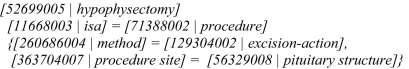Abstract
Objective
This study sought to develop and evaluate an approach for auditing the semantic completeness of the SNOMED CT contents using a formal concept analysis (FCA)–based model.
Design
We developed a model for formalizing the normal forms of SNOMED CT expressions using FCA. Anonymous nodes, identified through the analyses, were retrieved from the model for evaluation. Two quasi-Poisson regression models were developed to test whether anonymous nodes can evaluate the semantic completeness of SNOMED CT contents (Model 1), and for testing whether such completeness differs between 2 clinical domains (Model 2). The data were randomly sampled from all the contexts that could be formed in the 2 largest domains: Procedure and Clinical Finding. Case studies (n = 4) were performed on randomly selected anonymous node samples for validation.
Measurements
In Model 1, the outcome variable is the number of fully defined concepts within a context, while the explanatory variables are the number of lattice nodes and the number of anonymous nodes. In Model 2, the outcome variable is the number of anonymous nodes and the explanatory variables are the number of lattice nodes and a binary category for domain (Procedure/Clinical Finding).
Results
A total of 5,450 contexts from the 2 domains were collected for analyses. Our findings revealed that the number of anonymous nodes had a significant negative correlation with the number of fully defined concepts within a context (p < 0.001). Further, the Clinical Finding domain had fewer anonymous nodes than the Procedure domain (p < 0.001). Case studies demonstrated that the anonymous nodes are an effective index for auditing SNOMED CT.
Conclusion
The anonymous nodes retrieved from FCA-based analyses are a candidate proxy for the semantic completeness of the SNOMED CT contents. Our novel FCA-based approach can be useful for auditing the semantic completeness of SNOMED CT contents, or any large ontology, within or across domains.
Introduction
The structure of modern terminologies has advanced well beyond simple 1-dimensional subsumption relationships through the introduction of composite expressions (see The International Organization for Standardization formal definition 1 ); these relationships have long been sought in controlled medical terminologies. 2–5 SNOMED-CT, the most comprehensive clinically oriented medical terminology system, now provides a platform where composite expressions have the potential to be used in clinical situations. 6 SNOMED CT adopted a description logic (DL) foundation 7,8 that has allowed its curators to formally represent concept meanings and relationships. 9
Because compositional variants and precomposed concepts may introduce redundancy and hamper information retrieval, SNOMED CT defines a concept “normal form” or canonical representation as the maximal decomposition of concepts into a set of primitive defining supertypes. These normal forms are proposed as the formal representation of clinical meaning for SNOMED CT concepts to support authoring tasks, distribution, and other purposes, such as the comprehensive retrieval of precoordinated and postcoordinated SNOMED CT expressions from clinical records. 10,11
Given the size, complexity, and sophistication of SNOMED, the need arises for automated and reliable means to algorithmically assess the completeness, correctness, consistency, and competency 12 of the vocabulary and its adherence to good terminology practices. We introduce one such method in this article, based on formal concept analysis (FCA).
Formal concept analysis is a generic structure of lattice-building algorithms, based on mathematical lattice theory, which permits visualizing partial or incomplete order in an information lattice and its consequences. 13,14 It provides a candidate mechanism for developing completeness-evaluation algorithms. Our hypothesis is that reformulating the rules of composition and compositional transformations associated with SNOMED normal forms, in the language of lattice theory, may provide a novel approach for auditing large terminologies such as SNOMED CT. The objective of this study is to develop and evaluate an approach for auditing the semantic completeness of the SNOMED CT contents using a FCA-based model.
Background
Auditing the Medical Terminological Systems
A number of generalized approaches have been designed for auditing medical terminologies. 12,15 Here we review those approaches that have mainly been applied to SNOMED CT auditing.
Spackman 16 summarized the overall size of the SNOMED CT and its rates of change over a period of 3 years, and found that awareness of the rate of change in the terminology can help both terminology and application developers. However, simply invoking DL formalisms for terminology development may not prevent incorrect or incomplete representations in a medical terminology or ensure compliance with all principles of a sound ontology. 17 Indeed, Bodenreider et al. 18 pointed out that the descriptions of many concepts in SNOMED CT are minimal or incomplete, with possible “detrimental consequences on inheritance.” Investigators have suggested that using more complete logical and ontological practices would prevent certain families of modeling mistakes and improve the quality of a large terminology such as SNOMED CT. 18,19
Halper et al. 20 developed an ad-hoc approach for analysis of error concentrations in SNOMED CT through investigating the area taxonomy, derived from a partition of SNOMED's concepts based on their respective sets of relationships. Spackman et al. 21 examined SNOMED CT from the perspective of formal ontological principles. While they showed the usefulness of formal ontological principles for improving consistency of design decisions of SNOMED CT, they argued that “the applicability of some of the formal ontology principles in providing consistent guidance in the very large areas like clinical finding and procedure is still not as clear, and appears to require further elaboration and study.”
In addition, several studies developed auditing approaches by applying SNOMED CT to actual problems and use cases. For example, Green et al. 22 developed and evaluated a method for the structured representation of heart murmur findings using SNOMED CT postcoordination. Similarly, Richesson et al. 23 used SNOMED CT to represent consistently clinical research data based on the semantic characterization of data items on Case Report Forms.
Semantic Completeness
Semantic completeness is a property of any logical system, including well-formed terminologies, that invokes 3 components: (1) a procedure for constructing formulas that will yield propositions when interpreted using specific rules (well-formed formulas), (2) a definition of truth that relates to interpretations and models of logical systems (expressive completeness), and (3) a proof procedure that allows new well-formed formulas to be derived from old ones (deductive completeness). 24 A logical system is logically complete if every true, well-formed formula can be derived.
Applying the notion of completeness to ontologies, we find that it can be interpreted in a number of ways. For example, Fox et al. 25 defined the “functional completeness” of ontology as its ability to represent the information necessary for a function to perform its task, i.e., the completeness of an ontology is determined by its competency. Over a decade ago, Devanbu and Jones 26 asserted that a terminology system should satisfy 4 requirements: completeness, correctness, consistency, and competency. Completeness as indicated by Devanbu indicates that a terminology system should have the knowledge necessary to represent a domain. They used competency to indicate that the system should have efficient algorithms to perform the inferences needed for the application. Given that medical concept representation in modern medical terminologies may be regarded from the perspectives of both breadth of coverage and depth of representation, 27 we consider that the completeness of a medical terminology system should include 2 parts: complete content coverage (coverage completeness) and complete semantics (semantic completeness), which supports its competency. The former has historically been well addressed, 28–32 whereas few studies address the latter. In this study, we focus on the semantic completeness of the SNOMED CT contents.
Relevant Constructs of SNOMED CT
Precoordination and Postcoordination
Precoordination is the use of composite expressions of coded concepts within a terminology to define a new coded concept. For example, “hand joint pain” is a composite expression defined by [[is a = joint pain] and [finding site = hand joint structure]], manifest as a precoordinated expression in SNOMED CT. The ability to define composite expressions within a terminology opens a whole new realm of expressive possibility. The same techniques used to create precoordinated expressions can also be used to describe new external classes that have no equivalent concept code within the terminology. The creation of new classes externally is called postcoordination. Given that it is neither practical nor desirable to define precoordinated terms for every conceivable clinical situation, postcoordination becomes one promising solution to the problem of clinical content completeness. 33,34 For example, for all possible types and severity of fractures of all possible bones and their subdivisions, 34 or for all potential points on the chest that can be considered for point of maximum intensity and area of radiation of a heart murmur 22 ; the enumerated possibilities may be finite but are awkwardly large.
Primitive and Fully Defined Concepts
Due to the fact that composite expressions are built using concept codes and the basic fact that not all classes are amenable to formal definition, some of the concept codes in any terminology will remain implicitly defined. Concept codes that fall into this category are referred to as primitive. SNOMED CT concepts are either primitive or fully defined. In the language of description logics, the asserted conditions of a primitive concept are necessary but not sufficient, and the asserted conditions of a fully defined concept are both necessary and sufficient. All members of the set of sufficient conditions are also necessary conditions. For example, “gastric ulcer” is a fully defined concept given that the definition [[associated morphology = ulcer] and [finding site = stomach structure]] is asserted as both necessary and sufficient, whereas the aforementioned “hand joint pain” is a primitive concept given that its definition is asserted as necessary but not sufficient. We assert that the higher the proportion of fully defined concepts within a domain, the more complete are the semantics of that domain. We use fully defined concepts as an independent index to represent the semantic completeness of SNOMED CT in this study.
