Abstract
Sphingosine 1-phospate (S1P) has been demonstrated to protect against the formation of lipopolysaccharide (LPS)-induced lung edema when administered concomitantly with LPS. In the present study, we sought to determine the effectiveness of S1P to attenuate lung injury in a translationally relevant canine model of acute lung injury (ALI) when administered as rescue therapy. Secondarily, we examined whether the attenuation of LPS-induced physiological lung injury following administration of S1P was, at least in part, due to an alteration in local and/or systemic inflammatory cytokine expression. We prospectively examined 18 one-year old male beagles in which we instilled bacterial LPS (2–4 mg/kg) intratracheally followed in one hour with intravenous S1P (85 μg/kg) or vehicle and eight hours of high tidal volume mechanical ventilation. S1P attenuated the formation of shunt fraction (32%) and both the presence of protein (72%) and neutrophils (95%) in bronchoalveolar lavage (BAL) fluid compared to vehicle controls. Although lung tissue inflammatory cytokine production was found to vary regionally throughout the LPS-injured lung, S1P did not alter the expression pattern. Similarly, BAL cytokine production was not significantly altered by intravenous S1P in this model. Interestingly, S1P potentiated the LPS-induced systemic production of three inflammatory cytokines, TNF-α (6-fold), KC (1.2-fold), and IL-6 (3-fold), without resulting in end-organ dysfunction. In conclusion, intravenous S1P reduces inflammatory lung injury when administered as rescue therapy in our canine model of LPS-induced ALI. This improvement is observed in the absence of changes in local pulmonary inflammatory cytokine production and an augmentation of systemic inflammation.
INTRODUCTION
Acute lung injury (ALI) is a life-threatening complication of many systemic and primary pulmonary disorders that affects nearly 200,000 persons in the United States each year and conveys mortality between 30–40% 1–3. In ALI, inflammation, either as a direct result of injury to the lung itself or as a result of the systemic release of inflammatory mediators from injury distal to the lung, leads to an increase in lung microvascular permeability, enabling the leakage of intravascular fluid and protein into the interstitial and alveolar spaces resulting in pulmonary edema and impairment of respiratory function 3. Despite numerous studies providing insight into the pathophysiology of ALI, no clinical investigation has demonstrated therapeutic success targeting the pathogenesis of ALI. In fact, since ALI was first described by Ashbaugh and colleagues in 1967, despite numerous clinical investigations, only a single clinical intervention, low tidal volume ventilation, has been shown to improve mortality in ALI 4, 5.
While treatment of ALI with low tidal volume ventilation has provided a substantial improvement in outcome, the syndrome continues to result in unacceptably high mortality 2. In addition, low tidal volume ventilation represents a viable therapy only for patients with respiratory failure. No intervention has been proven to prevent the onset of ALI in at-risk patients or to reverse lung edema formation in patients early in the evolution of disease. As such, a treatment that would serve to prevent, reduce, or reverse the increase in pulmonary vascular permeability that is an essential factor in the development of ALI, would be highly desirable as a prophylactic and/or therapeutic intervention to improve outcome from this devastating condition.
Sphingosine 1-phosphate (S1P), a sphingolipid exhibiting a diverse range of signaling and biological functions, including a profound effect on pulmonary vascular permeability, is a prime candidate for such an intervention 6–8. S1P, generated by the phosphorylation of sphingosine by sphingosine kinase, decreases endothelial permeability to both water and solute in vitro via cytoskeletal reorganization and adherens junction assembly 6, 9, 10. In addition, S1P has been demonstrated in vitro to modulate cell proliferation, differentiation, and migration, promote cell survival, stimulate angiogenesis, modify vascular tone both systemically and in the lung, and alter inflammatory cell trafficking 8,11–16. S1P (1μM) administered to C57BL/6 mice with endotoxin (LPS)-induced lung injury decreases lung edema formation, solute transport across the alveolar capillary endothelium, and inflammatory cell infiltration into lung parenchyma 7. Extending these findings into a translationally relevant canine model, we recently demonstrated that intravenous (iv) S1P (85 μg/kg) administered concomitantly with intratracheal LPS reduces shunt formation, attenuates alveolar protein accumulation, and decreases regional alveolar edema formation compared to animals injured with LPS alone 17. Putative benefits of S1P in ALI may result from endothelial barrier enhancement, modulation of inflammation, alterations in pulmonary vascular tone, or a combination of mechanisms.
The primary objective of the present study was to determine the effectiveness of S1P to ameliorate lung injury in a translationally relevant canine model of ALI when administered as a rescue therapy, (i.e. after the induction of inflammatory lung injury). We found that intravenous S1P, when administered subsequent to the introduction of intratracheal LPS, attenuated the deterioration in arterial oxygenation and the accumulation of protein in the alveolar space compared to control intratracheal LPS-injured animals. Subsequently, we sought to determine whether the improvement in physiological lung injury imparted by S1P was in part due to a reduction in neutrophil influx into the lung compartment and/or to an alteration in inflammatory cytokine production in the injured host. We found that S1P reduced neutrophil accumulation in the alveolar compartment despite the lack of significant change in the lung tissue compartment. Furthermore, this reduction was not related to an alteration in lung tissue or alveolar air space inflammatory cytokines. Interestingly, intravenous S1P augmented LPS-induced systemic production of the inflammatory cytokines, IL-6, IL-8, KC, and TNF-α.
METHODS
Animal protocols were approved by the University of Pittsburgh and the Johns Hopkins University Institutional Animal Care and Use Committees.
Canine model preparation
The details of the model have been previously described 17. Briefly, one year-old male beagles weighing between 10–15 kg (Covance, Richmond, VA) were anesthetized with pentobarbital (25 mg/kg bolus and 5 mg/kg iv hourly) via a forelimb iv catheter and paralyzed with pancuronium (3 mg bolus and 0.5 mg hourly iv). The animal was intubated using standard oral intubation techniques with an 8.0 endotracheal tube and mechanically ventilated (ventilation parameters detailed below). The animal was placed in the supine position, and a skin incision was made in the right inguinal area using aseptic technique. The femoral artery and vein were exposed, and vascular introducers were placed in both the femoral artery and vein. A Swan-Ganz catheter was inserted through the femoral vein introducer and advanced into the pulmonary artery using standard flotation technique. Subsequently, tracheostomy was performed to reduce dead space and to improve lower airway access for fiberoptic bronchoscopy. Rectal temperature was monitored throughout the experiment and maintained at 36–37°C with a radiant heat lamp. Supportive care consisting of fluid replacement, mechanical ventilation, and maintenance of anesthesia was provided in the supine position for eight hours after injury induction.
Canine mechanical ventilation
Volume-controlled, time-cycled mechanical ventilation was delivered by piston ventilator (Lifecare PLV-102, Respironics Inc., or Harvard Apparatus large animal ventilator Model 613) with high tidal volume (Vt =17 cc/kg) designed to exacerbate the LPS-induced inflammatory response within the lung. Respiratory rate was set initially to achieve an end-tidal carbon dioxide (ETCO2) =30–35 mm Hg and subsequently adjusted to maintain pH >7.20 with maximum rate predetermined to be 24 breaths per minute. Fraction of inspired oxygen (FiO2) of 0.30 was increased as required to maintain oxygen saturation (SpO2) > 88% or PaO2 > 60 mmHg. Positive end-expiratory pressure (PEEP) was applied at 5 cm H2O and maintained constant throughout the experiment. Peak flow rates were adjusted to maintain the ratio of inspiration to expiration at 1:2 or 1:3.
