Abstract
Objective
To determine if the C421A single nucleotide polymorphism (SNP) in the ATP-binding cassette transporter ABCG2 increases prostate cancer risk or affects survival.
Patients, subjects and methods
Numerous studies have suggested that dietary, hormonal and environmental factors all play a role in the initiation in prostate cancer; among these, the carcinogenic heterocyclic amine 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), a known substrate of the ABCG2. A SNP of ABCG2, C421A, resulting in a glutamine to lysine change at amino acid 141, has been shown to result in decreased function of the protein. Due to the expression of ABCG2 in the prostate, together with the purported role of dietary carcinogens and steroids in the development and progression of prostate cancer, 311 individuals were genotyped for the ABCG2 C421A SNP, 170 patients with androgen-independent prostate cancer (AIPC) and 141 ‘healthy’ controls. We also evaluated the effect of this SNP on the intracellular accumulation of PhIP and testosterone in vitro.
Results
There were no significant differences in the prevalence of prostate cancer based on ABCG2 genetic variation in this population. However, survival was significantly longer for individuals with wild-type ABCG2, as compared with those hetero- or homozygous for the C421A SNP (7.4 years vs 5.3 years, P = 0.044).
Conclusion
Intracellular accumulation of PhIP was 80% higher in HEK293 cells transfected with Q141K ABCG2 than in wild-type cells, confirming that this SNP decreases transport of PhIP. In contrast, testosterone was not transported by either wild-type or variant transfected cells, nor did it act as in inhibitor of ABCG2 in subsequent transport assays.
Keywords: transport, prostate cancer, mutations, diet, survival, risk
Introduction
Prostate cancer is the most prevalent systemic neoplasm diagnosed in American men, and exposure to dietary and environmental carcinogens may play a role in the development of prostate cancer. One example of this is 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), the most abundant carcinogenic heterocyclic amine. Formed during the cooking and charring of meat, PhIP has been shown to induce tumours of the prostate, large intestine, and mammary glands in rats [1,2]. Human prostate and colon cancers have also been linked to PhIP exposure [3–6]. In addition, the role of endogenous hormones in cancer progression has been studied extensively [7]. As many of these compounds are purported to be transported by ATP-binding cassette, sub-family G (WHITE), member 2 (ABCG2), we sought to explore the possible link between ABCG2 and prostate cancer incidence.
ABCG2, also known as breast cancer resistance protein, (BCRP) or mitoxantrone-resistance protein (MXR), is a member of the ABC transporter family [8]. The ABCG2 gene encodes a 655-amino acid half-transporter, which is thought to dimerize or multimerize to form a functional protein [9]. ABCG2 is highly expressed in the gastrointestinal tract, brain endothelium, and placenta where is thought to affect oral absorption of drugs, form part of the blood-brain barrier or maternal-fetal barrier, respectively [10]. Furthermore, it is expressed in both normal prostate epithelial and endothelial cells [11,12]. In addition to actively effluxing a wide range of endogenous compounds, toxins and carcinogens out of cells, ABCG2 transports many anticancer drugs, such as topotecan, imatinib, flavopiridol, methotrexate, and mitoxantrone, as well as several nonchemotherapeutic agents [13]. As such, it is implicated in both the pharmacokinetic variability of substrate drugs and the development of multidrug resistance [14,15].
Several groups have conducted studies searching for single nucleotide polymorphisms (SNPs) in the ABCG2 gene, resulting in the discovery of the C421A SNP that results in a glutamine to lysine change at amino acid 141 [15]. The frequency of this SNP differs with ethnicity, ranging from 5% in African Americans to 34% in Han Chinese; American Caucasians are reported to have a 12% frequency of this variant allele [16]. Several studies have found this SNP to have functional consequences on the protein, leading to decreased drug efflux [17,18] or decreased surface expression [19,20]. The C421A SNP has also been shown to affect the pharmacokinetics of drugs such as diflomotecan [21] and 9-aminocamptothecin [22]. These data suggest that individuals with the C421A SNP might be exposed to higher concentrations of substrates, including sterols and PhIP, which we hypothesize may lead to increased risk of prostate cancer or poorer prognosis.
