 4 h (C), plasmin (P), (50 nM) or thrombin (Thr), (5 nM) in serum free medium for 1 h or 4 h. Total RNA was isolated from the cells, 10 μg of each RNA sample was used for Northern blot analysis, and the blot was hybridized with a radiolabeled TF cDNA probe and exposed to X-ray film. Ethidium bromide staining of 18S ribosomal RNA of the same samples was shown for RNA loading control.
Fig. 2.
The plasmin-mediated increase in TF activity in human mesothelial cells is independent of de novo protein synthesis. Confluent monolayers of HMC were preincubated with actinomycin-D (Act-D), 5 μg/ml or cycloheximide (CHX), 10 μg/ml for 1 h before they were treated with plasmin (P) (50 nM) or thrombin (Thr) (5 nM) for 6 h. At the end of the treatment, TF activity on cell surfaces was measured. Values are shown as mean ± SEM (n=3 to 4 experiments). * denotes that TF activity was significantly higher compared to TF activity measured in control unstimulated cells (p < 0.01). # denotes the values differs significantly (p < 0.01). ns, stands for not statistically significant difference.
We next sought to ensure that increased procoagulant activity we observed in HMC, particularly in plasmin-treated cells, reflects increased TF activity on the cell surface. Unstimulated, plasmin- and thrombin-stimulated cells were preincubated either with control IgG or anti-TF IgG prior to the addition of FVIIa and FX to the cells to measure TF activity. Anti-TF antibody completely attenuated both the plasmin- and thrombin-induced increase in TF activity as well as that of basal TF activity observed in unperturbed HMC. By contrast, control IgG had no effect on TF activity. It is unlikely that the increased TF activity we observed in plasmin-treated cells comes from amplification of TF activity in the presence of FVIIa or direct activation of FX by cell-bound plasmin. That is because plasmin treatment of fibroblasts which constitutively express TF failed to increase TF activity. Moreover, removal of the cell-bound plasmin by washing plasmin-treated HMC with ε-aminocaproic acid (EACA) prior to measuring TF activity or blockade of annexin II, a potential cell binding site for plasminogen/plasmin [24], with specific antibodies prior to the addition of plasmin failed to diminish the plasmin-mediated increase in cell surface TF activity by HMC (data not shown).
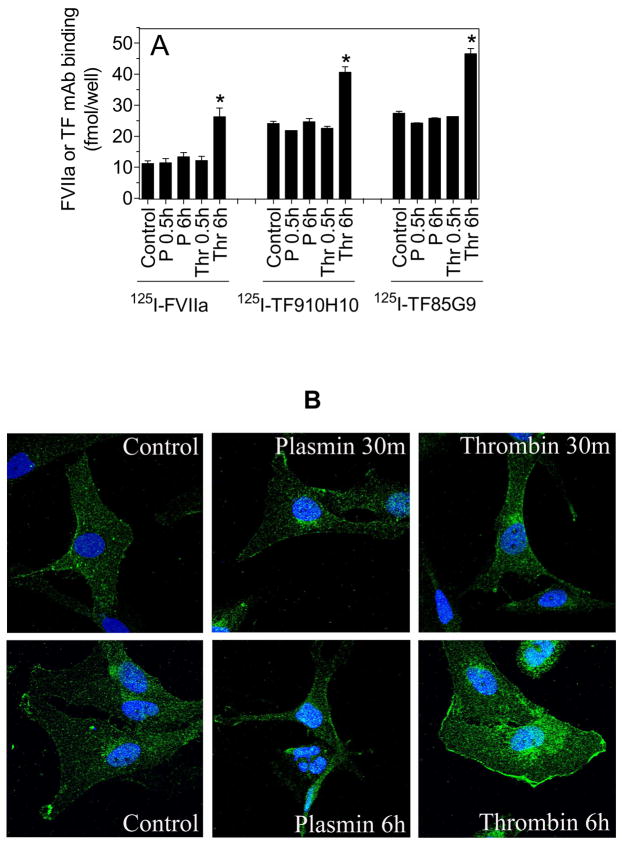
Recent studies from our laboratory showed that a number of proteases, including thrombin and plasmin, mobilize intracellular TF pools to the cell surface via PAR-mediated cell signaling but independent of de novo synthesis of TF [25]. However, the increase in cell surface TF activity by this mechanism appears to be limited (~25 to 50% increase over non-stimulated cells). Further, it is unclear whether such a mechanism exists in other cell types. Nonetheless, it is important to determine whether such a mechanism explains a 3 to 4-fold increased TF activity on HMC in response to plasmin treatment. To examine this possibility, we measured cell surface TF antigen levels quantitatively in control HMC and HMC stimulated with plasmin or thrombin. As shown in Fig. 3A, no significant differences were observed in the amount of FVIIa or TF mAb bound to unstimulated HMC and plasmin-stimulated HMC. In contrast, thrombin treatment (6 h) increased the cell surface TF antigen levels by 2-fold or more. In further studies, we employed immunofluorescence confocal microscopy to examine the cell surface and intracellular distribution of TF. Control unstimulated HMC stained weakly with TF antibody. The staining was mostly limited to the cell surface with little intracellular staining. No significant differences were observed in TF immunostaining between the unstimulated and plasmin-stimulated cells. In contrast to plasmin, thrombin-treated cells (for 6 h) stained more brightly with TF antibody and newly synthesized TF is evident in perinuclear staining of TF (Fig. 3B).
Fig. 3.
