Abstract
Recent evidence suggests that blockade of aberrant Hedgehog signaling can be exploited as a therapeutic strategy for pancreatic cancer. Our previous studies using the prototype Hedgehog small-molecule antagonist cyclopamine had shown the striking inhibition of systemic metastases on Hedgehog blockade in spontaneously metastatic orthotopic xenograft models. Cyclopamine is a natural compound with suboptimal pharmacokinetics, which impedes clinical translation. In the present study, a novel, orally bioavailable small-molecule Hedgehog inhibitor, IPI-269609, was tested using in vitro and in vivo model systems. In vitro treatment of pancreatic cancer cell lines with IPI-269609 resembled effects observed using cyclopamine (i.e., Gli-responsive reporter knock-down, down-regulation of the Hedgehog target genes Gli1 and Ptch, as well as abrogation of cell migration and colony formation in soft agar). Single-agent IPI-269609 profoundly inhibited systemic metastases in orthotopic xenografts established from human pancreatic cancer cell lines, although Hedgehog blockade had minimal effect on primary tumor volume. The only discernible phenotype observed within the treated primary tumor was a significant reduction in the population of aldehyde dehydrogenase–bright cells, which we have previously identified as a clonogenic tumor-initiating population in pancreatic cancer. Selective ex vivo depletion of aldehyde dehydrogenase–bright cells with IPI-269609 was accompanied by significant reduction in tumor engraftment rates in athymic mice. Pharmacologic blockade of aberrant Hedgehog signaling might prove to be an effective therapeutic strategy for inhibition of systemic metastases in pancreatic cancer, likely through targeting subsets of cancer cells with tumor-initiating (“cancer stem cell”) properties.
Introduction
Ductal adenocarcinoma of the pancreas, which constitutes more than 90% of pancreatic cancers, remains the fourth most common cause of cancer-related mortality in both females and males in the United States. In the year 2004, a total of 31,771 deaths were reported to be caused by pancreatic cancer (1). With an overall median survival of <6 months from the time of diagnosis and an average 5-year survival rate of <5%, it is one of the deadliest malignancies known to date (1).
At advanced, metastatic stages, pancreatic cancer can almost never be controlled by any of the available therapeutic options, mirrored by an extremely low estimated 5-year survival rate of <2%. In more than 80% of cases, metastases in locoregional lymph nodes or distant organ sites are already present at the time of initial diagnosis (1), which is commonly explained by the absence of specific early symptoms and lack of appropriate diagnostic tools for early detection (2).
Even in cases with early-stage, localized disease, in which surgical resection with curative intention can be done, almost all patients experience local recurrence later on or develop metastases to distant organ sites, and finally succumb to the debilitating effects of metastatic growth (3, 4).
Therefore, increasing efforts have been undertaken in recent years to develop therapeutic strategies that directly target metastatic tumor spread because such regimens would most likely have tremendous clinical impact.
Recently, aberrant reactivation of the Hedgehog signaling pathway has been described in several gastrointestinal tract cancers including pancreatic cancer (5, 6). Pharmacologic blockade of Hedgehog signaling with cyclopamine retarded the growth of s.c. pancreatic cancer xenografts (5, 6) and diminished in vitro invasion/migration and colony formation, as well as development of metastases in orthotopic xenograft models (7).
Moreover, recent evidence suggests that Sonic Hedgehog ligand (SHH) might be specifically overepxressed in a subpopulation of CD24+/CD44+/ESA+ pancreatic cancer cells with increased tumorigenic potential and other stem cell–like properties (8). Another recent report by our own group states that overexpression of Gli1, the major activating Hedgehog transcription factor, is observed at the mRNA level in a subset of SSC-low/aldehyde dehydrogenase (ALDH)–“bright” cells with increased clonogenic potential (7). Together, these studies suggest an element of Hedgehog dependence in a subset of pancreatic cancer cells with tumor-initiating properties, also referred to as “cancer stem cells.”.
Despite these encouraging preclinical data, the effects of pharmacologic Hedgehog inhibition have never been systematically evaluated in a clinical setting, and thus far, no pathway inhibitors are available for clinical use. This likely has resulted from the relatively poor oral bioavailability and short systemic half-life of cyclopamine, the prototype small-molecule Hedgehog inhibitor, rendering the maintenance of systemic drug levels sufficient for continuous pathway inhibition problematic (9). In the present study, we describe in vitro and in vivo data on a novel, water-soluble small-molecule inhibitor of the Hedgehog signaling pathway, IPI-269609, which was designed for potential subsequent clinical evaluation.
In line with previous results obtained using cyclopamine, inhibition of Hedgehog signaling with IPI-269609 led to marked inhibition of metastatic spread and tumor initiation in murine xenograft models of human pancreatic cancers. These effects were accompanied by a reduction of a subpopulation of cells with high ALDH-activity, as observed in vitro by flow cytometry and in vivo by immunohistochemistry, suggesting that the observed blockade of metastasis and tumor initiation might be mediated by targeting a subpopulation of pancreatic cancer cells with tumor-initiating properties.
Materials and Methods
Cell Lines
All pancreatic cancer cell lines used were grown in either DMEM (Invitrogen) supplemented with 10% (v/v) fetal bovine serum (FBS; Invitrogen), 1× Pen-Strep (Biofluids), 1× MEM vitamins solution (Sigma-Aldrich), 1× nonessential amino acid solution, and 1% sodium pyruvate solution (both from Biofluids), or in RPMI (Invitrogen) supplemented with 10% FBS and 1× Pen-Strep.
Shh-LightII (ATCC accession no. CRL-2795) cells were maintained in DMEM containing 4 mmol/L l-glutamine, 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, 10% bovine calf serum (Biomeda Corp.), 0.4 mg/mL geneticin, and 0.15 mg/mL zeocin (both from Invitrogen).
293-ShhN-pIND cells, stably transfected to secrete the palmitoylated truncated Hedgehog-ligand ShhN (10), were grown in DMEM, 10% FBS, 1× Pen-Strep, and 0.4 mg/mL geneticin until they reached ~80% confluency. Next, full growth medium was replaced with medium containing 2% FBS for 1 d. Conditioned medium was then collected and filtered (pore size, 0.22 µm) before use in reporter assays.
Cell lines were routinely tested for Mycoplasma infection using the MycoSensor PCR Assay Kit (Stratagene).
Hedgehog Inhibitors
Cyclopamine and IPI-269609 were provided by Infinity Pharmaceuticals (11, 12). The chemical structure of IPI-269609 is shown in Fig. 1D.
