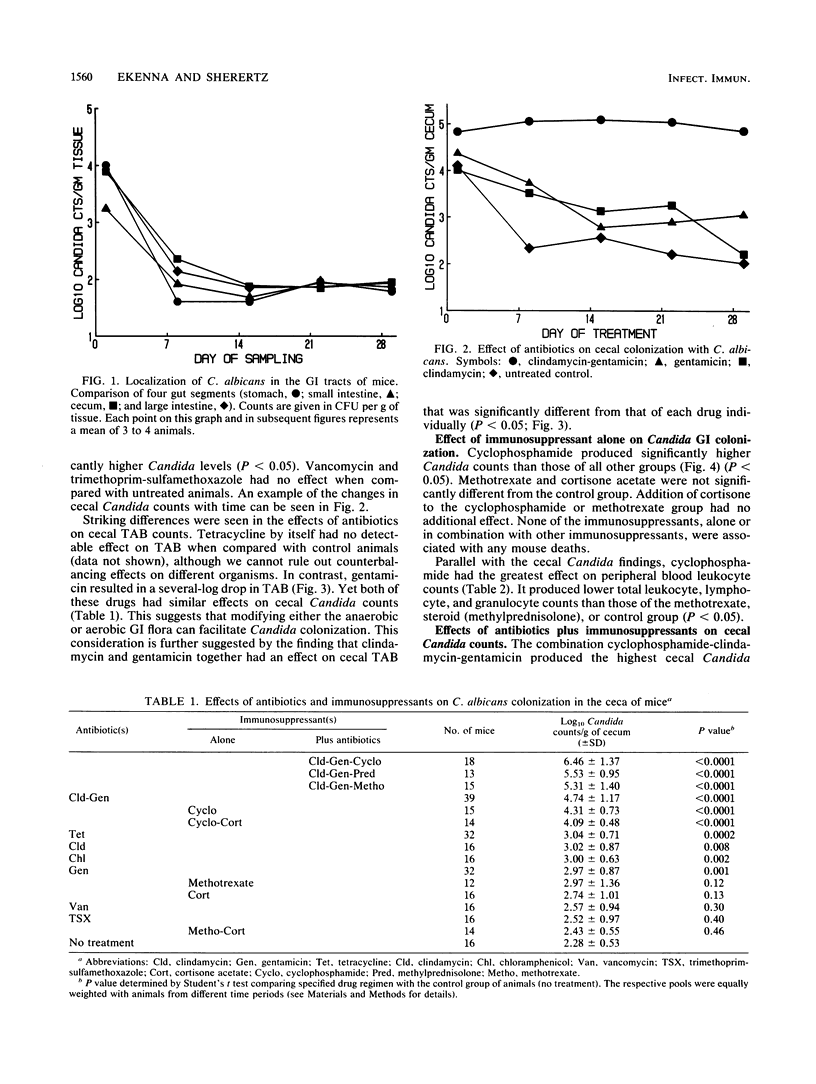
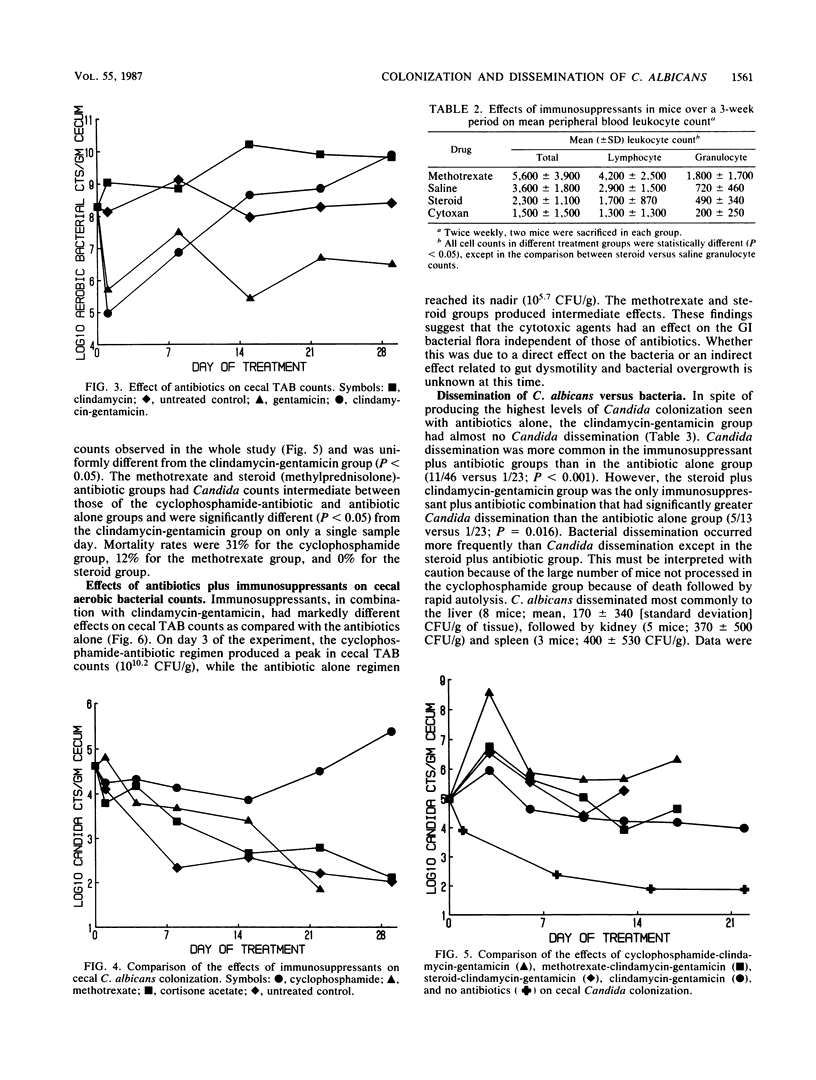
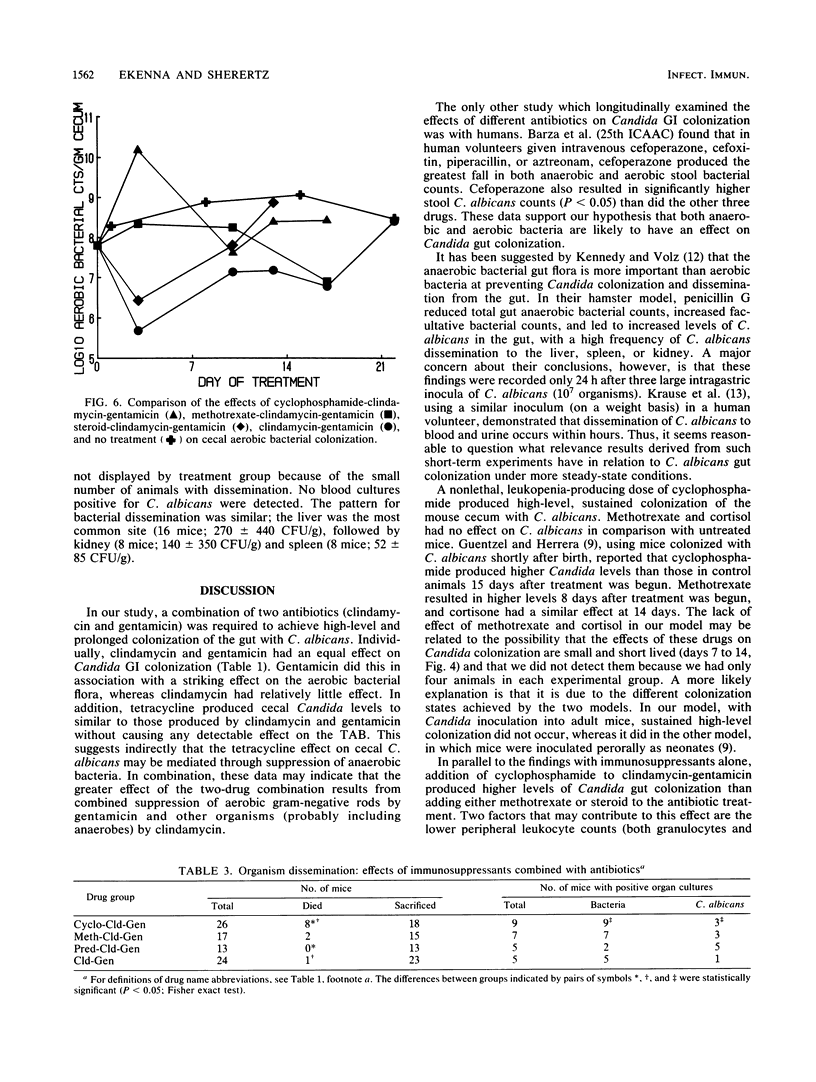
Abstract
Male ICR Swiss mice (2 to 3 months old) were fed Candida albicans in their drinking water for 3 days, followed by no treatment, antibiotics in their drinking water (daily), or immunosuppressants given by intraperitoneal injection (two to three times weekly) over a 3- to 4-week period. The organs of animals were processed to determine the numbers of C. albicans and total aerobic bacteria per g of tissue. Untreated animals had mean Candida counts during the 1-month period of 10(2.3) CFU/g of cecum. Animals in six of eight antibiotic-treated groups had mean cecal Candida counts higher than those of control animals (P less than 0.05), with clindamycin-gentamicin producing the highest counts (10(4.7) CFU/g). Cyclophosphamide produced counts (10(4.3) CFU/g) which were higher (P less than 0.05) than those resulting from methotrexate (10(3.0) CFU/g) or steroid (10(2.7) CFU/g) treatment. Cyclophosphamide-clindamycin-gentamicin treatment was associated with the highest (P less than 0.05) levels of Candida colonization (10(6.5) CFU/g). Mice receiving immunosuppressants plus clindamycin-gentamicin were more likely to disseminate C. albicans than were mice receiving antibiotics alone (P less than 0.001). Our findings suggest that colonization of the guts of mice by C. albicans can be facilitated by manipulating the aerobic, anaerobic, or both types of gut flora. The combined effect of immunosuppressants on both Candida gut colonization and dissemination appears multifactorial and deserves further investigation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodet C. A., 3rd, Jorgensen J. H., Drutz D. J. Antibacterial activities of antineoplastic agents. Antimicrob Agents Chemother. 1985 Sep;28(3):437–439. doi: 10.1128/aac.28.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodey G. P. Candidiasis in cancer patients. Am J Med. 1984 Oct 30;77(4D):13–19. [PubMed] [Google Scholar]