Plasmin does not increase TF antigen levels on human mesothelial cell surfaces. (A) HMC monolayers were stimulated with plasmin (P) (50 nM) or thrombin (Thr) (5 nM) for 0.5 h or 6 h. At the end of the treatment, the monolayers were chilled and were incubated with 125I-FVIIa (10 nM) in the absence or presence of anti-TF polyclonal antibodies (100 μg/ml) or 125I-TF mAB, either TF9-10H10 or TF8-5G9 (10 nM), in the presence or absence of 10-fold molar excess of corresponding unlabeled TF mAB at 4°C for 2 h. The specific binding of 125I-FVIIa or 125I-TF mAB was determined by subtracting the amount of radioligand bound to the cells in the presence of anti-TF IgG or unlabeled TF mAB, respectively, from the total radioligand associated with the cells. Data are represented as mean ± SEM (n=3). * denotes that the value is significantly higher compared to the value noted for control unstimulated cells (p < 0.01). (B) HMC were treated with control vehicle, plasmin (50 nM) or thrombin (5 nM) for 30 min or 6 h. The cells were then fixed, permeabilized and immunostained with anti-TF antibodies followed by Oregon green-conjugated secondary antibodies. Nuclei were stained with DAPI. The immunofluorescence was analyzed by confocal microscopy (Axio Observer microscope, 60X lens with oil, Zeiss LSM 510 Meta confocal system).
Plasmin treatment enhances TF activity of stimulated HUVEC
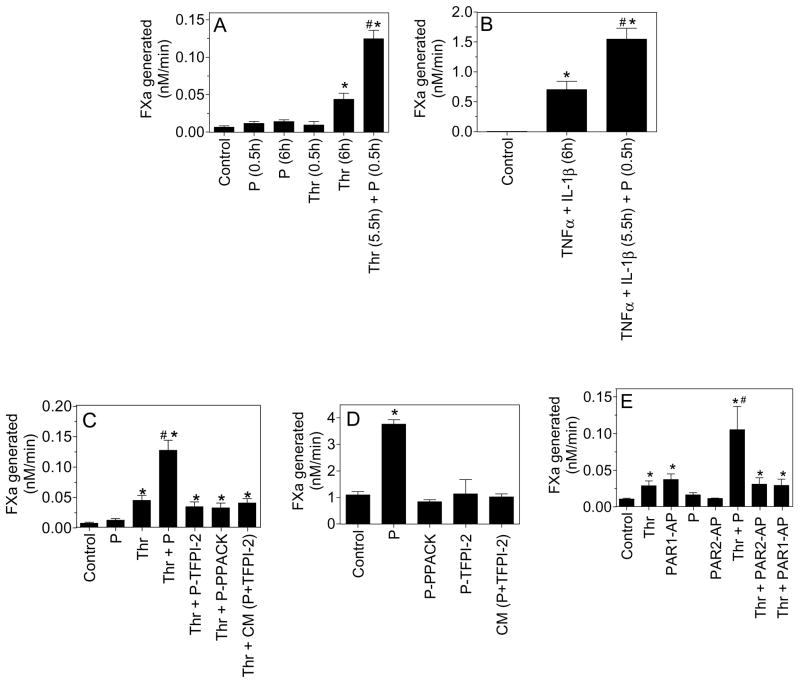
Unlike HMC, unperturbed endothelial cells, both in vivo and in culture, express little TF on their cell surfaces. Plasmin treatment (for 0.5 h and 6 h) had only a minimal effect in inducing TF expression in native HUVEC. Thrombin, as expected, induced TF expression in HUVEC, resulting in 3 to 5-fold increase in TF-FVIIa coagulant activity. Exposure of thrombin-stimulated HUVEC to plasmin for 30 min resulted in a further increase in TF activity (Fig. 4A). Compared to thrombin, cytokines were much more potent in inducing TF activity in HUVEC. TNFα + IL1-β-stimulation increased TF activity in HUVEC by about 50-fold over the unstimulated HUVEC, which was further increased by about 3-fold with plasmin treatment (Fig. 4B).
Fig. 4.
Plasmin enhances cell surface TF activity of activated endothelial cells and the requirement of plasmin’s proteolytic activity for the increased TF activity. (A) Confluent monolayers of HUVEC were treated with plasmin (P) or thrombin (Thr) for 0.5 h or 6 h, or first stimulated with thrombin for 5.5 h and then treated with plasmin for 0.5 h. * denotes significantly differs from the control and plasmin-treated cells (p < 0.001). # denotes significantly differs compared to thrombin-treated (6 h) cells. (B) HUVEC monolayers were stimulated with TNFα (20 ng/ml) + IL1-β (20 ng/ml) for 5.5 h and then exposed to plasmin for 0.5 h. * denotes significantly differs from the control vehicle-treated cells (p < 0.0001). # denotes significantly differs compared to TNFα + IL1-β-treated cells. (C) HUVEC monolayers stimulated with thrombin for 6 h and then treated for 30 min with either plasmin, plasmin preincubated with TFPI-2 KD1 (200 nM) (P-TFPI-2) for 30 min, plasmin inactivated with PPACK (P-PPACK) or the conditioned medium (CM) obtained from the cells that were treated with plasmin for 30 min (plasmin’s proteolytic activity in the CM was inhibited by TFPI-2, CM(P+TFPI-2)). * denotes significantly differs from the control vehicle-treated cells (p < 0.002). # denotes significantly differs from thrombin-, thrombin+P-TFPI-2-, thrombin+P-PPACK- or thrombin+CM(P-TFPI-2)- treated cells (p < 0.005). (D) Same as (C) except unperturbed HMC was substituted for thrombin-stimulated HUVEC. (E) Confluent monolayers of HUVEC were stimulated with plasmin, thrombin or PAR1/PAR2 agonist peptide for 6 h or first stimulated with thrombin for 5.5 h and then treated with plasmin, PAR1 or PAR2 agonist peptides (AP) for 30 min. At the end of the treatment, surface TF activity assay was measured as described in methods. The concentrations of reagent used were: thrombin, 5 nM; plasmin, 50 nM; PAR1-AP, 50 μM and PAR2-AP, 50 μM. * denotes significantly differs from the control vehicle-treated cells (p < 0.05). # denotes significantly differs from all other values shown in the graph (p < 0.01). Data shown in all panels represent mean ± SEM (n=3 to 6 experiments).