Endotoxin delivery
Escherichia coli lipopolysaccharide (LPS, O55:B5, Sigma L4005, St. Louis, MO), 2–4 mg/kg diluted in 50 cc saline, was delivered intrabronchially through a flexible18-gauge catheter introduced into each of five lobar bronchi (10 cc aliquots) via the working channel of a flexible fiberoptic bronchoscope (Olympus, Fujinon).
Sphingosine 1-phosphate administration
S1P (Sigma, S9666) was solubilized in methanol (10mM). One hour after intrabronchial instillation of LPS, nine (9) animals received 85 μg/kg of S1P diluted in 100 ml 0.9% normal saline infused under gravity into the right atrial port of the Swan-Ganz catheter over a period of 20min.
Gas exchange and respiratory mechanics
Pulse oximetry, ETCO2, and airway pressure were monitored continuously. Gas exchange was quantified at baseline, 30 min after LPS introduction, and hourly thereafter by measurement of arterial and mixed venous blood gases. Shunt fraction (Qs/Qt) was calculated using standard equations 18. Lung compliance was monitored hourly by measurement of peak airway pressures (Ppeak).
Hemodynamic Monitoring
Arterial blood pressure (Pa), pulmonary artery pressure, and central venous pressure were monitored continuously (Hewlett Packard, Merlin Model 68) and recorded hourly. Pulmonary artery occlusion pressure and thermodilution cardiac output were recorded at baseline, 30 min after LPS instillation and hourly thereafter. Intravenous saline was administered at 15 cc/kg/hr with 20 cc/kg bolus as needed for hypotension defined as mean Pa<60 mm Hg or for hemoconcentration defined as an increase in hemoglobin concentration greater than 1.5 g/dl. The target right atrial pressure was 5–8 mm Hg.
Physiological Outcomes
Global lung injury was characterized by venous admixture (Qs/Qt), as a measure of shunt formation, and lung compliance. BAL protein concentration and cell count were measured by wedging a flexible fiberoptic bronchoscope into a non-dependent segment of the middle lobe. Lavage was performed with 20 cc aliquots of saline (50% yield) at baseline and three, five, and seven hours after injury induction. BAL fluid was centrifuged at 5,000g for 30 min and the supernatant protein concentration was determined using BCA protein Assay Reagent Kit (Pierce Prod #23227, Rockford, IL).
For six animals in each group, the cell pellet was resuspended in 100μl normal saline and Crystal Violet (1:1 vol/vol). 20 μl of the suspension was applied on the surface of a hemocytometer for manual cell count (Fisher Scientific, Pittsburgh, PA). A cytospin was prepared on separate aliquot of BAL fluid. The slide was stained with Wright-Giemsa stain for manual differential cell count (Fisher Scientific).
Tissue Collection and Processing
Animals were euthanized at the conclusion of the study with a bolus dose of pentobarbital (10 mg/kg i.v.) followed by exsanguination. Immediately upon cessation of heartbeat, a sternotomy was performed and lung tissue samples were harvested from the right lung upper lobe in a gravitationally non-dependent (ULND) region, and from lower lobe non-dependent (LLND), transitional (LLT), and dependent (LLD) regions based on prior CT studies defining the regionally heterogeneous nature of lung injury in this model 17. Transitional regions represent those areas of the cross-sectional lung CT image displaying a ground glass pattern of alveolar infiltrate localized between the dependent flooded or collapsed alveolar spaces and the non-dependent lung regions free of alveolar infiltrate 17. Tissue samples were immediately frozen in liquid nitrogen and stored at −80°C for analysis.
Tissue Myeloperoxidase Determination
Soluble protein was extracted from regional lung tissue samples according to published protocol 19. Briefly, frozen tissue was thawed, homogenized in 20 mM potassium phosphate buffer (pH 7.4), and centrifuged at 6000 rpm for 10 min. The pellet was resuspended in 50 mM potassium phosphate buffer (pH 6.0) containing 0.5% HTAB (Sigma, H5882), sonicated, and subjected to three freeze thaw cycles. After centrifugation at 14000 rpm for 20 min, the pellet was discarded and the supernatant collected. MPO activity was assayed by combining tissue supernatant (1:50) with assay buffer containing 50 mM potassium phosphate (pH 6.0), 0.5% HTAB, 0.167 mg/ml O-diandisidine dihydrochloride (Sigma, D3252) and 0.05% hydrogen peroxide. Reaction products were quantified by measuring light absorbance at 460nm in one minute intervals for a total of 20 min (SOFTmax Pro 4.0, Molecular Devices, Sunnyvale, CA). MPO activity was quantified by fitting absorbance at 460nm to standard first-order enzyme kinetics using parametric data fitting software (MATLAB R2007a, The MathWorks, Inc., Natick, MA). MPO activity is reported as time to one-half maximal light absorption, t½, with shorter times reflective of increased enzyme activity.
Cytokine Determination
Serum, BAL supernatant, and regional lung tissue samples were assayed for cytokines and chemokines in six animals from each group using the canine LINCOplex kit (CCYTO-90K, Millipore) according to the manufacturer’s 96-well plate assay protocol. Briefly, 150 μl of each of 14 sonicated antibody-immobilized bead solutions were combined in a mixing bottle and diluted to 3 ml in LINCOplex assay buffer. Serum was diluted 1:3 in assay buffer for analysis. BAL supernatant was analyzed directly without dilution. Lung tissue samples were homogenized at high speed in PBS containing a protease inhibitor cocktail (Complete, Roche Molecular Diagnostics) and centrifuged at 5000 rpm for 4 min. The pellet was discarded and supernatant collected for analysis. Bead fluorescence was detected using a Luminex x100 instrument (BioRad Laboratories, Hercules, CA) and analyzed using Bio-Plex Manager 4.0 software (BioRad Laboratories, Hercules, CA). Serum TNF-α was measured by ELISA according to kit manufacturer protocol (CATA00, R&D Systems, Minneapolis, MN).
Statistical Analysis
All data are presented as means ± standard error. Two-way and one-way repeated measures analyses of variance (ANOVA) were used where appropriate to compare multiple groups within and between experiments. Post-hoc analyses using Scheffe tests were run when the ANOVA indicated that significant differences (p<0.05) exist between groups. Unpaired Students t-tests assuming equal variance or Mann-Whitney U-tests were applied where appropriate.
RESULTS
Experimental Groups
Eighteen one year-old male beagles (10–15 kg) received intrabronchial instillation of LPS (2–4 mg/kg) under direct bronchoscopic visualization. The animals then received either intravenous S1P (85 μg/kg) or vehicle (saline) one hour after LPS instillation (n=9 each group).