ABCG2 has also been shown to transport numerous compounds that have been linked to prostate cancer risk. Experiments performed by Janvilisri et al. [23] have suggested that ABCG2 interacts with sterols, and may play a role in the transport of cholesterol, oestradiol, testosterone and progesterone. Huss et al. [24] determined that dihydrotestosterone is also a substrate for ABCG2 and showed that efflux of this androgen can be competitively inhibited. ABCG2 also transports PhIP and appears to play a significant role in the disposition of this compound. In mice, systemic exposure to PhIP was 2.9-fold higher in ABCG2 knockouts than in wild-type mice, following oral administration [25].
Herein, we sought to determine the effects of the C421A SNP on prostate cancer incidence and its effect on survival after diagnosis. In addition, we evaluated the transport of testosterone and PhIP in cells expressing wild-type and variant ABCG2 to determine if they are differentially transported.
Patients, subjects and methods
All the patients were diagnosed with androgen-independent prostate cancer (AIPC) and were seen in the Medical Oncology Clinic at the National Cancer Institute (NCI), National Institutes of Health in Bethesda, MD, USA. Patients were seen in the context of various clinical research protocols for the treatment of AIPC. For this retrospective analysis, the patient population was limited to non-Hispanic Caucasian men. All patients are deceased. This analysis was approved by the NCI Institutional Review Board. Control samples were obtained from Valley Biomedical (Winchester, VA, USA). Control subjects were all non-Hispanic Caucasian men with no known diagnosis of prostate cancer.
For genotyping, isolation of genomic DNA was performed as described in Gardner et al. [26], and followed by amplification using the following PCR primers: 5′-TGGCAATCCTTGTATGAAGCAG-3′ and 5′-TTCACGTACAACACCACATTGCC-3′, for 40 cycles with an annealing temperature of 64 °C. Direct nucleotide sequencing was conducted using Big Dye Terminator Cycle Sequencing Ready Reaction kit V3.1 (Applied Biosystems, Foster City, CA, USA) and an ABI Prism 310 Genetic Analyser, utilizing the following sequencing primers: 5′-GCAGGTTCATCATTAGCTAGAAC-3′ and 5′-CCTACTTATGCTGATCATGAGC-3′.
The cell lines used were human embryonic kidney cells (HEK293) stably transfected with empty pcDNA3.1 vector (pcDNA3.1-HEK) or pcDNA3.1 vector containing full-length wild-type (482R-HEK) or Q141K (Q141K-HEK) ABCG2 have been previously characterized and were maintained as described [18,27]. MCF7 cells and MCF7FLV1000 were cultured in RPMI with 10% fetal bovine serum (FBS). MCF7FLV1000 (R482-ABCG2) were maintained in 1 μg/mL flavopiridol as described previously [28].
[14C]-PhIP and [3H]-testosterone accumulation assays pcDNA3.1-HEK, R482-HEK and Q141K-HEK cells (2.5 × 105 cells/well) were grown in monolayer in a 24-well tissue culture plate at 37 °C in the presence of 5% CO2 in Dulbecco’s Modified Eagle’s Medium supplemented with 10% FBS. The assay was initiated by incubating cells with either 10 μM [14C]-PhIP (10 mCi/mmole; Toronto Research Chemicals, Canada) or 30 nM [3H]-testosterone (Sigma-Aldrich, St. Louis, MO, USA) in the absence or presence of 10 μM ABCG2-specific inhibitor fumitremorgin C (FTC) (Developmental Therapeutics Program, Natural Products Extraction Laboratory, National Institutes of Health (NIH), Bethesda, MD, USA) at 32 °C for 30 min. After incubation, cells were washed with PBS and lysed by incubation with 0.3 mL/well trypsin/EDTA at 37 °C for 30 min. The cell lysates were transferred to scintillation vials containing 5 mL of Biosafe II scintillation fluid and the radioactivity was measured in a scintillation counter. Cells washed with PBS immediately after addition of the assay mix were used as the ‘0’-min time point. The value for accumulated [14C]-PhIP or [3H]-testosterone at the ‘0’-min was subtracted from a given time point as nonspecific binding to the cells. Drug accumulation of was expressed as nmoles/million cells. Each experiment was performed three times.