Plasmin proteolytic activity is required for increased TF activity
To investigate whether plasmin’s proteolytic activity is required for increasing TF activity on cell surfaces, thrombin-stimulated HUVEC were treated with either plasmin, plasmin pretreated with TFPI-2 Kunitz-type domain 1 (KD1), which specifically inhibits plasmin’s proteolytic activity [26] or active-site inhibited plasmin (PPACK-plasmin), and expression of cell surface TF activity was measured. As shown in Fig. 4C, proteolytically inactive plasmin failed to increase TF activity, suggesting that plasmin’s protease activity is a prerequisite for the plasmin-mediated increase in TF activity. Similar data was obtained in experiments in which HMC cells were used (Fig. 4D).
Since plasmin can affect various cellular processes through activation of PAR-mediated cell signaling [27–29], the proteolysis of the extracellular matrix [30;31] or by activating/liberating growth factors [32;33], we next examined all these possibilities in plasmin enhancement of cell surface TF activity. Pretreatment of HMC or stimulated HUVEC with PAR1 or PAR2 neutralizing antibodies prior to the addition of plasmin failed to attenuate the plasmin-induced increased TF activity. As expected, PAR1 antibodies inhibited thrombin-induced TF activity in HUVEC (data not shown). Further activation of PAR1 or PAR2 by PAR1 or PAR2 specific agonist peptides failed to mimic the plasmin-induced response in thrombin-stimulated HUVEC (Fig. 4E) indicating that it is unlikely that activation of PAR1 or PAR2 by plasmin is responsible for plasmin-mediated increased TF activity. To examine whether plasmin could proteolytically release a soluble mediator from HMC or HUVEC into the conditioned medium that could support the enhancing effect of plasmin, HUVEC or HMC were first treated with plasmin (50 nM) for 30 min and the conditioned medium collected. The proteolytic activity of plasmin in the conditioned medium was inhibited by incubating the conditioned medium with TFPI2-KD1 (200 nM) for 30 min, after which the conditioned medium was added to thrombin-stimulated HUVEC or quiescent HMC for 30 min. As shown in (Fig. 4C and 4D), the conditioned medium failed to increase TF activity thus excluding the possibility that a plasmin-released soluble mediator is being responsible for increased TF activity seen in plasmin-treated cells.
Another possibility for the increased TF activity by plasmin could be that plasmin directly interacts with TF and enhances its activity by cleaving TF or activating it without any apparent changes in the protein. No cleavage of cell-associated TF protein could be detected in HMC or stimulated HUVEC treated with plasmin (50 nM) for 1 h (data not shown). Similar data was obtained using soluble (sTF) or full-length relipidated TF as a source of TF (data not shown). In additional studies, we examined the effect of plasmin on the procoagulant activity of sTF and relipidated TF to determine whether plasmin could enhance TF activity in a cell-free system. Plasmin had no effect on the procoagulant activity of either soluble or relipidated TF.
Plasmin does not increase anionic phospholipid levels at cell surfaces
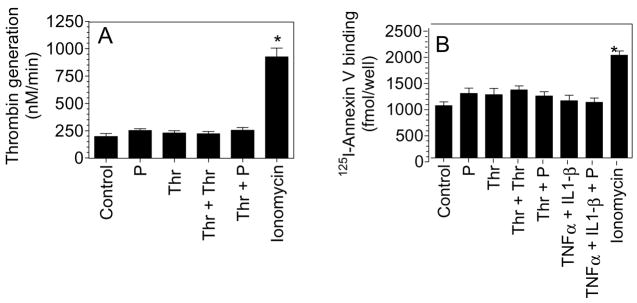
Since exposure of anionic phospholipids at the outer cell surface membrane can increase cell surface TF activity, we next sought to determine if plasmin treatment increases anionic phospholipids on the outer bilayer of the cell membrane. Plasmin and thrombin treatments of HUVEC had no significant effect on prothrombinase activity supported by HUVEC. More importantly, plasmin treatment of thrombin-stimulated cells failed to enhance the prothrombinase activity (Fig. 5A). Binding studies using radiolabeled annexin V, a Ca2+ dependent anticoagulant protein that preferentially binds to anionic phospholipids, confirmed the above findings (Fig. 5B). As a positive control, HUVEC were treated with calcium ionophore to increase the exposure of anionic phospholipid to the outer leaflet. Ionomycin-treated cells exhibited significantly increased prothrombinase activity and annexin V binding compared to unstimulated cells or cells stimulated with plasmin, thrombin or cytokines (Fig. 5A and Fig. 5B).
Fig. 5.
Plasmin treatment does not lead to increased anionic phospholipids at the cell surface. HUVEC monolayers were stimulated with plasmin or thrombin for 6 h or first stimulated with thrombin or TNFα + IL1-β for 6 h and then treated with plasmin for 30 min. As a positive control, HUVEC were treated with calcium ionomycin (10 μM) for 3 min. (A) Cell surface prothrombinase activity was measured by adding FXa (1 nM), FVa (10 nM) and prothrombin (1.4 μM) and measuring the rate of thrombin generation. (B) Stimulated cells were incubated with 125I-Annexin V (20 nM) for 2 h at 4°C, and at the end of 2 h surface binding of radioligand was determined. The concentration of plasmin, thrombin, TNFα and IL1-β used in this experiment was same as denoted for other figures. * denotes significantly differs from the control vehicle-treated cells (p < 0.05). Data are represented as mean ± SEM (n=3 to 5 experiments).
