Abstract
Background
Staphylococcus aureus is the most common cause of healthcare-associated pneumonia. Despite the significant morbidity and mortality associated with the disease, animal models of S. aureus pneumonia are rare.
Materials and Methods
We examined the pathogenicity of four different strains of S. aureus (both methicillin-sensitive and resistant as well as Panton-Valentine leukocidin positive and negative) in four strains of immunocompetent inbred and outbred mice (FVB/N, C57Bl/6, Balb/c, ND4, n=148). The immunologic basis for the development of murine S. aureus pneumonia was then determined by selectively depleting neutrophils, lymphocytes, or pulmonary macrophages prior to the onset of infection. An additional cohort of animals was rendered immunosuppressed by induction of abdominal sepsis via cecal ligation and puncture 2, 4 or 7 days prior to the onset of pneumonia.
Results
Nearly all immunocompetent mice survived, regardless of which strain of S. aureus was used or which strain of mouse was infected. Among animals with immune depletion or prior immunosuppression, survival was decreased only following neutrophil depletion (26% vs. 90% alive at 7 days, p<0.0001). Compared to immunocompetent animals, neutrophil-depleted mice with S. aureus pneumonia had delayed pulmonary bacterial clearance at 16 and 40 hours but had no difference in levels of bacteremia. Neutrophil-depleted mice also had elevated levels of pulmonary MCP-1 (822 pg/ml vs. 150 pg/ml, p<0.05). In contrast, pulmonary histologic appearance was similar in both groups as was dry/wet lung weight.
Conclusions
These results suggest that neutrophils play a critical role in the host response to S. aureus pneumonia, and the survival differences observed in neutrophil-depleted mice are associated with alterations in bacterial clearance and pulmonary cytokine response.
Keywords: Pneumonia, sepsis, Staphylococcus aureus, bacteria, infection, neutrophil, MCP-1, murine, model, survival
INTRODUCTION
S. aureus is the most common cause of healthcare-associated, hospital-acquired, and ventilator-associated pneumonia in the United States (1). More than 50% of S. aureus infections in the intensive care unit are caused by methicillin resistant (MRSA) strains (2;3). S. aureus infections are especially important in surgical patients as they can cause soft tissue infections and bacteremia in addition to pneumonia (4). S. aureus infections have become more challenging to treat recently as increasingly resistant strains have gained in dominance, including strains found in the community (5;6) and those which contain the highly virulent Panton-Valentine leukocidin (PVL) (7).
Compared to other common and lethal microbes, there are relatively few animal models of S. aureus pneumonia. Initial descriptions of a mouse model of S. aureus pneumonia demonstrated that 6 × 108 colony forming units (CFU) of bacteria had to be inoculated to cause lethality (8). However, bacteria did not replicate in vivo at this dose, and the mortality observed may have been related to toxicity of bacterial cell components rather than active infection. Broad-spectrum immunosuppression with systemic cyclophosphamide and impairment of mucociliary clearance with intranasal formalin also allow for development of S. aureus pneumonia, independent of bacterial toxin production (9). Injection of PVL positive MRSA causes a rapidly fatal (20% survive 24 hours) necrotizing pneumonia in immunocompetent mice via transcription of genes coding for secreted and cell wall-anchored proteins including lung inflammatory factor staphylococcal protein A (10). Additionally, intranasal inoculation of 4–8 × 108 (but not 8 × 107) CFU of S. aureus Newman, a human clinical isolate, causes a rapidly fatal model of pneumonia in immunocompetent C57Bl/6 mice with evidence of bacterial growth in vivo associated with production of sortase A (11). Intranasal injection of 2 × 108 CFUs S. aureus also causes early pneumonia in neonatal mice, which is dependent on the agr and sar A loci, but survival in this model beyond 24 hours has not been described (12).
In order to develop a strategy for treatment of S. aureus pneumonia, it is critical to understand both pathogen-related and host-related elements that lead to morbidity and mortality. Currently, treatment of S. aureus pneumonia primarily consists of targeted therapy in the form of antibiotics. However, when antibiotics fail, treatment becomes non-specific (such as supplemental oxygen) and is independent of the host response to the pathogen, often with resultant poor outcomes. While pathogen-related factors accounting for S. aureus virulence in animal models are beginning to be understood, the factors underlying the varied host response to this organism are less well-defined. We therefore used a series of cell-specific and generalized immune depletion strategies to determine what is responsible for resistance to S. aureus infection in a variety of mouse strains and examined the mechanisms through which survival may be mediated in pneumonia caused by this organism.