Determination of cell surface expression of ABCG2
The cell surface expression of ABCG2 in pcDNA3.1-HEK, R482-HEK and Q141K-HEK cells was determined as described previously [29]. Briefly the cells were incubated in a 1 : 3500 dilution of unlabelled 5D3 antibody (eBioscience, San Diego, CA, USA) for 2 h in 2% BSA. Subsequently, the cells were washed and then incubated with APC-labelled anti-mouse secondary antibody (1 : 35, Leinco Technologies, Inc., St. Louis, MO, USA). APC fluorescence was detected on a FACSort flow cytometer (Becton Dickinson, San Jose, CA, USA) equipped with a 635-nm red diode laser and a 561-nm bandpass filter. For all samples, at least 10 000 events were collected. Debris was eliminated by gating on forward scatter vs side scatter and dead cells were excluded based on propidium iodide staining.
125I-iodoarylazidoprazosin (IAAP) accumulation assay
MCF7 cells and MCF7FLV1000 cells were incubated with 3–5 nM of 125I-IAAP (Perkin Elmer Life Sciences, Wellesley, MA, USA), in the presence of 20 μM FTC, 40 μM testosterone (Sigma) or vehicle control for 30 min at 32 °C as described previously [30]. After incubation, cells were washed with PBS and lysed by incubation with 0.3 mL/well trypsin/EDTA at 37 °C for 30 min. The cell lysates were transferred to scintillation vials containing 5 mL of Biosafe II scintillation fluid and the radioactivity was measured in a scintillation counter. Cells washed with PBS immediately after addition of the assay mix were used as the ‘0’-min time point. The value for accumulated [125I]-IAAP at ‘0’-min was subtracted from a given time point as nonspecific binding to the cells. Drug accumulation was expressed as nmoles/million cells. Each experiment was performed three times.
Statistical analyses
The statistical difference in genotype distribution between the two groups was evaluated using Mehta’s version of Fisher’s exact test [31]. Survival was calculated as the time from the date of diagnosis to the date of death and the statistical significance is based on the two-tailed log-rank P-value, interpolated from the generated Kaplan—Meier curve. PhIP-accumulation data was compared using a two-sided t-test to compare groups of interest, and a P < 0.05 was considered to indicate statistical significance.
Results
ABCG2 C421A SNP frequency
We determined the frequency of the variant A allele to be 12% in healthy non-Hispanic Caucasian controls, with 19% of the genotyped individuals heterozygous for the C421A SNP (n = 27/141). In non-Hispanic Caucasian patients with prostate cancer, the frequency of the A allele was 9%, with 16% of genotyped individuals heterozygous for this SNP (n = 27/170) as shown in Table 1. As such, there was no significant difference in the frequency of the C421A SNP between healthy individuals and those with prostate cancer (P = 0.34). The odds ratio for prostate cancer in patients with the A allele was 0.7 (95% CI 0.42–1.19). Allele frequencies for both groups were in Hardy—Weinberg equilibrium.
Table 1.
Comparison of genotypic frequencies of ABCG2 C421A SNP in patients diagnosed with AIPC and healthy volunteers (control). There was no significant difference in allele frequency between the groups (P = 0.34)
| N | ABCG2 C421A genotypic frequency, n (%) |
|||
|---|---|---|---|---|
| CC | CA | AA | ||
| AIPC | 170 | 142 (83.5) | 27 (15.9) | 1 (0.6) |
| Control | 141 | 111 (78.7) | 27 (19.2) | 3 (2.1) |
Correlation of ABCG2 C421A genotype with survival
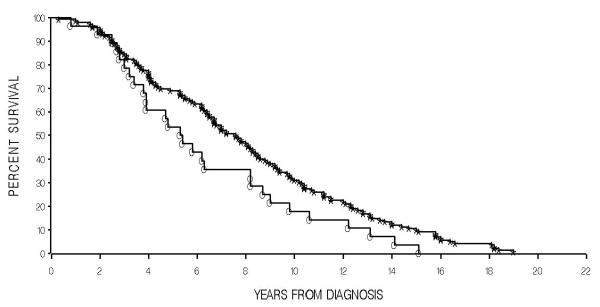
Although the C421A SNP does not seem to predict for prostate cancer, amongst patients with the disease, the A allele appears to predict for lower overall survival. The median overall survival for the 149 patients with C/C genotype was 7.4 years vs 5.3 years for patients with a C/A (28 patients) or A/A (two) genotype. The difference in survival in this retrospective analysis is significant (P = 0.044), as shown in Fig. 1. Demographics did not differ significantly among patients carrying wild-type and variant alleles as shown in Table 2.
