Abstract
The efficient use of nutrients is important in development and aging. In this study, we asked if the protein repair methyltransferase has a related or additional role in energy metabolism and stress response in the nematode Caenorhabditis elegans. Worms lacking the pcm-1 gene encoding this enzyme exhibit reduced longevity as SDS-isolated dauer larvae and as arrested L1 larvae under starvation stress, while overexpression leads to increased adult longevity. These findings led us to question whether pcm-1 deficient C. elegans may have inappropriate metabolic responses to stress. We assayed dauer and dauer-like larvae for starvation survival and observed a 2-fold reduction of median survival time for pcm-1 mutants compared to N2 wild-type worms. Under these conditions, pcm-1 deficient dauer larvae had reduced fat stores, suggesting that PCM-1 may have a role in the initiation of the correct metabolic responses to stress starvation. We show expression of the pcm-1 gene in neurons, body wall and reproductive tissues. Upon heat shock and dauer formation-inducing conditions, we observe additional pcm-1 expression in body wall muscle nuclei and actomyosin filaments and in hypodermal cells. These results suggest that this enzyme may be important in stress response pathways, including proper decision making for energy storage.
Keywords: pcm-1, L-isoaspartyl-O-methyltransferase, Caenorhabditis elegans, dauer larvae formation, survival, dauer recovery, fat storage, GFP expression
1. Introduction
The L-isoaspartyl-O-methyltransferase is an evolutionarily-conserved enzyme that initiates the repair of age-damaged aspartate and asparagine residues (Ingrosso et al., 2000; Clarke, 2003; Lanthier and Desrosiers, 2004, O’Connor, 2006). L-Aspartic acid and L-asparagine residues are prime targets of protein damage, as they undergo spontaneous deamidation, isomerization, and racemization reactions that result in altered L-isoaspartyl, D-aspartyl, and D-isoaspartyl residues (Brennan and Clarke, 1995). A deficiency of protein repair can lead to cell deterioration and premature aging in many species (O’Connor, 2006). The L-isoaspartyl-O-methyltransferase is encoded by the pcm-1 gene in the worm Caenorhabditis elegans (Kagan et al., 1997).
C. elegans is a simple multicellular organism with a short generation time and a well-defined life cycle, most of which is post-reproductive. C. elegans has the ability to bypass the growth pathway during unfavorable environmental conditions and enter into an alternate larval state known as the dauer stage (Golden and Riddle, 1984). The dauer larvae is a specialized larvae with extensive morphological and neuronal reconstructions, which allow dauer larvae to live for months when an adult C. elegans can only live for a few weeks (Klass and Hirsh, 1976; Golden and Riddle, 1984). The dauer larvae has been of interest to us because damaged proteins may accumulate during its extended life span, presenting an additional survival challenge, especially to pcm-1-deficient larvae.
In mice lacking the L-isoaspartyl protein repair methyltransferase, there are many severe phenotypes including seizures shortly after birth, decreased adult life span, enlarged brains and an altered metabolic state (Farrar and Clarke, 2005a; Farrar et al., 2005b). However, in lower organisms deficient in protein repair, such as bacteria and nematodes, phenotypes are generally only observed when the organism is stressed (Kagan et al., 1997; Niewmierzycka and Clarke, 1999;Kindrachuk et al., 2003; Hicks et al., 2005; Gomez et al., 2007). For instance, C. elegans adult pcm-1 mutants do not have reduced lifespan and do not show an increase in damaged proteins when compared to N2 wild-type nematodes (Kagan et al., 1997; Niewmierzycka and Clarke, 1999; Banfield et al., 2008). However, under environmental conditions that induce dauer and L1 diapauses, we have seen that the genetic loss of pcm-1 has been associated with a reduced ability to form dauer larvae, an SDS dauer larval sensitivity phenotype, a 40% reduced dauer larvae survival after SDS isolation, defects in autophagy during dauer formation and a reduced arrested-L1 larval lifespan (Kagan et al., 1997; Gomez et al., 2007; Banfield et al., 2008).
Remarkably, overexpression of PCM-1 in C. elegans leads to a 62% extended adult life span even when its absence does not reduce it (Banfield et al., 2008). In the fly Drosophila, overexpression of the repair methyltransferase has no effect on life span at 25 °C, but under heat shock conditions (29 °C) longevity was extended 32–39% (Chavous et al., 2001). These results, combined with previous studies demonstrating that aged pcm-1 mutants in C. elegans do not accumulate significantly more damaged proteins than wild-type cells, suggest that PCM-1 may have physiological roles in addition to, or in place of, its function in recognizing damaged proteins for repair.
Based on the phenotypes mentioned, pcm-1 mutants have been classified as dauer-defective mutants, daf-d. Previous epistasis analysis of pcm-1 with genes involved in insulin signaling and dauer formation show that PCM-1 may be involved in signaling pathways parallel or upstream of the nematode insulin-receptor homolog and dauer constitutive gene, daf-2, and downstream of other dauer formation genes daf-7 and daf-11 in dauer morphogenesis (Banfield et al., 2008). Additional support for placement of pcm-1 is given by microarray data which shows pcm-1 is upregulated during the dauer stage and in daf-2 mutants (McElwee et al., 2004). It is noteworthy that mutations in the gene for the DAF-2 insulin-like receptor lead to elevated autophagy during dauer formation, an increased dauer lifespan, and large stores of fat during dauer formation (Kimura et al., 1997; Melendez et al., 2003; Banfield et al., 2008), and mutations in genes encoding pathway components result in adult longevity and stress resistance (Ayyadevara et al., 2008).
Since pcm-1 is upregulated in the dauer stage as opposed to the adult stage (McElwee et al., 2004), it appears that pcm-1 phenotypes will be more pronounced under conditions of starvation stress. Our first goal was to determine if a population of non-SDS isolated dauer and dauer-like larvae of the pcm-1 genotype could survive as well as wild-type animals. We show that pcm-1 dauer and dauer-like larvae have an average of 46% decrease in wild-type survival ability; however, pcm-1 mutants do not have a reduced ability to recover and exit dauer diapause upon introduction to a food source, indicating that perhaps the inappropriate use of nutrient stores during starvation causes the pcm-1 survival defect. To test this theory, we used Sudan Black, a lipid stain, to qualitatively assess fat storage in pcm-1 and wild-type dauer larvae. We found that pcm-1 dauer larvae have decreased amounts of fat at the onset of dauer formation. The initial low levels of fat upon entry into the dauer stage may be the life limiting factor for pcm-1 mutants. Additionally, we found that pcm-1 expression occurs in the pharynx, body wall, and nervous and reproductive tissues under environmental conditions that favor growth and reproduction. However, under heat shock and in the dauer larval stage, pcm-1 expression in body wall muscle was found in nuclei and actomyosin filaments and hypodermal cell tissues. These results support the theory that PCM-1 may be important in maintenance of cellular integrity in compromised environments.