Normal (or Canonical) Forms
As introduced in the technical documentation of SNOMED CT, 11 a normal form is a view that can be generated by maximally decomposing any valid expression by applying a set of logical transformation rules. The purpose of generating normal forms is to facilitate complete and accurate retrieval of precoordinated and postcoordinated SNOMED CT expressions from clinical records or other resources. Two alternative normal forms are proposed: the long normal form and the short normal form. Both normal form transformation algorithms are described in the technical document. For example, the long normal form for “hypophysectomy” is shown in ▶.
Figure 1.
A long normal form for “hypophysectomy.” The semantics of the long normal form may be interpreted as that hypophysectomy is a subtype of procedure which is defined by the conditions “method = excision-action” and “procedure site = pituitary structure.” The square brackets indicate pair of a concept identifier with its preferred name (separated by bar “|”). The curly brackets indicate the conditions used for defining “procedure.”
Basic Notions of FCA
Many published articles and books describe the features of FCA in detail. 13,35,36 Here we briefly introduce some basic notions and features to help explain the modeling process in the next section. A (1-valued) formal context is defined as a triple comprising a set of formal objects, a set of formal attributes, and binary relations expressing which attributes describe each object. Usually, a formal context can be represented by a cross table. In many use cases, we may find that the relations between the objects and the attributes are a set of values rather than binary relations. Thus a many-valued formal context could also be expressed in a cross table. However, for the FCA application, many-valued formal contexts are transformed into a 1-valued context by transformational scaling. 13,35
Graphically, a formal context could be visualized by a line diagram of a concept lattice. A concept lattice consists of the set of formal concepts of a formal context and the subconcept–superconcept relations between the formal concepts. Each node in a concept lattice represents a formal concept for which the meaning is interpreted by a set of formal objects (extension) and a set of formal attributes (intension). In other words, the extension covers all objects belonging to this concept and its child nodes, while the intension comprises all defining attributes for this concept and its parent nodes. The labels for each node are usually displayed on the lattice; the FCA literature refers to these labels as own objects and own attributes, respectively. Retrieving the extension and the intension of a node (i.e., a formal concept) from a concept lattice is achieved by a trace down or trace up using well-specific rules. A node without a label for its own object in a concept lattice is called an anonymous node.
The notion of anonymous nodes is not specific to our FCA approach. For example, the Generalized Architecture for Languages, Encyclopedias, and Nomenclatures in medicine (GALEN) common reference model, developed by the University of Manchester, does not enumerate all sanctioned variants, e.g., it does not pre-enumerate all possible left-handed and right-handed variants of anatomical structures. Instead, GALEN defines anonymous concepts by expressions such as (SolidStructure which <isPairedOrUnpaired leftRightPaired>) (representing a bilateral solid structure). 37–39 Similarly, the Web Ontology Language (OWL) supports the representation of an anonymous class (i.e., anonymous concept), which may be or may not be an equivalent class of a named class. 40 However, we argue that the FCA-based approach is DL-language independent, and it encodes the problem of multiple relations in the definition of (many-valued) multicontexts and allows the transformation of a multicontext into a meaningful structure of concept lattices. 41 Thus FCA provides a flexible and scalable way to capture the semantic completeness (represented by anonymous nodes) of modern terminologies.
Formal concept analysis has been advocated as a mechanism to represent and process context knowledge in domains such as the description of patient cases, interpretation of therapeutic decisions, and the representation of rules. 42 It provides a means to represent the semantics underlying the meaning of a concept definition 35 and has been applied to many knowledge representation areas, such as ontology building, 43,44 ontology mapping and merging, 45,46 lexical databases, and taxonomy modeling. 47,48
Methods
An FCA-based Model for the Normal Forms of the SNOMED CT Expressions
Expanding the Semantic Space for a Specific Domain
To do retrieval or analysis of stored clinical data, the stored expressions and the query expression would both be compared in their normal form when evaluating equivalence or subsumption. 10 However, in many real use cases, we found that the attributes (or slots) of a normal form for a specific expression were not sufficient to meet the requirements of postcoordination in a clinical statement. For instance, consider the composition expression “[hypophysectomy] + [approach = transfrontal approach]” retrieved from a clinical record. When converting this expression into normal form, we find that the normal form of “hypophysectomy” only contains 3 attributes—“isa”, “procedure site”, and “method;” there is no slot for “approach” (▶).
The absence of an expected normal form to support information retrieval and other use cases suggests that all subconcepts within a domain should be “semantically completed”; specifically, the domain should be expanded or populated with the additional concepts necessary to create the normal forms needed for retrieval. For our purposes in this report, we regard any SNOMED CT concept that contains subconcepts as a candidate domain. Still using “hypophysectomy” as an example, when we retrieve the normal forms of its 20 subclasses we find that there are 7 attributes used in these normal forms (▶). For convenience, according the children of “hypophysectomy” the status of a domain, these attributes could be shared to describe any postcoordinated expressions retrieved from clinical statements within that domain. Using the expanded semantic space, we find that this domain does contain a slot “approach” that could be used for converting the composition expression “[hypophysectomy] + [approach = transfrontal approach]” into a normal form.
Table 1.
Table 1 The Normal Forms of All Concepts in the Domain “Hypophysectomy”
| Expression | isA | Procedure Site | Procedure Site, Indirect | Procedure Site, Direct | Direct Morphology | Method | Approach |
|---|---|---|---|---|---|---|---|
| Hypophysectomy | Procedure | Pituitary structure | – | – | – | Excision - action | – |
| Excision of lesion of pituitary gland | Procedure | – | Pituitary structure | Pituitary structure | Morphologically abnormal structure | Excision - action | Surgical action | – |
| Excision of pituitary gland NOS | Excision of pituitary gland NOS | Pituitary structure | – | – | – | Excision - action | – |
| Excisional biopsy of hypophysis | Excisional biopsy of hypophysis | Pituitary structure | – | – | – | Excision biopsy | – |
| Excisional biopsy of pituitary gland by transfrontal approach | Procedure | Pituitary structure | – | – | – | Excision biopsy | Transfrontal approach |
| Excisional biopsy of pituitary gland by transsphenoidal approach | Procedure | Pituitary structure | – | – | – | Excision biopsy | Transsphenoidal approach |
| Hypophysectomy NEC | Hypophysectomy NEC | Pituitary structure | – | – | – | Excision - action | – |
| Other specified excision of pituitary gland | Other specified excision of pituitary gland | Pituitary structure | – | – | – | Excision - action | – |
| Partial excision of pituitary gland by transfrontal approach | Procedure | Pituitary part | – | – | – | Excision - action | Transfrontal approach |
| Partial excision of pituitary gland by transsphenoidal approach | Procedure | Pituitary part | – | – | – | Excision - action | Transsphenoidal approach |
| Partial hypophysectomy | Procedure | Pituitary part | – | – | – | Excision - action | – |
| Removal of normal pituitary gland | Removal of normal pituitary gland | Pituitary structure | – | – | – | Excision - action | – |
| Selective transsphenoidal pituitary adenomectomy | Selective transsphenoidal pituitary adenomectomy | Pituitary structure | – | – | – | Excision - action | Transsphenoidal approach |
| Sublabial hypophysectomy | Procedure | Pituitary structure | – | – | – | Excision - action | Transsphenoidal approach | Sublabial approach |
| Total excision of pituitary gland by transfrontal approach | Procedure | Entire pituitary gland | – | – | – | Excision - action | Transfrontal approach |
| Total excision of pituitary gland by transsphenoidal approach | Procedure | Entire pituitary gland | – | – | – | Excision - action | Transsphenoidal approach |
| Total hypophysectomy | Procedure | Entire pituitary gland | – | – | – | Excision - action | – |
| Transcranial hypophysectomy | Procedure | Pituitary structure | – | – | – | Excision - action | Transcranial approach |
| Transethmoidal hypophysectomy | Procedure | Pituitary structure | – | – | – | Excision - action | Transsphenoidal approach | Transethmoidal approach |
| Transseptal hypophysectomy | Transseptal hypophysectomy | Pituitary structure | – | – | – | Excision - action | Transsphenoidal approach |
| Transsphenoidal hypophysectomy | Procedure | Pituitary structure | – | – | – | Excision - action | Transsphenoidal approach |
NEC = not elsewhere classified; NOS = not otherwise specified.