Intrabronchial LPS induces severe lung injury in vehicle-treated animals
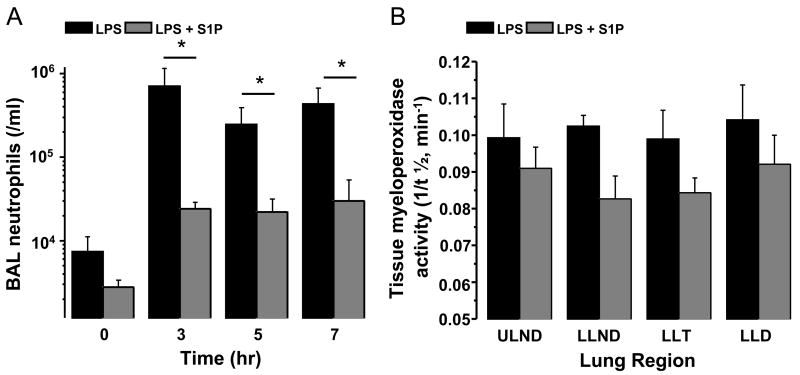
In the nine animals who received intrabronchial LPS followed by vehicle, PaO2/FiO2 declined throughout the duration of the experiment (from 561.0 ± 15.3 to 294.0 ± 31.3, Table 1). Shunt fraction was quantified by venous admixture (Qs/Qt), which rapidly and progressively increased throughout the duration of the experiment (0.2 ± 0.72% to 27.7± 4.5% after 8 hours, Figure 1A). In conjunction with the rise in shunt fraction, bronchoalveolar lavage (BAL) protein increased steadily over seven hours (5.48-fold over baseline, Figure 1B). BAL neutrophils (number of cells/ml BAL) increased to a maximum of 94.8-fold over baseline in three hours followed by a sustained increase over baseline ranging from 33-fold to 58-fold for the remainder of the experiment (n=6, Figure 2A). Myeloperoxidase activity in the lung varied by region but on average converted one half of the substrate by 10.0±0.7 min (n=3, Figure 2B).
Table 1.
Physiological parameters in canine LPS-induced ALI
| LPS | LPS + S1P | |||||
|---|---|---|---|---|---|---|
| Baseline | 90 min | 8 hours | Baseline | 90 min | 8 hours | |
| PaO2/FiO2 | 561.0 ± 15.3 | 310.1 ± 17.9† | 294.0 ± 31.3† | 568.7 ± 22.7 | 326.1 ± 29.1† | 321.1 ± 24.9† |
| PaCO2 (mmHg) | 38.2 ± 1.8 | 41.9 ± 3.1 | 35.9 ± 3.1 | 31.5 ± 1.3§ | 40.2 ± 2.4 | 36.9 ± 3.1 |
| CI (l/min/m2) | 2.04 ± 0.21 | 2.51 ± 0.29 | 2.16 ± 0.32 | 1.90 ± 0.17 | 2.53 ± 0.12† | 1.72 ± 0.17‡ |
| MAP (mm Hg) | 98.4 ± 4.2 | 105.9 ± 3.44 | 106.0 ± 3.95 | 112.9 ± 4.9§ | 118.1 ± 5.00§ | 111.8 ± 8.15 |
| SVR (dyne s/cm5) | 3997.7 ± 480.1 | 3648.7 ± 567.4 | 4413.4 ± 813.0 | 4818.0 ± 420.1 | 3589.5 ± 245.0† | 5034.3 ± 417.5‡ |
| MPAP (mmHg) | 12.2 ± 1.03 | 14.1 ± 1.24 | 18.7 ± 1.52†‡ | 12.44 ± 0.9 | 16.0 ± 0.90† | 17.8 ± 1.37† |
| PAOP (mmHg) | 3.44 ± 0.66 | 5.11 ± 0.48† | 6.33 ± 0.75† | 4.22 ± 1.17 | 7.33 ± 0.85†§ | 6.29 ± 0.9 |
| PVR (dyne s/cm5) | 286.9 ± 22.7 | 242.5 ± 35.0 | 463.7 ± 54.4†‡ | 290.5 ± 43.9 | 226.2 ± 31.0 | 480.2 ± 65.4†‡ |
| Ppeak (mmHg) | 12.3 ± 1.10 | 15.9 ± 1.02† | 17.0 ± 0.84† | 12.9 ± 0.48 | 16.1 ± 0.53† | 17.0 ± 0.64† |
| IVF (ml/kg/hr) ** | 28.9 ± 1.5 | 28.3 ± 1.46 | ||||
VE – minute ventilation; CI – cardiac index; MAP – mean arterial pressure; SVR – systemic vascular resistance; MPAP – mean pulmonary artery pressure; PAOP – pulmonary artery occlusion pressure; PVR – pulmonary vascular resistance; Ppeak – peak airway pressure; IVF – intravenous fluid
total intravenous fluid administration
p<0.05 compared to Baseline,
p<0.05 compared to 90 min,
p<0.05 compared to LPS
Figure 1. Intravenous S1P administered subsequent to injury reduces shunt formation and bronchoalveolar lavage (BAL) protein accumulation in a canine model of intrabronchial LPS-induced lung injury.
Panel A depicts the evolution of venous admixture (Qs/Qt) in anesthetized beagles subject to high tidal volume mechanical ventilation after intrabronchial administration of bacterial LPS (2–4 mg/kg, black squares, n=9) or intrabronchial LPS plus intravenous S1P one hour later (85 μg/kg, gray circles, n=9). Beagles receiving S1P demonstrated significant reductions in shunt formation beginning 5.5 hours after LPS instillation compared to those receiving LPS alone. Panel B depicts the time-dependent accumulation of protein in the BAL fluid of anesthetized beagles subject to high tidal volume mechanical ventilation after intrabronchial administration of bacterial LPS (2–4 mg/kg, black bars, n=9) or intrabronchial LPS plus intravenous S1P one hour later (85 μg/kg, gray bars, n=9). Mean ± SEM, *p≤0.05 LPS compared to LPS+S1P, † p≤0.05 compared to 90 min, ‡p<0.05 compared to baseline, ††p<0.05 compared to baseline, 3, and 5 hr. Qs/Qt is significantly elevated relative to baseline for all t ≥30 min.
Figure 2. Intravenous S1P administered subsequent to injury attenuates BAL neutrophil accumulation in a canine model of intrabronchial LPS-induced lung injury without significant effect on regional tissue myeloperoxidase (MPO) activity.
Panel A depicts the time-dependent accumulation of neutrophils in the BAL fluid of anesthetized beagles subject to high tidal volume mechanical ventilation after intrabronchial administration of bacterial LPS (2–4 mg/kg, black bars, n=6) or intrabronchial LPS plus intravenous S1P one hour later (85 μg/kg, gray bars, n=6). Beagles receiving S1P demonstrated significant reductions in BAL neutrophil accumulation after LPS instillation compared to those receiving LPS alone. Panel B depicts regional lung tissue MPO activity in beagles subject to eight hours of high tidal volume mechanical ventilation after intrabronchial administration of bacterial LPS (2–4 mg/kg, black bars, n=3) or intrabronchial LPS plus intravenous S1P one hour later (85 μg/kg, gray bars, n=6). S1P reduced lung tissue MPO activity (i.e. prolonged t½) by an average of 18% (p=0.1). Mean ± SEM, *p≤0.05 LPS compared to LPS+S1P.
Rescue S1P attenuates LPS-induced lung injury
In nine animals that received intravenous S1P one hour after intrabronchial LPS, shunt fraction increased for 90 min following LPS to 25.1±4.4%, similar to the increase observed in the vehicle control group over this time period (Figure 1A). Subsequently, shunt fraction in S1P-treated animals decreased to a minimum of 17.0±3.1% by the completion of the experiment, a marked improvement (32%) compared with vehicle-treated animals (Figure 1A). The development of shunt due to intrabronchial administration of LPS, therefore, was attenuated in S1P-treated animals with significant reduction evident after five hours of S1P administration (p<0.05, Figure 1A). Corresponding to the attenuation in shunt formation, intravenous S1P abrogated the increase in BAL protein induced by LPS, resulting in a 72% decrease in BAL protein content compared to vehicle treated animals five hours after LPS instillation (p<0.05, Figure 1B). Similarly, S1P-treated animals experienced a nearly 10-fold reduction in BAL neutrophils after intrabronchial LPS compared to vehicle treated controls (n=6 each, p<0.05, Figure 2A) and displayed a trend toward a decrease in lung tissue MPO activity that did not reach statistical significance (t½=11.8±0.6 min, p=0.1, Figure 2B).