MATERIALS AND METHODS
Bacteria
Strains of S. aureus used included 292, 295, 301, and 313, all of which were isolated from patients in the BJC HealthCare system (St. Louis, MO). Further description of the strains is as follows: 292 (MSSA, multilocus sequence type 45), 295 (MRSA, PVL negative, multilocus sequence type 8, SCC IV), 313 (MRSA, PVL negative, multilocus sequence type 5, SCC II), and 301 (MRSA, PVL positive, multilocus sequence type 8, SCC IV). Bacteria were maintained at −80°C as frozen stock cultures. They were then cultured on blood agar medium for 24 hours prior to inoculation into trypticase soy broth which was then incubated overnight. Cells were then centrifuged, washed twice in sterile PBS and resuspended twice for 10 minutes at 6000g in sterile 0.9% NaCl. The inoculum was then adjusted to an absorbance of 0.5 at 600nm.
Pneumonia model
Surgeries were performed on six to twelve week-old FVB/N, ND4 (Harlan BioProducts, Indianapolis, IN), C57Bl/6, BALB/c, or Rag-1−/− mice (Jackson Laboratory, Bar Harbor, ME) that lack mature lymphocytes (13). FVB/N, C57Bl/6 and BABL/c mice were chosen since they are commonly used inbred strains while ND4 mice were chosen since they are a commonly used outbred strain. Midline cervical incision was performed under isoflurane anesthesia using a technique previously described using Pseudomonas aeruginosa (14;15). Each animal received an intratracheal injection of 40 μL of a solution containing S. aureus after which the mouse was held vertically for 10 seconds to enhance delivery into the lung. The final density of the inoculum was 5×108 CFU/ml as determined by serial dilution and colony counts, corresponding to a dose of 2×107 CFU/injection. Sham-operated mice were handled identically except they received 40 μL of 0.9% NaCl only. Mice were either sacrificed at pre-determined timepoints (for functional studies) or assessed daily for survival for 7 days. Of note, animals were assessed daily for the presence of wound infections although none were detected. Mice were maintained on an alternating 12-hr light-dark schedule in a pathogen-free environment and received standard mouse feed ad libitum. All studies complied with National Institutes of Health laboratory animals use guidelines and were approved by the Washington University Animal Studies Committee.
“Two-hit” model
Death following sepsis frequently results from a combination of two insults. Frequently, neither is sufficient to decrease survival. However, the first “hit” results in immunosuppression of the host and the second insult (which ordinarily is not lethal) results in a substantial decrease in survival. Published “two hit” models using cecal ligation and puncture (CLP), a model of polymicrobial intra-abdominal infection, followed by pneumonia with different organisms demonstrate the time window where animals are maximally susceptible to the second insult vary widely (16–18). In this study, FVB/N mice underwent CLP by the method of Baker et al. with a single 29 gauge needle puncture (19). Mice were given 1ml of 0.9% NaCl subcutaneously at the time of operation for fluid resuscitation, as well as antibiotics in the form of a single dose of 1mg imipenem 6 hours after operation. Seven day survival of CLP alone or CLP followed by sham pneumonia (intratracheal injection of 0.9% NaCl) ranged from 0–15% depending on length of time between CLP and sham pneumonia (p=ns). Mice then had intratracheal instillation of S. aureus 2, 4 or 7 days following CLP.
Cellular depletion
Neutrophil depletion was performed in FVB/N mice which received intraperitoneal injections on two successive days prior to surgery of 1 mL of rabbit anti-mouse PMN polyclonal antibody (Accurate Chemical & Scientific, Westbury, NY) diluted 1:10 in 0.9% NaCl (20). Neutrophil depletion was verified by manual differential of leukocytes on a smear with Wright’s stain performed in a blinded fashion demonstrating an absolute neutrophil count of less than 300/mm3.
Pulmonary macrophage depletion was performed in FVB/N mice via administration of liposomes composed of phosphatidylcholine and cholesterol containing PBS-dissolved dichloromethylene diphosphonate (clodronate, Boehringer Mannheim, Mannheim, Germany) 48 hours prior to S. aureus challenge as previously described (21). Since there was no difference in survival in any inbred or outbred strains of mice given S. aureus, the decision to use FVB/N mice for depletion and “two hit” experiments was due to our laboratory’s extensive use of this strain in the past (14;15).
Cultures and cytokine analysis
FVB/N animals were anesthetized with ketamine/xylazine either 16 or 40 hours following injection of S. aureus. These timepoints were chosen to sample animals a) before any animals died and b) shortly before most neutrophil-depleted animals died. Blood was taken from the inferior vena cava through a midline laparotomy. Bronchoalveolar lavage (BAL) fluid was obtained by cannulation of the trachea followed by lavaging the lungs with 1ml of 0.9% NaCl. Blood and BAL cultures were serially diluted and grown overnight at 37° C on blood agar plates. Growth was identified 24 hours after plating. Log transformation of calculated colony counts was then used for further analysis (22). In a different cohort of mice, BAL fluid was also characterized for cytokine analysis using a commercially available kit via cytometric bead array (Mouse Inflammation Kit, which tests for MCP-1, IFN-γ, TNF-α, IL-6, IL-10 and IL-12, Cat. # 552364 BD Biosciences, San Jose, CA). Following obtaining blood and BAL samples, animals were sacrificed while under anesthesia.