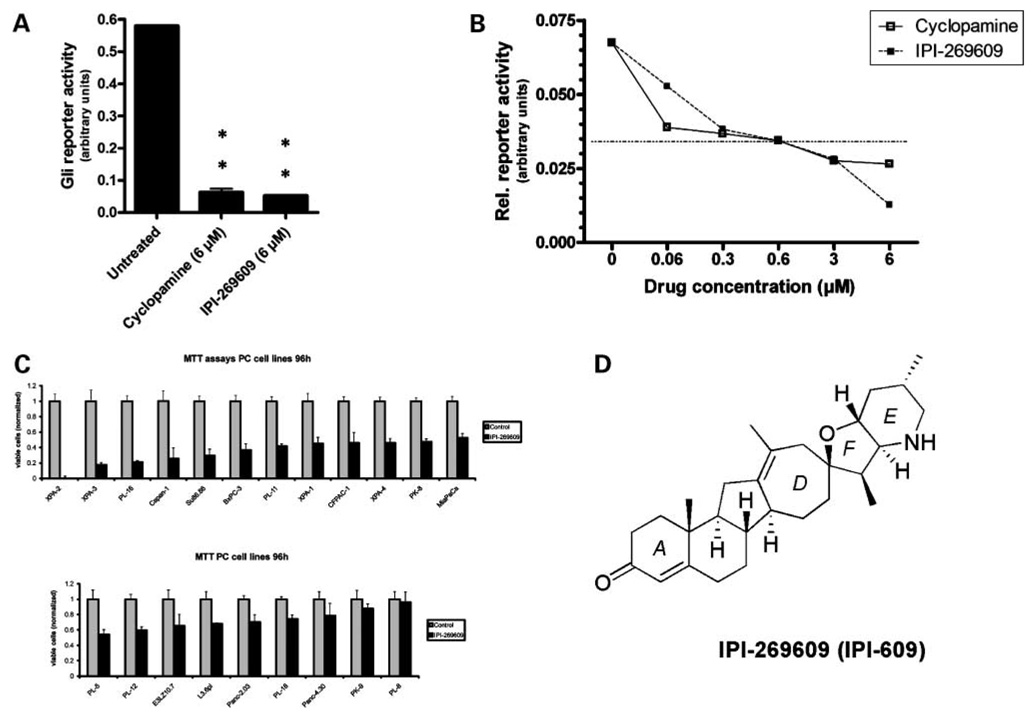
Figure 1.
IPI-269609 inhibits Hedgehog signaling in vitro. In Gli-responsive reporter assays, IPI-269609 blocked the pathway activity of LightII cells stimulated with conditioned medium containing ShhN (A). (**, P < 0.01, compared with controls.) In LightII reporter assays, IPI-269609 showed a dose-response curve similar to that of cyclopamine (B). Hedgehog pathway inhibition led to a wide range of in vitro growth inhibition as observed by 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assays in a panel of human pancreatic cancer cell lines (C). IPI-269609 is a semisynthetic analogue of the plant-derived natural product cyclopamine. IPI-269609 has a molecular weight of 423 Da and differs from cyclopamine in that it has a seven-member D-ring and a conjugated A-ring ketone. The combination of these structural modifications is responsible for its enhanced pharmaceutical properties relative to cyclopamine (D).
Cell Growth Assays
Cell growth assays using 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide reagent (Promega) were done as described previously (7, 13).
Gli-Responsive Reporter Assays
Shh-LightII cells were seeded into 24-well plates at 50,000 in 1 mL of full medium per well. On the next day, full medium was removed and the cells were washed once with PBS. Next, low-serum medium, containing 0.5% BCS only, was mixed 1:1 (v/v) with conditioned medium containing ShhN, supplemented with cyclopamine or IPI-269609 at the given concentrations or equivalent amounts of solvent only, and added to the wells. After incubation at 37°C for another 48 h, the medium was removed and adherent cells were washed with PBS and lysed on the plate with 100 µL of 1× passive lysis buffer (Promega). The samples were further processed following the standard protocol provided with the Dual-Luciferase Reporter Assay System (Promega).
Finally, luminescence was determined on a luminometer (Wallac Victor2 1420, Perkin-Elmer) for each sample. All experiments were set up in triplicates, and means and SDs calculated.
Drug Treatment of Pancreatic Cancer Cell Lines, RNA Extraction, and Quantitative Real-time Reverse Transcription-PCR
Determination of steady-state mRNA expression levels was carried out as described previously (7). In brief, pancreatic cancer cells in 100-mm cell culture dishes in the log growth phase were serum starved at 0.5% FBS for 24 h, and then drug at the given concentrations or solvent only was added in medium containing 0.5% FBS and the plates were incubated for another 72 h.
Cells were lysed in the culture dishes with lysis buffer RLT (Qiagen) containing 2-mercaptoethanol (Sigma-Aldrich); lysates were collected using a rubber policeman (Sarstedt) and homogenized with QiaShredder spin columns (Qiagen). For RNA extraction from tumor samples, the tissue was homogenized with a rotor-stator homogenizer (Polytron PT1200C, Kinematica AG). Total RNA was extracted using the RNeasy Mini kit (Qiagen) according to the standard protocol provided by the manufacturer with on-column DNA digestion.
Wound Assays
In vitro wound healing assays were done using near-confluent E3LZ10.7 and Capan-1 cells, which were treated with either 6 µmol/L of IPI-269609 or an equivalent amount of solvent in full culture medium. An area of cells was scraped off using 200-µL pipette tips, and microscopic photographs were taken at 0, 12, and 24 h.
Soft Agar Assays
Colony formation in soft agar was determined as previously described with minor modifications (7). Briefly, 10,000 pancreatic cancer cells were dissolved as a single-cell suspension in full medium containing 0.7% agarose (Invitrogen) and seeded into six-well plates. The bottom layer contained 1% agarose and the top layer consisted of full medium only. IPI-269609 was added to each layer at final concentrations of 3an d 6 µmol/L, respectively; addition of equivalent amounts of solvent served as controls. Plates were incubated at 37°C for 4 wk. After staining with 0.05% crystal violet (Sigma-Aldrich) in 80% PBS/10% ethanol/10% glacial acetic acid solution at 37°C for 2 h, colonies were visualized by trans-UV illumination and quantified using Quantity One analysis software (Bio-Rad).
H&E Stainings
Formalin-fixed, parafin-embedded tissue sections were stained with H&E in the reference histology lab of the Johns Hopkins University School of Medicine.
Flow Cytometry Analysis of ALDH Activity
Determination of in vitro ALDH activity after treatment with IPI-269609 (6 µmol/L) or solvent only in low-serum conditions (0.5% FBS) was carried out as previously described (7).
Treatment of S .c. Pancreatic Cancer Xenografts
All animal experiments described here conformed to the guidelines of the Animal Care and Use Committee of Johns Hopkins University. Mice were maintained in accordance to the guidelines of the American Association of Laboratory Animal Care.