Modeling the Normal Forms Using the FCA
Invoking the description-logic structures of SNOMED CT, the attribute name-value model was applied to describe the composite expression in SNOMED CT. The name part of the attribute name-value pair is a conceptId that refers to a concept and the value part of the attribute name-value pair is an expression. 11 A normal form, in fact, is a decomposed structure for a nested expression using a set of specific transformation rules. ▶ shows the long normal forms of all concepts in the domain “hypophysectomy” defined in the SNOMED CT.
In the language of the FCA, the data in ▶ can be interpreted as a formal context, which actually is referred to as a many-valued formal context. ▶ may be understood as a structure that contains a set of objects (whose names are heading of rows, i.e., the concept names of a domain), a set of attributes (whose names are heading of columns, i.e., the name part of the attribute name-value pairs), and a set containing all attribute values described by the entries in the table cells (i.e., the value part of the attribute name-value pairs).
For the FCA application, the many-valued formal context can be transformed to a 1-valued context, or Boolean form, by transformation scaling. Here, we take a 2-step approach for the transformation. The first step, called plain scaling, substitutes each attribute in the original many-valued context with a set of columns representing each one of the allowed values for the attribute. This corresponds to the notion that is called reification in the DL community, i.e., transforming one relation and its object value into a relationship. 49 Still using the data in ▶ as our example, we obtain 22 Boolean columns containing the same information that substitutes for the 7 original attributes or column headings in ▶ (▶). The Xs in ▶ indicate when an object has a defined attribute value. We concatenated the attribute value and the original attribute name to synthesize a readable column name, e.g., “Procedure(isA)”.
Table 2.
Table 2 A Completed Formal Context of the Domain “Hypophysectomy”
| Procedure(isA) | Hypophysectomy NEC(isA) | Removal of normal pituitary gland(isA) | Transseptal hypophysectomy(isA) | Excision of pituitary gland NOS(isA) | Excisional biopsy of hypophysis(isA) | Selective transsphenoidal pituitary adenomectomy(isA) | Other specified excision of pituitary gland(isA) | Pituitary structure(Procedure site) | Pituitary part(Procedure site) | Entire pituitary gland(Procedure site) | Pituitary structure(Procedure site–Direct) | Pituitary structure(Procedure site–Indirect) | Morphologically abnormal structure (Direct morphology) | Excision - action(Method) | Surgical action(Method) | Excision biopsy(Method) | Transfrontal approach(Approach) | Transcranial approach(Approach) | Transsphenoidal approach(Approach) | Sublabial approach(Approach) | Transethmoidal approach(Approach) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hypophysectomy | X | X | X | X | ||||||||||||||||||
| Excision of lesion of pituitary gland | X | X | X | X | X | X | X | |||||||||||||||
| Excision of pituitary gland NOS | X | X | X | X | X | |||||||||||||||||
| Excisional biopsy of hypophysis | X | X | X | X | X | X | ||||||||||||||||
| Excisional biopsy of pituitary gland by transfrontal approach | X | X | X | X | X | X | X | |||||||||||||||
| Excisional biopsy of pituitary gland by transsphenoidal approach | X | X | X | X | X | X | X | |||||||||||||||
| Hypophysectomy NEC | X | X | X | X | X | |||||||||||||||||
| Other specified excision of pituitary gland | X | X | X | X | X | |||||||||||||||||
| Partial excision of pituitary gland by transfrontal approach | X | X | X | X | X | X | X | |||||||||||||||
| Partial excision of pituitary gland by transsphenoidal approach | X | X | X | X | X | X | X | |||||||||||||||
| Partial hypophysectomy | X | X | X | X | X | |||||||||||||||||
| Removal of normal pituitary gland | X | X | X | X | X | |||||||||||||||||
| Selective transsphenoidal pituitary adenomectomy | X | X | X | X | X | X | X | |||||||||||||||
| Sublabial hypophysectomy | X | X | X | X | X | X | X | |||||||||||||||
| Total excision of pituitary gland by transfrontal approach | X | X | X | X | X | X | X | |||||||||||||||
| Total excision of pituitary gland by transsphenoidal approach | X | X | X | X | X | X | X | |||||||||||||||
| Total hypophysectomy | X | X | X | X | X | |||||||||||||||||
| Transcranial hypophysectomy | X | X | X | X | X | |||||||||||||||||
| Transethmoidal hypophysectomy | X | X | X | X | X | X | X | |||||||||||||||
| Transseptal hypophysectomy | X | X | X | X | X | X | X | |||||||||||||||
| Transsphenoidal hypophysectomy | X | X | X | X | X | X |
Xs in shade indicate those relations completed by the hierarchical knowledge of the SNOMED CT.
Abbreviations as in ▶.
The second step in transformation scaling is to complete the context using hierarchical knowledge within SNOMED CT. For example, in the column “Procedure site” of ▶, there are 3 different values, including “Pituitary structure,” “Pituitary part,” and “Entire pituitary gland.” When we retrieve the relationships (including both direct and indirect relationships using the transitive closure of “isa” relationship) among them, we may find that “Pituitary part” and “Entire pituitary gland” are subconcepts of “Pituitary structure.” Thus, for the transformation to a 1-valued context, those objects having the values “Pituitary part” and “Entire pituitary gland,” besides being X'ed for “Pituitary part(Procedure site)” and “Entire pituitary gland(Procedure site)” as taken in the first step, are also X'ed for “Pituitary structure(Procedure site).” For each column of ▶, the same transformation using the transitive closure is applied to partially complete the context. In addition, the relationships among all the column headings are also retrieved. For ▶, we may find that “Procedure site - Direct” and “Procedure site - Indirect” are the subproperties of “Procedure site.” Thus, for the transformation to 1-valued context, those objects having the attributes “Procedure site - Direct” and “Procedure site - Indirect” are included for completing the context for the attribute “Procedure site.” In ▶, only 1 concept, “Excision of lesion of pituitary gland,” has 2 attributes defined. Besides being X'ed for “Pituitary structure(Procedure site - Direct)” and “Pituitary structure(Procedure site - Indirect),” the concept is also X'ed for “Pituitary structure(Procedure site)” because the former 2 properties are both subproperties of “Pituitary structure(Procedure site).” These inferred Xs that derive from completing the context using hierarchical associations in the base terminologies are shaded in ▶ for exposition, although they are not further distinguished in FCA analyses.
Visualizing the Modeled Domain Using Concept Lattice
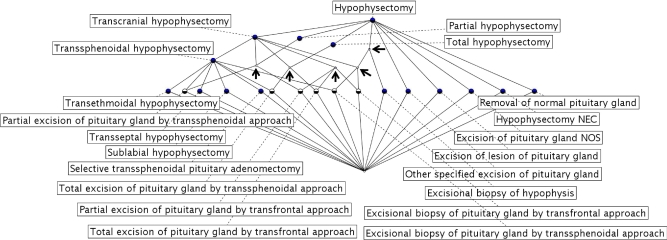
Besides the cross table representation, there is a graphical representation of formal contexts using the line-diagram form for concept lattice. ▶ shows a line diagram of the concept lattice for the context given in ▶. The lattice contains exactly the same information as the cross table. Each node in the diagram represents a formal concept of the context and the ascending paths of line edge between the 2 nodes represent the subconcept and superconcept relations. For the readability of the lattice, we only display the labels for objects (i.e., the concept names of the domain “hypophysectomy” defined in the SNOMED CT) in ▶.
Figure 2.
A line diagram of concept lattice for the domain “hypophysectomy.” The arrows indicate those nodes that were called “anonymous node.”
Retrieving the Information about Anonymous Nodes
Inspection of ▶ reveals 5 nodes without a label attached, indicated by arrows. These nodes are the anonymous nodes. We propose that the anonymous nodes provide interesting and important information about the semantic (in)completeness of SNOMED CT contents. ▶ shows the information about the anonymous nodes retrieved from the concept lattice given in ▶; note that the column Own Object is populated by None for every row in ▶, which indicates that these nodes are anonymous by definition. For example, consider Node 2, which includes 3 extension objects. When we analyze the 3 objects, we find that they share the common attribute “Transfrontal approach(Approach),” indicated by the attribute label in this anonymous node. This suggests that an object label “Transfrontal hypophysectomy” is missing, which would cause a kind of semantic incompleteness of the domain contents. By analyzing the other 4 anonymous nodes, it is not difficult to conclude that they share common attributes, such as “Total excision,” “Partial excision,” “Excision biopsy(Method),” etc., some of which are indicated by the attribute labels and some of which are missing from both their object labels and attribute labels.