Intrabronchial LPS induces a regionally variable pulmonary inflammatory response
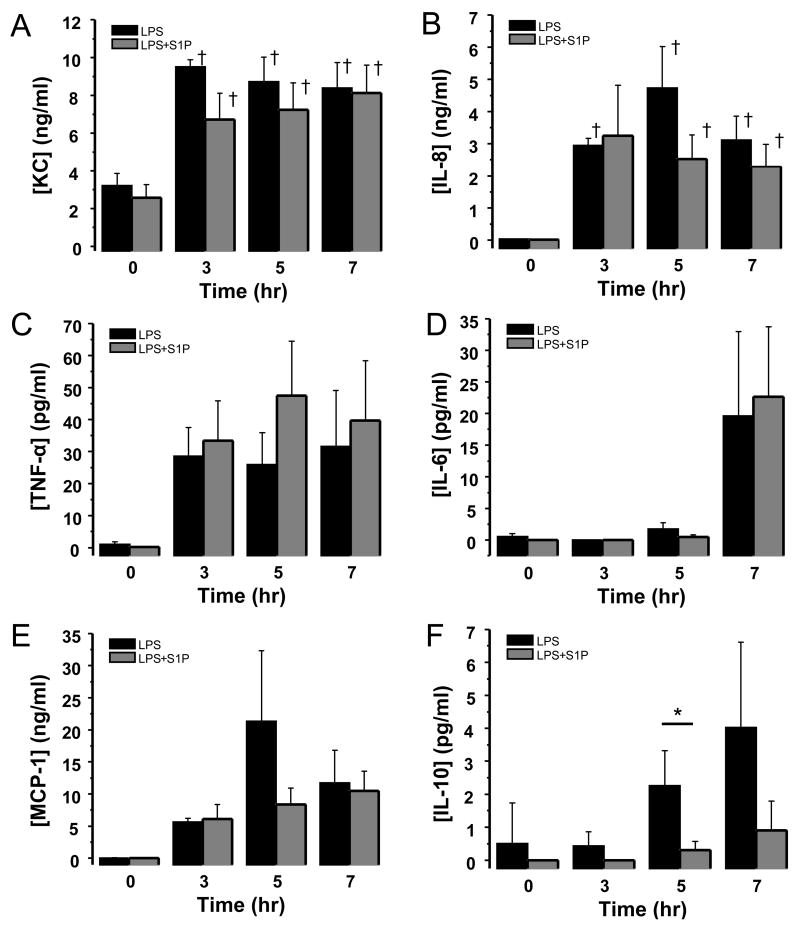
Bronchoalveolar lavage (BAL) was performed at baseline and subsequently three, five, and seven hours after LPS instillation. BAL fluid was analyzed for inflammatory cytokine and chemokine protein production in a subset of animals (n=6 each group) using the canine LINCOplex kit (Millipore CCYTO-90K). Intratracheal LPS administration resulted in significant increases in BAL inflammatory chemokines KC (2.9-fold), IL-8 (236-fold), and MCP-1 (337-fold) over the eight hour experiment (Figure 3). In addition, BAL TNF-α and IL-10 production increased throughout the experiment (54-fold and 8-fold relative to baseline at 7 hr respectively, Figure 3), and IL-6 production peaked seven hours following LPS administration (93-fold compared to baseline, Figure 3).
Figure 3. Intravenous S1P administered subsequent to injury has no effect on bronchoalveolar lavage (BAL) inflammatory cytokine production in a canine model of intrabronchial LPS-induced lung injury.
Depicted are the time-dependent productions of KC (panel A), IL-8 (panel B), TNF-α (panel C), IL-6 (panel D), MCP-1 (panel E), and IL-10 (panel F) in the BAL fluid of anesthetized beagles subject to high tidal volume mechanical ventilation after intrabronchial administration of LPS (2–4 mg/kg, black bars, n=6) or intrabronchial LPS plus intravenous S1P one hour later (85 μg/kg, gray bars, n=6). Production of each chemokine or cytokine increased relative to baseline without significant alteration by administration of S1P. Mean ± SEM, *p=0.053, †p≤0.01 relative to baseline.
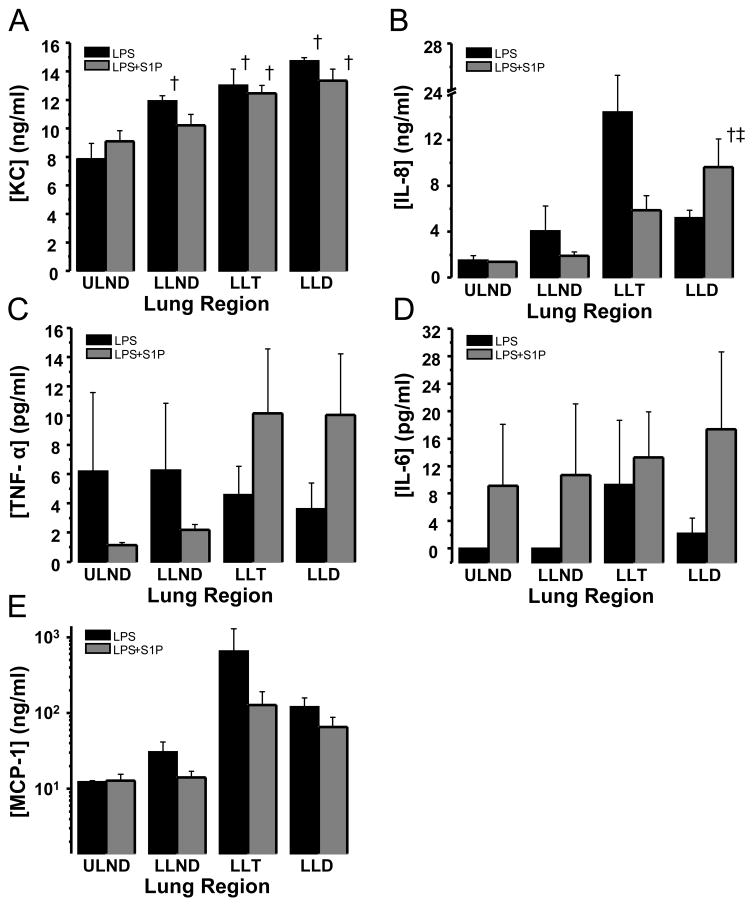
Lung tissue production of KC (1.87-fold), IL-8 (3.38-fold), and MCP-1 (9.8-fold) increased after LPS administration in dependent lung regions relative to non-dependent regions (LLD vs. ULND). In contrast, regional differences were not detected in either TNF-α or IL-6 production (Figure 4) and IL-10 was undetectable in lung tissue homogenate supernatants.
Figure 4. Intrabronchial LPS induces regional lung tissue inflammatory cytokine production.