To generate s.c. murine xenografts, human pancreatic cancer cells in the log growth phase were trypsinized, washed with PBS (Gibco-Life Technologies, Inc.), and counted on a hemocytometer (Hausser Scientific).
Next, 2.5 × 106 cells per injection site, suspended in a total volume of 200 µL [PBS/Matrigel (BD Biosciences), 1:1 (v/v), prechilled to 4°C], were s.c. injected into the flanks of male athymic CD1 nu/nu mice (Charles River) 6 to 12 wk of age.
Two weeks after the injection of tumor cells, with the use of digital callipers (Fisher Scientific), s.c. tumor volumes (V) were determined as V = 1/2(ab2), where a is the biggest and b is the smallest orthogonal tumor diameter; mice were distributed into four groups with similar tumor volumes. Each group was then randomly assigned to one of four treatment regimens: (a) controls, (b) IPI-269609 given as single daily doses of 20 mg/kg p.o., (c) gemcitabine 100 mg/kg i.p. every 4th day, and (d) combination of gemcitabine and IPI-269609. Gemcitabine and IPI-269609 were dissolved in sterile physiologic sodium chloride solution (0.9%) at concentrations of 15 and 2.5 mg/mL, respectively. Groups A and C received equivalent amounts of NaCl solution p.o.; groups A and B received mock i.p. injections of NaCl. The IPI-269609 dose of 20 mg/kg/d p.o. was picked based on initial experiments showing robust Hedgehog pathway blockade determined as down-regulation of steady-state Gli1 mRNA levels by quantitative real-time reverse transcription-PCR in s.c. pancreatic cancer xenografts.
Tumor volumes as primary outcome and body weight of mice to assess potential toxicity were determined every 5th day. The study was terminated after 25 d of drug treatment.
Generation of Orthotopic Xenografts and Drug Treatment
The generation of murine orthotopic xenografts of human pancreatic cancer by surgical implantation has also been described previously (7).
Briefly, s.c. xenograft tumors were harvested under sterile conditions, cut into cubes of 1-mm edge length, and stored in PBS until orthotopic implantation. Male athymic CD1 nu/nu mice, 6 to 12 wk of age, were anesthesized using gas anesthesia with isofluorane (Vedco), and spleen and adherent pancreas visualized through a subcostal left incision. Using microscissors (Roboz Surgical Instruments), a small pocket was prepared inside the pancreas, into which one of the previously prepared tumor chunks was inserted. The incision in the pancreas was closed with a 8-0 nylon monofilament suture; the abdominal wall was sutured with 6-0 nylon monofilament string; and the skin adapted using AutoClips (Braintree Scientific).
Three weeks after surgical implantation of E3LZ10.7 xenograft tumor chunks, the presence of intrapancreatic tumors was confirmed by ultrasound scan (Vevo660, Visual Sonics) and measured in three orthogonal axes, a, b, and c; tumor volumes were determined as V = (abc) / 2. Mice were distributed into two groups with similar average tumor volumes and randomly assigned to receive either IPI-269609 (20 mg/d p.o.) or mock treatment with solvent only for 30 d.
In the case of Capan-1, based on the idea to asses maximum effect on primary tumor growth by continuous pathway knockdown and longer treatment period, drug application was initiated by s.c. implantation of two 14D Alzet osmotic pumps (Alzet) 2 wk after orthotopic tumor implantation, and treatment was continued for 6 wk with exchange of pumps every 14 d. For application with Alzet pumps, IPI-269609 was dissolved in 30% (2-hydroxy-propyl)-β-cyclodextrin (Sigma-Aldrich) in sterile water at 40 g/L; control animals received pumps with 30% cyclodextrin in water only.
At the end of treatment, all animals were euthanized, primary tumor sizes determined, and necropsy was done to determine grossly visible metastases. Spleen, liver, one kidney, lungs, stomach, and intestine were harvested and fixed in formalin. In the case of spleen, only the organ portion free of direct tumor invasion per continuitatem was harvested. For each mouse, one random section through these organs was evaluated histologically for presence of micrometastases.
ALDH Immunohistochemistry
Immunohistochemical analysis was done using a standard technique. In brief, 4-µm sections from formalin-fixed, paraffin-embedded tissues were baked, deparaffinized, and hydrated by a graded series of ethanol washes. Antigen retrieval was done in a steamer in citrate buffer (pH 6.0) at 90+ °C for 25 min. Sections were then probed with primary ALDH antibody (BD Biosciences; 1:100) for 1 h at room temperature, followed by a blocking step with Dual Endogenous Enzyme Block (DAKO). The reaction was developed using the EnVision+ visualization system (DAKO) with chromogen 3,3′-diaminobenzidine and counterstained with hematoxylin.
Relative quantification of cells with high ALDH expression was done using Frida analysis software.7
Pretreatment with IPI-269609 for Peri-implantational Hedgehog Blockade
To further dissect the contribution of Hedgehog signaling to tumor initiation, the Hedgehog pathway was blocked with IPI-269609 only at a 7-d interval shortly before and after s.c. injection of pancreatic cancer cells into nude mice. In particular, pancreatic cancer cell line E3LZ10.7 or Capan-1 was treated with 6 µmol/L IPI-269609 or an equivalent amount of solvent in vitro for 3d. At the same time, application of IPI-269609 to nude mice was initiated using 7D s.c. osmotic Alzet pumps (Alzet). Cells were counted using a hemocytometer with trypan blue counterstain. Only suspensions with >95% viable cells were used for injection. A total of 5 × 106 cells were s.c. injected into the flanks of male CD1 nu/nu athymic mice in a total volume of 200 µL of PBS/Matrigel (1:1, v/v). Drug application to mice went on for a total of 7 d (i.e., for another 4 d after tumor cell injection). Tumor volumes were determined 7 and 14 d after injection using digital callipers, as described above.
Statistical Analysis
Kruskal-Wallis analysis was done using SPSS version 14.0 (SPSS, Inc.) for Microsoft Windows; two-tailed t test and Mann-Whitney U test were done using Prism version 4.00 (GraphPad Software, Inc.). P < 0.05 was regarded as statistically significant.
Results
IPI-269609 Inhibits Hedgehog Signaling In vitro
LightII cells were incubated with conditioned medium containing ShhN (ref. 10; Fig. 1A) at low-serum conditions for 48 hours. Treatment with 6 µmol/L IPI-269609 significantly inhibited the Gli-responsive reporter activity of LightII cells as compared with solvent-treated controls.
As shown in Fig. 1B, IPI-269609 impeded the reporter activity of ShhN-stimulated LightII cells in a dose-dependent manner. The degree of reporter inhibition in vitro was comparable to that of cyclopamine and tended to be slightly more pronounced at higher concentrations.