Table 3.
Table 3 The Information about the Anonymous Nodes Retrieved from the Concept Lattice of the Domain “Hypophysectomy”
| Anonymous Node | Own Object | Own Attribute | Extension |
|---|---|---|---|
| Node 1 | None | None | 1. Total excision of pituitary gland by transsphenoidal approach |
| 2. Total excision of pituitary gland by transfrontal approach | |||
| Node 2 | None | Transfrontal approach(Approach) | 1. Total excision of pituitary gland by transfrontal approach |
| 2. Partial excision of pituitary gland by transfrontal approach | |||
| 3. Excisional biopsy of pituitary gland by transfrontal approach | |||
| Node 3 | None | None | 1. Partial excision of pituitary gland by transsphenoidal approach |
| 2. Partial excision of pituitary gland by transfrontal approach | |||
| Node 4 | None | None | 1. Excisional biopsy of pituitary gland by transsphenoidal approach |
| 2. Excisional biopsy of pituitary gland by transfrontal approach | |||
| Node 5 | None | Excision biopsy(Method) | 1. Excisional biopsy of pituitary gland by transsphenoidal approach |
| 2. Excisional biopsy of pituitary gland by transfrontal approach | |||
| 3. Excisional biopsy of hypophysis |
“None” indicates that no label was found for own object(s) or own attribute(s) of an anonymous node.
A Protégé plug-in on FCA-based ontology visualization is available at: http://informatics.mayo.edu/LexGrid/index.php?page= fca.
An Evaluation of the FCA-based Approach
We performed 2 modalities of evaluation: (1) quantitative and (2) inspection and interpretation of results.
Evaluation by Statistical Model
Our hypothesis in this study is that the anonymous nodes retrieved from the FCA model would be an index of the semantic (in)completeness of the SNOMED CT contents. Therefore, the research questions here are: (1) Can the number of anonymous nodes characterize the semantic completeness of SNOMED CT domains? (2) Can our approach using the number of anonymous nodes audit the difference of semantic completeness across domains?
We used the January 2006 version of SNOMED CT files as provided directly by CAP (College of American Pathologists, Northfield, IL. http://www.cap.org), the original producer of the nomenclature. We implemented a method for transforming SNOMED CT expressions into normal forms, consistent with the algorithms described in SNOMED CT technical document. 11 In this study, we focused on the 2 largest domains of SNOMED CT: Clinical Finding (SCTID_404684003) and Procedure (SCTID_71388002). Through a stratified sampling, 2 sets of sample contexts were collected randomly from the direct subbranches of the 2 domains. ▶ shows the total number of subconcepts in that version of SNOMED CT, the total number of contexts that could be formed, and the number of contexts (the sample size) that we randomly selected for each subbranch. Because computing FCA context is computationally intensive, we randomly selected about 10% of the total number of contexts for each subbranch.
Table 4.
Table 4 The Stratified Sampling for 2 Largest Domains: Clinical Finding and Procedure
| SCTID | Concept Name | SubCls Num | Context Num | Sample Size |
|---|---|---|---|---|
| 404684003 | Clinical finding | |||
| 64572001 | Disease (disorder) | 74769 | 15573 | 1557 |
| 118234003 | Finding by site | 67355 | 13635 | 1527 |
| 250171008 | Clinical history and observation findings | 20314 | 3138 | 314 |
| 118240005 | Finding by method | 8397 | 1013 | 101 |
| 225552003 | Wound finding | 4791 | 1061 | 106 |
| 102957003 | Neurological finding | 4170 | 703 | 70 |
| 307824009 | Administrative statuses | 2396 | 278 | 28 |
| 417893002 | Deformity | 888 | 163 | 20 |
| 419026008 | Effect of exposure to physical force | 628 | 87 | 20 |
| 365860008 | General clinical state finding | 613 | 70 | 20 |
| 267038008 | Edema | 370 | 57 | 20 |
| 418799008 | Finding reported by subject or history provider | 330 | 41 | 20 |
| 127357005 | Finding related to physiologic substance | 88 | 3 | 3 |
| 207577006 | [X]Additional symptom, signs and abnormal clinical and laboratory findings classification terms | 86 | 13 | 13 |
| 285153007 | Sequelae of external causes and disorders | 62 | 13 | 13 |
| 384740007 | Finding of grade | 53 | 11 | 11 |
| 80631005 | Clinical stage finding | 28 | 5 | 5 |
| 217020008 | Medical and surgical procedures as the cause of abnormal reaction of patient or later complication, without mention of misadventure at the time of procedure | 16 | 5 | 5 |
| 69449002 | Drug action | 11 | 0 | 0 |
| 365858006 | Prognosis/outlook finding | 6 | 0 | 0 |
| 405533003 | Adverse incident outcome categories | 5 | 0 | 0 |
| Subtotal | 185376 | 35869 | 3853 | |
| 71388002 | Procedure | |||
| 128927009 | Procedure by method | 38404 | 7781 | 778 |
| 362958002 | Procedure by site | 33848 | 7298 | 730 |
| 363691001 | Procedure by device | 6647 | 1057 | 106 |
| 108252007 | Laboratory procedure | 9531 | 946 | 95 |
| 362961001 | Procedure by intent | 4157 | 680 | 68 |
| 243120004 | Regimes and therapies (regime/therapy) | 3034 | 436 | 44 |
| 127777001 | Provider-specific procedure | 1981 | 321 | 32 |
| 14734007 | Administrative procedure | 2446 | 308 | 31 |
| 408767007 | Procedure with a clinical finding focus | 1223 | 154 | 20 |
| 373311009 | Procedure by approach | 1099 | 135 | 20 |
| 399248000 | Procedure related to anesthesia and sedation | 666 | 114 | 20 |
| 408766003 | Procedure with a procedure focus | 1296 | 114 | 20 |
| 410533009 | Procedure by priority | 216 | 22 | 22 |
| 389067005 | Community health procedure | 24 | 4 | 4 |
| 225288009 | Environmental care procedure | 20 | 5 | 5 |
| 266705004 | Preoperative/postoperative procedures | 12 | 4 | 4 |
| 389084004 | Staff related procedure | 11 | 3 | 3 |
| 371883000 | Outpatient procedure | 0 | 0 | 0 |
| 373111004 | Procedure in coronary care unit | 0 | 0 | 0 |
| 410606002 | Social service procedure | 6 | 0 | 0 |
| 7922000 | General treatment | 0 | 0 | 0 |
| Subtotal | 104621 | 19382 | 2002 | |
| Total | 289997 | 55251 | 5855 |
Context Num = number of the contexts that could be formed in each subbranch; Sample Size = number of contexts randomly selected; SubCls Num = number of subconcepts in each subbranch.
Because a concept in SNOMED CT may have multiple superconcepts, and a concept could be selected more than 1 time from a different subbranch of a domain in the sampling process, we removed these repeated sample contexts. In addition, many primitive SNOMED CT concepts have very minimal definitions (e.g., limited to 1 isa relation), so we also removed those contexts only having the isa relations as the formal attributes.
For each sample context, we transformed the data into 1-valued contexts and completed the context—i.e., we remodeled the data using the FCA methods above. For each context in the resultant matrix and concept lattice, we counted the number of fully defined concepts within the objects (definedObjNum), the number of lattice nodes (latticeNodeNum), and the number of anonymous nodes (anonymousNodeNum), and recorded its domain type (domain: Procedure/Clinical Finding). For example, consider the context of the domain “hypophysectomy” given in ▶ in the sampling process. The set of data related with this context is definedObjNum = 14, latticeNodeNum = 27, anonymousNodeNum = 5, domain = “Procedure.”