Fig. 1.
Increased survival in patients diagnosed with AIPC carrying the wild-type ABCG2 C421A allele. Individuals with C/C genotype (stars, 142 patients) had a median survival of 7.4 years, as compared with individuals with a C/A or A/A genotype (open circles, 28) with a median survival of 5.3 years (P = 0.044).
Table 2.
Comparison of age and Gleason score for patients with AIPC, according to ABCG2 C421A SNP
| ABCG2 C421A SNP |
Median (mean, range) |
|
|---|---|---|
| Age, years | Gleason score | |
| All | 8 (4–10) | |
| at diagnosis | 61 (61, 40–78) | |
| at death | 70 (69, 43–86) | |
| C/C | 8 (4–10) | |
| at diagnosis | 61 (61, 40–78) | |
| at death | 70 (69, 43–86) | |
| C/A or A/A | 8 (6–10) | |
| at diagnosis | 65 (62, 45–76) | |
| at death | 72 (69, 50–81) | |
Effect of C421A SNP on in vitro PhIP transport
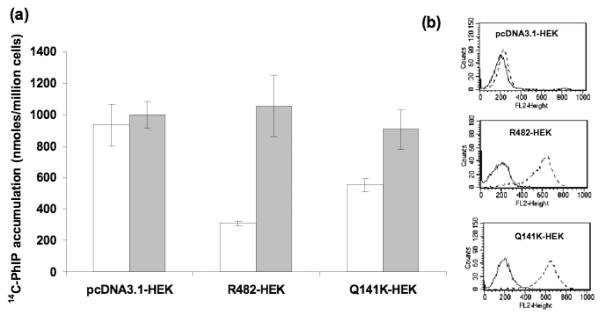
Comparison of the pcDNA3.1-HEK cells that do not express ABCG2 with the R482-HEK cells that express wild-type ABCG2 showed a three-fold decrease in intracellular accumulation of [14C]-PhIP, in the absence of FTC (P < 0.001) as shown in Fig. 2a. The cells expressing the C421A variant form of ABCG2 (Q141K-HEK) had a reduced ability to efflux [14C]-PHIP, resulting in a 1.8-fold increase in intracellular accumulation of this substrate compared with the wild-type cells (P < 0.001). The presence of 10 μM FTC inhibited the activity of both wild-type and C421A ABCG2, resulting in intracellular accumulation of [14C]-PHIP comparable with the control (pcDNA3.1 HEK) cells. Comparative ABCG2 expression levels were confirmed in the R482-HEK and Q141K-HEK cell lines, using flow cytometry as shown in Fig. 2b.
Fig. 2.
C421A SNP results in decreased intracellular PhIP accumulation in vitro. Green and rede bars represent PhIP accumulation with and without the presence of FTC, respectively. pcDNA3.1-HEK cells do not express ABCG2. R482-HEK cells express wild-type ABCG2 and Q141K-HEK cells are homozygous variant for the C421A SNP. Each bar represents the mean accumulation (n = 3) and the error bars indicate the S D. Flow cytometric experiments showing equal cell surface expression of ABCG2 in wild-type and variant transfected cell lines.