Using 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide assays on a panel of 21 pancreatic cancer cell lines, including several low-passage cell lines generated at our institution, we found a wide range of growth inhibition (0% to almost 100%) on treatment with 6 µmol/L IPI-269609 in low-serum conditions for 96 hours (Fig. 1C), which is in line with previous findings by our own group and by others (5–7).
For further in vitro characterization and for in vivo experiments, two pancreatic cancer cell lines were selected, E3LZ10.7 and Capan-1. These cell lines were selected based on the variable histologic spectrum of resulting xenografts (poorly differentiated in E3LZ10.7, moderately differentiated in the case of Capan-1), the variable spectrum of response to IPI-269609 in vitro (Fig. 1C), and their ability to form orthotopic xenografts in nude mice.
Hedgehog Inhibition with IPI-269609 Diminishes Migration and Colony Formation of Pancreatic Cancer Cells In vitro
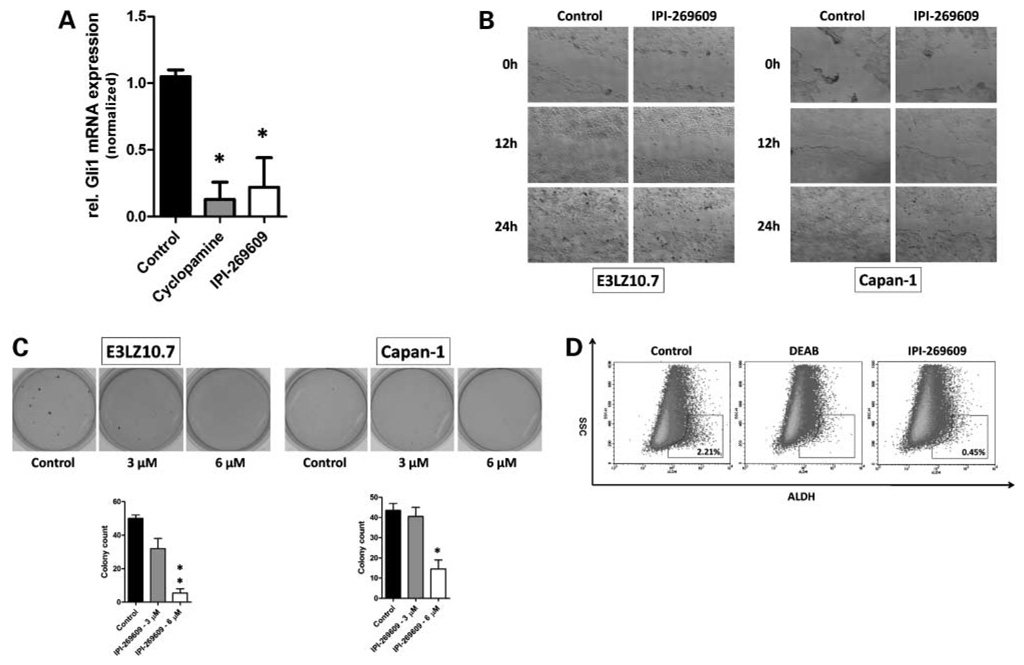
Incubation with IPI-269609 (6 µmol/L) for 48 hours resulted in down-regulation of the Hedgehog target gene Gli1 at the mRNA level in vitro (Fig. 2A).
Figure 2.
Treatment of pancreatic cancer cell lines with IPI-269609 in vitro resembles the biological effects of Hedgehog pathway blockade. Similarly to cyclopamine, incubation with IPI-269609 (6 µmol/L) caused a marked down-regulation of Gli1 steady-state mRNA levels in the pancreatic cancer cell line E3LZ10.7 (A). In vitro treatment with IPI-269609 reduced cell migration as observed in wound assays in E3LZ10.7 and Capan-1 (B), as well as colony formation and anchorage-independent growth in soft agar (C). IPI-269609 reduced the fraction of SSC-low/ALDH-bright cells in the E3LZ10.7 cell line. Treatment with the ALDH inhibitor diethylamino-benzaldehyde (DEAB) served as a negative control (D). The graph shows a representative of three independent experiments (*, P < 0.05; **, P < 0.01).
Moreover, the in vitro effects of Hedgehog inhibition on cell migration and clonogenecity, which had previously been found using the “standard” Hedgehog inhibitor cyclopamine, were also recapitulated when using the novel inhibitor IPI-269609 for pathway blockade. Namely, cell migration, as observed in wound healing assays, and colony formation in soft agar were both dramatically reduced on IPI-269609–mediated Hedgehog inhibition (Fig. 2B and C).
IPI-269609 Diminishes a Subpopulation of ALDH-Bright Pancreatic Cancer Cells In vitro
Inhibition of Hedgehog signaling in pancreatic cancer cell lines had previously been shown to reduce a subpopulation of SSC-low/ALDH-bright cells with tumor initiating (clonogenic) properties (7). In line with these previous results, we found that incubation of E3LZ10.7 with IPI-269609 (6 µmol/L) at low-serum conditions significantly reduced this fraction of SSC-low/ALDH-bright cells ~4-fold (Fig. 2D).
Treatment of S .c. E3LZ10.7 Xenografts
After observing that treatment with the novel small-molecule inhibitor IPI-269609 fully resembled the in vitro effects of Hedgehog inhibition with cyclopamine, we next sought to evaluate the in vivo effects of IPI-269609 treatment on pancreatic cancer growth and metastasis.
For this purpose, preestablished s.c. xenografts of E3LZ10.7 (n = 4 per treatment arm) were randomly assigned to receive (a) mock treatment, (b) IPI-269609 20 mg/d p.o., (c) gemcitabine 100 mg/kg i.p. every 4th day, or (d) combination treatment with IPI-269609 and gemcitabine at the given doses. Drug treatment was initiated 2 weeks after s.c. injection of the tumor cell suspension, at an average s.c. tumor volume of 60 to 70 mm3, and continued for 25 days.