2. Materials and methods
2.1. Worm strains and culture conditions
Animals were maintained using standard methods (Brenner, 1974). All nematode culture stocks were kept at 20 °C on 60 mm Petri dishes holding 10 ml of nematode growth agar medium (NGM) and fed Escherichia coli OP50. Liquid culture stocks were maintained in baffled flasks with 350 ml S medium, shaken at 150 rpm at 20 °C and fed OP50. The wild-type N2 animals were obtained from the Caenorhabditis Genetics Center. The mutation used in this study was Linkage Group V: pcm-1(qa201) generated by Tc-1 mutagenesis and back crossed eight times to him-8, then five times to N2 (Banfield et al., 2008; Kagan et al., 1997). The pcm-1(tm363) strain was a gift from Shohei Mitani (Tokyo Women’s Medical University, Tokyo). The pcm-1(qa201) mutant was used for all experiments unless explicitly indicated otherwise.
2.2. Dauer Lifespan Analysis
A well fed culture of gravid C. elegans was hypochlorite treated. The eggs were then resuspended in M9 media and allowed to hatch overnight at 20 °C without food. The next day, the L1 larvae were counted and separated into experimental flasks at a density of 4000 animals/ml in a media composed of 1/3 fresh S medium and 2/3 sterilely-filtered old nematode culture media presumably rich in C. elegans pheromone. Larvae were fed minimally (approximately 1 ml of concentrated OP50 (from 3.4 l of culture) per 350 ml of C. elegans culture) and kept at 25 °C to induce dauer formation. Dauer formation occurred approximately 60 hours post hypochlorite treatment. When we use old media that is presumably highly concentrated with pheromone, we see nearly 100% dauer or dauer-like larvae formation. The dauer larvae and dauer-like larvae were split into aging flasks with M9 at a density of 2–3 × 103 animals/ml and maintained at 25 °C. Every 5–7 days, samples were taken from the aging flask using glass sampling tools, and a small volume of the aging culture was spotted onto an NGM agar plate for visualization. The total number of dead and alive animals was scored immediately, based on movement in response to touch stimulus. All measurements were done in triplicate. A similar procedure as described above was used for determining the lifespan of dauer larvae fractionated by SDS sensitivity as described byBanfield et al. (2008).
To statistically analyze our data, we used two methods. First, the exact binomial test was used to compute a p-value that would indicate the probability that N2 and pcm-1 survival was not different. Secondly, we estimated the median survival by careful interpolation of the values from the survival curves of at least 3 separate experimental populations. Maximal survival values were defined as the points in which all of the animals in our triplicate samples had died. After the median and maximal values had been computed for each of the separate experimental trials, we averaged the values to obtain a standard deviation to describe the variation seen in separate populations. Student’s t-tests were done to compute p-values to describe the significance of the differences between the different populations. T-test were also used to make comparisons between N2 and pcm-1 at specific points indicated as asterisks when p<0.05 on survival curves.
2.3. Dauer Recovery Assay
In addition to assaying survival of the dauer and dauer-like larvae, the ability for dauer larvae to recover beyond the dauer larval stage upon introduction to food at various ages was also considered. Only dauer larvae fractionated by SDS sensitivity were used in the recovery assay. All dauer larvae assayed in the survival assay were spotted onto an empty portion of an NGM plate away from the OP50 bacterial lawn. The recovery assay tested the ability of both larvae scored as dead and those scored as alive, to move from the original spot, migrate to the bacterial lawn, and develop to at least the post dauer stage 1 (PD1) 48 hours after the initial survival score. Most larvae were young adults by the time of the recovery score and the number of recovered animals never exceeded the number of larvae initially scored as alive. Hence, the percent recovery was calculated as the number of recovered animals divided by the number alive at the survival score.
2.4. Sudan Black staining of dauer larvae
Protocols to stain dauer larvae were done as described previously (Kimura et al., 1997; Ogg et al., 1997; Wolkow et al., 2000). In brief, 1 ml of 2.5 – 3 ×103 animals/ml of aging dauer culture was placed into a glass vial. The animals were collected by centrifugation. The nematode pellet was washed three times with phosphate buffered saline (PBS). Next, 0.75 ml of 1% paraformaldehyde in PBS was added, and rocked at room temperature for 15 minutes. Three freeze-thaws were performed before the animals were rocked for 5 min, incubated on ice for 10 min, and centrifuged. The sample was then subjected to three PBS washes, and three ethanol washes with 25%, 50%, and 70% ethanol, respectively. The ethanol washes consisted of rocking in ethanol for 3 minutes, centrifugation, and aspiration of the supernatant. Samples were stained overnight in 1 ml filtered 50% saturated Sudan Black dissolved in 70% ethanol. The animals were re-hydrated through three ethanol washes of 70%, 50%, 25% ethanol respectively and two PBS washes. The stained animals were mounted on 2% dry agarose pads and visualized with a Zeiss Axiovert microscope (Carl Zeiss MicroImaging, Thornwood, NY, U.S.A.).
2.5. Cloning the pcm-1 gene and promoter
A pcm-1 promoter green fluorescent protein (GFP) fusion, Ppcm-1::GFP, was made amplifying the 3 kb genomic region at coordinates 5966000…5969000 upstream of the pcm-1 start site. C. elegans genomic DNA was amplified using Expand Long Template PCR system (Roche) and primers: F, 5′ GCGCATGCGGTGTTTGTGTGATAGAAGTG, R, 5′ GCGGATCCCAGGCTATCCTCGCGTTTG 3′. The promoter insert was ligated into the BamHI and SphI cloning sites of the pPD92.62 vector. A PCM-1::GFP construct was made via a PCR fusion procedure as described (Hobert, 2002). The primer sequences used to construct the fusions are: 5′ CTGCAGGAGTGCGGTGCTAATTTC 3′, TGTTCGATCGGGAAACACGAGCCA, CTTTGGCCAATCCCGGGGATCATTGCGGTTCCATTGTTCTTCGC, and 5′ GCGAAGAACAATGGAACCGCAATGATCCCCGGGATTGGCCAAAG 3′. The PCM-1::GFP construct was generated at the Worm Core Facility (University of Utah).