PDI is not responsible for plasmin-mediated increase in TF activity
Recent studies suggest that TF activity on certain cell types could be regulated by protein disulfide isomerase (PDI) [34;35], but evidence for this is not incontrovertible [36]. To test whether exposure of HMC or HUVEC to plasmin leads to PDI secretion which could thereby influence TF activity, cells were treated with control medium or the medium containing plasmin (50 nM) or thrombin (5 nM) for varying time periods, and the conditioned media and cell extracts were subjected to immunoblot analysis to detect PDI. Only traces of PDI (<1% of PDI found in total cell lysates) are present in the conditioned media. No differences in PDI levels were found between the conditioned media collected from cells treated with the control vehicle or plasmin. Preincubation of HMC or stimulated HUVEC with PDI neutralizing antibodies failed to diminish the plasmin-mediated increase in TF activity (data not shown).
Plasmin cleavage of TFPI
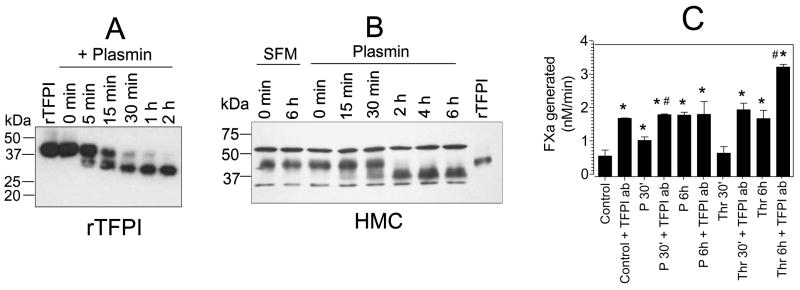
Earlier studies showed that plasmin can cleave and inactivate TFPI [37–39]. TFPI is the major inhibitor of TF-mediated coagulation. Both HMC and HUVEC were shown to express TFPI [40;41]. Thus, plasmin proteolysis of cell associated TFPI may lead to significant reduction in TFPI anticoagulant activity, which could result in increased TF activity. Therefore we examined the proteolytic effect of plasmin on rTFPI and the cell-associated TFPI. Plasmin readily cleaved rTFPI in a time-dependent fashion and more than 90% of rTFPI was cleaved into lower mol. wt. form in 30 min (Fig. 6A). Plasmin also cleaved HMC-associated TFPI, however at a slower rate (Fig. 6B). Plasmin cleavage of cell associated TFPI was detectable at 15 min and almost all the cell-associated TFPI was cleaved by 2 h. To test whether plasmin proteolysis of the cell-associated TFPI is responsible for the increased TF activity in plasmin-treated cells, both unstimulated and plasmin-stimulated cells were treated with anti-TFPI antibodies prior to measuring the cell surface TF activity. As shown in Fig. 6C, TFPI antibodies enhanced TF activity of unstimulated HMC by three fold, i.e., to the same extent as obtained with HMC treated with plasmin for 6 h. A 30 min plasmin treatment enhanced TF activity of HMC by about 2-fold, which was further increased by about 50% if the cells were also treated with anti-TFPI antibodies. There is no difference in TF activity in HMC treated with plasmin for 6 h and unstimulated HMC treated with anti-TFPI antibodies. Anti-TFPI antibodies failed to enhance TF activity further of HMC treated with plasmin for 6 h. In contrast, TF activity of thrombin-stimulated cells (for 6 h) was increased by two-fold if the stimulated cells were treated with anti-TFPI antibodies. These data suggest that increased TF activity observed in plasmin-treated cells stems from inactivation of cell associated TFPI by plasmin by limited proteolysis.
Fig. 6.
Proteolytic cleavage of TFPI by plasmin. Full length recombinant TFPI (A) or monolayers of HMC (B) were incubated with 50 nM of plasmin at 37°C for varying time periods. For rTFPI, at varying time intervals an aliquot was removed from the reaction mixture and added to SDS-PAGE buffer. For HMC, at each time interval, a monolayer from a single well from 24-well culture dish was washed with serum-free medium and the cells were solubilized in SDS-PAGE buffer. The samples were subjected to a non-reducing SDS-PAGE and immunoblotted with anti-TFPI polyclonal antibody. A non-specific band seen in the immunoblot acted as an internal loading control. Concentration of rTFPI, 30 ng/ml. (C) Effect of anti-TFPI antibodies on HMC TF activity. Monolayers of HMC were treated with control vehicle, plasmin (P) or thrombin (Thr) for 30 min or 6 h and then with anti-TFPI (100 μg/ml) for 30 min. The cells were washed twice to remove the unbound antibody before measuring the TF activity. * denotes significantly differs from the control vehicle-treated cells (p < 0.02). # denotes significantly differs from the value obtained from a matched treatment in which cells were not treated with TFPI antibodies (p < 0.05).
Discussion
The mesothelium expresses components of both procoagulant and fibrinolytic pathways [10;42;43] and the local equilibrium between their activities is a critical for homeostatic control of fibrin generation and turnover in the pleural space [4]. Most types of pleural injury disrupt this equilibrium towards procoagulant side, leading to fibrin deposition in the pleural space. Pleural fibrin deposition is a common complication of pleural inflammation and occurs in a wide variety of pleural diseases [3]. Tissue factor is the principal initiator of the coagulation cascade in vivo [44] and earlier studies have shown that HMC are capable of expressing TF [5;9]. Further, TF procoagulant activity has been found in pleural fluids from patients with exudative pleuritis [4] and TF antigen was detected in HMC of pleura overlying inflamed lung [7]. These observations strongly suggest that induction of TF by HMC may play a critical role in activating the coagulation cascade in pleural space and initiating the deposition of fibrin characteristic of complicated inflammatory effusions. At present our knowledge about potential mechanisms by which TF is induced in HMC is limited. In the present study, we demonstrate that thrombin and plasmin, two major proteases generated in coagulation and fibrinolysis and which occur in pleural injury, induce TF expression in HMC. Interestingly, the mechanisms by which they induce TF expression in HMC differ markedly.