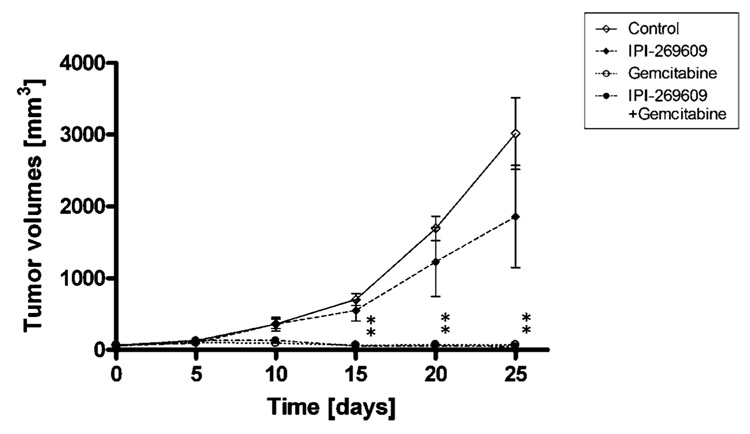
As shown in Fig. 3, s.c. tumor growth was slightly inhibited in IPI-269609–treated animals as compared with controls with an ~40% smaller average tumor volume at the end of treatment; however, these differences did not reach statistical significance, possibly due to the small number of animals per treatment arm and considerable interindividual variation in xenograft tumor sizes. Treatment with gemcitabine or the combination of IPI-269609 plus gemcitabine, on the other hand, led to marked inhibition of s.c. tumor growth as compared with mock-treated controls [all P < 0.01 beginning after 15 days of drug treatment (D15)]. No difference was observed between treatment with gemcitabine alone and combination therapy with IPI-269609 plus gemcitabine.
Figure 3.
Treatment of s.c. E3LZ10.7 xenografts with IPI-269609. Although after 25 d of drug treatment the average xenograft tumor size was ~40% smaller in IPI-269609 (20 mg/kg/d p.o.) treated animals as compared with controls, this difference was not statistically significant. Growth inhibition by gemcitabine (100 mg/kg i.p. every 4th day) was much more pronounced. There was no difference in growth inhibition between animals treated with gemcitabine alone and those treated with the combination of IPI-269609 plus gemcitabine (n = 4 per group). *, P < 0.05.
At the given doses, we did not observe any signs of toxicity; the animals were alert and active; histologies of the examined organs looked normal (data not shown); and there was no weight loss as determined by total body weights (data not shown).
IPI-269609 Treatment Abrogates Metastasis in Orthotopic Pancreatic Cancer Xenografts
Previous studies have shown that cyclopamine has minimal effects on s.c. tumor growth while profoundly inhibiting systemic metastases (7). Therefore, we wanted to examine whether IPI-269609 was capable of suppressing the development of distant organ metastases of pancreatic cancer xenografts in vivo. Using a surgical implantation technique, orthotopic E3LZ10.7 xenografts were generated in male CD1 nu/nu athymic mice, and treatment of established intrapancreatic tumors was initiated 3 weeks after intra-pancreatic implantation. Mice received either IPI-269609 (20 mg/kg/d) or mock treatment for a total of 30 days.
The tumor take rate for E3LZ10.7 was 100% with our surgical implantation method.
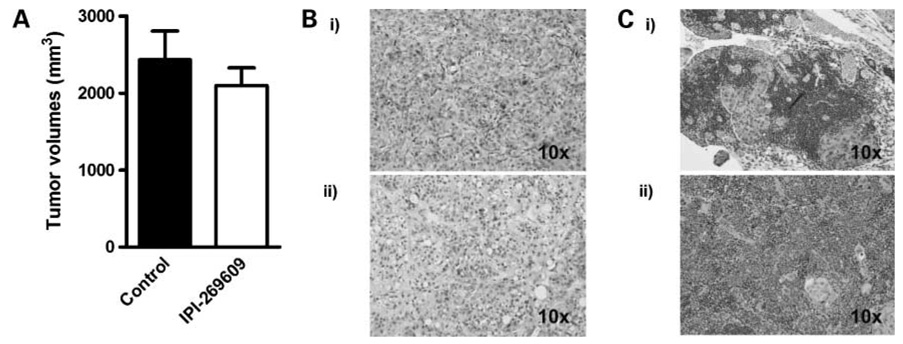
Whereas Hedgehog blockade with IPI-269609 had no effect on intrapancreatic “primary” tumor growth (Fig. 4A), we observed complete abrogation of distant metastases in IPI-269609–treated animals, whereas metastases in at least one distant organ site were observed in 5 of 5 (100%) of mock-treated animals (Table 1). Again, there was no growth retardation, behavioral abnormalities, or other obvious signs of toxicity at the given dose (data not shown).
Figure 4.
Treatment of orthotopic E3LZ10.7 xenografts with IPI-269609. A, treatment with IPI-269609 (20 mg/kg/d p.o.) for 30 d did not affect the growth of orthotopic E3LZ10.7 xenografts. B, H&E stainings of orthotopic E3LZ10.7 treated with IPI-269609 (i) or solvent only (ii ). C, examples of histologies of E3LZ10.7 metastases to lymph node (i) and spleen (ii ); H&E staining.
Table 1.
Numbers of animals with orthotopic E3LZ10.7 xenografts in which metastases to distant organ sites were found
| Group |
Control, n (%) |
IPI-269609, n (%) |
|---|---|---|
| No. animals | 5 | 5 |
| Lymph nodes | 2 of 5 (40) | 0 of 5 (0) |
| Spleen | 4 of 5 (80) | 0 of 5 (0) |
| Liver | 2 of 5 (40) | 0 of 5 (0) |
| Intestine | 0 of 5 (0) | 0 of 5 (0) |
| Lungs | 0 of 5 (0) | 0 of 5 (0) |
| Peritoneum | 2 of 5 (40) | 0 of 5 (0) |
| Kidneys | 2 of 5 (40) | 0 of 5 (0) |
Histologically, the orthotopic xenograft tumors resembled poorly differentiated pancreatic ductal adenocarcinomas; there were no visible differences between IPI-269609–treated and control animals (Fig. 4B and C).
These effects were confirmed using another pancreatic cancer cell line, Capan-1. Metastases in at least one distant organ site were found in 5 of 5 (100%) of control animals, but only two regional lymph node metastases were found in mice treated with IPI-269609 (Table 2).
Table 2.
Numbers of animals with orthotopic Capan-1 xenografts in which metastases to distant organ sites were found
| Group |
Control, n (%) |
IPI-269609, n (%) |
|---|---|---|
| No. animals | 5 | 5 |
| Lymph nodes | 4 of 5 (80) | 2 of 5 (40) |
| Spleen | 5 of 5 (100) | 0 of 5 (0) |
| Liver | 4 of 5 (80) | 0 of 5 (0) |
| Intestine | 5 of 5 (100) | 0 of 5 (0) |
| Lungs | 1 of 5 (20) | 0 of 5 (0) |
| Peritoneum | 1 of 5 (20) | 0 of 5 (0) |
| Kidneys | 1 of 5 (20) | 0 of 5 (0) |
At the end of treatment, Capan-1 tumor volumes tended to be smaller in IPI-269609–treated mice, but again this difference did not reach statistical significance (P = 0.095) due to considerable interindividual variation in tumor size (data not shown). Again, no signs of toxicity or body weight loss were observed in this cohort.