Depicted are productions of KC (panel A), IL-8 (panel B), TNF-α (panel C), IL-6 (panel D), and MCP-1 (panel E) in regional lung tissue samples harvested from anesthetized beagles subject to high tidal volume mechanical ventilation eight hours after intrabronchial administration of bacterial LPS (2–4 mg/kg, black bars, n=3) or intrabronchial LPS plus intravenous S1P one hour later (85 μg/kg, gray bars, n=6). LPS induced regionally heterogeneous inflammation most pronounced in the gravitationally dependent (LLD) and transitional (LLT) regions of the lower lobe. However, regional inflammation was not significantly altered by administration of S1P. Mean ± SEM, †p≤0.01 relative to upper lobe non-dependent (ULND) lung tissue, ‡ p≤0.05 relative to lower lobe non-dependent (LLND) lung tissue.
Rescue S1P does not alter lung compartment inflammatory cytokine production
Intravenous S1P attenuated BAL IL-10 production (7.46-fold decrease at 5h, p=0.053) without significant impact on production of TNF-α, IL-6, KC, IL-8, or MCP-1 in either BAL (Figure 3) or lung tissue (Figure 4).
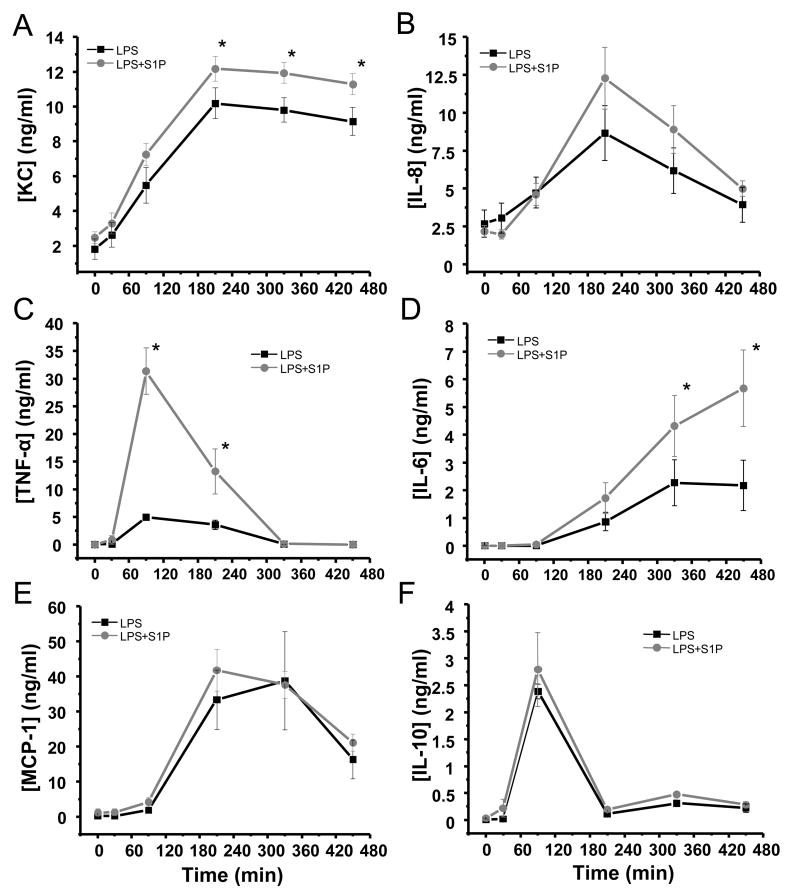
Intrabronchial LPS incites a systemic inflammatory response
Intratracheal LPS induced systemic cytokine release in a time-dependent manner (Figure 5). Serum KC rose to its peak (10.2±0.7 ng/ml) within 4 hours of instillation and remained elevated throughout the duration of the experiment (Figure 5). IL-8 production peaked at 3.5 hours (8.7±1.8 ng/ml) and subsequently returned to near baseline (3.9±1.2 ng/ml) by the completion of the protocol, and MCP-1 peaked after 5 hours (38.7±1.4 ng/ml) before its decline (Figure 5). TNF-α peaked at 90 minutes and rapidly returned to baseline; whereas, IL-6 rose gradually throughout the course of the study starting after 90 minutes (peak 2.2±0.9 ng/ml at 7.5h, Figure 5). IL-10 production followed a time course similar to that of TNF-α, peaking after 90 min (2.4±1.3 ng/ml) and returning rapidly to pre-LPS levels (Figure 5). All LPS-injured animals experienced a transient period of reduced systemic vascular resistance accompanied by an increase in cardiac output characteristic of the systemic inflammatory response syndrome (SIRS) which subsequently returned to near baseline (Table 1). The systemic inflammatory response was not associated with significant end-organ dysfunction as defined by serum biochemical assessments of kidney and liver function (data not shown). Circulating leukocytes decreased over the first 2.5 hours after LPS instillation, recovering by the end of the experiment (Table 2).
Figure 5. Intravenous S1P administered subsequent to injury augments systemic inflammatory cytokine production in a canine model of intrabronchial LPS-induced lung injury.
Depicted are the time-dependent productions of KC (panel A), IL-8 (panel B), TNF-α (panel C), IL-6 (panel D), MCP-1 (panel E), and IL-10 (panel F) in serum from anesthetized beagles subject to high tidal volume mechanical ventilation after intrabronchial administration of LPS (2–4 mg/kg, black squares, n=6) or intrabronchial LPS plus intravenous S1P one hour later (85 μg/kg, gray circles, n=6). Intravenous S1P augmented the systemic production of KC, TNF-α, and IL-6 induced by intrabronchial LPS compared to LPS alone with no significant alterations in the production of IL-8, MCP-1, or IL-10. Mean ± SEM, *p≤0.05 between groups.
Table 2.
Peripheral blood cell profiles in canine LPS-induced ALI
| LPS | LPS + S1P | |||||
|---|---|---|---|---|---|---|
| Baseline | Nadir | 450 min | Baseline | Nadir | 450 min | |
| WBC (×103)* | 5.32 ± 0.50 | 1.30 ± 0.23† | 5.68 ± 0.86‡ | 4.22 ± 1.02 | 1.02 ± 0.10† | 4.33 ± 0.81‡ |
| ANC (×103)* | 2.35 ± 0.45 | 0.40 ± 0.16† | 2.78 ± 0.46‡ | 1.98 ± 0.62 | 0.22 ± 0.08† | 1.97 ± 0.54‡ |
| ALC (×103)** | 2.02 ± 0.08 | 0.58 ± 0.05† | 1.60 ± 0.27‡ | 1.45 ± 0.35 | 0.45 ± 0.08† | 1.42 ± 0.28‡ |
| Platelets (×103)*** | 340 ± 68 | 207 ± 13† | 202 ± 13§ | 157 ± 17†§ | ||
| Hgb (g/dl) | 11.2 ± 0.4 | 12.8 ± 0.6† | 10.4 ± 0.8 | 12.6 ± 1.1 | ||
WBC – white blood cell; ANC – absolute neutrophil count; ALC – absolute lymphocyte count; Hgb - hemoglobin
Nadir at 150 min,
Nadir at 270 min,
Nadir at 450 min
p<0.05 compared to Baseline,
p<0.05 compared to Nadir,
p<0.05 compared to LPS
Rescue S1P augments systemic inflammatory cytokine release
Interestingly, intravenous S1P administration augmented the systemic cytokine release induced by LPS. Serum KC peaked at 1.2±0.9 ng/ml in S1P treated animals 3.5 hours after LPS instillation (p<0.05 compared to LPS-injured animals, Figure 5). TNF-α spiked dramatically to 31.4±4.6 ng/ml after 90 min in S1P treated canines (p<0.05 compared to LPS injured animals), and IL-6 levels were 3-fold higher in S1P-treated animals compared to LPS-injured canines after 7.5 hours (p<0.05, Figure 5). IL-8, IL-10, and MCP-1 production followed similar patterns in S1P-treated animals and those injured with LPS (Figure 5). Despite the augmentation of systemic inflammatory cytokine production, intravenous S1P had no effect on end-organ function (data not shown) or circulating leukocytes compared to animals injured with LPS alone (Table 2).