Previous studies demonstrated that serum stimulation of quiescent HMC enhanced TF expression by about 3-fold [7]. In contrast to serum, plasma did not induce TF expression in quiescent HMC [7]. At present, the factor(s) in serum that are responsible for the induction of TF expression in HMC are unknown. It is conceivable that the activation of coagulation and fibrinolytic pathway proteins in serum are responsible for the induction of TF expression in HMC. However, in the present study we observed both thrombin and plasmin are capable of inducing TF expression not only in quiescent HMC but also in HMC cultured in serum. This suggests that mechanisms by which thrombin and plasmin induce TF expression differ from that of serum-induced TF expression.
An interesting finding from our study is that plasmin enhances TF activity in HMC without increasing TF mRNA or antigen levels. Plasmin also enhanced cell surface TF activity on endothelial cells but these cells had to be stimulated first with other agonists to induce TF expression. It is pertinent to note here that culturing HMC in serum is sufficient for HMC to constitutively express low but significant amount of TF on their cell surfaces whereas unperturbed endothelial cells cultured in serum containing medium express little or no TF. Overall our data suggest that plasmin enhances cell surface TF activity by modulating the activity of preexisting TF on the cell surface.
A number of earlier studies indicated that only a small fraction of the TF found on the cell surface is actually procoagulant whereas the vast majority is non-functional (cryptic) [45;46]. Perturbation of cells with various stimuli – for example calcium ionophores, oxidizing agents, or those that induce apoptosis or cell lysis – was shown to transform cryptic TF to active, procoagulant TF [46]. It is unclear how coagulant active TF differs from the cryptic form and mechanisms involved in decryption of TF. Most evidence to date suggests that an increase in the exposure of anionic phospholipids at the cell surface is responsible for this functional transformation of TF [46–50]. Recent studies suggest that the cryptic form of TF contains unpaired cysteine thiols at Cys 186 and Cys 209 and that activation of TF involves the formation of the Cys186-Cys209 disulfide bond, a process mediated by PDI [34]. However, validity of the later mechanism has yet to be convincingly established [36]. It is conceivable that plasmin-enhanced cell surface TF activity in our studies could have stemmed from increased anionic phospholipids on the cell surface in response to plasmin. However, we found no significant increase in anionic phospholipids on the cell surface in cells treated with plasmin, compared to the control or thrombin-treated cells. Our studies also indicate that it is unlikely that PDI-dependent mechanism is responsible for plasmin-induced increased TF activity as we reported earlier in tumor cells and fibroblasts [36], PDI is exclusively localized within the cell in both HMC and HUVEC (data not shown). Further, we did not find a significant release of PDI into the conditioned medium in cells treated with plasmin. Finally, pretreatment of HMC or HUVEC with PDI neutralizing antibodies failed to attenuate the plasmin-induced increase in cell surface TF activity.
Others and we have shown earlier that plasmin could activate PAR1 on cell surfaces [27;29]. However, potential activation of PAR1 by plasmin is unlikely to explain the enhancement of cell surface TF activity by plasmin. Thrombin is much more potent than plasmin in activating PAR1 and thrombin does not mimic plasmin in increasing TF activity rapidly and at the post-translational level. Further, in contrast to plasmin, PAR1 and PAR2 activation peptide agonists failed to increase TF activity further in thrombin-stimulated HUVEC. Thus, it is unlikely that activation of PAR1 or PAR2 is responsible for the plasmin effect.
Earlier studies showed that pleural mesothelial cells express TFPI and that TFPI was present in exudative pleural effusions from patients with pneumonia, emphysema or pleural carcinoma [40]. It had been reported that plasmin cleaves and inactivates TFPI [37;38], the major inhibitor of TF-mediated coagulation pathway. Since both HMC and HUVEC express TFPI and plasmin can cleave and inactivate TFPI, it is possible that increased cell surface TF activity we observed following the plasmin treatment in these cells could reflect plasmin cleavage of cell associated TFPI. Although cell associated TFPI, compared to rTFPI, was somewhat resistant to plasmin, plasmin did cleave TFPI associated with HMC in a time-dependent manner. Plasmin proteolysis of TFPI inactivates TFPI and therefore releases TF from its regulatory control.