Two quasi-Poisson regression models were developed to answer our research questions. 43 One is to test whether the anonymous nodes can explain the semantic completeness of the SNOMED CT contents (Model 1), and the other is to test whether semantic completeness differs between domains (Model 2). Poisson regression assumes that a process or outcome occurs infrequently following the Poisson distribution, determined by a dependent variable (x) and a response variable (Y) which has an expected value of 1: log(E(Y)) = a+bx; it is well suited to low-frequency count data, which is the nature of the data in this study. Quasi-Poisson regression differs from Poisson in that its expected value need not be 1; or frequency counts on average are a bit larger than 1. We used a Poisson model for anonymous nodes because they are rare (non-Gaussian); we used the quasi-Poisson variant because our observed occurrences were not singular (i.e., did not have an expected value of 1). Furthermore, we used Poisson regression techniques rather than simple proportion comparisons so that we could adjust for confounding factors, such as the number of nodes in a sublattice, or compute different point estimates of effect across domains, i.e., so that we could build a multivariate model.
We created 2 regression models for our published analyses. In Model 1, the outcome variable was definedObjNum, which is used here as a proxy to represent the semantic completeness of the SNOMED CT contents, with the explanatory variables being log-transformation latticeNodeNum (to dampen skewing) and anonymousNodeNum. In Model 2, the outcome variable is anonymousNodeNum and the explanatory variables are log-transformed latticeNodeNum and domain (binary: Procedure/Clinical Finding). The log transformation of variable latticeNodeNum empirically optimized the goodness of fit statistics (Akaike Information Criterion) 50 for model selection relative to other possible transformations. We performed the regression analyses using the open-source statistical software R, version 2.3.1. 51
Validation by Case Studies
For providing inspection and interpretation evidence, a small set of sample contexts was randomly selected from those contexts that have 1 anonymous node. The authors of this article reviewed and analyzed the anonymous nodes and described their findings. The latest version (20070131) of SNOMED CT was used for validation.
Results
Statistical Results
A total of 3,853 contexts in the domain of Clinical Finding and 2002 contexts in the domain of Procedure were computed and collected (▶). By removing repeated sample contexts and those contexts only having the isa relations as the formal attributes, we obtained 3,586 contexts from the domain Clinical Finding and 1,864 contexts from the domain Procedure, yielding 5,450 unique contexts for analyses. ▶ shows the basic characteristics of the dataset.
Table 5.
Table 5 Basic Characteristics of the Dataset
| Variable | Mean | Min | Max | SD |
|---|---|---|---|---|
| definedObjNum | 20.7 | 0 | 4674 | 148.2 |
| latticeNodeNum | 159.3 | 1 | 66985 | 1848.2 |
| anonymousNodeNum | 101.1 | 0 | 56673 | 1475.6 |
anonymousNodeNum = number of anonymous nodes; definedObjNum = number of fully defined concepts within the objects; latticeNodeNum = number of lattice nodes; SD = standard deviation.
The regression results of Model 1 are detailed in ▶. The results showed that, after normalizing for the number of lattice nodes, the number of anonymous nodes had significant negative effects on the number of fully defined concepts within the objects of a context (p < 0.001). The dispersion parameter (expected value) of the model was 7.96. The finding reveals that the number of anonymous nodes may explain the semantic completeness of the SNOMED CT contents. In other words, the larger the number of anonymous nodes within a specific domain, the smaller the number of fully defined concepts within that domain; we suggest this indicates that SNOMED CT contents are quantifiably semantically incomplete.
Table 6.
Table 6 Results of the Quasi-Poisson Regression Model: Model 1
| Dependent Variable | Independent Variable | Coefficients | Standard Error | t Value | p |
|---|---|---|---|---|---|
| definedObjNum | Intercept | −0.77 | 0.028 | −27.13 | <0.001 |
| Log(latticeNodeNum) | 0.86 | 0.0044 | 192.96 | <0.001 | |
| anonymousNodeNum | −9.16e-6 | 8.06e-7 | −11.37 | <0.001 | |
| Overall model: residual deviance: 44587 on 5447 degrees of freedom (dispersion parameter for quasi-Poisson family taken to be 7.96) | |||||
The regression results of Model 2 are detailed in ▶. The results showed that, adjusting for the number of lattice nodes, the contexts from the domain Clinical Finding have fewer anonymous nodes than those from the domain Procedure (p < 0.001). The dispersion parameter of the model was 4.26. The finding reveals that the semantic completeness is significantly different between the domains Clinical Finding and Procedure when the number of anonymous nodes is used as the representation of the semantic completeness of the SNOMED CT contents.
Table 7.
Table 7 Results of the Quasi-Poisson Regression Model: Model 2
| Dependent Variable | Independent Variable | Coefficients | Standard Error | t Value | p |
|---|---|---|---|---|---|
| anonymousNodeNum | Intercept | −1.92 | 0.014 | −136.33 | <0.001 |
| Log(latticeNodeNum) | 1.18 | 0.0015 | 790.85 | <0.001 | |
| domain(ClinicalFinding) | −0.20 | 0.0059 | −34.13 | <0.001 | |
| Overall model: residual deviance: 29276 on 5447 degrees of freedom (dispersion parameter for quasi-Poisson family taken to be 4.26) | |||||
The variable domain was coded as binary (Procedure/ClinicalFinding).
Case Study Results
▶ provides a list of top 20 contexts (i.e., domains) ranked by the proportion of anonymous nodes (i.e., the ratio of the number of anonymous nodes over the number of lattice nodes); the anonymous nodes are identified.
Table 8.
Table 8 Top 20 Contexts with the Anonymous Nodes Identified (Ranked by the Proportion of Anonymous Nodes)
| SCTID | Concept Name | Lattice NodeNum (LNN) | Anonymous NodeNum (ANN) | Proportion (ANN/LNN) | Domain |
|---|---|---|---|---|---|
| 129233004 | Procedure on bone (organ) | 30678 | 27613 | 90.0% | Procedure |
| 118699001 | Procedure on pelvis | 42631 | 36885 | 86.5% | Procedure |
| 118710009 | Procedure on lower extremity | 18977 | 16262 | 85.7% | Procedure |
| 118745001 | Procedure on joint | 15793 | 13483 | 85.4% | Procedure |
| 928000 | Disorder of musculoskeletal system | 66985 | 56673 | 84.6% | Clinical finding |
| 129152004 | Procedure on back | 1828 | 1524 | 83.4% | Procedure |
| 417163006 | Traumatic and/or nontraumatic injury | 59739 | 48249 | 80.8% | Clinical finding |
| 71861002 | Implantation | 8162 | 6440 | 78.9% | Procedure |
| 118943001 | Disorder of pelvis | 34179 | 26666 | 78.0% | Clinical finding |
| 414252009 | Finding of back | 12198 | 9508 | 77.9% | Clinical finding |
| 118712001 | Procedure on thigh | 1040 | 810 | 77.9% | Procedure |
| 373196008 | Operative procedure on bone of lower extremity | 1519 | 1179 | 77.6% | Procedure |
| 76069003 | Disorder of bone | 23408 | 18156 | 77.6% | Clinical finding |
| 230896003 | Intracranial vascular operation | 519 | 399 | 76.9% | Procedure |
| 118953000 | Bone finding | 24216 | 18516 | 76.5% | Clinical finding |
| 38629001 | Operative procedure on the arteries of the thorax and abdomen | 2796 | 2130 | 76.2% | Procedure |
| 112698002 | Operation on joint | 6062 | 4614 | 76.1% | Procedure |
| 2119009 | Repair of blood vessel | 5139 | 3832 | 74.6% | Procedure |
| 120166004 | Mediastinum repair | 2249 | 1661 | 73.9% | Procedure |
| 239364005 | Maxillofacial bone operation | 1336 | 979 | 73.3% | Procedure |
anonymousNodeNum (ANN) = number of anonymous nodes; latticeNodeNum (LNN) = number of lattice nodes; proportion(ANN/LNN) = the ratio of anonymousNodeNum over latticeNodeNum.
Four domains (i.e., contexts) that have 1 anonymous node were randomly selected for human-based review. Two domains are from Clinical Finding domain, and 2 are from Procedure domain. ▶ shows the information of 4 anonymous nodes from 4 specific domains. Of note, while 3 of the potential missing relationships shown remain missing in the current version of SNOMED CT, one of the discovered anonymous nodes from the 2006 version has been corrected in the 2007 version.
Table 9.