Histology
Neutrophil-depleted and WT mice were sacrificed 16 or 40 hours after instillation of S. aureus. Lungs were then harvested, fixed in formalin and stained with hematoxylin and eosin. Slides were evaluated by a pathologist (MJD) who was blinded to sample identity for both severity and distribution of pneumonia using a subjective grading scale developed for this study. Scores were from 0–4 (no abnormality to most severe pneumonia) and 0–3 (no abnormality to most widespread pneumonia).
Lung Weights
A different cohort of neutrophil-depleted and WT mice were sacrificed 40 hours after instillation of S. aureus, and lungs were harvested. A “wet” weight was immediately obtained. Lungs were then dried in a 60 degree oven for 7 days and reweighed (23). The ratio of dry/wet weight was then calculated.
Statistics
Group survival differences were analyzed using the chi square test. Blood and bronchoalveolar lavage data were compared by Mann-Whitney test. Data analysis was performed using Prism 3.0 (GraphPad Software, San Diego, CA). A p value <0.05 was considered to be statistically significant.
RESULTS
S. aureus pneumonia does not affect survival in WT inbred or outbred mice, regardless of whether bacteria are methicillin sensitive or resistant or carry PVL
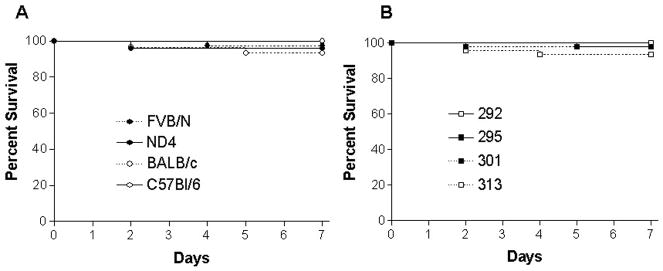
Intratracheal instillation of S. aureus was performed on FVB/N, C57Bl/6, Balb/C and ND4 mice. A total of 148 animals from these four strains of mice were injected with four strains of S. aureus (Table 1A). When stratified by mouse genetic background independent of which strain of S. aureus was given, the percentage of animals alive at 7 days was 97% in FVB/N animals (37/38), 100% in C57Bl/6 animals (30/30), 93% in BALB/c animals (28/30), and 96% in ND4 animals (48/50, Fig 1A). When stratified by which strain of S. aureus was given independent of which type of mouse was infected, the percentage of animals alive was 100% with 292 (10/10), 98% with 295 (46/47), 93% with 313 (42/45), and 98% with 301 (45/46, Fig 1B). Of note, S. aureus strains 295 and 301 were used to infect all four genetic strains of mice (range 5–15 mice/group) while strain 292 was used exclusively in FVB/N mice.
TABLE 1.
| Table 1A Survival by mouse strain and bacterial strain
| ||
|---|---|---|
| Mouse Strain | Bacterial Strain | Survival |
| FVB/N | 292 | 100% (10/10) |
| 295 | 100% (10/10) | |
| 301 | 100% (8/8) | |
| 313 | 90% (9/10) | |
| ND4 | 295 | 100% (10/10) |
| 301 | 100% (5/5) | |
| 313 | 94% (33/35) | |
|
| ||
| Balb/c | 295 | 93% (14/15) |
| 301 | 93% (14/15) | |
|
| ||
| C57Bl/6 | 295 | 100% (12/12) |
| 301 | 100% (18/18) | |
| Table 1B Survival in depletion and “two hit” experiments
| ||
|---|---|---|
| Mouse Model | Strain/Experiment | Survival |
| Rag-1−/− | 313 | 100% (6/6) |
| 301 | 88% (7/8) | |
|
| ||
| Pulmonary macrophage depletion | 313 | 100% (10/10) |
|
| ||
| FVB/N PMN depleted | 313 | 26% (7/27) |
| FVB/N WT | 313 | 96% (23/24) |
|
| ||
| Two-Hit | 313 2 days after CLP | 76% (29/38) |
| Sham 2 days after CLP | 86% (24/28) | |
| 313 4 days after CLP | 100% (18/18) | |
| Sham 4 days after CLP | 100% (17/17) | |
| 313 7 days after CLP | 100% (17/17) | |
| Sham 7 days after CLP | 88% (14/16) | |
FIG. 1.
Percentage of animals alive at 7 days by genetic background or bacterial strain of mice that received intratracheal injection of S. aureus. Regardless of whether mice (n=148) were (A) inbred or outbred or received (B) MSSA or MRSA (PVL positive or negative), essentially all immunocompetent animals survived seven days.