Histologically, these tumors resembled moderately differentiated ductal adenocarcinomas, with no differences in IPI-269609–treated versus control group (data not shown).
Abrogation of ALDH-Bright Pancreatic Cancer Cells In vivo
Previous studies by others have shown that a subpopulation of cells with high ALDH activity shows increased clonogenic potential in a variety of malignancies (14–17), and we have recently shown that Hedgehog inhibition reduces this population in pancreatic cancer cell lines in vitro (7).
To evaluate whether Hedgehog inhibition with IPI-269609 reduced the fraction of cells with high ALDH activity in pancreatic cancer xenografts in vivo, orthotopic E3LZ10.7 xenografts (n = 3 per treatment arm) were treated with IPI-269609 for 5 days using s.c. osmotic Alzet pumps, and ALDH expression was assessed by immunohistochemistry.
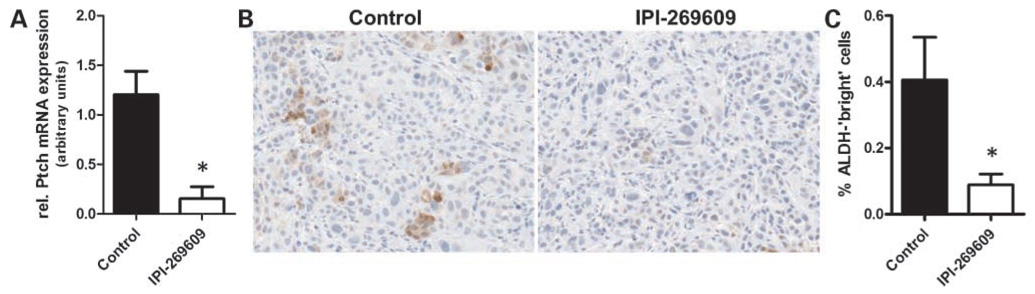
Hedgehog inhibition was mirrored by down-regulation of intratumoral steady-state Ptch mRNA levels (P = 0.021; Fig. 5A). Of note, Hedgehog pathway inhibition with IPI-269609 was accompanied by a significant reduction of cells with high ALDH activity in vivo in these tumors as observed using immunohistochemistry (Fig. 5B and C).
Figure 5.
Application of IPI-269609 caused significant down-regulation of the Hedgehog target gene Ptch1 at the mRNA level in vivo in E3LZ10.7 xenografts (A), which was accompanied by reduction of cancer cells with high ALDH activity as visualized by immunohistochemistry (B). ALDH-positive cells were quantified using Frida analysis software (C; *, P < 0.05).
Pretreatment with IPI-269609 Diminishes Tumorigenicity In vivo
The experimental results obtained thus far, as described above, seemed to indicate that Hedgehog inhibition had profound influence on inhibiting tumor metastases, likely mediated in part by targeting a subpopulation of tumor cells with high ALDH activity, with minimal effect on the “bulk” primary tumors. Because metastasis is functionally equivalent to tumor initiation at a distant site, we hypothesized that ex vivo pretreatment of cells with IPI-269609 could preferentially eliminate the tumorigenic subpopulation and block xenograft engraftment.
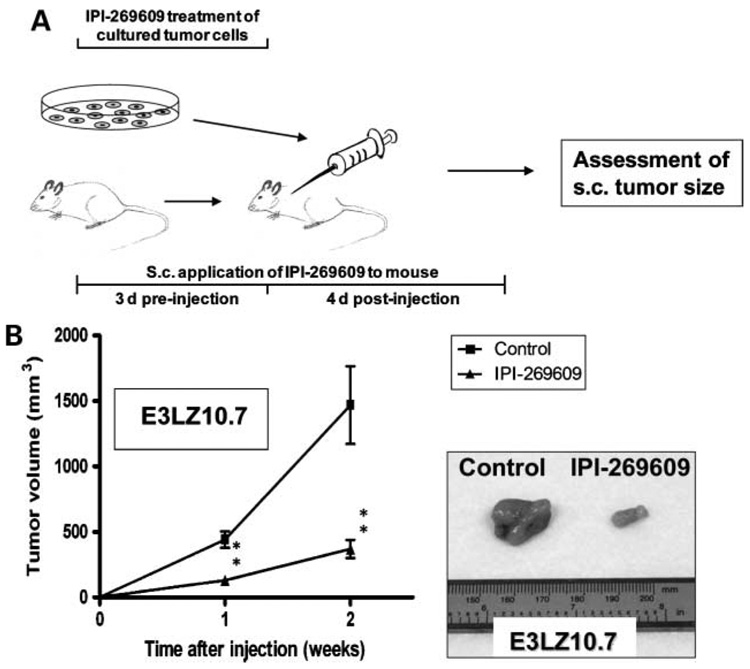
To further corroborate this hypothesis, we speculated whether blockade of Hedgehog signaling solely during the time of tumor initiation was sufficient to affect the growth of pancreatic cancer xenografts. For this purpose, pancreatic cancer cell line E3LZ10.7 or Capan-1 was incubated with IPI-269609 (6 µmol/L) or solvent for 3 days preceding s.c. injection into nude mice. At the same time, Hedgehog signaling was blocked with IPI-269609 in nude mice using s.c. Alzet pumps. Drug application with pumps went on over a total period of 7 days (i.e., another 4 days after tumor cell injection; Fig. 6A).
Figure 6.
Peri-implantational Hedgehog blockade is sufficient to cause in vivo growth inhibition of pancreatic cancer xenografts. A, treatment scheme used: Hedgehog signaling in pancreatic cancer cell lines was suppressed by treatment with IPI-269609 in vitro for 3 d. At the same time, continuous application of IPI-269609 to nude mice using s.c. osmotic pumps was initiated and continued for another 4 d after implantation of tumor cells. Mock treatment with solvent only was used as control, and only cell suspensions with >95% viable cells were used for injection. S.c. tumor growth was measured 1 and 2 wk after cancer cell injection. Pretreatment with IPI-269609 led to significantly reduced growth of s.c. E3LZ10.7 and xenografts in nude mice (B; n = 6 per group). **, P < 0.01.
The aim of pretreating pancreatic cancer cells in vitro was to eliminate ALDH-bright cells before injection into nude mice. Application of IPI-269609 to nude mice was done to abrogate any potential contribution of host stroma cells to pathway activation by secretion of Hedgehog ligand, and therefore to block Hedgehog signaling as completely as possible during tumor initiation. After that, no further drug was applied, but simply the growth of s.c. xenograft tumors was followed.
Surprisingly, this peri-implantational Hedgehog block-ade was sufficient to cause significant growth retardation in xenografts of both Capan-1 (data not shown) and E3LZ10.7 (Fig. 6B). In fact, growth retardation was more pronounced than observed in treatment of preestablished xenografts as described above.