Effect of C421A SNP on in vitro testosterone transport
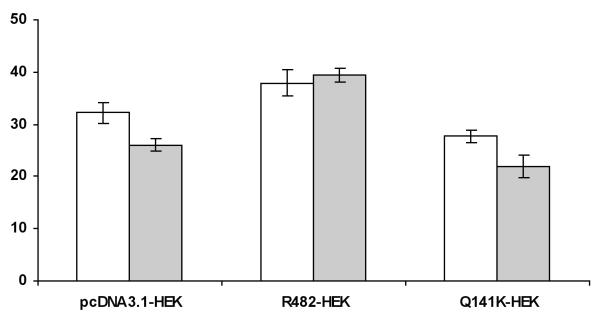
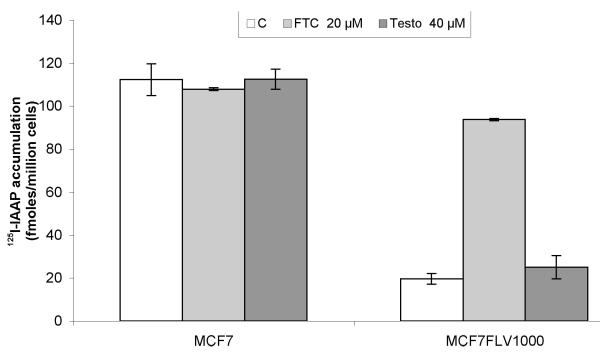
Transport assays using transfected R482-HEK and Q141K-HEK cells showed that testosterone was not transported by either wild-type or variant ABCG2. There was no significant difference in intracellular drug accumulation between these cell lines and the empty vector cells (pcDNA3.1-HEK) as seen in Fig. 3. To determine whether testosterone could inhibit ABCG2 transport, MCF7 and MCF7/FLV1000 cells were incubated with radiolabelled IAAP, a probe ABCG2 substrate, in combination with testosterone or FTC (an inhibitor of ABCG2). In MCF7/FLV1000 cells, which overexpress ABCG2, testosterone did not inhibit drug efflux, as shown in Fig. 4.
Fig. 3.
Lack of testosterone transport by wild-type or C421A variant ABCG2. Green and red bars represent testosterone accumulation with and without the presence of FTC, respectively. pcDNA3.1-HEK cells do not express ABCG2. R482-HEK cells express wild-type ABCG2 and Q141K-HEK cells are homozygous variant for the C421A SNP. Each bar represents the mean accumulation (n = 3) and the error bars indicate the S D.
Fig. 4.
Testosterone does not inhibit ABCG2 transport of IAAP. Red, green and light-red bars represent intracellular accumulation of 125I-IAAP, a probe substrate of ABCG2, in the presence of vehicle control, 20 μM FTC and 40 μM testosterone, respectively. In MCF7/FLV1000 cells, which overexpress ABCG2, FTC inhibits drug efflux from the cell. However, testosterone does not significantly affect drug efflux.
Discussion
It was hypothesized that individuals carrying the C421A SNP may be at increased risk of prostate cancer, from a possible increase in exposure to carcinogens and androgens both systemically and in the prostate gland itself, due to the localization of this transporter. The frequency of the ABCG2 C421A SNP was analysed in 170 patients with AIPC, along with 141 healthy controls. There was no statistically significant difference in allele frequency between these groups, and the present findings are in accordance with previously published allele frequencies in volunteers [16]. Hence, it is unlikely that the ABCG2 C421A SNP is associated with an increased risk of prostate cancer in non-Hispanic Caucasian Americans.
Comparing survival from the date of the initial prostate cancer diagnosis, suggests that individuals with the wild-type ABCG2 genotype, encoding for the fully functional protein, had increased survival (7.4 years) as compared with those carrying one or more C421A variant alleles (5.3 years), which encode for a protein with decreased function. The patients analysed in the present study span numerous clinical trials and were treated with a wide range of agents (including thalidomide, docetaxel and suramin), so these findings are thought to be independent of treatment.
Conversely, a recent smaller study, evaluating the effect of this SNP in patients with prostate cancer who received docetaxel together with either estramustine or vinorelbine, found an increase in 15-month survival from the start of treatment on study, for individuals carrying the variant allele [32]. The authors suggest that this increase in survival for patients with the C421A SNP might be due to increased intracellular accumulation of docetaxel, leading to improved response. However, docetaxel has not been shown to be a substrate for ABCG2.