S. aureus pneumonia does not affect survival following CLP in a “two-hit” model of sepsis
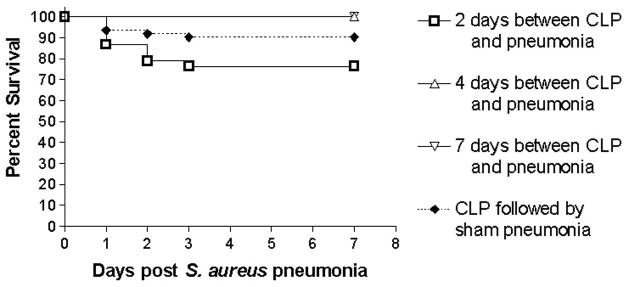
Mice subjected to a minimally lethal model of CLP subsequently received an intratracheal injection of strain 313 S. aureus 2, 4, or 7 days after the onset of intra-abdominal sepsis (n=134 total, Table 1B). Animals that were inoculated 2 days following CLP had similar survival (76% vs. 86%) compared to mice subjected to CLP followed by sham pneumonia (p>0.05, Figure 2). All animals survived when S. aureus was introduced 4 or 7 days after CLP.
FIG. 2.
Percentage of animals alive at 7 days in mice subjected to “two hit” model of sepsis. Animals were subjected to CLP followed by intratracheal injection of S. aureus a variable number of days later. Regardless of interval between CLP and S. aureus, survival was statistically similar to animals subjected to CLP followed by sham pneumonia.
Neutrophil depletion, but not lymphocyte or pulmonary macrophage depletion, decreases S. aureus pneumonia-induced survival
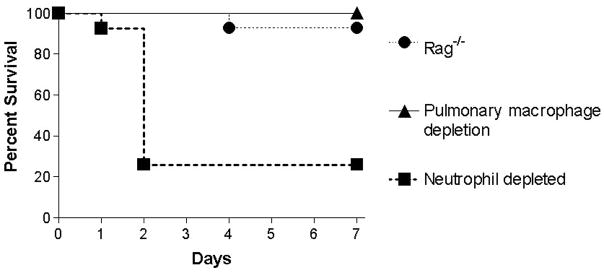
Neutrophil-depleted FVB/N mice had decreased survival, with only 26% alive 7 days following intratracheal instillation of S. aureus strain 313. This was significantly different than immunocompetent littermate mice given the same infection (90% alive at 7 days, p<0.0001, Fig. 3, Table 1B). Of note, neutrophil depletion itself had no impact on survival in neutrophil-depleted mice subjected to sham pneumonia. In contrast, 92% of Rag-1−/− mice (n=14) were alive 7 days following S. aureus pneumonia, as were all pulmonary macrophage depleted mice (n=10). Of note, the decision to use strain 313 in this experiment and subsequent experiments is because MRSA is more lethal than MSSA, and the majority of S. aureus strains causing clinical disease are PVL negative.
FIG. 3.
Percentage of animals alive at 7 days of immunodepleted animals that had intratracheal injection of S. aureus. Mice that had neutrophil depletion via polyclonal antibody (n=27) had decreased survival within two days while lymphocyte-deficient Rag-1−/− mice (n=14) and animals that had pulmonary macrophage depletion (n=10) essentially all survived (p<0.0001).
Comparison of neutrophil-depleted and immunocompetent mice subjected to S. aureus pneumonia
Having determined that there was a marked survival difference between neutrophil-depleted and immunocompetent animals given S. aureus pneumonia, mice from each group were compared to identify functional differences that might explain the survival difference after instillation of strain 313.
BAL cultures were taken in each group 16 or 40 hours post pneumonia (n=6/group/timepoint), to examine bacterial clearance before significant mortality occurred in either group. Culture results demonstrated significantly higher levels of bacteria in the lungs of neutrophil-depleted mice at both 16 and 40 hours (p<0.05 at both timepoints, Fig. 4). In contrast, no significant differences were identified in systemic blood cultures (n=6/group/timepoint). Cultures at 16 hours demonstrated only a single neutrophil-depleted animal had trace levels of bacteremia while the other 11 animals had no detectable bacteria in their bloodstream. At 40 hours, 3/6 animals in each group had trace levels of bacteremia (ranging from 10 to 50 CFU/ml), regardless of whether they were immunocompetent (p=ns).
FIG. 4.
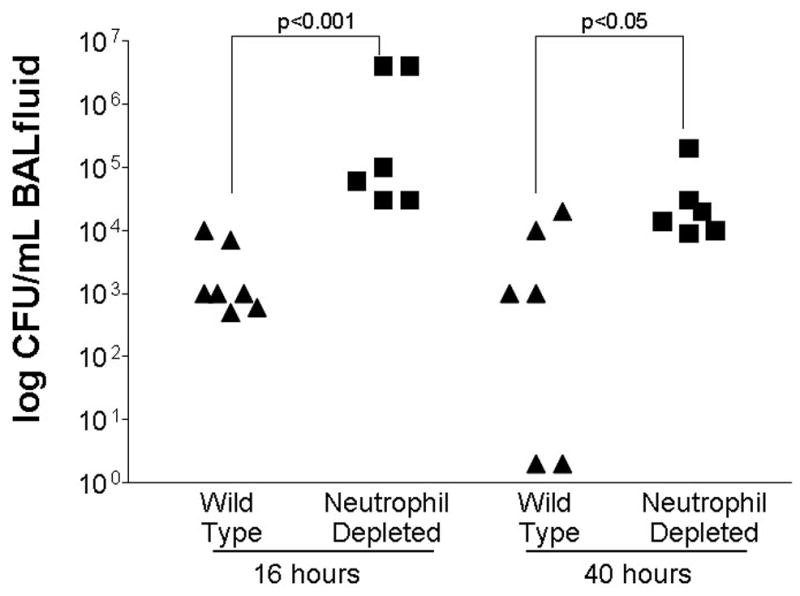
Bacterial clearance in neutrophil-depleted and immunocompetent (“wild type”) mice following intratracheal injection of S. aureus (n=6–7 animals/group/timepoint). Neutrophil-depleted mice had higher concentrations of bacteria in BAL fluid at both 16 and 40 hours. Data has been log transformed for presentation.