2.6. Transgenic worm construction and analysis
All transgenic lines were created at the C. elegans Worm Core Research Facility (University of Utah). The PCM-1::GFP fusion constructs were injected at 5 ng/μl along with 80 ng/μl pRF4 and pBlueScript KS at 35 ng/μl and the Ppcm-1::GFP construct was injected at 50 ng/μl along with 80 ng/μl pRF4 and salmon sperm DNA at 25 ng/μl into the gonad arms of N2 adult hermaphrodites. Transgenic animals were identified by the Rol phenotype and stable lines were isolated. The PCM-1 promoter lines generated were named xtEx46–51 (Ppcm-1::GFP, pRF4) and the PCM-1::GFP fusions generated were named xtEx103-9(pcm-1::GFP, pRF4). Cells were identified by comparing the position of GFP-positive areas viewed by simultaneous fluorescence and differential interference contrast (DIC) microscopy. Transgenic dauer larvae were induced by culturing larvae on plates with 40 μl of crude pheromone/2 ml NGM media at 20 °C for about 60 h. Transgenic animals were heat shocked by placing L4 larvae and young adult worms at 34 °C for 3–4 h. Larvae were mounted on 2% dry agarose pads and observed on a Zeiss Axiovert Microscope at 40 power magnification.
3. Results
3.1. pcm-1 dauer and dauer-like larvae have a reduced dauer larval life span
It had previously been shown that pcm-1 dauer larvae exhibit a 40% decrease in longevity after isolation by the SDS method (Banfield et al., 2008). This treatment is usually effective because dauer larvae are defined by their resistance to SDS due to their impermeable cuticle that forms during dauer development (Golden and Riddle, 1984). However, SDS treatment kills about 70% of pcm-1 dauer larvae because they have dauer formation defects (Banfield et al., 2008). In order to know if the pcm-1 dauer larvae, which had not been fractionated by their resistance to SDS, would have a similar survival phenotype, a dauer lifespan procedure excluding SDS isolation was performed. Here, the survival of all dauer and dauer-like larvae was analyzed at 25 °C.
Based on careful eye-examination, at approximately 60 h post dauer induction, nearly 100% of N2 and pcm-1 larvae appeared to have formed dauer larvae based on the characteristic phenotypes of dark gut granules, elongated thin bodies, and no pharyngeal pumping (Golden and Riddle, 1984). Because no SDS separation was used, we must assume that some of the population were in fact dauer-like larvae (Albert et al., 1988). However, we note that there was no recovery of dauer larvae to larval stages beyond the dauer state over the course of three replicate dauer and dauer-like larval lifespan experiments.
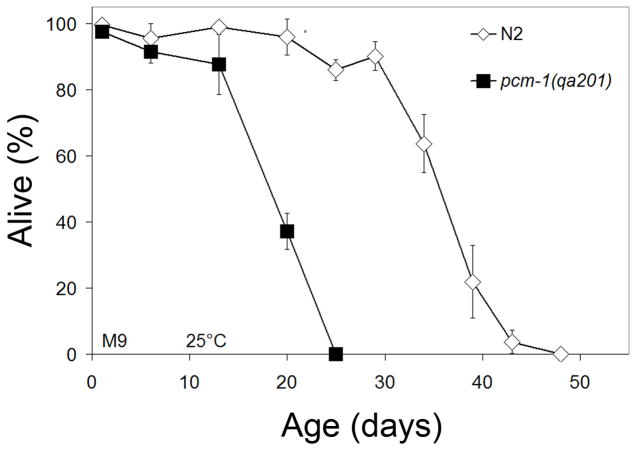
Using the non-SDS isolated dauer lifespan procedure, we found that pcm-1 dauer and dauer-like larvae display an average of 46% decrease in lifespan in comparison to N2 dauer and dauer-like larvae (Fig. 1, Table 1). In three experiments, it was found that N2 dauer and dauer-like larvae have a median and maximal lifespan of 27.7 ± 9.1 and 38.8 ± 9.0 days respectively, while pcm-1 dauer and dauer-like larvae have median and maximal lifespans of 14.2± 3.5 and 20.4 ± 4.5 days. A t-test comparison of the average maximal lifespans of both strains from all three replicate experiments gave a p-value of 0.035.
Fig. 1.
Dauer and Dauer-like Larvae Survival. Non-SDS isolated dauer and dauer-like larvae were aged in M9 at 25 °C and survival was assayed at various time points. Error bars indicate standard deviation from three replicate samples of 25–30 animals from the total population and asterisks indicate p-values less than 0.05 when comparing N2 and pcm-1 survival at the specified time point. Similar results were obtained in two replicate experiments at 25 °C.
Table 1.
Summary of survival experiments for dauer larvae. In each experiment, triplicate samples of 25–30 worms were analyzed at each time point as shown in Fig. 1.
| Experiment 1 | Experiment 2 | Experiment 3 | ||||
|---|---|---|---|---|---|---|
| N2 | pcm-1 | N2 | pcm-1 | N2 | pcm-1 | |
| Median life span (days)† | 35.6 | 18.2 | 29.6 | 12.4 | 17.8 | 11.8 |
| Maximal life span (days) § | 48 | 25 | 38 | 16 | 30 | 20 |
| % Reduction of maximal life span in mutanta | 48 | 58 | 33 | |||
| p-value* | 0.002 | 0.004 | 0.008 | |||
Median survival was estimated by careful analysis and interpolation of the dauer survival plots.
Maximal life span was the point at which the total population was dead.
% Reduction refers to the percent decrease observed in pcm-1 survival in comparison to wild-type
Exact Binomial two-tailed test p-value comparing all points of the N2 and pcm-1 survival curves.
To further confirm the importance of PCM-1 for normal dauer longevity, we used an additional pcm-1 deletion mutant, pcm-1(tm363). In C. elegans, the pcm-1 gene overlaps the C10F3.4 gene in an antiparallel arrangement. The C10F3.4 protein is a homolog of a plant GDP-L-galactose phosphorylase; its role in C. elegans remains to be determined (Linster et al., 2007). Because the pcm-1(qa201) deletion removes potential promoter elements and an alternatively spliced exon from the C10F3.4 gene, it was important to confirm the dauer larvae longevity defects in the pcm-1(tm363) allele that only removes intronic portions of C10F3.4. In an independent experiment, we found that pcm-1(tm363) dauer larvae had a maximum lifespan of 45 days at 24 °C in comparison to the pcm-1(qa201) which lived 30 days. N2 dauer larvae survived to day 60, longer than both pcm-1 allele mutants (data not shown). These results suggest that the dauer survival defect is caused by the absence of PCM-1. Previous results had shown that mutations in the active site of PCM-1 in transgenic constructs containing both the pcm-1 and C10F3.4 genes that would not affect the C10F3.4 protein led to a failure to rescue the dauer formation phenotype of pcm-1 mutants and a failure to display the adult longevity phenotype (Banfield et al., 2008).