Table 9 The Information of 4 Anonymous Nodes from 4 Specific Domains
| Sample Domains |
Anonymous Node Information |
||
|---|---|---|---|
| SCTID | Concept Name | OwnAttributes | Extensions |
| Samples from clinical finding domain | |||
| 269176007 | Open wound of shoulder region and upper limb with tendon involvement | Upper arm structure (body structure)(Finding site (attribute)) | 1. Open wound of upper arm with tendon involvement (disorder) |
| 2. Multiple open wounds of upper arm with tendon involvement (disorder) | |||
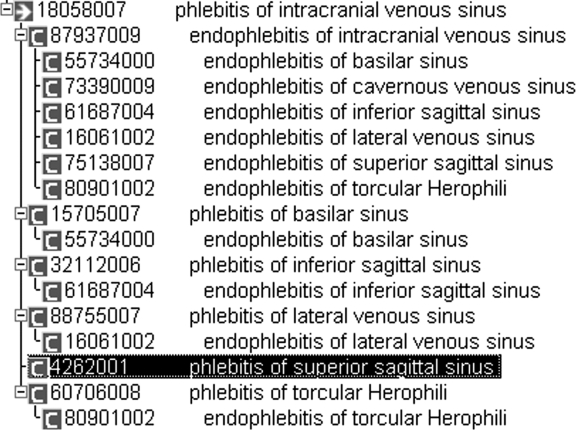
| 18058007 | Phlebitis of intracranial venous sinus | Superior sagittal sinus structure (body structure)(Finding site (attribute)) | 1. Phlebitis of superior sagittal sinus (disorder) |
| 2. Endophlebitis of superior sagittal sinus (disorder) | |||
| Samples from procedure domain | |||
| 23304006 | Operation on vas deferens | Excision - action (qualifier value)(Method (attribute)) | 1. Removal of valve of vas deferens (procedure) |
| 2. Bilateral vasectomy (procedure) | |||
| 14421005 | Serologic test for herpes virus | Human herpes simplex virus type 2 antibody (substance)(Component (attribute)) | 1. Herpes simplex virus 2 antibody pattern determination (procedure) |
| 2. Herpes simplex virus 2 antibody assay (procedure) | |||
- • Sample domain 1: Open wound of shoulder region and upper limb with tendon involvement (SCTID_269176007)This is a subdomain of Clinical Finding and it contains 8 concepts. One of them is a fully defined concept. The anonymous node identified has an own attribute “Upper arm structure (body structure)” and 2 extensions: the concept “Open wound of upper arm with tendon involvement (disorder)” and the concept “Multiple open wounds of upper arm with tendon involvement (disorder).” This may imply that a super concept of the 2 concepts is missing and worth adding as a first-class concept. In addition, we found that the sibling concepts of these 2 concepts are not consistently distinguished by the “single” and “multiple” properties. This representation persists in the latest version of SNOMED CT (▶).
Figure 3.
The sample domain “Open wound of shoulder region and upper limb with tendon involvement (SCTID_269176007)” in 20070131 version of SNOMED CT. This figure is a part of screenshot of CliniClue 2006—Terminology Browser (http://www.clinical-info.co.uk).
- • Sample domain 2: Phlebitis of intracranial venous sinus (SCTID_18058007)This is a subdomain of Clinical Finding and it contains 13 concepts; 4 of them are fully defined concepts. The anonymous node identified has an own attribute “Superior sagittal sinus structure (body structure),” and 2 extensions: the concept “Phlebitis of superior sagittal sinus (disorder)” and the concept “Endophlebitis of superior sagittal sinus (disorder).” We found that the assignment of the latter concept as the subconcept of the former one is missing. This is particularly striking since such a relationship is asserted for all of its siblings. This singular exception persists in the latest version of SNOMED CT. (▶).
Figure 4.
The sample domain “Phlebitis of intracranial venous sinus (SCTID_18058007)” in 20070131 version of SNOMED CT. This figure is a part of screenshot of CliniClue 2006—Terminology Browser (http://www.clinical-info.co.uk).
- • Sample domain 3: Operation on vas deferens (SCTID_23304006)This is a subdomain of “Procedure” and contains 12 concepts, 7 of which are fully defined concepts. The anonymous node identified has an own attribute “Excision - action (qualifier value)” and 2 extensions: the concept “Removal of valve of vas deferens (procedure)” and the concept “Bilateral vasectomy (procedure).” This may imply that a superconcept of the 2 concepts is missing. While we consider that the concept “vas deferens excision” is worth adding, we found that there is an existing superconcept “vas deferens excision (SCTID_120013000)” for the 2 concepts, which, however, is not a subconcept of “Operation on vas deferens (SCTID_23304006).” This is obviously an error, which indeed has been fixed in the latest version (▶).
Figure 5.
The sample domain “Operation on vas deferens (SCTID_23304006)” in 20070131 version of SNOMED CT. This figure is a part of screenshot of CliniClue 2006—Terminology Browser (http://www.clinical-info.co.uk).
- • Sample domain 4: Serologic test for herpes virus (SCTID_14421005)This is a subdomain of Procedure, and it contains 7 concepts. None of them are fully defined concepts. The anonymous node identified has an own attribute “Human herpes simplex virus type 2 antibody (substance)” and 2 extensions: the concept “Herpes simplex virus 2 antibody pattern determination (procedure)” and the concept “Herpes simplex virus 2 antibody assay (procedure).” This implies that a superconcept of the 2 concepts is missing and the superconcept may be named as “Serologic test for herpes simplex virus 2.” This is still not fixed in the latest version (▶).
Figure 6.
The sample domain “Serologic test for herpes virus (SCTID_14421005)” in 20070131 version of SNOMED CT. This figure is a part of screenshot of CliniClue 2006—Terminology Browser (http://www.clinical-info.co.uk).
Discussion
About the FCA Model
In this study, we used a high-level SNOMED CT concept and its subconcepts as a proxy to select a specific domain, within which all subconcepts were transformed into their normal forms. As a consequence, the semantic space of the domain in question is expanded (by completing the context) and the details of the semantic definitions of all concepts within the domain could be collected to support the normalization process for instances of postcoordinated expressions of the domain.
The normal form is the central structure for the formal representation of SNOMED CT concepts. We consider that the normal form is also the intermediate layer for the tasks of semantic integration, such as comparing, merging, and classifying precoordinated and postcoordinated expressions. By formalizing the normal forms of a specific domain using the language of the FCA, the model provides the potential to establish an automatic way to perform these semantic integration tasks.
A transformational scaling is needed to transform a many-valued formal context into a 1-valued or Boolean context. By this kind of transformation—in particular, completing the context—the semantic space of the domain is expanded further and made more complete. We consider that it is a feature of the FCA scaling model that every fine-grained element defined in SNOMED CT is exploited. In addition, we found that a step to “complete the context” from relationships that are not otherwise exhaustively asserted is also required by using the hierarchical knowledge of the SNOMED CT. By this kind of completion, the model acquires a robust representation of the semantics explicitly and implicitly contained in SNOMED CT.
As a consequence of transforming the 1-value table into the concept lattice, new entities are synthesized that lack any “own object” labels; these new entities are called anonymous nodes. When formal structures are represented graphically, they induce associative structures in a user's mind, which could provide an opportunity for the discovery of tacit knowledge, emergent structure, and the continuing evolution of fully defined meanings. 47 In this study, when the formal structure of the normal forms was visualized as a concept lattice, the anonymous nodes attracted our attention. As shown in ▶, 5 anonymous nodes emerge and their information (▶) could be retrieved and provided to the SNOMED CT curators and developers for consideration. We suggest that it would be difficult to acquire this kind of knowledge from other existing approaches. For instance, while a node labeled by “Transsphenoidal hypophysectomy” exists in the lattice, an object label “Transfrontal hypophysectomy” is probably missing from an anonymous node in the same level. We suggest that this kind of specific knowledge is a practical mechanism to show foci of incompleteness within SNOMED CT, and thus could be useful for auditing SNOMED CT or any other large terminology.