Cytokine levels in BAL fluid were also measured 40 hours following S. aureus pneumonia (n=7/group, Table 2). Levels of Monocyte chemotactic protein-1 (MCP-1) were significantly higher in neutrophil-depleted mice. There was also a non-statistically significant trend (p=0.07 and 0.09) toward higher levels of IFN-γ and Interleukin (IL)-6 in neutrophil-depleted mice as well. Of note, neither IL-10 nor IL-12 was detectable in BAL fluid in any animals given S. aureus pneumonia.
TABLE 2.
Cytokine levels in pulmonary BAL fluid
| Cytokine | Immunocompetent (SEM) | Neutrophil-depleted (SEM) | p value |
|---|---|---|---|
| TNF-α (pg/ml) | 199 (53) | 249 (57) | 0.62 |
| IFN-λ (pg/ml) | 15 (26) | 67 (4) | 0.07 |
| MCP-1 (pg/ml) | 150 (225) | 822 (48) | 0.007 |
| IL-6 (pg/ml) | 33 (13) | 63 (9) | 0.09 |
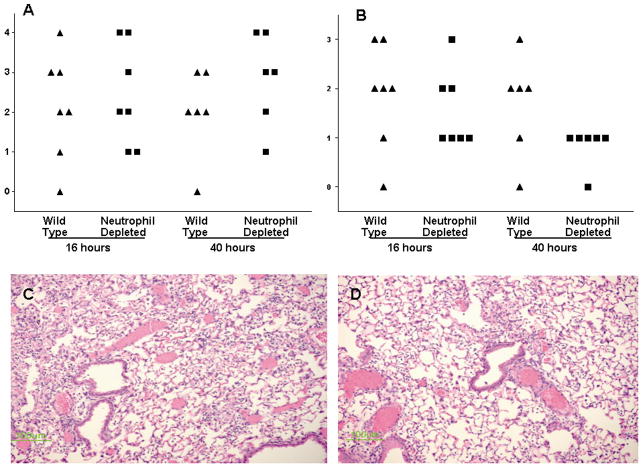
Despite the differences in bacterial clearance and pulmonary cytokines, there were no gross differences in pulmonary histologic appearance (either severity or distribution of pneumonia) between neutrophil-depleted and immunocompetent mice subjected to S. aureus pneumonia (Fig. 5). Additionally, the mean dry lung weight of neutrophil-depleted mice subjected to pneumonia was 22.4% of fresh weight, while this value was 22.9% in immunocompetent mice with the same insult (n=7–8, p=ns).
FIG. 5.
Histologic severity and distribution of pneumonia in neutrophil-depleted and immunocompetent (“wild type”) mice following intratracheal injection of S. aureus (n=6–7 animals/group/timepoint). Marked interanimal variation was seen in both severity (A) and anatomic distribution (B) without significant differences seen between neutrophil-depleted or immunocompetent animals (p>0.05). Severity was graded on a 1–4 scale, with 1 representing normal lung and 4 representing severe pneumonia. Distribution was graded on a 1–3 scale with 1 representing no pneumonia and 3 representing diffuse pneumonia. Representative micrographs of immunocompetent (C) and neutrophil-depleted (D) mice with grade 3 severity pneumonia 16 hours after intratracheal injection of S. aureus demonstrate similar histologic appearance.
DISCUSSION
Our study demonstrates that neutrophils are critical in mediating survival following intratracheal injection of S. aureus in mice, whereas the absence of lymphocytes or pulmonary macrophages as well as immunosuppression via prior sepsis, is insufficient to cause lethality following intratracheal S. aureus inoculation. The decreased survival in neutrophil-depleted animals is associated with decreased pulmonary bacterial clearance and increased pulmonary cytokine secretion.
Recently published studies have focused predominantly on bacterial components necessary to induce pneumonia in immunocompetent animals (10;11). We were unable to induce a lethal infection in immunocompetent mice despite using four strains of S. aureus that were MSSA or MRSA, PVL negative or positive and had three distinct multilocus sequence types. This suggests that the factors necessary to produce a lethal infection in immunocompetent mice are not straightforward, as altering a number of variables in our study did not lead to this result.