3.2. Dauer larvae maintain a high recovery after long periods of arrest
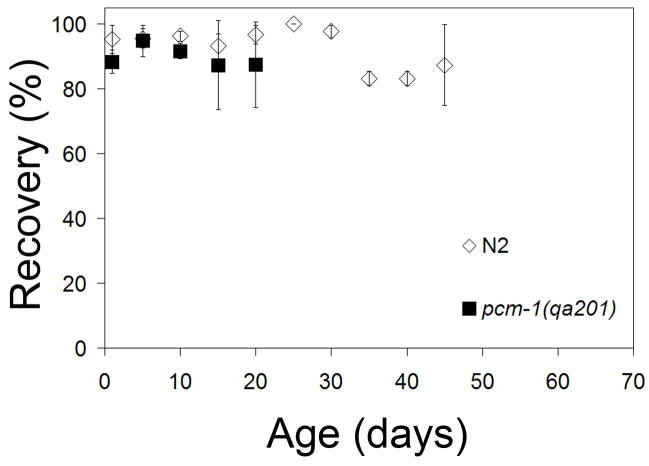
Recovery was defined as the ability for a dauer larvae scored as alive in the life span assay to migrate to a lawn of a bacterial food source and resume development. In this assay, we examined dauer larvae isolated by SDS treatment so no dauer-like larvae were present. The number of recovered animals was determined by comparing the percent of animals that had exited dauer larval arrest to the number of dauer larvae scored as alive in the initial assessment 48 h earlier. Two days after being plated on NGM with E. coli OP50, we found that nearly 100% of N2 and the pcm-1(qa201) mutant developed into L4 larvae or young adult animals regardless of the amount of time they had originally been starved (Fig. 2). N2 dauer larvae exhibited a slight decrease in recovery ability after 35 days of arrest; only about 80% of dauer larvae were able to recover. No differences were seen in the ability of N2 and pcm-1 dauer larvae to recover upon introduction to food (Fig. 2). It is possible that worms that did not respond to touch during the original survival score could have recovered. However, because the number that recovered was always less than the number scored alive, it appears that animals originally scored as dead were indeed dead.
Fig. 2.
Dauer Larvae Recovery. Starved dauer larvae were placed on OP50-seeded, NGM plates at various ages and incubated at 20 °C for two days. Recovery was defined as the ability to exit dauer arrest and is expressed as a percentage of the number of animals originally scored as alive. Error bars indicate the standard deviation obtained from three replicate measurements of the total population.
3.3. pcm-1 deficient dauer and dauer-like larvae have decreased fat stores
The daf-2(e1370) mutation causes an increase in both dauer longevity and dauer fat accumulation in comparison to wild-type (Banfield et al., 2008, Kimura et al., 1997). Hence, it was hypothesized that a decrease in dauer larvae fat accumulation may be one contributing factor to the decrease in pcm-1 dauer longevity. To test this hypothesis, dauer larvae of different ages were stained with Sudan Black, a fat-binding dye.
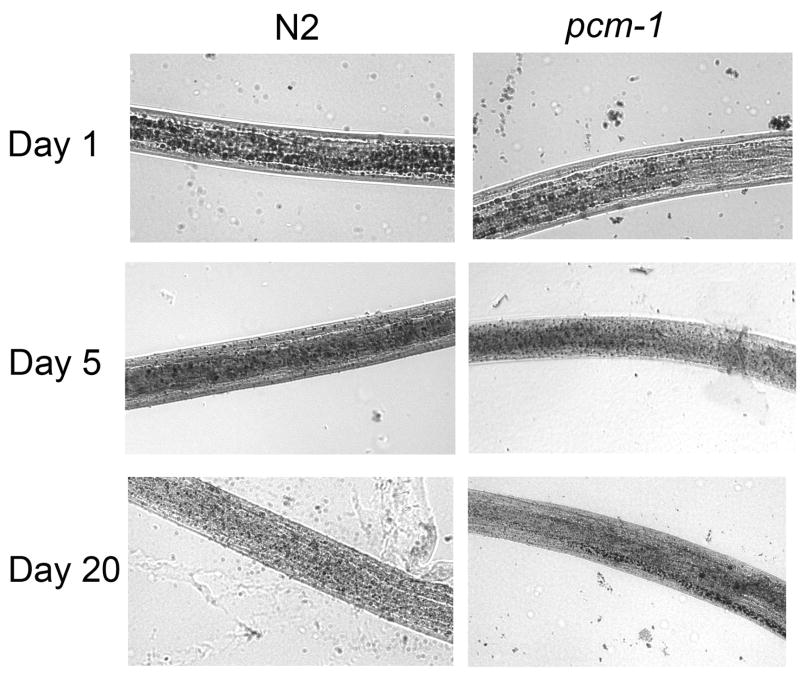
One day old pcm-1 dauer larvae exhibit reduced amounts of Sudan Black staining in comparison to N2 one day old dauer larvae (Fig. 3). By measuring the relative number of granules and their size in multiple images of dauer larvae, we estimated that pcm-1 fat stores were only about 24% of those of wild type animals (Table 2). As expected, as the dauer larvae age, N2 fat stores decrease with age (Fig. 3). At six days, wild type larvae had only about 29% of the fat reserves of wild type animals at one day, and pcm-1 larvae only had 5% of these reserves (Table 2). These trends continue to 25 days when the level of fat reserves in wild type and mutant dauer larvae are less than 3% of those of N2 dauer larvae at day one (Table 2). These data suggests that the fat stores are actively being used as a source of energy to maintain the vitality of the dauer larvae. It is possible, that because pcm-1 larvae do not have this initial large source of energy, their longevity is truncated. Decreased fat stores relative to wild type dauer larvae are seen in pcm-1 larvae with age through 20 days (Table 2). It is interesting to note the abnormalities in the appearance of fat in the pcm-1 dauer larvae as they age. At day 20, many pcm-1 dauer larvae show a characteristic line of dark fat accumulation on one side of their body, while fat is nearly absent throughout the remaining portions of the animal (Fig. 3). This characteristic line of fat was not observed in all pcm-1 animals tested, but was seen exclusively in pcm-1 mutants.