While the anonymous nodes may represent missing concepts, an interesting question is whether it is sensible or necessary to represent all anonymous nodes as concepts in a biomedical terminology. Clearly the appropriateness will vary with the purpose of the terminology and the navigability of hierarchies. We argue that explicit representation of the knowledge retrieved from anonymous nodes with the support of lattice-based visualization may provide a mechanism for terminology curators to define rules that may inform which anonymous nodes would deserve representation. For example, the fact that there is a concept “Transsphenoidal hypophysectomy” may be an argument for a rule to represent “Transfrontal hypophysectomy.” These kinds of issues have also been discussed in a study of SNOMED CT about classifying diseases with respect to anatomy 52 and in a work on the compositionality of the Gene Ontology by Ogren et al. 53
Regression Models and Results
We developed a quasi-Poisson regression model to test whether the number of anonymous nodes could explain the semantic completeness of SNOMED CT contents. The number of fully defined concepts within a context was used as the outcome variable to represent the semantic completeness of the SNOMED CT contents. We believe that this is reasonable because the modeling of a fully defined concept expresses the full meaning of the concept. In other words, the higher the percentage of the SNOMED CT concept codes that were fully defined, the more complete are the semantics of the SNOMED CT contents. Improving this kind of logic definition has become one of main goals of the SNOMED CT curators. 16 The regression results confirm our hypothesis that the number of anonymous nodes correlates negatively with the number of fully defined concepts within a context (i.e., the semantic completeness) after normalizing for the number of lattice nodes of the context. We consider that the adjusting variable here is necessary because the size of the contexts indicated by the number of lattice nodes was different and obviously confounding.
While the FCA model proposed in this study could provide specific information about the anonymous nodes for auditing the semantic completeness of a specific domain in SNOMED CT, we also developed an approach for measuring the differences in the semantic completeness among different domains. In our Model 2, the number of anonymous nodes was used as the outcome variable. The results show that the contexts from the domain Clinical Finding have fewer anonymous nodes than the domain Procedure, after adjusting for the size of those contexts. Furthermore, the findings indicate that the semantic completeness of the 2 largest domains in the SONMED CT is significantly different, i.e., the contexts from the domain Clinical Finding is more semantically complete than those from the domain Procedure. Thus we believe that the approach also could be used to audit or compare the semantic completeness of any arbitrarily defined domains or subdomains.
We used quasi-Poisson regression models in this study rather than the more conventional Poisson regression model because we found that an overdispersion (having an expected value >1) existed in the data for outcome variables definedObjNum and anonymousNodeNum, which are count data, nonnegative and highly skewed. A possible reason for the overdispersion in these data is that both the number of objects that are fully defined concepts and the number of anonymous nodes do not occur independently. In fact, these sampled data were extracted from the contexts that were nested. The dispersion parameters in the 2 models were 7.96 and 4.26, indicating that use of a quasi-Poisson regression model increased the standard error metric by the square root of each dispersion parameter, 50 penalizing significance measures. However, this means that our finding of significant difference in semantic completeness across domains is more likely to be real, since this significance metric is highly conservative by absorbing the full “penalty” of dispersion estimates higher than 1.
Practical Significance
We consider that the proportion of anonymous nodes (i.e., the ratio of the number of anonymous nodes over the number of lattice nodes) in a specific domain may provide a practical measure for the semantic completeness of the domain. In our sampled contexts (i.e., domains), the domain “Procedure on bone (organ)” has the highest proportion of anonymous nodes, up to 90% (▶). This indicates the curators may need to pay more attention to the domain for further investigation.
We performed case studies and reviewed 4 domains that have 1 anonymous node identified. This analysis demonstrated that the anonymous nodes are useful not only to identify missing concepts from a domain, but also to identify the semantic inconsistency and errors within a domain. Through referencing the latest version of SNOMED CT, we validated that some errors identified by our approach have been fixed in the latest version (e.g., sample domain 3), and some errors have not been fixed. We consider that this analysis provides anecdotal evidence for the effectiveness of our FCA approach to auditing the SNOMED CT.
Limitations
There are several limitations in this study. First, our approach using the anonymous nodes retrieved from the FCA-based model should complement other auditing approaches for SNOMED CT. In addition, the approach was based on a model specific to SNOMED CT. Validation of the approach in other DL-based clinical terminologies will be necessary in the future. Second, the FCA approach should not be applied for evaluating semantic completeness to terminological systems that have very minimal definitions (e.g., limited to one isa relation). In this study, we have removed those contexts only having the isa relations as the formal attributes. Third, while the number of anonymous nodes correlates well with semantic completeness of SNOMED CT contents, we reviewed 4 domains to demonstrate the practical significance of our FCA approach. A more systematic review beyond our anecdotal examination would be the next step for future study. Fourth, SNOMED CT uses “role groups” for grouping “attribute-value pairs” to simplify the terminology's concept model. 8 The model in this study dealt only with the attribute-value pairs and did not consider the issue of role groups. We consider that the semantics of role groups is a higher level abstraction for representing the knowledge of SNOMED CT expressions and further studies are needed to address how to formalize the role groups using FCA as a tool. In addition, the FCA model only formalized the long normal forms, and did not include a representation of SNOMED CT context model. Finally, the sample size in this study was only about 10% of all contexts that could be formed, and while efficient for testing our model, it is possible that the sample is not representative of all contexts.
Conclusion
In this study, we developed a novel approach for auditing the semantic completeness of SNOMED CT using an FCA-based model. We demonstrate that the anonymous nodes retrieved from the FCA model can explain the semantic completeness of SNOMED CT contents indicated by the fully defined concepts. Our novel FCA-based approach may be useful for auditing the semantic completeness of SNOMED CT not only for a specific domain, but also for different domains.
Acknowledgments
The authors thank Harold R. Solbrig, Thomas M. Johnson, and James D. Buntrock for their critical input and support. The authors also thank the reviewers for their insightful and constructive criticism, which materially improved this work.
Footnotes
Supported in part by R01 LM07319.
References
- 1.ISO 17115 Health Informatics—Vocabulary for Terminological Systems1st edition. Geneva, Switzerland: The International Organization for Standardization; 2007. http://www.iso.org .
- 2.Chute CG, Cohn SP, Campbell JR, ANSI Healthcare Informatics Standards Board Vocabulary Working Group and the Computer-Based Patient Records Institute Working Group on Codes and Structures A framework for comprehensive health terminology systems in the United States: development guidelines, criteria for selection, and public policy implications J Am Med Inform Assoc 1998;5:503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cimino JJ. Desiderata for controlled medical vocabularies in the twenty-first century Methods Inf Med 1998;37:394-403. [PMC free article] [PubMed] [Google Scholar]
- 4.ISO 1087-1: Terminology Work—Vocabulary, Part 1: Theory and Application: Technical Committee TC 37/SC 1; ISO Standards—Terminology (Principles and Coordination). 1996. The International Organization for Standardization (Geneva, Switzerland. http://www.iso.org).
- 5.ISO 1087-2: Terminology Work—Vocabulary, Part 2: Computer Applications: Technical Committee TC 37/SC 1; ISO Standards—Computer Applications for Terminology. 1996. The International Organization for Standardization (Geneva, Switzerland. http://www.iso.org).
- 6.SNOMEDhttp://www.snomed.org/ 1998. Accessed February 20, 2007.
- 7.Spackman KA, Campbell KE. Compositional concept representation using SNOMED: towards further convergence of clinical terminologies Proc AMIA Symp 1998:740-744. [PMC free article] [PubMed]
- 8.Spackman KA, Dionne R, Mays E, Weis J. Role grouping as an extension to the description logic of Ontylog, motivated by concept modeling in SNOMED Proc AMIA Symp 2002:712-716. [PMC free article] [PubMed]
- 9.Yu AC. Methods in biomedical ontology J Biomed Inform 2006;39:252-266. [DOI] [PubMed] [Google Scholar]
- 10.Spackman KA. Normal forms for description logic expressions of clinical concepts in SNOMED RT Proc AMIA Symp 2001:627-631. [PMC free article] [PubMed]
- 11. SNOMED Clinical Terms Guide: Transforming Expressions to Normal Forms. July 2005 release. The International Health Terminology Standards Development Organization. Copenhagen, Denmarkhttp://www.ihtsdo.org 2001. Accessed November 2008.
- 12.Cornet R, Abu-Hanna A. Two DL-based methods for auditing medical terminological systems Proc AMIA Symp 2005:166-170. [PMC free article] [PubMed]
- 13.Granter B, Wille R. Formal Concept Analysis: Mathematical FoundationsNew York: Springer; 1999.
- 14.Kalfoglou Y, Dasmahapatra S, Chen-Burger Y. FCA in knowledge technologies: experiences and opportunities. In: Concept Lattices: Second International Conference on Formal Concept Analysis, ICFCA 2004, Sydney, Australia, February 23–26, 2004, Proceedings. Eklund P (Ed.), Vol. 2961, 2004, ISBN 978-3-540-21043-6.