A limitation in extrapolating the present data to in vivo events is lack of data on concentrations of plasmin that are generated in intrapleural or intravascular pathophysiologic conditions. Despite high PAI-1 levels [4;11] and lack of readily detectable plasmin [4] in exudative pleural effusions, it is evident that substantial amounts of plasmin are generated in pleural effusions due to infections or malignancy as high levels of fibrin degradation products, plasmin-derived peptides and plasmin-inhibitor complexes were found in pleural fluids [4;11;14]. In a rabbit model, about 90% of the plasminogen-related protein in malignant pleural effusions was associated with the heavy chain of plasmin within plasmin-α2-antiplasmin complexes [14]. Thus, it is possible that sufficient amounts of plasmin could be generated and cleave HMC-associated TFPI in pleural inflammation, resulting in increased TF activity that promotes pleural loculation. Plasmin-mediated cleavage of cell-associated TFPI may also have implications in other disease settings. For example, plasmin cleavage of lung-associated TFPI in sepsis may contribute to vascular and perivascular fibrin deposition noted in the advanced stages of sepsis [51]. Recent studies showed that TFPI activity was decreased abruptly in septic baboon lungs and the decrease is coincided with the release of tPA and the peak of plasmin generation [52]. It is likely that plasmin-mediated cleavage of cell-associated TFPI is responsible for the diminished TFPI activity abruptly in lungs in early phases of sepsis. Degradation of TFPI by plasmin on endothelial cells and monocytes could also contribute to thrombotic complications associated with thrombolytic therapy for acute myocardial infarction [53]. Here it may be pertinent to note that activation of the fibrinolytic system by Altephase (tPA) in acute myocardial infarction decreased surface-associated TFPI on circulating monocytes [54]. Plasmin-dependent proteolysis of TFPI on cells expressing TF may be responsible for thrombotic complications associated with thrombolytic therapy. In summary our present data suggest that plasmin could activate TF activity by limited proteolysis of cell associated TFPI. We speculate that this mechanism may contribute to fibrotic repair that may follow acute pleural injury and thrombotic complications associated with sepsis or thrombolytic therapy.
Acknowledgments
This work was supported by grants from the National Institutes of Health, HL58869 (to L.V.M. Rao), HL65550 (U. Pendurthi) and HL76406 (S. Idell). We acknowledge the help of Ramesh Nayak in performing confocal microscopy.
Footnotes
Disclosure of Conflict of Interests
The authors state that they have no conflict of interests.
References
- 1.Boggs DS, Kinasewitz GT. Review: pathophysiology of the pleural space. Am J Med Sci. 1995;309:53–9. doi: 10.1097/00000441-199501000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Herbert A. Pathogenesis of pleurisy, pleural fibrosis, and mesothelial proliferation. Thorax. 1986;41:176–89. doi: 10.1136/thx.41.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mutsaers SE, Prele CM, Brody AR, Idell S. Pathogenesis of pleural fibrosis. Respirology. 2004;9:428–40. doi: 10.1111/j.1440-1843.2004.00633.x. [DOI] [PubMed] [Google Scholar]
- 4.Idell S, Girard W, Koenig KB, Mclarty J, Fair DS. Abnormalities of pathways of fibrin turnover in the human pleural space. Am Rev Respir Dis. 1991;144:187–94. doi: 10.1164/ajrccm/144.1.187. [DOI] [PubMed] [Google Scholar]
- 5.Idell S, Zwieb C, Kumar A, Koenig KB, Johnson AR. Pathwyas of fibrin turnover of human pleural mesothelial cells in vitro. Am J Respir Cell Mol Biol. 1992;7:414–26. doi: 10.1165/ajrcmb/7.4.414. [DOI] [PubMed] [Google Scholar]
- 6.Sitter T, Toet K, Fricke H, Schiffl H, Held E, Kooistra T. Modulation of procoagulant and fibrinolytic system components of mesothelial cells by inflammatory mediators. Am J Physiol. 1996;271:R1256–R1263. doi: 10.1152/ajpregu.1996.271.5.R1256. [DOI] [PubMed] [Google Scholar]
- 7.Bottles KD, Laszik Z, Morrissey JH, Kinasewitz GT. Tissue factor expression in mesothelial cells: induction both in vivo and in vitro. Am J Respir Cell Mol Biol. 1997;17:164–72. doi: 10.1165/ajrcmb.17.2.2438. [DOI] [PubMed] [Google Scholar]
- 8.Verhagen HJM, Heijnen-snyder GJ, Vink T, Pronk A, van Vroonhoven TJMV, Eikelboom BC, Sixma JJ, De Groot PG. Tissue factor expression on mesothelial cells is induced during invitro culture-manipulation of culture conditins creates perspectives. Thromb Haemost. 1995;74:1906–102. [PubMed] [Google Scholar]
- 9.Kumar A, Koenig KB, Johnson AR, Idell S. Expression and assembly of procoagulant complexes by human pleural mesothelial cells. Thromb Haemost. 1994;71:587–92. [PubMed] [Google Scholar]
- 10.Glauser FL, Otis PT, Levine RI, Smith WR. Coagulation factors and fibrinogen in pleural effusions. Respiration. 1976;33:396–402. doi: 10.1159/000193755. [DOI] [PubMed] [Google Scholar]
- 11.Philip-Joet F, Alessi MC, Philip-Joet C, Aillaud M, Barriere JR, Arnaud A, Juhan-vague I. Fibrinolytic and inflammatory processes in pleural effusions. Eur Respir J. 1995;8:1352–6. doi: 10.1183/09031936.95.08081352. [DOI] [PubMed] [Google Scholar]