Fig. 3.
Dauer and Dauer-like Larvae Fat Accumulation. N2 and pcm-1 dauer and dauer-like larvae after various times of arrest were stained with Sudan Black and observed at 40 power magnification. Representative images are shown for three independent experiments analyzing worms at day 1 (2 experiments), day 5, day 6, day 10, day 13, day 16, day 20, and day 25. In each case, we observe a progressive decrease in Sudan Black staining in larvae from day 1 of dauer arrest; pcm-1 mutant larvae also appear to have an decrease in initial fat stores in comparison to N2 larvae.
Table 2.
Quantitation of Fat Reserves in Aging Dauer Larvae
| Day 1 | Day 6 | Day 13 | Day 20 | Day 25 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N2 | pcm-1 | N2 | pcm-1 | N2 | pcm-1 | N2 | pcm-1 | N2 | pcm-1 | |
| Estimated relative total volume of fat droplets† | 6.2 ± 5.0 | 1.5 ± 2.0 | 1.8 ± 1.4 | 0.3 ± 0.5 | 0.8 ± 0.5 | 0.3 ± 0.4 | 0.2 ± 0.2 | 0.05 ± 0.06 | 0.2 ± 0.4 | 0.1 ± 0.1 |
|
| ||||||||||
| number of worms measured | 11 | 11 | 16 | 19 | 19 | 15 | 15 | 12 | 18 | 17 |
|
| ||||||||||
| p-value* | 0.01 | 0.0002 | 0.003 | 0.04 | 0.3 | |||||
Blinded images of the indicated number of Sudan Black stained larvae were analyzed for the relative number of fat globules (abundance) and for their average diameter. The relative total volume was estimated by multiplying the abundance value by the cube of the average diameter ± the standard deviation.
Two-tailed student’s T-test p-value comparing N2 and pcm-1 relative volumes
We believe that the difference seen here in fat accumulation is due to a defect in fat metabolism as opposed to decreased nutrient intake because there is no difference in pharyngeal pumping between N2 and pcm-1(qa201) mutants (data not shown). We also note that the decrease in fat granules in the pcm-1 mutants was not accompanied by a decrease in overall body size of the dauer larvae (data not shown).
3.4. pcm-1 is expressed in nervous, reproductive and muscle tissues
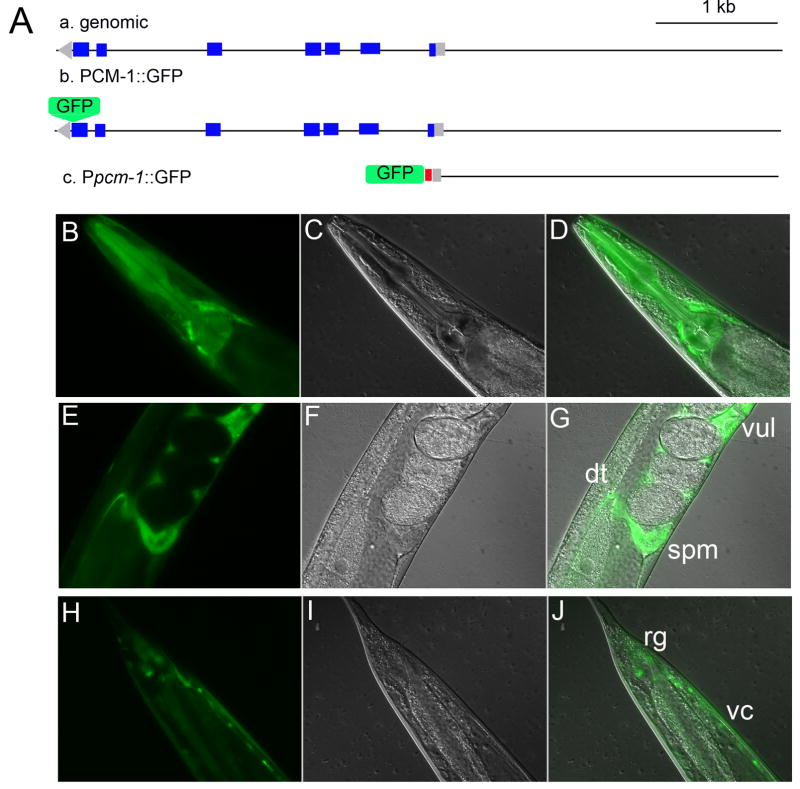
In order to analyze where and in what cell types PCM-1 expression occurs, transgenic animals containing GFP fusions were analyzed at various developmental stages (Fig. 4). Two GFP fusions were used, a PCM-1::GFP fusion and a promoter pcm-1 GFP fusion (Ppcm-1::GFP) (Fig. 4A). In L4 larvae expressing the PCM-1::GFP construct, there appears to be high levels of cytoplasmic expression in the body wall, vulva, enteric and pharengyl muscles, all neural cords, neuronal cell bodies (head region, lateral interneurons and tail region), gonad, distal tip and spermatheca (Fig. 4B–4J). This expression pattern remained the same for all normal larval stages observed, excluding embryos in which no pcm-1 expression was observed. Interestingly, we did not detect significant expression in intestinal tissues. In transgenic animals with the Ppcm-1::GFP fusion construct containing a nuclear localization signal (Fig. 4A), we found GFP expression in non-identified head neurons, the ventral nerve cord and rectal ganglia in all developmental stages (data not shown). Similar results were reported for these constructs by Dr. Colin Thacker, who generated these strains at Worm Core Facility at the University of Utah (http://web.mac.com/colinthacker/iWeb/UofUWormCore/Resources_files/Worm%20Core%20strains.htm).
Fig. 4.
PCM-1 Expression Pattern. Two constructs were prepared for observing the expression pattern of PCM-1: a pcm-1 promoter GFP fusion, and a PCM-1::GFP fusion. (A) pcm-1 genomic DNA is in the anti-sense direction (a). The PCM-1::GFP fusion was created to included genomic regions that previously rescued pcm-1 dauer formation defects (Banfield et al., 2008) (b). The Ppcm-1::GFP fusion was made by fusing 3 kb upstream of the pcm-1 start site with GFP containing a nuclear localization signal (NLS), which is shown in red (c). Blue represents coding regions while gray represents non-coding regions. (B–D) GFP, DIC II, and merged images of an adult animal containing PCM-1::GFP transgene shows GFP positive areas in unidentified head neurons. PCM-1::GFP expression is also seen in the distal tip (dt), vulva (vul) and spermatheca (spm) (E–G) of adult nematodes as well as in the root ganglia (rg) and nerve cords (vc) (H–J) (A). Transgenic animals were analyzed for GFP expression at 40 power magnification.