- 15.Cimino JJ. Auditing the Unified Medical Language System with semantic methods J Am Med Inform Assoc 1998;5:41-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spackman KA. Rates of change in a large clinical terminology: three years experience with SNOMED CT clinical terms Proc AMIA Symp 2005:714-718. [PMC free article] [PubMed]
- 17.Ceusters W, Smith B, Flanagan J. Ontology and medical terminology: why description logics are not enough. In: Proceedings of TEPR 2003—Towards an Electronic Patient Record. San Antonio, Texas, May 10–14, 2003. CD-ROM publication.
- 18.Bodenreider O, Smith B, Kumar A, Burgun A. Investigating subsumption in DL-based terminologies: a case study in SNOMED CTIn: Hahn J, editor. KR-MED 2004 Proceedings. Wilstler, Canada: AMIA; 2004. pp. 12-20.
- 19.Ceusters W, Smith B, Kumar A, Dhaen C. Ontology-based error detection in SNOMED-CT Medinfo 2004;11:482-486. [PubMed] [Google Scholar]
- 20.Halper M, Wang Y, Min H, Chen Y, Hripcsak G, Perl Y, Spackman KA. Analysis of error concentrations in SNOMED AMIA Symp Proc 2007:314-318. [PMC free article] [PubMed]
- 21.Spackman K, Reynoso G. Examining SNOMED from the perspective of formal ontological principlesIn: Hahn J, editor. KR-MED 2004 Proceedings. Wilstler, Canada: AMIA; 2004. pp. 81-87.
- 22.Green JM, Wilcke JR, Abbott J, Rees LP. Development and evaluation of methods for structured recording of heart murmur findings using SNOMED-CT post-coordination J Am Med Inform Assoc 2006;13:321-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richesson RL, Andrews J, Krischer J. Use of SNOMED CT to represent clinical research data: a semantic characterization of data items on Case Report Forms in vasculitis research J Am Med Inform Assoc 2006;13:536-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cordi V, Mascardi V. Checking the completeness of ontologies: a case study from the semantic web. In: Proceedings of the Italian Conference on Computational Logic (CILC-2004). 2004. Panegai and G. Rossi eds., Quaderno del Dipartimento di Matematica, vol. 390, University of Parma, 2004.
- 25.Fox MS, Gruninger M. On ontologies and enterprise modeling International Conference on Enterprise Integration Modelling Technology 97. New York: Springer-Verlag; 1997.
- 26.Devanbu PT, Jones MA. The use of description logics in KBSE systems: experience reportIn: Fadini B, Osterweil L, Lamsweerde AV, editors. Proceedings of the 16th International Conference on Software Engineering. Los Alamitos, CA: IEEE Computer Society Press; 1994. pp. 23-25.
- 27.Rassinoux AM, Miller RA, Baud RH, Scherrer JR. Compositional and enumerative designs for medical language representation Proc AMIA Annu Fall Symp 1997:620-624. [PMC free article] [PubMed]
- 28.Chute CG, Cohn SP, Campbell KE, Oliver DE, Campbell JR, Computer-Based Patient Record Institute's Work Group on Codes & Structures The content coverage of clinical classifications J Am Med Inform Assoc 1996;3:224-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell JR, Carpenter P, Sneiderman C, Cohn S, Chute CG, Warren J, CPRI Work Group on Codes and Structures Phase II evaluation of clinical coding schemes: completeness, taxonomy, mapping, definitions, and clarity J Am Med Inform Assoc 1997;4:238-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Humphreys BL, McCray AT, Cheh ML. Evaluating the coverage of controlled health data terminologies: report on the results of the NLM/AHCPR large scale vocabulary test J Am Med Inform Assoc 1997;4:484-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penz JF, Brown SH, Carter JS, et al. Evaluation of SNOMED coverage of Veterans Health Administration terms Medinfo 2004;11:540-544. [PubMed] [Google Scholar]
- 32.Elkin PL, Brown SH, Husser CS, et al. Evaluation of the content coverage of SNOMED CT: ability of SNOMED clinical terms to represent clinical problem lists Mayo Clin Proc 2006;81:741-748. [DOI] [PubMed] [Google Scholar]
- 33.Rosenbloom ST, Miller RA, Johnson KB, Elkin PL, Brown SH. Interface terminologies: facilitating direct entry of clinical data into electronic health record systems J Am Med Inform Assoc 2006;13:277-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elkin PL, Brown SH, Lincoln MJ, Hogarth M, Rector A. A formal representation for messages containing compositional expressions Int J Med Inform 2003;71:89-102. [DOI] [PubMed] [Google Scholar]
- 35.Diaz-Agudo B, Gonzalez-Calero PA. Formal concept analysis as a support technique for CBR Knowledge-Based Syst 2001;14:163-171. [Google Scholar]
- 36.Priss U. Formal concept analysis in information science Annual Review of Information Science and Technology 2006;40:521-543. [Google Scholar]
- 37.Rector AL, Bechhofer S, Goble CA, Horrocks I, Nowlan WA, Solomon WD. The GRAIL concept modelling language for medical terminology Artif Intell Med 1997;9:139-171. [DOI] [PubMed] [Google Scholar]
- 38.Rector AL, Rogers JE. Ontological and Practical Issues in Using a Description Logic to Represent Medical Concepts: Experience from GALEN. Technical Reports, School of Computer Science PrePrint, University of Manchester: CSPP-35:1-35, 2006http://www.opengalen.org 1997. Accessed November 2008.
- 39.Zhang S, Bodenreider O. Aligning representations of anatomy using lexical and structural methods Proc AMIA Annu Fall Symp 2003:753-757. [PMC free article] [PubMed]
- 40. W3C Recommendation. OWL Web Ontology Language Reference. http://www.w3.org/TR/owl-ref/ 2003. Accessed November 2008.
- 41.Cimiano P, Hotho A, Stumme G, Tane J. Conceptual Knowledge Processing with Formal Concept Analysis and Ontologies Proceedings of the Second International Conference on Formal Concept Analysis (ICFCA 04). New York: Springer; 2004. pp. 189-207.
- 42.Schnabel M. Representing and processing medical knowledge using formal concept analysis Methods Inf Med 2002;41:160-167. [PubMed] [Google Scholar]
- 43. The IEEE P1600.1 Standard Upper Ontology Working Group (SUO WG). Home page. http://suo.ieee.org/ 2002. Accessed February 20, 2007.
- 44.Jiang G, Ogasawara K, Endoh A, Sakurai T. Context-based ontology building support in clinical domains using formal concept analysis Int J Med Inform 2003;71:71-81. [DOI] [PubMed] [Google Scholar]
- 45.Kalfoglou Y, Schorlemmer M. IF-Map: an ontology-mapping method based on information-flow theory LNCS 2800 Journal of Data Semantics 198–127. New York: Springer; 2003.
- 46.Stumme G, Maedche A. Merging ontologies by means of formal concept analysis. First International Workshop on Databases, Documents, and Information Fusion. Magdeburg, Germany: April 2001.
- 47.Priss U, Old LJ. Modeling lexical databases with formal concept analysis J Universal Comput Sci 2004;10:967-984. [Google Scholar]
- 48.Priss U. Formalizing botanical taxonomiesIn: De Moor A, Lex W, Ganter B, editors. Conceptual Structures for Knowledge Creation and Communication. . Proceedings of the 11th International Conference on Conceptual Structures. New York: Springer Verlag; 2003. pp. 309-322.
- 49. W3C Technical Report. Design Issues: Architectural and philosophical points. http://www.w3.org/DesignIssues/Reify.html 2003. Accessed March 13, 2008.
- 50.Maindonald J, Braun J. Data analysis and graphics using R: an example-based approachCambridge: Cambridge University Press; 2003.
- 51. The R Project for Statistical Computing. Home Page. http://www.r-project.org/ 2003. Accessed February 20, 2007.
- 52.Burgun A, Bodenreider O, Mougin F. Classifying diseases with respect to anatomy: a study in SNOMED CT AMIA Annu Symp Proc 2005:91-95. [PMC free article] [PubMed]
- 53.Ogren PV, Cohen KB, Hunter L. Implications of compositionality in the gene ontology for its curation and usage Pac Symp Biocomput 2005:174-185. [PubMed]