DISCUSSION
Using a translationally relevant large animal model of inflammatory ALI, we recently demonstrated that intravenous S1P (85 μg/kg) administered concomitantly with intratracheal LPS reduced shunt formation, attenuated alveolar protein accumulation, and decreased regional alveolar edema formation in beagles compared to animals injured with LPS alone 17. The current study has extended these findings to now demonstrate that intravenous S1P reduces physiological lung injury when administered subsequent to injury induction as a “rescue” therapy, an important step in preclinical evaluation of potentially beneficial therapeutics for ALI as the stimulus for development of lung injury often occurs prior to clinical presentation. Not only is shunt fraction reduced with intravenous S1P infused one hour after LPS instillation into the airways, but alveolar permeability to protein and influx of neutrophils into the alveolar space are decreased by S1P over the ensuing seven hours of high tidal volume mechanical ventilation (Figures 1 and 2). In addition, computed tomographic imaging of one animal injured with intratracheal LPS and treated with rescue S1P revealed a reduction in regional lung edema (data not shown) similar to that previously described with preventive S1P therapy 17. These observations are important for translation of sphingosine 1-phosphate into a clinically relevant therapeutic in the intensive care unit.
The endothelial barrier enhancing properties of S1P are well described in the literature 20–22. Recent evidence suggests that S1P in the airspace may also disrupt the alveolar epithelial barrier through action on apical surface S1P3 receptors 23. In our model of LPS-induced lung injury, we have shown that S1P is protective against the formation of permeability lung edema when administered both as preventive therapy 17 and, now, subsequent to the initiation of the inflammatory response. Furthermore, despite compromise of the alveolar barrier and thus decompartmentalization within the lung induced by LPS, we were unable to demonstrate the presence of S1P in the airspace using BAL samples from our previous model (data not published). A growing body of evidence now points to regulatory effects of S1P on cytokine and chemokine production by epithelium, endothelium, lymphocytes, macrophages, and dendritic cells as well as on inflammatory cell chemotaxis toward such stimuli 12, 13, 24–31. Recent evidence suggests that S1P and LPS may interact to enhance inflammatory cytokine production from dendritic cells and epithelial cells in vitro 32, 33. Our data represent the first quantification of the inflammatory chemokine and cytokine response to LPS and S1P in vivo, and, thus, provide insight into the possible role that S1P-induced modulation of the inflammatory response may play in the evolution or resolution of acute inflammatory lung injury.
Intratracheal LPS resulted in increased production of KC, IL-8, TNF-α, and IL-6 in both lung tissue and in the alveolar space (Figures 3 and 4). Inflammatory cytokine production is regionally heterogeneous with greater production in gravitationally dependent regions of the lung compared to gravitationally non-dependent regions corresponding to the distribution of lung edema in this model as we previously described 17. This observation is consistent with previous observations describing regionally heterogeneous expression of inflammatory mediators and other cellular responses within injured lungs compared to uninjured control lungs from the same animals protected with low-tidal volume ventilation, measured with genomic microarrays 34. Taken together, these data suggest that modification of inflammatory responses regionally within the lung may improve lung edema formation and thus outcome in ALI. Intravenous S1P, however, had no effect on LPS-induced regional lung tissue cytokine production nor did it alter cytokine levels in the BAL with the exception of reducing the production of the anti-inflammatory molecule, IL-10 (Figures 3 and 4). The relative inactivity of this compound on cytokine production in the lung may be explained in part by the lack of direct exposure of alveolar epithelial cells and resident alveolar macrophages and dendritic cells to circulating S1P in this model and highlights, perhaps, the importance of compartmentalization of cytokine production as it pertains to the evolution and resolution of lung injury. Alternatively, the cytokine response to intrabronchial LPS may, in fact, overwhelm any effect of intravenously delivered S1P.
Interestingly, neutrophil accumulation in the BAL was attenuated by intravenous S1P (Figure 2) despite the lack of effect on inflammatory cytokine production within the lung (Figures 3 and 4) or on lung tissue neutrophil recruitment (Figure 2). Notably, lung tissue cytokine production and myeloperoxidase (MPO) activity were measured at study termination only, that is, after eight hours of ventilatory support. It may be possible that tissue differences exist earlier in the evolution of the injury which we are unable to discern at study termination, though circulating neutrophil counts were similar in LPS-injured and S1P-treated groups (Table 2) suggesting similar degrees of pulmonary neutrophil sequestration in each group. This contrasts our previous observation of reduced lung tissue MPO activity in mice 24 hours after injury with intratracheal LPS and treatment with S1P in contrast to mice receiving LPS alone 7. In the current model, however, we are unable to assess the presence of neutrophils in the lung at 24 hours, and our statistical power is limited by small numbers. It remains possible that neutrophil clearance from the lung is enhanced by S1P and that differences would become evident at later time points as the injury resolves. Perhaps, the decrease in BAL neutrophils represents reduced chemotaxis in response to KC and IL-8 as demonstrated in vitro by Kawa and colleagues 12, a finding further supported in a recent article describing S1P-induced reduction of neutrophil expression of CXCR1 and subsequent chemotaxis to IL-8 35. Alternatively, the gradient of IL-8 and KC from the alveolar space to the intravascular space may lead to retention of neutrophils in the vascular compartment rather than promoting transmigration into the lung interstitium and ultimately the alveolar space. In addition, S1P has been demonstrated to reduce TNF-α induced adhesion molecule expression by endothelial cells in vitro 36. It is possible that alterations in endothelial cell surface adhesion molecule expression by S1P may impair neutrophil transmigration out of the vasculature thereby contributing to the physiological improvement observed in this model of LPS-induced lung injury. Finally, the potent endothelial barrier enhancement induced by S1P may serve to trap neutrophils within the vascular compartment thereby preventing their transmigration into the alveolar space.
Intrabronchial LPS triggered a systemic inflammatory response initiated by TNF-α followed by production of KC, IL-8, MCP-1, and IL-6. In addition, the anti-inflammatory cytokine, IL-10 was also transiently up-regulated by intrabronchial LPS. All animals experienced a transient increase in cardiac output and reduction in vascular resistance characteristic of the systemic inflammatory response syndrome (SIRS) which recovered by the end of the support period (Table 1). In contrast to the lung compartment, intravenous S1P treatment dramatically augmented systemic production of TNF-α, KC, and IL-6 during the support period. In addition, we identified a trend toward increased circulating IL-8 without significant alteration in circulating IL-10. These observations are consistent with published in vitro data describing endothelial and peripheral blood dendritic cell cytokine expression profiles in response to S1P and LPS 30–32. The exacerbation of systemic inflammatory cytokine release induced by S1P in this model is intriguing given the physiological improvements observed in shunt fraction and alveolar permeability and lack of significant alterations in physiological indices of SIRS (Table 1) or in biochemical evidence of end-organ function. It is not clear from these data, or from the literature, what independent effect S1P may have on inflammatory cytokine expression in vivo in the absence of an underlying inflammatory insult. Certainly, these data will be important for future investigation into the mechanisms responsible for the protective effects of S1P in inflammatory lung injury. IL-6 is known to be up-regulated in patients with lung injury 4, 37, but its physiological consequences remain unknown. The results of our study are consistent with a growing body of evidence in the literature suggesting that IL-6 may, in fact, be protective in lung injury 38–41, and it is possible that S1P works in part through IL-6 in mitigating its protection against lung injury. Certainly, further work is warranted in this regard to further elucidate what, if any, anti-inflammatory or reparative effect of IL-6 may be operative in this model.