In the present study, variant ABCG2 lead to increased intracellular accumulation of PhIP, as compared with wild-type ABCG2, in stably transfected HEK293 cells. If this phenomenon occurs in vivo, it is possible that an increase in carcinogen exposure contributes to the decline in survival in individuals with prostate cancer, though it does not appear to increase the risk of developing prostate cancer. Due to the localization of the transporter, this SNP could be increasing both systemic and localized prostate exposure to ABCG2 substrates.
Assessment of ATPase activity led Janvilisri et al. [23] to originally conclude that testosterone interacts with ABCG2, but the mechanism was not demonstrated. A functional steroid-binding element has subsequently been identified in ABCG2 [33]. The present data in the HEK cells expressing wild-type and C421A ABCG2 suggest that testosterone is neither transported by nor inhibits transport by ABCG2.
Huss et al. [24] have identified putative malignant prostate stem cells in biopsy samples as those expressing ABCG2, but lacking androgen receptor (AR). These cells survived androgen deprivation and retained their proliferative potential in the absence of androgen, in both the prostates of patients with advanced disease and in primary xenografts in castrated animals. When ABCG2 transport was decreased (through addition of an inhibitor) in cells expressing little detectable AR protein, a significant increase (up to 25-fold) of AR protein occurred, due to stabilization of intracellular AR. Inhibition of ABCG2 led to an increase of dihydrotestosterone accumulation and increased cellular proliferation. As such, it is possible that in individuals with AIPC, the hetero- or homozygous forms of ABCG2 do not sufficiently efflux dihydrotestosterone out of these prostate cancer cells, leading to increased proliferation and subsequent decreased survival. Further investigation is necessary to confirm this possible link between ABCG2 C421A genotype and dihydrotestosterone.
Identification of ABCG2 substrates is a relatively new area of interest, so it is possible that other, as yet unidentified, compounds contribute to the decline in prostate cancer survival observed in individuals carrying one or more C421A variant alleles. Though PhIP is differentially transported by wild-type and variant ABCG2, clinical studies are required to conclusively demonstrate causality. Finally, as the present study was performed only in patients with AIPC (the typical group seen at the NCI), the link between the C421A SNP and prostate cancer survival, together with the lack of correlation with disease risk, must be confirmed in a larger population.
Acknowledgements
This project has been funded in whole or in part with federal funds from the NCI, NIH, under contract N01-CO-12400.# The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. #E.R. Gardner
This research was supported by the Intramural Research Program of the NIH, NCI, Center for Cancer Research.
Abbreviations
- NCI
National Cancer Institute
- PhIP
2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine
- ABCG2
ATP-binding cassette, sub-family G (WHITE), member 2
- SNP
single nucleotide polymorphism
- AIPC
androgen-independent prostate cancer
- HEK
human embryonic kidney
- FBS
fetal bovine serum
- FTC
fumitremorgin C (ABCG2-specific inhibitor)
- IAAP
iodoarylazidoprazosin
- NIH
National Institutes of Health
- AR
androgen receptor
Footnotes
Conflict of Interest
None declared. Source of funding: Intramural NCI.