In contrast, these results expand our understanding of the host response to S. aureus pneumonia, a topic on which there have been fewer published studies than those examining pathogen-specific mechanisms, although it is known that overexpression of elafin, an anti-elastase/antimicrobial molecule, improves clearance of S. aureus in a non-lethal model of pneumonia (24). Since the host response to infection is drastically different depending on which genetic background is used, we used multiple strains of animals in these studies (25). Our findings indicate that murine resistance to S. aureus pneumonia is not simply a case of differing response depending on the animals genetic background, since similar results were seen with both inbred and outbred murine strains and regardless of whether they are Th1 or Th2 predominant (26). However, neutrophils appear to be critical to the development of lethal S. aureus pneumonia while lymphocytes and pulmonary macrophages do not appear to play a major role in mediating host survival. It has previously been shown that immune suppression with cyclophosphamide converts a fully survivable S. aureus insult into one that is uniformly lethal (9). Cyclophosphamide is a common chemotherapeutic agent that is also used for treatment of autoimmune disorders. While the drug is well-known to cause neutropenia (27), it has a number of unrelated side effects as well (28–30). As such, it was unclear if the neutropenia associated with cyclophosphamide was responsible for the development of murine pneumonia. By using a neutrophil-specific antibody, we were able to determine that neutrophil depletion is sufficient for the development of lethal S. aureus pneumonia. Further, the lack of effect seen with cellular depletion studies using Rag-1−/− mice and liposomes directed against pulmonary macrophages allows a more focused understanding of the cell types responsible for mediating survival in S. aureus pneumonia.
It was somewhat surprising that the “two hit” model failed to result in substantial lethality. The approach of inducing immunosuppression via nonlethal CLP followed by pneumonia is clinically relevant and has been demonstrated to work with multiple organisms (16–18). A similar approach of using two sublethal injuries has yielded substantial lethality following the second “hit” in other models of critical illness as well (31). Although no survival studies have been performed using S. aureus pneumonia in “two hit” models, bacterial clearance is decreased if S. aureus is given after prior infection with respiratory syncytial virus (32). We did not examine bacterial clearance in any model other than neutrophil-depletion reasoning that delayed clearance in a model where all animals survived would be of limited interest. However, based upon the results of our immune depletion studies, we would predict that the “two hit” model of sepsis would not cause a marked delay in bacterial clearance since CLP is notable for lymphopenia caused by apoptosis (33) without accompanying neutropenia, whereas only neutrophil-depletion resulted in decreased survival following intratracheal injection of S. aureus.
Our results also indicate some potential mechanisms through which host response is altered via neutrophil-depletion to yield lethal S. aureus pneumonia. Pulmonary bacterial clearance is decreased at both 16 and 40 hours compared to immunocompetent mice. This defect is compartmentalized since there is scant bacteremia in either immunocompetent or neutrophil-depleted mice at either timepoint, despite the fact that most of the latter mice die 2 days following intratracheal injection of S. aureus. Additionally, there is a markedly greater pulmonary cytokine response in neutrophil-depleted animals. MCP-1 levels are more than 5-fold greater, and there are non-significant trends towards increased levels of IL-6 and IFN-γ as well. Interestingly, the difference in survival was not associated with histological differences in pneumonia, as both histologic severity and distribution of pneumonia was similar in neutrophil-depleted and immunocompetent animals. It is difficult to know why histology was not associated with survival. It is possible that this means that survival is mediated, at least in part, by systemic factors. Although levels of bacteremia were similar between neutrophil-depleted and immunocompetent animals at 16 and 40 hours, this does not rule out a role for systemic factors not directly associated with bacterial burden. Alternatively, there might have been differences in oxygenation or ventilation between the groups that would not have been identified simply by examining lung histology and dry/wet weight.
The role MCP-1 plays in the pathophysiology of pneumonia is complex and appears to be organism-specific. While no studies correlating S. aureus pneumonia and MCP-1 have been performed in mice, this has been examined in a number of related models. Pulmonary MCP-1 is increased following administration of the superantigen staphylococcal enterotoxin B; however, this is unrelated to IFN-γ, since MCP-1 levels are similar in control and IFN-γ-knockout mice (34). Pseudomonas aeruginosa pneumonia increases MCP-1 levels, associated with increased numbers of alveolar macrophages. Anti-MCP-1 reduces alveolar macrophages and hepatocyte growth factor levels in BAL fluid and increases lung injury while administration of MCP-1 has the opposite effect and attenuates lung injury (35). In contrast, MCP-1 levels are elevated in idiopathic pneumonia following allogeneic bone marrow transplantation and neutralization of MCP-1 results in decreased lung injury (36). Additionally, while MCP-1 levels are elevated following Streptococcus pneumoniae pneumonia, these are correlated to bacterial load but do not appear to have functional significance since MCP-1 knockout mice are indistinguishable from control mice with regard to inflammatory response or lethality (37). There is less data surrounding the role of MCP-1 following pneumonia in neutrophil-depleted animals. However, neutropenic rats have similar levels of MCP-1 as immunocompetent rats following intratracheal injection of lipopolysaccharide despite an abrogation of both early and late increases in pulmonary monocyte/macrophages seen in immunocompetent animals (38).