- Celle G., Dodero M., Bogliolo G., Mansi C., Pannacciulli I. Chronic treatment with azathioprine and cyclophosphamide in rats: structural and functional effects on small intestine and liver. G E N. 1977 Jan-Mar;31(3):179–184. [PubMed] [Google Scholar]
- Cohen R., Roth F. J., Delgado E., Ahearn D. G., Kalser M. H. Fungal flora of the normal human small and large intestine. N Engl J Med. 1969 Mar 20;280(12):638–641. doi: 10.1056/NEJM196903202801204. [DOI] [PubMed] [Google Scholar]
- Donaldson R. M., Jr Small bowel bacterial overgrowth. Adv Intern Med. 1970;16:191–212. [PubMed] [Google Scholar]
- Ecknauer R., Löhrs U. The effect of a single dose of cyclophosphamide on the jejunum of specified pathogenfree and germfree rats. Digestion. 1976;14(3):269–280. doi: 10.1159/000197940. [DOI] [PubMed] [Google Scholar]
- Eras P., Goldstein M. J., Sherlock P. Candida infection of the gastrointestinal tract. Medicine (Baltimore) 1972 Sep;51(5):367–379. doi: 10.1097/00005792-197209000-00002. [DOI] [PubMed] [Google Scholar]
- Gorbach S. L., Nahas L., Lerner P. I., Weinstein L. Studies of intestinal microflora. I. Effects of diet, age, and periodic sampling on numbers of fecal microorganisms in man. Gastroenterology. 1967 Dec;53(6):845–855. [PubMed] [Google Scholar]
- Guentzel M. N., Herrera C. Effects of compromising agents on candidosis in mice with persistent infections initiated in infancy. Infect Immun. 1982 Jan;35(1):222–228. doi: 10.1128/iai.35.1.222-228.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibullah C. M., Chhuttani P. N., Sehgal A. K. Effect of oral cyclophosphamide on the rat intestine. Indian J Med Sci. 1979 Jul;33(7):180–184. [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Antimicrobial activity of 21 anti-neoplastic agents. Br J Cancer. 1984 Mar;49(3):367–369. doi: 10.1038/bjc.1984.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. J., Volz P. A. Ecology of Candida albicans gut colonization: inhibition of Candida adhesion, colonization, and dissemination from the gastrointestinal tract by bacterial antagonism. Infect Immun. 