Despite the strengths of our observations, certain limitations of our study warrant discussion. First, the administration of S1P only one hour after injury induction may not represent true rescue therapy. The data suggest that the inflammatory response and barrier disruption in the lung are underway already 60 min after LPS instillation as shunt fraction is rapidly rising between 30 and 90 min. The impairment in oxygenation follows the accumulation of alveolar edema resulting from permeability in response to the inflammatory stimulus. As such, administration of S1P one hour after LPS instillation represents a point after which the inflammatory response has begun in the lung and, thus, rescue therapy. Longer follow-up may be important as upregulation of inflammatory cytokines may lead downstream to injurious consequences beyond the interval of observation in this study. Our ability to extend the canine model beyond the eight hours currently studied is limited by both expense and logistical complexity. Importantly however, we have previously reported improvement in physiological measures of lung injury in mice up to 24 hrs after LPS instillation and treatment with iv S1P 7. Reductions in cardiac output have been reported to decrease shunt fraction. In our study, despite the indication in Table 1 that cardiac index appeared to be depressed more in the S1P-treated group than in the LPS-injury group, both groups, in fact, experienced a similar evolution of cardiac index over time (data not shown, p=NS by 2-way ANOVA). Furthermore, no clear relationship existed between shunt fraction and cardiac index in our model. Finally, one may question the translational relevance of an endotoxin model of lung injury and our use of high tidal volume mechanical ventilation. It is not our intention to model pneumonia or clinical sepsis; rather it is to model inflammatory lung injury in general. The use of endotoxin allows for better control and reproducibility of the inflammatory response, and the high tidal volume is designed to exacerbate the inflammatory response within the lung and accentuate the physiological injury. Dogs tend to breathe at higher tidal volumes than do humans 42. Therefore, interpretation of the tidal volume employed in this study as it related to clinical relevance in humans is difficult. Of note, in the past, we have been unable to adequately ventilate our dogs at tidal volumes less than 10–11 cc/kg. The translational strength of the model lies in the use of a large animal with regionally heterogeneous lung injury which more closely approximates human ALI than do rodent models, as well as the ability to control and measure important hemodynamic parameters.
Despite these limitations, these results suggest that S1P may represent a viable therapy for the treatment of ALI when administered as rescue therapy. Based on our previously published observations 17 and the data presented herein, the physiological improvement in this model of intratracheal LPS-induced ALI afforded by S1P appears to be due in large part to the reduction in endothelial permeability. The contribution of the modulation of the inflammatory response remains unclear. Alterations in neutrophil chemotaxis into the alveolar space may contribute to the reduction in permeability observed in this and in our previous study of the preventive effects of S1P in this model. Further study to characterize the margination of neutrophils within the lung, their movement across the pulmonary endothelium, and the contributions of IL-6 to the evolution and resolution of acute lung injury are warranted to further explore the mechanisms underlying the therapeutic benefit of S1P. Further investigating the mechanisms underlying the preclinical benefits of S1P may provide deeper insight into the pathogenesis of acute lung injury and present valuable information for the development of novel therapeutic agents for this devastating syndrome.
Acknowledgments
This work was supported by grants from the NHLBI F32 HL74569, P50 HL073994, P01 HL 58604, and K08 HL083101. The authors gratefully acknowledge the contributions of Stephen F. Badylak, MD, PhD, DVM and the McGowan Institute for Regenerative Medicine, Tim Burman for expert technical assistance, and Respironics, Inc. for the donation of the PLV-102 mechanical ventilator.
ABBREVIATIONS
- ALI
acute lung injury
- ANOVA
analysis of variance
- BAL
bronchoalveolar lavage
- CT
computed tomography
- CXCR-1
C-X-C chemokine receptor-1
- ETCO2
end-tidal carbon dioxide
- FiO2
fraction of inspired oxygen
- IL
interleukin
- iv
intravenous
- KC
C-X-C chemokine ligand-1
- LLD
lower lobe dependent
- LLND
lower lobe non-dependent
- LLT
lower lobe transitional
- LPS
lipopolysaccharide
- MCP
monocyte chemotactic protein
- MPO
myeloperoxidase
- Pa
arterial pressure
- PEEP
positive end-expiratory pressure
- PPEAK
peak airway pressure
- Qs/Qt
venous admixture or shunt fraction
- S1P
sphingosine 1-phosphate
- SaO2
arterial oxygen saturation
- SIRS
systemic inflammatory response syndrome
- TNF
tumor necrosis factor
- ULND
upper lobe non-dependent
- Vt
tidal volume
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Goss CH, Brower RG, Hudson LD, Rubenfeld GD. Incidence of acute lung injury in the United States. Crit Care Med. 2003;31(6):1607–1611. doi: 10.1097/01.CCM.0000063475.65751.1D. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 4.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 5.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2(7511):319–323. doi: 10.1016/s0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 6.Garcia JG, Liu F, Verin AD, et al. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108(5):689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng X, Hassoun PM, Sammani S, et al. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med. 2004;169(11):1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- 8.Szczepaniak WS, Leelavanichkul K, Pitt BR, Choi AMK, St Croix CM, McVerry BJ. Sphingosine 1-phosphate induces dose-dependent pulmonary vascoconstriction in the intact mouse lung. Am J Respir Crit Care Med. 2007;175:A404. [Google Scholar]
- 9.Schaphorst KL, Chiang E, Jacobs KN, et al. Role of sphingosine-1 phosphate in the enhancement of endothelial barrier integrity by platelet-released products. Am J Physiol Lung Cell Mol Physiol 2003. 2003;285(1):L258–267. doi: 10.1152/ajplung.00311.2002. [DOI] [PubMed] [Google Scholar]
- 10.Schaphorst KL, Jacobs KN, Verin AD, Garcia JGN. Sphingosine 1-phosphate increases F-actin/adherens junction linkage and enhances barrier protection. Amer J Resp Crit Care Med. 2001;163:A615. [Google Scholar]
- 11.Hla T. Signaling and biological actions of sphingosine 1-phosphate. Pharmacol Res. 2003;47(5):401–407. doi: 10.1016/s1043-6618(03)00046-x. [DOI] [PubMed] [Google Scholar]
- 12.Kawa S, Kimura S, Hakomori S, Igarashi Y. Inhibition of chemotactic motility and trans-endothelial migration of human neutrophils by sphingosine 1-phosphate. FEBS Letters. 1997;420:196–200. doi: 10.1016/s0014-5793(97)01516-0. [DOI] [PubMed] [Google Scholar]