References
- 1.Ito N, Hasegawa R, Sano M, et al. A new colon and mammary carcinogen in cooked food, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) Carcinogenesis. 1991;12:1503–6. doi: 10.1093/carcin/12.8.1503. [DOI] [PubMed] [Google Scholar]
- 2.Shirai T, Sano M, Tamano S, et al. The prostate: a target for carcinogenicity of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) derived from cooked foods. Cancer Res. 1997;57:195–8. [PubMed] [Google Scholar]
- 3.Bogen KT, Keating GA. U.S. dietary exposures to heterocyclic amines. J Expo Anal Environ Epidemiol. 2001;11:155–68. doi: 10.1038/sj.jea.7500158. [DOI] [PubMed] [Google Scholar]
- 4.Cross AJ, Peters U, Kirsh VA, et al. A prospective study of meat and meat mutagens and prostate cancer risk. Cancer Res. 2005;65:11779–84. doi: 10.1158/0008-5472.CAN-05-2191. [DOI] [PubMed] [Google Scholar]
- 5.Sinha R, Kulldorff M, Chow WH, Denobile J, Rothman N. Dietary intake of heterocyclic amines, meat-derived mutagenic activity, and risk of colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 2001;10:559–62. [PubMed] [Google Scholar]
- 6.Sinha R, Peters U, Cross AJ, et al. Meat, meat cooking methods and preservation, and risk for colorectal adenoma. Cancer Res. 2005;65:8034–41. doi: 10.1158/0008-5472.CAN-04-3429. [DOI] [PubMed] [Google Scholar]
- 7.Hsing AW, Reichardt JK, Stanczyk FZ. Hormones and prostate cancer: current perspectives and future directions. Prostate. 2002;52:213–35. doi: 10.1002/pros.10108. [DOI] [PubMed] [Google Scholar]
- 8.Sarkadi B, Homolya L, Szakacs G, Varadi A. Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol Rev. 2006;86:1179–236. doi: 10.1152/physrev.00037.2005. [DOI] [PubMed] [Google Scholar]
- 9.Xu J, Liu Y, Yang Y, Bates S, Zhang JT. Characterization of oligomeric human half-ABC transporter ATP-binding cassette G2. J Biol Chem. 2004;279:19781–9. doi: 10.1074/jbc.M310785200. [DOI] [PubMed] [Google Scholar]
- 10.Lepper ER, Nooter K, Verweij J, Acharya MR, Figg WD, Sparreboom A. Mechanisms of resistance to anticancer drugs: the role of the polymorphic ABC transporters ABCB1 and ABCG2. Pharmacogenomics. 2005;6:115–38. doi: 10.1517/14622416.6.2.115. [DOI] [PubMed] [Google Scholar]
- 11.Fetsch PA, Abati A, Litman T, et al. Localization of the ABCG2 mitoxantrone resistance-associated protein in normal tissues. Cancer Lett. 2006;235:84–92. doi: 10.1016/j.canlet.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 12.Pascal LE, Oudes AJ, Petersen TW, et al. Molecular and cellular characterization of ABCG2 in the prostate. BMC Urol. 2007;7:6. doi: 10.1186/1471-2490-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obligacion R, Murray M, Ramzan I. Drug-metabolizing enzymes and transporters: expression in the human prostate and roles in prostate drug disposition. J Androl. 2006;27:138–50. doi: 10.2164/jandrol.05113. [DOI] [PubMed] [Google Scholar]
- 14.Cascorbi I. Role of pharmacogenetics of ATP-binding cassette transporters in the pharmacokinetics of drugs. Pharmacol Ther. 2006;112:457–73. doi: 10.1016/j.pharmthera.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Robey RW, Polgar O, Deeken J, To KW, Bates SE. ABCG2: determining its relevance in clinical drug resistance. Cancer Metastasis Rev. 2007;26:39–57. doi: 10.1007/s10555-007-9042-6. [DOI] [PubMed] [Google Scholar]
- 16.de Jong FA, Marsh S, Mathijssen RH, et al. ABCG2 pharmacogenetics: ethnic differences in allele frequency and assessment of influence on irinotecan disposition. Clin Cancer Res. 2004;10:5889–94. doi: 10.1158/1078-0432.CCR-04-0144. [DOI] [PubMed] [Google Scholar]
- 17.Mizuarai S, Aozasa N, Kotani H. Single nucleotide polymorphisms result in impaired membrane localization and reduced ATPase activity in multidrug transporter ABCG2. Int J Cancer. 2004;109:238–46. doi: 10.1002/ijc.11669. [DOI] [PubMed] [Google Scholar]