- 12.Gieseler F, Luhr I, Kunze T, Mundhenke C, Maass N, Erhart T, Denker M, Beckmann D, Tiemann M, Schulte C, Dohrmann P, Cavaille F, Godeau F, Gespach C. Activated coagulation factors in human malignant effusions and their contribution to cancer cell metastasis and therapy. Thromb Haemost. 2007;97:1023–30. [PubMed] [Google Scholar]
- 13.Lu XG, Mao JS, Tong JF, Zhu L, Liu J, Gong XB, Huang J. Fibrinolytic characteristics and their significance in malignant, tuberculous and cirrhotic pleural and ascitic fluids. Int J Lab Hematol. 2007;29:132–8. doi: 10.1111/j.1751-553X.2006.00835.x. [DOI] [PubMed] [Google Scholar]
- 14.Hatton MW, Southward SM, Ross BL, Clarke BJ, Singh G, Richardson M. Relationships among tumor burden, tumor size, and the changing concentrations of fibrin degradation products and fibrinolytic factors in the pleural effusions of rabbits with VX2 lung tumors. J Lab Clin Med. 2006;147:27–35. doi: 10.1016/j.lab.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Ozdemir O, Emri S, Karakoca Y, Sayinalp N, Akay H, Dundar S, Baris I. Fibrinolytic system in plasma and pleural fluid in malignant pleural mesothelioma. Thromb Res. 1996;84:121–8. doi: 10.1016/0049-3848(96)00167-3. [DOI] [PubMed] [Google Scholar]
- 16.Rao LVM. Characterization of anti-tissue factor antibody and its use in immunoaffinity purification of human tissue factor. Thromb Res. 1988;51:373–84. doi: 10.1016/0049-3848(88)90373-8. [DOI] [PubMed] [Google Scholar]
- 17.Pendurthi UR, Rao LVM, Williams JT, Idell S. Regulation of tissue factor pathway inhibitor expression in smooth muscle cells. Blood. 1999;94:579–86. [PubMed] [Google Scholar]
- 18.Pendurthi UR, Williams JT, Rao LVM. Acidic and basic fibroblast growth factors suppress transcriptional activation of tissue factor and other inflammatory genes in endothelial cells. Arterioscler Thromb Vasc Biol. 1997;17:940–6. doi: 10.1161/01.atv.17.5.940. [DOI] [PubMed] [Google Scholar]
- 19.Hansen CB, van Deurs B, Petersen LC, Rao LVM. Discordant expression of tissue factor and its activity in polarized epithelial cells. Asymmetry in anionic phospholipid availability as a possible explanation. Blood. 1999;94:1657–64. [PubMed] [Google Scholar]
- 20.Le DT, Rapaport SI, Rao LVM. Relations between factor VIIa binding and expression of factor VIIa/tissue factor catalytic activity on cell surfaces. J Biol Chem. 1992;267:15447–54. [PubMed] [Google Scholar]
- 21.Mandal SK, Pendurthi UR, Rao LV. Cellular localization and trafficking of tissue factor. Blood. 2006;107:4746–53. doi: 10.1182/blood-2005-11-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartha K, Brisson C, Archipoff G, de la SC, Lanza F, Cazenave JP, Beretz A. Thrombin regulates tissue factor and thrombomodulin mRNA levels and activities in human saphenous vein endothelial cells by distinct mechanisms. J Biol Chem. 1993;268:421–9. [PubMed] [Google Scholar]
- 23.Wu SQ, Aird WC. Thrombin, TNF-alpha, and LPS exert overlapping but nonidentical effects on gene expression in endothelial cells and vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2005;289:H873–H885. doi: 10.1152/ajpheart.00993.2004. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Hajjar KA. Annexin II: A plasminogen-plasminogen activator co-receptor. Frontiers in Bioscience. 2002;7:d341–8. doi: 10.2741/kim. [DOI] [PubMed] [Google Scholar]
- 25.Mandal SK, Pendurthi UR, Rao LV. Tissue factor trafficking in fibroblasts: involvement of protease-activated receptor-mediated cell signaling. Blood. 2007;110:161–70. doi: 10.1182/blood-2006-10-050476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chand HS, Schmidt AE, Bajaj SP, Kisiel W. Structure-function analysis of the reactive site in the first Kunitz-type domain of human tissue factor pathway inhibitor-2. J Biol Chem. 2004;279:17500–7. doi: 10.1074/jbc.M400802200. [DOI] [PubMed] [Google Scholar]
- 27.Pendurthi UR, Ngyuen M, Andrade-Gordon P, Petersen LC, Rao LVM. Plasmin induces Cyr61 gene expression in fibroblasts via protease activated receptor-1 and p44/42 mitogen-activated protein kinase-dependent signaling pathway. Arterioscler Thromb Vasc Biol. 2002;22:1421–6. doi: 10.1161/01.atv.0000030200.59331.3f. [DOI] [PubMed] [Google Scholar]
- 28.Tarui T, Majumdar M, Miles LA, Ruf W, Takada Y. Plasmin-induced migration of endothelial cells. Potential target for the anti-angiogenic action of angiostatin. J Biol Chem. 2002;277:33564–70. doi: 10.1074/jbc.M205514200. [DOI] [PubMed] [Google Scholar]
- 29.Majumdar M, Tarui T, Shi B, Akakura N, Ruf W, Takada Y. Plasmin-induced migration requires signaling through protease-activated receptor 1 and integrin alpha(9)beta(1) J Biol Chem. 2004;279:37528–34. doi: 10.1074/jbc.M401372200. [DOI] [PubMed] [Google Scholar]
- 30.Pepper MS. Role of the matrix metalloproteinases and plasminogen activator-plasmin system in angiogenesis. Arterioscler Thromb Vasc Biol. 2001;21:1104–17. doi: 10.1161/hq0701.093685. [DOI] [PubMed] [Google Scholar]
- 31.Lijnen HR. Plasmin and matrix metalloproteinases. Thromb Haemost. 2001;86:324–33. [PubMed] [Google Scholar]