3.5. The expression of pcm-1 changes upon heat shock and in dauer larvae
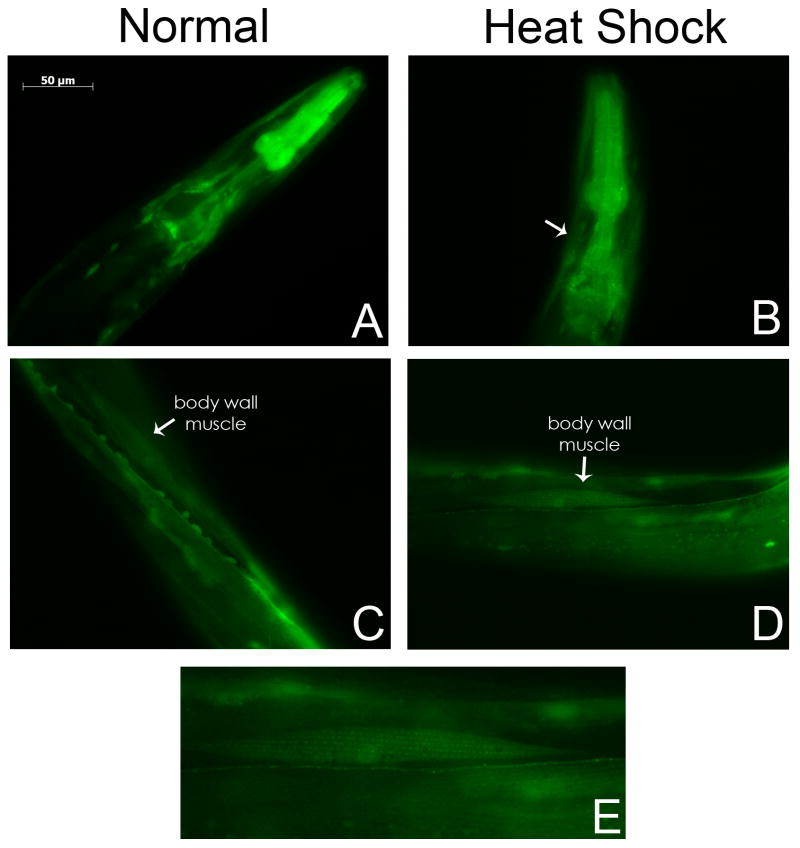
To investigate the role of pcm-1 under stress, transgenic L4 and young adult animals were incubated at 34 °C for 3 hours before analysis. Transgenic animals that have not been heat shocked, display PCM-1 localization in the cytoplasm of body wall muscles (Fig. 5A). After heat shock, we have observed that the localization of PCM-1 in the body wall is redistributed into nuclei and muscle fibers in the head and around the pharyngeal bulb (Fig. 5A–5B) and in body wall muscles throughout the midbody (Fig. 5C–5E). The striated pattern of expression is similar to that of proteins in actomyosin filaments (Willis et al., 2006) and has not been seen in worms that have not been heat shocked. Transgenic animals with the Ppcm-1:: GFP fusion construct were also tested under heat shock conditions. However, we did not find a similar change in expression pattern (data not shown). This discrepancy could be due to the fact that promoter fusions may lack regulatory elements that are important for tissue expression.
Fig. 5.
PCM-1 is Expressed in Muscle Tissue after Heat Shock Treatment. Transgenic L4 and young adult animals were incubated at 34 °C for 3 hours before analysis. PCM-1::GFP expression is observed in a striated pattern in body wall muscles of the head of treated animals but not in untreated animals (A–B). Similar striated muscle expression is also seen throughout the midbody of treated animals (C–D). Expression of PCM-1 can also be seen in the nuclei of the body wall muscles. One of the muscles from panel D is enlarged for detail in panel E. Arrows in panels B, C, and D indicate GFP positive muscles. Pictures were taken with identical exposure times and microscope settings.
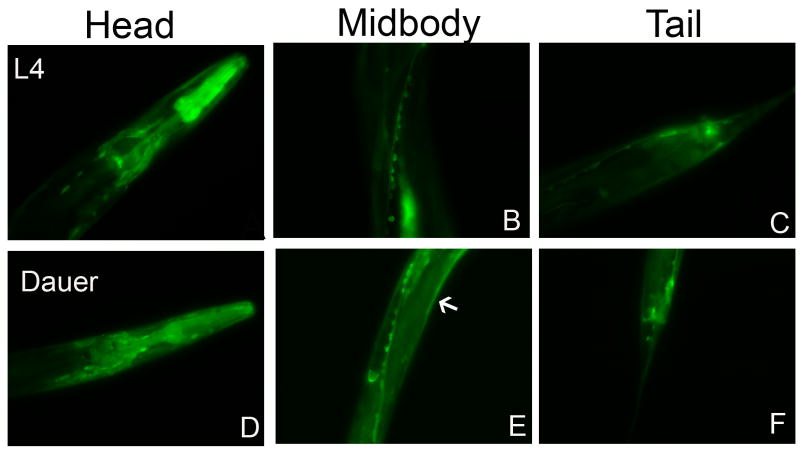
Due to the roles of pcm-1 in dauer survival, it was of interest to see if the localization of PCM-1 changes upon dauer formation. Transgenic PCM-1::GFP dauer larvae were analyzed and showed similar tissue PCM-1::GFP expression as was seen in L4 larvae and young adults (Fig. 6). Although we have not performed quantitative experiments, it appears that the expression of PCM-1 is more ubiquitous, spread out through the body wall and hypodermal cells in dauer larvae.
Fig. 6.
PCM-1 Dauer Larvae Expression. PCM-1::GFP animals were allowed to lay eggs on pheromone plates. GFP positive L4 (panels A–C) and dauer larvae (panels D–F) progeny were compared. The expression pattern of PCM-1::GFP is similar in dauer larvae as in L4, with the exception that dauer larvae show more ubiquitous expression including hypodermal cells (as indicated by an arrow in panel E). Pictures were taken with identical exposure times and microscope settings.