Discussion
Aberrant reactivation of Hedgehog signaling in pancreatic cancer was first described in 2003 by two groups, including our own (5, 6). Hedgehog activation seems to mainly be mediated through aberrant ligand overexpression by pancreatic cancer cells (5), but more recently, alternative mechanisms have been proposed that might contribute to increased Hedgehog pathway activity in pancreatic cancer as well (e.g., through silencing of the pathway inhibitor human Hedgehog interacting protein due to promoter hypermethylation; ref. 18).
Using s.c. xenografts in nude mice as an in vivo model of pancreatic cancer and cyclopamine as a model small-molecule Hedgehog inhibitor, these initial reports already suggested pharmacologic blockade of Hedgehog signaling as a potential new treatment option for pancreatic cancer. Moreover, these studies also indicated that growth inhibition of pancreatic cancer xenografts might be more pronounced when Hedgehog blockade was begun concomitantly with injection of tumor cells into nude mice (5), rather than when applied to already established xenografts (6). Indeed, a recent report by our own group showed that Hedgehog blockade with cyclopamine led to a marked inhibition of colony formation in vitro and prevented the development of metastases in vivo using orthotopic xenografts, and that treatment of pancreatic cancer cell lines led to reduction of a SSC-low/ALDH-bright subpopulation that show tumor-initiating properties in vitro (7).
In the present study, we show that the novel small-molecule Hedgehog inhibitor IPI-269609 fully resembles the properties of cyclopamine to block Hedgehog signaling in pancreatic cancer cells in vitro and in vivo. Cell motility and in vitro colony formation were impaired by treatment with IPI-269609 in vitro; moreover, distant metastases in vivo, as observed using orthotopic murine xenografts of human pancreatic cancer, were essentially abolished, similar to our previous observations using cyclopamine (7). This is in line with previous reports suggesting that Hedgehog signaling mediates epithelial-to-mesenchymal transition and thus confers increased motility and invasive capacity to cancer cells (7, 19, 20).
We had previously shown that Hedgehog inhibition with cyclopamine reduced the fraction of SSC-low/ALDH-bright cancer cells (7), which had originally been identified in hematologic malignancies and are characterized by significantly higher rates of engraftment in nonobese diabetic/severe combined immunodeficient mice (14–17). In the present study, we found that similar to cyclopamine, treatment with IPI-269609 reduces this subpopulation in pancreatic cancer cell lines in vitro. Moreover, we show for the first time that Hedgehog blockade seems to reduce the fraction of ALDH-overexpressing cancer cells in vivo as observed using immunohistochemistry, and suggest that this might be a major mechanism by which Hedgehog blockade prevents tumor initiation and development of metastases. In this model, the last steps of the metastatic cascade are interpreted as initiation of distinct novel tumors at distant organ sites.
The findings presented here support a recent report by Bar et al. (21), who describe that cyclopamine treatment reduced the fraction of ALDH-bright glioblastoma cells and abrogated its ability to form neurospheres in vitro as well as its potential to grow tumors in athymic mice.
To date, we have never been able to clearly predict the response of pancreatic cancer cell lines or xenografts to pharmacologic Hedgehog pathway inhibition based on the extent of Hedgehog ligand expression.8 Therefore, it is interesting to speculate whether staining of ALDH-bright cells as presented here might be suitable to serve as a surrogate biomarker to predict overall prognosis or sensitivity to Hedgehog pathway inhibition in the setting of pancreatic cancer in the future.
Our observations are, to some degree, in line with another recent report by Li et al. (8), which describes identification of a subpopulation of pancreatic cancer cells with stem cell–like properties; CD24+/CD44+/ESA+ cells were found to have significantly increased tumorigenic capacity as compared with bulk tumor cells, as well as to show other properties of stem cells, like self-renewal and the ability to produce differentiated progeny. Of note, this putative pancreatic cancer stem cell fraction was also characterized by ~10-fold overexpression of Sonic Hedgehog ligand SHH as compared to “bulk” tumor cells and ~50-fold SHH overexpression as compared with surrounding nonmalignant pancreas tissue, implying that increased Hedgehog signaling might be a defining feature of this putative pancreatic cancer stem cell population.
Whichever concept will be adopted in the future, the mentioned observations together with the experimental results presented in this present study support the hypothesis that targeting the Hedgehog pathway might prove to be a valid therapeutic strategy that could be particularly effective in preventing formation of metastasis. In other words, targeting aberrant Hedgehog signaling with small-molecule inhibitors might provide the tool to effectively target the subpopulation of putative cancer stem cells in the future (22).
Clinical evaluation of pharmacologic Hedgehog blockade as a novel cancer treatment strategy has in the last years been hampered by the lack of suitable substances that might serve as future drugs for use in humans. The novel small-molecule Hedgehog pathway inhibitor IPI-269609 was designed with the intention to overcome this shortcoming. It is readily water soluble; modification of the galenics, in the present study for example addition of cyclodextrin, can be used to further increase its solubility. The drug showed strong pathway knockdown in vitro and in vivo, and at the applied doses there were no signs of toxicity, weight loss, or other signs of adverse events in mice during the described treatment periods. IPI-269609 might therefore be an interesting candidate substance for future clinical testing.
A 2004 study by Romer et al. (23) reported in vitro inhibition of Hedgehog pathway activity as well as profound retardation of tumor growth and prolonged survival in a PTCH1+/−/p53−/− transgenic mouse model of medulloblastoma by a small-molecule inhibitor, which had previously been identified in a high-throughput screening (24). However, the efficacy of this compound in the setting of pancreatic cancer has yet to be confirmed, and clinical data about the adverse effects and toxicity in humans are still not available to date.
Of note, another small-molecule Hedgehog pathway antagonist, GDC-0449 (Genentech), showed a favorable toxicity profile and promising initial efficacy in patients with different solid cancers (i.e., in basal cell carcinomas, in phase I clinical trials; ref. 25) and is currently being evaluated as combination regimen with chemotherapy and bevacizumab as first-line therapy in metastatic colorectal cancer patients in a phase II trial,9 with additional phase II trials likely to soon be initiated.
If pharmacologic Hedgehog blockade will in fact be successfully translated into clinical application at some time in the future, it is quite possible that combination of this therapeutic strategy with other drugs will be superior to single-agent therapy. Combination with already established drug regimens like gemcitabine, which aims at reducing bulk tumor mass, or other novel, targeted therapies are likewise imaginable (7, 26). Although we did not find any synergistic effect of combination therapy with gemcitabine plus IPI-269609 in growth inhibition of s.c. E3LZ10.7 xenografts, possibly due to the relatively pronounced growth inhibitory effect of gemcitabine alone in this model at the given dose, it is entirely possible that potential synergistic effects could be unmasked by administration of lower, suboptimal gemcitabine doses.