- 32.Lyons RM, Gentry LE, Purchio AF, Moses HL. Mechanism of activation of latent recombinant transforming growth factor-b1 by plasmin. J Cell Biol. 1990;110:1361–7. doi: 10.1083/jcb.110.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grainger DJ, Wakefield L, Bethell HW, Farndale RW, Metcalfe JC. Release and activation of platelet latent TGF-b in blood clots during dissolution with plasmin. Nature Medicine. 1995;1:932–7. doi: 10.1038/nm0995-932. [DOI] [PubMed] [Google Scholar]
- 34.Chen VM, Ahamed J, Versteeg HH, Berndt MC, Ruf W, Hogg PJ. Evidence for activation of tissue factor by an allosteric disulfide bond. Biochem. 2006;45:12020–8. doi: 10.1021/bi061271a. [DOI] [PubMed] [Google Scholar]
- 35.Ahamed J, Versteeg HH, Kerver M, Chen VM, Mueller BM, Hogg PJ, Ruf W. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proc Natl Acad Sci U S A. 2006;103:13932–7. doi: 10.1073/pnas.0606411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pendurthi UR, Ghosh S, Mandal SK, Rao LV. Tissue factor activation: is disulfide bond switching a regulatory mechanism? Blood. 2007;110:3900–8. doi: 10.1182/blood-2007-07-101469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li A, Wun TC. Proteolysis of tissue factor pathway inhibitor (TFPI) by plasmin: effect on TFPI activity. Thromb Haemost. 1998;80:423–7. [PubMed] [Google Scholar]
- 38.Stalboerger PG, Panetta CJ, Simari RD, Caplice NM. Plasmin proteolysis of endothelial cell and vessel wall associated tissue factor pathway inhibitor. Thromb Haemost. 2001;86:923–8. [PubMed] [Google Scholar]
- 39.Li Y, Spencer FA, Becker RC. Plasmin-mediated proteolysis of vascular endothelial cell heparin releasable tissue factor pathway inhibitor. J Thromb Thrombolysis. 2003;15:19–23. doi: 10.1023/a:1026136216869. [DOI] [PubMed] [Google Scholar]
- 40.Bajaj MS, Pendurthi U, Koenig K, Pueblitz S, Idell S. Tissue factor pathway inhibitor expression by human pleural mesothelial and mesothelioma cells. Eur Respir J. 2000;15:1069–78. doi: 10.1034/j.1399-3003.2000.01515.x. [DOI] [PubMed] [Google Scholar]
- 41.Bajaj MS, Kuppuswamy MN, Saito H, Spitzer SG, Bajaj SP. Cultured normal human hepatocytes do not synthesize lipoprotein-associated coagulation inhibitor: Evidence that endothelium is the principal site of its synthesis. Proc Natl Acad Sci U S A. 1990;87:8869–73. doi: 10.1073/pnas.87.22.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Hinsbergh VW, Kooistra T, Scheffer MA, Hajo vB, van Muijen GN. Characterization and fibrinolytic properties of human omental tissue mesothelial cells. Comparison with endothelial cells. Blood. 1990;75:1490–7. [PubMed] [Google Scholar]
- 43.Glauser FL, Otis PT, Levine RI, Smith WR. In vitro pleural fluid clottability and fibrinogen content. Chest. 1975;68:205–8. doi: 10.1378/chest.68.2.205. [DOI] [PubMed] [Google Scholar]
- 44.Rapaport SI, Rao LVM. The tissue factor pathway: How it has become a “prima ballerina”. Thromb Haemost. 1995;74:7–17. [PubMed] [Google Scholar]
- 45.Rao LVM, Pendurthi UR. Tissue factor on cells. Blood Coag Fibrin. 1998;9(suppl 1):S27–S35. [PubMed] [Google Scholar]
- 46.Bach RR. Tissue factor encryption. Arterioscler Thromb Vasc Biol. 2006;26:456–61. doi: 10.1161/01.ATV.0000202656.53964.04. [DOI] [PubMed] [Google Scholar]
- 47.Le DT, Rapaport SI, Rao LVM. Studies of the mechanism for enhanced cell surface factor VIIa/tissue factor activation of factor X in fibroblast monolayers after their exposure to N-ethylmalemide. Thromb Haemost. 1994;72:848–55. [PubMed] [Google Scholar]
- 48.Bach R, Rifkin DB. Expression of tissue factor procoagulant activity: regulation by cytosolic calcium. Proc Natl Acad Sci U S A. 1990;87:6995–9. doi: 10.1073/pnas.87.18.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolberg AS, Monroe DM, Roberts HR, Hoffmann MR. Tissue factor de-encryption:ionophore treatment induces changes in tissue factor activity by phosphatidylserine-dependent and -independent mechanisms. Blood Coag Fibrinol. 1999;10:201–10. [PubMed] [Google Scholar]
- 50.Carson SD, Perry GA, Pirruccello SJ. Fibroblast tissue factor: Calcium and ionophore induce shape changes, release of membrane vesicles, and redistribution of tissue factor antigen in addition to increased procoagulant activity. Blood. 1994;84:526–34. [PubMed] [Google Scholar]
- 51.Welty-wolf KE, Carraway MS, Ortel TL, Piantadosi CA. Coagulation and inflammation in acute lung injury. Thromb Haemost. 2002;88:17–25. [PubMed] [Google Scholar]
- 52.Tang H, Ivanciu L, Popescu N, Peer G, Hack E, Lupu C, Taylor FB, Jr, Lupu F. Sepsis-induced coagulation in the baboon lung is associated with decreased tissue factor pathway inhibitor. Am J Pathol. 2007;171:1066–77. doi: 10.2353/ajpath.2007.070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson SH, Bell MR, Rihal CS, Bailey KR, Holmes DR, Jr, Berger PB. Infarct artery reocclusion after primary angioplasty, stent placement, and thrombolytic therapy for acute myocardial infarction. Am Heart J. 2001;141:704–10. doi: 10.1067/mhj.2001.114971. [DOI] [PubMed] [Google Scholar]
- 54.Ott I, Malcouvier V, Schomig A, Neumann FJ. Proteolysis of tissue factor pathway inhibitor -1 by thrombolysis in acute myocardial infarction. Circulation. 2002;105:278–81. doi: 10.1161/hc0302.103591. [DOI] [PubMed] [Google Scholar]