1985 Sep;49(3):654–663. doi: 10.1128/iai.49.3.654-663.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause W., Matheis H., Wulf K. Fungaemia and funguria after oral administration of Candida albicans. Lancet. 1969 Mar 22;1(7595):598–599. doi: 10.1016/s0140-6736(69)91534-7. [DOI] [PubMed] [Google Scholar]
- Moss A. A., Goldberg H. I., Brotman M. Idiopathic intestinal pseudo-obstruction. Am J Roentgenol Radium Ther Nucl Med. 1972 Jun;115(2):312–317. doi: 10.2214/ajr.115.2.312. [DOI] [PubMed] [Google Scholar]
- Mulligan M. E., Citron D. M., McNamara B. T., Finegold S. M. Impact of cefoperazone therapy on fecal flora. Antimicrob Agents Chemother. 1982 Aug;22(2):226–230. doi: 10.1128/aac.22.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerowitz R. L., Pazin G. J., Allen C. M. Disseminated candidiasis. Changes in incidence, underlying diseases, and pathology. Am J Clin Pathol. 1977 Jul;68(1):29–38. doi: 10.1093/ajcp/68.1.29. [DOI] [PubMed] [Google Scholar]
- Pope L. M., Cole G. T. Comparative studies of gastrointestinal colonization and systemic spread by Candida albicans and nonlethal yeast in the infant mouse. Scan Electron Microsc. 1982;(Pt 4):1667–1676. [PubMed] [Google Scholar]
- Pope L. M., Cole G. T., Guentzel M. N., Berry L. J. Systemic and gastrointestinal candidiasis of infant mice after intragastric challenge. Infect Immun. 1979 Aug;25(2):702–707. doi: 10.1128/iai.25.2.702-707.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimpff S. C., Greene W. H., Young V. M., Fortner C. L., Cusack N., Block J. B., Wiernik P. H. Infection prevention in acute nonlymphocytic leukemia. Laminar air flow room reverse isolation with oral, nonabsorbable antibiotic prophylaxis. Ann Intern Med. 1975 Mar;82(3):351–358. doi: 10.7326/0003-4819-82-3-351. [DOI] [PubMed] [Google Scholar]
- Trier J. S., Bjorkman D. J. Esophageal, gastric, and intestinal candidiasis. Am J Med. 1984 Oct 30;77(4D):39–43. [PubMed] [Google Scholar]