This study has a number of limitations. Primarily, while our results lead to a clearer understanding of the host response to S. aureus pneumonia, it is unclear what relevance they have toward disease in humans. S. aureus pneumonia is more common in immunocompromised patients than immunocompetent patients; however, the disease can clearly be acquired in a community setting. We were unable to induce lethal pneumonia in immunocompetent mice regardless of which strain of bacteria or mice were used. This suggests that simply injecting MRSA is insufficient to cause severe pneumonia in mice, and even the addition of PVL, which has severe virulence in patients, does not guarantee a lethal model. Further, generalized immunosuppression via prior CLP, a clinically relevant model of sepsis, failed to induce lethality when followed by intratracheal injection of S. aureus. However, since we did not perform an extensive dose response curve, our results may simply have been due to injection of an insufficient number of bacteria to cause lethal pneumonia (i.e. the inoculum used might have been sufficient to decrease survival in a subset of immunosuppressed animals but a higher dose might have shown the same effect in immunocompetent or animals with generalized immunosuppression although it should be noted that injections of 5.2×107 or 7.6×107 CFU were insufficient to decrease survival). Additionally, BAL cytokine samples were obtained at 40 hours. This timepoint was chosen since it was shortly before neutrophil-depleted mice died. It is possible that looking at earlier timepoints would have yielded additional mechanistic insights. Also, the finding that MCP-1 levels are elevated following intratracheal injection of S. aureus in neutrophil-depleted mice is associative and knockout or antibody experiments would have to be performed to demonstrate its functional significance.
Despite these limitations, these results represent an advance in our understanding of the host response to murine S. aureus pneumonia. Survival is mediated by neutrophil depletion, but not by lymphocytes or pulmonary macrophages, and decreased survival is associated with a defect in pulmonary (but not systemic) bacterial clearance and elevated MCP-1. Further understanding of the host response to S. aureus pneumonia may prove as important as understanding the microbial components responsible for infection, and future research should target both pathogen and host responses to this increasingly common and lethal infection.
Acknowledgments
This work was supported by funding from the National Institutes of Health (GM066202, GM072808, GM008795, GM044118, GM055194) and the Alan A. and Edith L. Wolff Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005 December;128(6):3854–62. doi: 10.1378/chest.128.6.3854. [DOI] [PubMed] [Google Scholar]
- 2.Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005 February 15;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 3.Rello J, Ollendorf DA, Oster G, Vera-Llonch M, Bellm L, Redman R, Kollef MH. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002 December;122(6):2115–21. doi: 10.1378/chest.122.6.2115. [DOI] [PubMed] [Google Scholar]
- 4.Gillet Y, Issartel B, Vanhems P, Lina G, Vandenesch F, Etienne J, Floret D. Severe staphylococcal pneumonia in children. Arch Pediatr. 2001 September;8(Suppl 4):742s–6s. doi: 10.1016/s0929-693x(01)80190-1. [DOI] [PubMed] [Google Scholar]
- 5.Zetola N, Francis JS, Nuermberger EL, Bishai WR. Community-acquired meticillin-resistant Staphylococcus aureus: an emerging threat. Lancet Infect Dis. 2005 May;5(5):275–86. doi: 10.1016/S1473-3099(05)70112-2. [DOI] [PubMed] [Google Scholar]
- 6.Kazakova SV, Hageman JC, Matava M, Srinivasan A, Phelan L, Garfinkel B, Boo T, McAllister S, Anderson J, Jensen B, Dodson D, Lonsway D, McDougal LK, Arduino M, Fraser VJ, Killgore G, Tenover FC, Cody S, Jernigan DB. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med. 2005 February 3;352(5):468–75. doi: 10.1056/NEJMoa042859. [DOI] [PubMed] [Google Scholar]
- 7.Gillet Y, Issartel B, Vanhems P, Fournet JC, Lina G, Bes M, Vandenesch F, Piemont Y, Brousse N, Floret D, Etienne J. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002 March 2;359(9308):753–9. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 8.DeMaria TF, Kapral FA. Pulmonary infection of mice with Staphylococcus aureus. Infect Immun. 1978 July;21(1):114–23. doi: 10.1128/iai.21.1.114-123.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimura K, Miyazaki S, Tateda K, Matsumoto T, Tsujimoto S, Yamaguchi K. Factors affecting the course and severity of transnasally induced Staphylococcus aureus pneumonia in mice. J Med Microbiol. 1999 November;48(11):1005–10. doi: 10.1099/00222615-48-11-1005. [DOI] [PubMed] [Google Scholar]