4. Discussion
We show that pcm-1 is necessary in dauer and dauer-like larvae for normal longevity, but is not needed for these larvae to recover when refed. It has previously been shown that pcm-1 is important for L1 survival but not for adult longevity (Gomez et al., 2007; Banfield et al., 2008). Hence, in C. elegans the pcm-1 gene product may effect development and cellular homeostasis more than it does adult aging. In fact, hormesis studies in C. elegans show that stress resistance and adult longevity may be genetically separable (Cypser et al., 2006). The PCM-1 protein repair enzyme may serve a second function in cells in regulating signaling pathways. Our results from overexpressing PCM-1 are consistent with a function of the methyltransferase to attenuate signaling in the DAF-2 pathway, although we do not see potentiating effects on this pathway in the null pcm-1 mutant (Banfield et al., 2008), such as shortened adult life span (Paradis et al., 1999).
The dauer recovery data presented here is interesting because it has been shown previously that L1 larval recovery is age dependent (Gomez et al., 2007). However, the ability of dauer larvae to recover remains intact regardless of age. These data support the concept that dauer larvae are “non-aging” (Klass and Hirsh, 1976), although daf-2 mutants do demonstrate enhanced dauer survival (Banfield et al., 2008). Additionally, daf-2 mutants accumulate massive amounts of fat in the dauer stage (Ogg et al., 1997). Taken together with the fact that pcm-1 dauer larvae have greatly decreased dauer fitness and low initial reservoirs of fat, these data support the dependence of dauer longevity on fat storage (Klass and Hirsh, 1976). The decline of fat stores in both wild-type and mutant worms signals that the fat is being utilized in the starving dauer larvae. The slightly abnormal fat deposits in pcm-1 at later times of dauer diapause may indicate that the larvae are attempting to recover from dauer phase and/or inappropriately reverse the metabolic changes initiated to enter the dauer stage. It is known that dauer recovery is accompanied by the reversal of an alternate dauer larval metabolic state (Burnell et al., 2005; Houthoofd et al., 2002).
It is unclear how pcm-1 affects fat storage and if its broadened expression under stress serves a protective role in C. elegans. The pronounced expression of pcm-1 expression in neurons is consistent with a role in the regulation and integration of signaling pathways that lead to longevity and dauer formation (Inoue et al., 2000; Wolkow et al., 2000). pcm-1 was previously determined to be a member of Mount 6, a group or “mountain” of genes transcribed at high levels in neuronal cells (Kim et al., 2001). Neural and reproductive tissue pcm-1 expression pattern in worms parallels the pattern found in rat where the homolog is highly expressed in the brain and testes (Mizobuchi et al., 1994).
In dauer larvae, pcm-1 expression appeared to increase in hypodermal cells and under heat shock pcm-1 expression appears in body muscle in a striated pattern similar to that of actomyosin. Muscle deterioration is a biomarker for aging organisms and mutations such as daf-2 delay the onset of muscle deterioration (Glenn et al., 2004; Herndon et al., 2002). It appears plausible that to counteract deterioration in these tissues under stress, pcm-1 expression is turned on. The change in pcm-1 expression in dauer larvae is in accord with previous biochemical assays and microarray analysis which shows that enzyme and mRNA levels are approximately two-fold higher during this stage (Kagan et al., 1997; McElwee et al., 2004).
In summary, we have shown that dauer and dauer-like larvae require the pcm-1 gene for normal longevity. However, pcm-1 is not required for recovery of dauer larvae, a process that appears to not be highly dependent on the length of arrest. In addition, pcm-1 dauer larvae have reduced initial stores of fat and then show slight aberrations in fat compartmentalization at later time points. pcm-1 in C. elegans is expressed mainly in nervous, muscle and reproductive tissues, but the localization of PCM-1 changes in muscles upon stress. This study provides insight into how pcm-1 may modulate dauer longevity and the role it may play in cell maintenance under stress. However, a mechanism of how pcm-1 acts under stressful physiological conditions has yet to be elucidated.
Acknowledgments
We sincerely thank Colin Thacker of the Worm Core Facility (University of Utah) for generating the pcm-1 PCR fusion and transgenic lines. Shilpa Gandre and Ayako Hasegawa of the Alex van der Bliek lab provided guidance. Brian D. Young, Dorothy M. Trogler and Shilpi Khare offered various forms of assistance. We also thank Shohei Mitani for providing the pcm-1(tm363) strain. Some strains used in this study were obtained from the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR). This work was supported in part by the United States Public Service Institutional Award T32 GM07185 to K. L. B., National Institutes of Health grants AG018000 and GM026020 to S.G.C with a supplement to T.A.G.
References
- Albert PS, Riddle DL. Mutants of Caenorhabditis elegans that form dauer-like larvae. Dev Biol. 1988;126:270–293. doi: 10.1016/0012-1606(88)90138-8. [DOI] [PubMed] [Google Scholar]
- Ayyadevara S, Alla R, Thaden JJ, Shmookler Reis RJ. Remarkable longevity and stress resistance of nematode PI3K mutants. Aging Cell. 2008;7:13–22. doi: 10.1111/j.1474-9726.2007.00348.x. [DOI] [PubMed] [Google Scholar]
- Banfield KL, Gomez TA, Lee W, Clarke S, Larsen PL. Protein repair and hormone-signaling pathways specify dauer and adult longevity and dauer development in Caenorhabditis elegans” J. Gerontol. Biol Sci. 2008;63A:798–808. doi: 10.1093/gerona/63.8.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan TV, Clarke S. Deamidation and isoaspartate formation in model synthetic peptides: the effects of sequence and solution environment. In: Aswad DW, editor. Deamidation and isoaspartate formation in peptides and proteins. CRC Press; Boca Raton: 1995. pp. 65–90. [Google Scholar]