- 13.Mandala S, Hajdu R, Bergstrom J, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296(5566):346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 14.Pyne S, Pyne N. Sphingosine 1-phosphate signalling via the endothelial differentiation gene family of G-protein-coupled receptors. Pharmacol Ther. 2000;88(2):115–131. doi: 10.1016/s0163-7258(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 15.Salomone S, Potts EM, Tyndall S, et al. Analysis of sphingosine 1-phosphate receptors involved in constriction of isolated cerebral arteries with receptor null mice and pharmacological tools. Br J Pharmacol. 2008;153(1):140–147. doi: 10.1038/sj.bjp.0707581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsiao SH, Constable PD, Smith GW, Haschek WM. Effects of exogenous sphinganine, sphingosine, and sphingosine-1-phosphate on relaxation and contraction of porcine thoracic aortic and pulmonary arterial rings. Toxicol Sci. 2005;86(1):194–199. doi: 10.1093/toxsci/kfi167. [DOI] [PubMed] [Google Scholar]
- 17.McVerry BJ, Peng X, Hassoun PM, Sammani S, Simon BA, Garcia JG. Sphingosine 1-phosphate reduces vascular leak in murine and canine models of acute lung injury. Am J Respir Crit Care Med. 2004;170(9):987–993. doi: 10.1164/rccm.200405-684OC. [DOI] [PubMed] [Google Scholar]
- 18.Nunn J. Nunn’s Applied Respiratory Physiology. Butterworth-Heinemann; 1987. pp. 165–168. [Google Scholar]
- 19.Goldblum SE, Wu KM, Jay M. Lung myeloperoxidase as a measure of pulmonary leukostasis in rabbits. J Appl Physiol. 1985;59(6):1978–1985. doi: 10.1152/jappl.1985.59.6.1978. [DOI] [PubMed] [Google Scholar]
- 20.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91(4):1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- 21.McVerry BJ, Garcia JG. Endothelial cell barrier regulation by sphingosine 1-phosphate. J Cell Biochem. 2004;92(6):1075–1085. doi: 10.1002/jcb.20088. [DOI] [PubMed] [Google Scholar]
- 22.McVerry BJ, Garcia JG. In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cell Signal. 2005;17(2):131–139. doi: 10.1016/j.cellsig.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Gon Y, Wood MR, Kiosses WB, et al. S1P3 receptor-induced reorganization of epithelial tight junctions compromises lung barrier integrity and is potentiated by TNF. Proc Natl Acad Sci USA. 2005;102(26):9270–9275. doi: 10.1073/pnas.0501997102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Chandru H, Boggaram V. The role of sphingosine 1-phosphate in the TNF-alpha induction of IL-8 gene expression in lung epithelial cells. Gene. 2007;391(1–2):150–160. doi: 10.1016/j.gene.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goetzl EJ, Graler MH. Sphingosine 1-phosphate and its type 1 G protein-coupled receptor: trophic support and functional regulation of T lymphocytes. J Leukoc Biol. 2004;76(1):30–35. doi: 10.1189/jlb.1103567. [DOI] [PubMed] [Google Scholar]
- 26.Goetzl EJ, Wang W, McGiffert C, Huang MC, Graler MH. Sphingosine 1-phosphate and its G protein-coupled receptors constitute a multifunctional immunoregulatory system. J Cell Biochem. 2004;92(6):1104–1114. doi: 10.1002/jcb.20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graeler M, Goetzl EJ. Activation-regulated expression and chemotactic function of sphingosine 1-phosphate receptors in mouse splenic T cells. FASEB J. 2002;16(14):1874–1878. doi: 10.1096/fj.02-0548com. [DOI] [PubMed] [Google Scholar]
- 28.Graeler M, Shankar G, Goetzl EJ. Cutting edge: suppression of T cell chemotaxis by sphingosine 1-phosphate. J Immunol. 2002;169(8):4084–4087. doi: 10.4049/jimmunol.169.8.4084. [DOI] [PubMed] [Google Scholar]
- 29.Graler MH, Huang MC, Watson S, Goetzl EJ. Immunological effects of transgenic constitutive expression of the type 1 sphingosine 1-phosphate receptor by mouse lymphocytes. J Immunol. 2005;174(4):1997–2003. doi: 10.4049/jimmunol.174.4.1997. [DOI] [PubMed] [Google Scholar]
- 30.Lin CI, Chen CN, Chen JH, Lee H. Lysophospholipids increase IL-8 and MCP-1 expressions in human umbilical cord vein endothelial cells through an IL-1-dependent mechanism. J Cell Biochem. 2006;99(4):1216–1232. doi: 10.1002/jcb.20963. [DOI] [PubMed] [Google Scholar]
- 31.Lin CI, Chen CN, Lin PW, Lee H. Sphingosine 1-phosphate regulates inflammation-related genes in human endothelial cells through S1P1 and S1P3. Biochem Biophys Res Commun. 2007;355(4):895–901. doi: 10.1016/j.bbrc.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 32.Oz-Arslan D, Ruscher W, Myrtek D, et al. IL-6 and IL-8 release is mediated via multiple signaling pathways after stimulating dendritic cells with lysophospholipids. J Leukoc Biol. 2006;80(2):287–297. doi: 10.1189/jlb.1205751. [DOI] [PubMed] [Google Scholar]
- 33.Eskan MA, Rose BG, Benakanakere MR, et al. TLR4 and S1P receptors cooperate to enhance inflammatory cytokine production in human gingival epithelial cells. Eur J Immunol. 2008;38(4):1138–1147. doi: 10.1002/eji.200737898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simon BA, Easley RB, Grigoryev DN, et al. Microarray analysis of regional cellular responses to local mechanical stress in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;291(5):L851–861. doi: 10.1152/ajplung.00463.2005. [DOI] [PubMed] [Google Scholar]
- 35.Rahaman M, Costello RW, Belmonte KE, Gendy SS, Walsh MT. Neutrophil sphingosine 1-phosphate and lysophosphatidic acid receptors in pneumonia. Am J Respir Cell Mol Biol. 2006;34(2):233–241. doi: 10.1165/rcmb.2005-0126OC. [DOI] [PubMed] [Google Scholar]
- 36.Kimura T, Tomura H, Mogi C, et al. Sphingosine 1-phosphate receptors mediate stimulatory and inhibitory signalings for expression of adhesion molecules in endothelial cells. Cell Signal. 2006;18(6):841–850. doi: 10.1016/j.cellsig.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 37.Flores C, Ma SF, Maresso K, Wade MS, Villar J, Garcia JG. IL6 gene-wide haplotype is associated with susceptibility to acute lung injury. Transl Res. 2008;152(1):11–17. doi: 10.1016/j.trsl.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 38.Ward NS, Waxman AB, Einarsson O, Elias JA. Interleukin-6 protection in hyperoxic lung injury. Chest. 1999;116(1 Suppl):26S. [PubMed] [Google Scholar]
- 39.Ward NS, Waxman AB, Homer RJ, et al. Interleukin-6-induced protection in hyperoxic acute lung injury. Am J Respir Cell Mol Biol. 2000;22(5):535–542. doi: 10.1165/ajrcmb.22.5.3808. [DOI] [PubMed] [Google Scholar]
- 40.Waxman AB, Mahboubi K, Knickelbein RG, et al. Interleukin-11 and interleukin-6 protect cultured human endothelial cells from H2O2-induced cell death. Am J Respir Cell Mol Biol. 2003;29(4):513–522. doi: 10.1165/rcmb.2002-0044OC. [DOI] [PubMed] [Google Scholar]
- 41.Calfee CS, Eisner MD, Ware LB, et al. Trauma-associated lung injury differs clinically and biologically from acute lung injury owing to other clinical disorders. Crit Care Med. 2007;35(10):2243–2250. doi: 10.1097/01.ccm.0000280434.33451.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Venegas JG, Hales CA, Strieder DJ. A general dimensionless equation of gas transport by high-frequency ventilation. J Appl Physiol. 1986;60(3):1025–1030. doi: 10.1152/jappl.1986.60.3.1025. [DOI] [PubMed] [Google Scholar]