- 18.Morisaki K, Robey RW, Ozvegy-Laczka C, et al. Single nucleotide polymorphisms modify the transporter activity of ABCG2. Cancer Chemother Pharmacol. 2005;56:161–72. doi: 10.1007/s00280-004-0931-x. [DOI] [PubMed] [Google Scholar]
- 19.Imai Y, Nakane M, Kage K, et al. C421A polymorphism in the human breast cancer resistance protein gene is associated with low expression of Q141K protein and low-level drug resistance. Mol Cancer Therapeutics. 2002;1:611–6. [PubMed] [Google Scholar]
- 20.Kondo C, Suzuki H, Itoda M, et al. Functional analysis of SNPs variants of BCRP/ABCG2. Pharm Res. 2004;21:1895–903. doi: 10.1023/b:pham.0000045245.21637.d4. [DOI] [PubMed] [Google Scholar]
- 21.Sparreboom A, Gelderblom H, Marsh S, et al. Diflomotecan pharmacokinetics in relation to ABCG2 421C>A genotype. Clin Pharmacol Ther. 2004;76:38–44. doi: 10.1016/j.clpt.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Zamboni WC, Ramanathan RK, McLeod HL, et al. Disposition of 9-nitrocamptothecin and its 9-aminocamptothecin metabolite in relation to ABC transporter genotypes. Invest New Drugs. 2006;24:393–401. doi: 10.1007/s10637-006-6335-5. [DOI] [PubMed] [Google Scholar]
- 23.Janvilisri T, Venter H, Shahi S, Reuter G, Balakrishnan L, van Veen HW. Sterol transport by the human breast cancer resistance protein (ABCG2) expressed in Lactococcus lactis. J Biol Chem. 2003;278:20645–51. doi: 10.1074/jbc.M301358200. [DOI] [PubMed] [Google Scholar]
- 24.Huss WJ, Gray DR, Greenberg NM, Mohler JL, Smith GJ. Breast cancer resistance protein-mediated efflux of androgen in putative benign and malignant prostate stem cells. Cancer Res. 2005;65:6640–50. doi: 10.1158/0008-5472.CAN-04-2548. [DOI] [PubMed] [Google Scholar]
- 25.van Herwaarden AE, Jonker JW, Wagenaar E, et al. The breast cancer resistance protein (Bcrp1/Abcg2) restricts exposure to the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Cancer Res. 2003;63:6447–52. [PubMed] [Google Scholar]
- 26.Gardner ER, Burger H, van Schaik RH, et al. Association of enzyme and transporter genotypes with the pharmacokinetics of imatinib. Clin Pharmacol Ther. 2006;80:192–201. doi: 10.1016/j.clpt.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Robey RW, Honjo Y, Morisaki K, et al. Mutations at amino-acid 482 in the ABCG2 gene affect substrate and antagonist specificity. Br J Cancer. 2003;89:1971–8. doi: 10.1038/sj.bjc.6601370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robey RW, Medina-Perez WY, Nishiyama K, et al. Overexpression of the ATP-binding cassette half-transporter, ABCG2 (Mxr/BCrp/ABCP1), in flavopiridol-resistant human breast cancer cells. Clin Cancer Res. 2001;7:145–52. [PubMed] [Google Scholar]
- 29.Robey RW, Shukla S, Steadman K, et al. Inhibition of ABCG2-mediated transport by protein kinase inhibitors with a bisindolylmaleimide or indolocarbazole structure. Mol Cancer Ther. 2007;6:1877–85. doi: 10.1158/1535-7163.MCT-06-0811. [DOI] [PubMed] [Google Scholar]
- 30.Shukla S, Robey RW, Bates SE, Ambudkar SV. The calcium channel blockers, 1,4-dihydropyridines, are substrates of the multidrug resistance-linked ABC drug transporter, ABCG2. Biochemistry. 2006;45:8940–51. doi: 10.1021/bi060552f. [DOI] [PubMed] [Google Scholar]
- 31.Mehta CR, Patel NR. A network algorithm for performing Fisher’s Exact Test in r × c contingency tables. J Am Stat Assoc. 1983;78:427–34. [Google Scholar]
- 32.Hahn NM, Marsh S, Fisher W, et al. Hoosier Oncology Group randomized phase II study of docetaxel, vinorelbine, and estramustine in combination in hormone-refractory prostate cancer with pharmacogenetic survival analysis. Clin Cancer Res. 2006;12:6094–9. doi: 10.1158/1078-0432.CCR-06-1188. [DOI] [PubMed] [Google Scholar]
- 33.Velamakanni S, Janvilisri T, Shahi S, van Veen HW. A functional steroid-binding element in an ATP-binding cassette multidrug transporter. Mol Pharmacol. 2008;73:12–7. doi: 10.1124/mol.108.038299. [DOI] [PubMed] [Google Scholar]