- Brenner S. The Genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnell AM, Houthoofd K, O’Hanlon K, Vanfleteren JR. Alternate metabolism during the dauer stage of the nematode Caenorhabditis elegans. Exp Gerontol. 2005;11:850–856. doi: 10.1016/j.exger.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Chavous DA, Jackson FR, O’Connor CM. Extension of the Drosophila lifespan by overexpression of a protein repair methyltransferase. Proc Natl Acad Sci U S A. 2001;98:14814–14818. doi: 10.1073/pnas.251446498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S. Aging as war between chemical and biochemical processes: protein methylation and the recognition of age-damaged proteins for repair. Ageing Res Rev. 2003;2:263–285. doi: 10.1016/s1568-1637(03)00011-4. [DOI] [PubMed] [Google Scholar]
- Cypser JR, Tedesco P, Johnson TE. Hormesis and aging in Caenorhabditis elegans, Exp. Gerontol. 2006;41:935–939. doi: 10.1016/j.exger.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar C, Houser CR, Clarke S. Activation of the PI3K/Akt signal transduction pathway and increased levels of insulin receptor in protein repair-deficient mice. Aging Cell. 2005a;4:1–12. doi: 10.1111/j.1474-9728.2004.00136.x. [DOI] [PubMed] [Google Scholar]
- Farrar C, Clarke S. Diet-dependent survival of protein repair-deficient mice. J Nutr Biochem. 2005b;16:554–561. doi: 10.1016/j.jnutbio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Glenn CF, Chow DK, David L, Cooke CA, Gami MS, Iser WB, Hanselman KB, Goldberg IG, Wolkow CA. Behavioral deficits during early stages of aging in Caenorhabditis elegans result from locomotory deficits possibly linked to muscle frailty. J Gerontol A Biol Sci Med Sci. 2004;59:1251–1260. doi: 10.1093/gerona/59.12.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden JW, Riddle DL. The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Dev Biol. 1984;102:368–378. doi: 10.1016/0012-1606(84)90201-x. [DOI] [PubMed] [Google Scholar]
- Gomez TA, Banfield K, Trogler DM, Clarke S. The L-isoaspartyl O-methyltransferase in Caenorhabditis elegans larval longevity and autophagy. Dev Biol. 2007;303:493–500. doi: 10.1016/j.ydbio.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419:808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- Hicks WM, Kotlajich MV, Visick JE. Recovery from long-term stationary phase and stress survival in Escherichia coli require the L-isoaspartyl protein carboxyl methyltransferase at alkaline pH. Microbiology. 2005;151:2151–2158. doi: 10.1099/mic.0.27835-0. [DOI] [PubMed] [Google Scholar]
- Hobart O. PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques. 2002;32:728–730. doi: 10.2144/02324bm01. [DOI] [PubMed] [Google Scholar]
- Houthoofd K, Braeckman BP, Lenaerts I, Brys K, De Vreese A, Van Eygen S, Vanfleteren JR. Ageing is reversed, and metabolism is reset to young levels in recovering dauer larvae of C. elegans. Exp Gerontol. 2002;37:1015–1021. doi: 10.1016/s0531-5565(02)00063-3. [DOI] [PubMed] [Google Scholar]
- Ingrosso D, D’Angelo S, di Carlo E, Perna AF, Zappia V, Galletti P. Increased methyl esterification of altered aspartyl residues in erythrocyte membrane proteins in response to oxidative stress. Eur J Biochem. 2000;267:4397–4405. doi: 10.1046/j.1432-1327.2000.01485.x. [DOI] [PubMed] [Google Scholar]
- Inoue T, Thomas JH. Targets of TGF-beta signaling in Caenorhabditis elegans dauer formation. Dev Biol. 2000;217:192–204. doi: 10.1006/dbio.1999.9545. [DOI] [PubMed] [Google Scholar]
- Kagan RM, Niewmierzycka A, Clarke S. Targeted gene disruption of the Caenorhabditis elegans L-isoaspartyl protein repair methyltransferase impairs survival of dauer stage nematodes. Arch Biochem Biophys. 1997;348:320–328. doi: 10.1006/abbi.1997.0362. [DOI] [PubMed] [Google Scholar]
- Kim SK, Lund J, Kiraly M, Jiang M, Stuart JM, Eizinger A, Wylie BN, Davidson GS. A gene expression map for Caenorhabditis elegans. Science. 2001;293:2087–2092. doi: 10.1126/science.1061603. [DOI] [PubMed] [Google Scholar]
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- Klass M, Hirsh D. Non-ageing developmental variant of Caenorhabditis elegans. Nature. 1976;260:523–525. doi: 10.1038/260523a0. [DOI] [PubMed] [Google Scholar]
- Lanthier J, Desrosiers RR. Protein L-isoaspartyl methyltransferase repairs abnormal aspartyl residues accumulated in vivo in type-I collagen and restores cell migration. Exp Cell Res. 2004;293:96–105. doi: 10.1016/j.yexcr.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Linster CL, Gomez TA, Christensen KC, Adler LN, Young BD, Brenner C, Clarke SG. Arabidopsis VTC2 encodes a GDP-L-galactose phosphorylase, the last unknown enzyme in the Smirnoff-Wheeler pathway to ascorbic acid in plants. J Biol Chem. 2007;282:18879–18885. doi: 10.1074/jbc.M702094200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwee JJ, Schuster E, Blanc E, Thomas JH, Gems D. Shared transcriptional signature in Caenorhabditis elegans dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. J Biol Chem. 2004;279:44533–44543. doi: 10.1074/jbc.M406207200. [DOI] [PubMed] [Google Scholar]
- Melendez A, Talloczy Z, Seaman M, Eskelinen EL, Hall DH, Levine B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science. 2003;301:1387–1391. doi: 10.1126/science.1087782. [DOI] [PubMed] [Google Scholar]
- Mizobuchi M, Murao K, Takeda R, Kakimoto Y. Tissue-specific expression of isoaspartyl protein carboxyl methyltransferase gene in rat brain and testis. J Neurochem. 1994;62:322–328. doi: 10.1046/j.1471-4159.1994.62010322.x. [DOI] [PubMed] [Google Scholar]
- Niewmierzycka A, Clarke S. Do damaged proteins accumulate in Caenorhabditis elegans L-isoaspartate methyltransferase (pcm-1) deletion mutants? Arch Biochem Biophys. 1999;364:209–218. doi: 10.1006/abbi.1999.1114. [DOI] [PubMed] [Google Scholar]
- O’Connor CM. Protein L-isoaspartyl, D-aspartyl O-methyltransferases: Catalysts for protein repair. In: Clarke SG, Tamanoi F, editors. The Enzymes: Protein Methyltransferases. Vol. 24. Academic Press; New York: 2006. pp. 385–433. [DOI] [PubMed] [Google Scholar]
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- Paradis S, Ailion M, Toker A, Thomas JH, Ruvkun G. A PDK1 homolog is necessary and sufficient to transduce AGE-1 PI3 kinase signals that regulate diapause in Caenorhabditis elegans. Genes Develop. 1999;13:1438–1452. doi: 10.1101/gad.13.11.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis JH, Munro E, Lyczak R, Bowerman B. Conditional dominanta mutations in the Caenorhabditis elegans gene act-2 identify cytoplasmic and muscle roles for a redundant actin isoform. Mol Biol Cell. 2006;17:1051–1064. doi: 10.1091/mbc.E05-09-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkow CA, Kimura KD, Lee MS, Ruvkun G. Regulation of C. elegans life-span by insulin-like signaling in the nervous system. Science. 2000;290:147–150. doi: 10.1126/science.290.5489.147. [DOI] [PubMed] [Google Scholar]