Taken together, the results presented here support the concept that pharmacologically targeting aberrant Hedge-hog signaling is a valid approach for the development of novel therapeutic regimens for pancreatic cancer. The availability of suitable pathway inhibitors that might be evaluated in a clinical setting constitutes another step toward translation of this strategy into clinical application in the future.
Acknowledgments
We thank Dr. Christine Iacobuzio-Donahue (Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD) for kindly providing the low-passage pancreatic cancer cell line E3LZ10.7; Dr. Philip A. Beachy (Howard Hughes Medical Institute, Department of Molecular Biology and Genetics, Johns Hopkins University School of Medicine; present address: Department of Developmental Biology and Institute for Stem Cell Biology and Regenerative Medicine, Stanford, CA 94305-5329) for the generous gifts of the stably transfected cell lines 293-ShhN-pIND and Light2; and Marc Halushka for his help in using Frida software for analysis of immunohistochemical stainings.
Grant support: NIH grant R01CA113669 (A. Maitra), the Sol Goldman Pancreatic Cancer Research Center, and Michael Rolfe Foundation. G. Feldmann was supported by a fellowship grant within the postdoc program of the German Academic Exchange Service (DAAD).
Footnotes
Unpublished data.
Disclosure of Potential Conflicts of Interest
K. McGovern, Infinity Pharmaceuticals employee. Infinity Pharmaceuticals donated cyclopamine and IPI-269609 for this study and approved the manuscript prior to publication, as regulated by a material transfer agreement. IPI-269609 is covered by U.S. patent no. US 7,230,004 B2, owned by Infinity Discovery, Inc., Cambridge, MA. The other authors reported no potential conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Goggins M. Identifying molecular markers for the early detection of pancreatic neoplasia. Semin Oncol. 2007;34:303–310. doi: 10.1053/j.seminoncol.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lohr JM. Medical treatment of pancreatic cancer. Expert Rev Anticancer Ther. 2007;7:533–544. doi: 10.1586/14737140.7.4.533. [DOI] [PubMed] [Google Scholar]
- 4.Ujiki MB, Talamonti MS. Guidelines for the surgical management of pancreatic adenocarcinoma. Semin Oncol. 2007;34:311–320. doi: 10.1053/j.seminoncol.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Berman DM, Karhadkar SS, Maitra A, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–851. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 6.Thayer SP, di Magliano MP, Heiser PW, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldmann G, Dhara S, Fendrich V, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67:2187–2196. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 9.Lipinski RJ, Hutson PR, Hannam PW, et al. Dose- and route-dependent teratogenicity, toxicity, and pharmacokinetic profiles of the Hedgehog signaling antagonist cyclopamine in the mouse. Toxicol Sci. 2008;104:189–197. doi: 10.1093/toxsci/kfn076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taipale J, Chen JK, Cooper MK, et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 11.Adams J, Castro AC, Foley MA, et al. inventors; Infinity Discoveries, Inc., Cambridge, MA, assignee. Cyclopamine analogues and methods of use thereof. United States. 2007. 2007 June 12; [Google Scholar]
- 12.Tremblay M, McGovern K, Nevalainen M, et al. Synthesis of novel, chemically stable D-homo-cyclopamine analogs via a cyclopropanation/ring-expansion sequence; Tilton (NH): Gordon Research Conference on Natural Products.2007. [Google Scholar]
- 13.Sui G, Bonde P, Dhara S, et al. Epidermal growth factor receptor and hedgehog signaling pathways are active in esophageal cancer cells from rat reflux model. J Surg Res. 2006;134:1–9. doi: 10.1016/j.jss.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 14.Storms RW, Green PD, Safford KM, et al. Distinct hematopoietic progenitor compartments are delineated by the expression of aldehyde dehydrogenase and CD34. Blood. 2005;106:95–102. doi: 10.1182/blood-2004-09-3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hess DA, Meyerrose TE, Wirthlin L, et al. Functional characterization of highly purified human hematopoietic repopulating cells isolated according to aldehyde dehydrogenase activity. Blood. 2004;104:1648–1655. doi: 10.1182/blood-2004-02-0448. [DOI] [PubMed] [Google Scholar]
- 16.Pearce DJ, Taussig D, Simpson C, et al. Characterization of cells with a high aldehyde dehydrogenase activity from cord blood and acute myeloid leukemia samples. Stem Cells. 2005;23:752–760. doi: 10.1634/stemcells.2004-0292. [DOI] [PubMed] [Google Scholar]
- 17.Hess DA, Wirthlin L, Craft TP, et al. Selection based on CD133 and high aldehyde dehydrogenase activity isolates long-term reconstituting human hematopoietic stem cells. Blood. 2006;107:2162–2169. doi: 10.1182/blood-2005-06-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin ST, Sato N, Dhara S, et al. Aberrant methylation of the human Hedgehog interacting protein (HHIP) gene in pancreatic neoplasms. Cancer Biol Ther. 2005;4:728–733. doi: 10.4161/cbt.4.7.1802. [DOI] [PubMed] [Google Scholar]
- 19.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Zhou BP, Hung MC. Wnt, hedgehog and snail: sister pathways that control by GSK-3β and β-Trcp in the regulation of metastasis. Cell Cycle. 2005;4:772–776. doi: 10.4161/cc.4.6.1744. [DOI] [PubMed] [Google Scholar]
- 21.Bar EE, Chaudhry A, Lin A, et al. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25:2524–2533. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lou H, Dean M. Targeted therapy for cancer stem cells: the patched pathway and ABC transporters. Oncogene. 2007;26:1357–1360. doi: 10.1038/sj.onc.1210200. [DOI] [PubMed] [Google Scholar]
- 23.Romer JT, Kimura H, Magdaleno S, et al. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/−)p53(−/−) mice. Cancer Cell. 2004;6:229–240. doi: 10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 24.Williams JA, Guicherit OM, Zaharian BI, et al. Identification of a small molecule inhibitor of the hedgehog signaling pathway: effects on basal cell carcinoma-like lesions. Proc Natl Acad Sci U S A. 2003;100:4616–4621. doi: 10.1073/pnas.0732813100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garber K. Hedgehog drugs begin to show results. J Natl Cancer Inst. 2008;100:692–697. doi: 10.1093/jnci/djn169. [DOI] [PubMed] [Google Scholar]
- 26.Jinawath A, Akiyama Y, Sripa B, Yuasa Y. Dual blockade of the Hedgehog and ERK1/2 pathways coordinately decreases proliferation and survival of cholangiocarcinoma cells. J Cancer Res Clin Oncol. 2007;133:271–278. doi: 10.1007/s00432-006-0166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]