- 10.Labandeira-Rey M, Couzon F, Boisset S, Brown EL, Bes M, Benito Y, Barbu EM, Vazquez V, Hook M, Etienne J, Vandenesch F, Bowden MG. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science. 2007 February 23;315(5815):1130–3. doi: 10.1126/science.1137165. [DOI] [PubMed] [Google Scholar]
- 11.Wardenburg JB, Patel RJ, Schneewind O. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect Immun. 2007 February;75(2):1040–4. doi: 10.1128/IAI.01313-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heyer G, Saba S, Adamo R, Rush W, Soong G, Cheung A, Prince A. Staphylococcus aureus agr and sarA functions are required for invasive infection but not inflammatory responses in the lung. Infect Immun. 2002 January;70(1):127–33. doi: 10.1128/IAI.70.1.127-133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992 March 6;68(5):869–77. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 14.Coopersmith CM, Stromberg PE, Davis CG, Dunne WM, Amiot DM, Karl IE, Hotchkiss RS, Buchman TG. Sepsis from Pseudomonas aeruginosa pneumonia decreases intestinal proliferation and induces gut epithelial cell cycle arrest. Crit Care Med. 2003 June;31(6):1630–7. doi: 10.1097/01.CCM.0000055385.29232.11. [DOI] [PubMed] [Google Scholar]
- 15.Coopersmith CM, Stromberg PE, Dunne WM, Davis CG, Amiot DM, Buchman TG, Karl IE, Hotchkiss RS. Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. JAMA. 2002 April 3;287(13):1716–21. doi: 10.1001/jama.287.13.1716. [DOI] [PubMed] [Google Scholar]
- 16.Muenzer JT, Davis CG, Dunne BS, Unsinger J, Dunne WM, Hotchkiss RS. Pneumonia after cecal ligation and puncture: a clinically relevant “two-hit” model of sepsis. Shock. 2006 December;26(6):565–70. doi: 10.1097/01.shk.0000235130.82363.ed. [DOI] [PubMed] [Google Scholar]
- 17.Murphey ED, Lin CY, McGuire RW, Toliver-Kinsky T, Herndon DN, Sherwood ER. Diminished bacterial clearance is associated with decreased IL-12 and interferon-gamma production but a sustained proinflammatory response in a murine model of postseptic immunosuppression. Shock. 2004 May;21(5):415–25. doi: 10.1097/00024382-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Steinhauser ML, Hogaboam CM, Kunkel SL, Lukacs NW, Strieter RM, Standiford TJ. IL-10 is a major mediator of sepsis-induced impairment in lung antibacterial host defense. J Immunol. 1999 January 1;162(1):392–9. [PubMed] [Google Scholar]
- 19.Baker CC, Chaudry IH, Gaines HO, Baue AE. Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery. 1983 August;94(2):331–5. [PubMed] [Google Scholar]
- 20.Eliason JL, Hannawa KK, Ailawadi G, Sinha I, Ford JW, Deogracias MP, Roelofs KJ, Woodrum DT, Ennis TL, Henke PK, Stanley JC, Thompson RW, Upchurch GR., Jr Neutrophil depletion inhibits experimental abdominal aortic aneurysm formation. Circulation. 2005 July 12;112(2):232–40. doi: 10.1161/CIRCULATIONAHA.104.517391. [DOI] [PubMed] [Google Scholar]
- 21.Thepen T, Van RN, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med. 1989 August 1;170(2):499–509. doi: 10.1084/jem.170.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wells CL, Hess DJ, Erlandsen SL. Impact of the indigenous flora in animal models of shock and sepsis. Shock. 2004 December;22(6):562–8. doi: 10.1097/01.shk.0000145935.24344.2d. [DOI] [PubMed] [Google Scholar]
- 23.Zhao M, Fernandez LG, Doctor A, Sharma AK, Zarbock A, Tribble CG, Kron IL, Laubach VE. Alveolar macrophage activation is a key initiation signal for acute lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2006 November;291(5):L1018–L1026. doi: 10.1152/ajplung.00086.2006. [DOI] [PubMed] [Google Scholar]
- 24.McMichael JW, Maxwell AI, Hayashi K, Taylor K, Wallace WA, Govan JR, Dorin JR, Sallenave JM. Antimicrobial activity of murine lung cells against Staphylococcus aureus is increased in vitro and in vivo after elafin gene transfer. Infect Immun. 2005 June;73(6):3609–17. doi: 10.1128/IAI.73.6.3609-3617.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De MA, Torres MB, Reeves RH. Genetic determinants influencing the response to injury, inflammation, and sepsis. Shock. 2005 January;23(1):11–7. doi: 10.1097/01.shk.0000144134.03598.c5. [DOI] [PubMed] [Google Scholar]
- 26.Ma B, Blackburn MR, Lee CG, Homer RJ, Liu W, Flavell RA, Boyden L, Lifton RP, Sun CX, Young HW, Elias JA. Adenosine metabolism and murine strain-specific IL-4-induced inflammation, emphysema, and fibrosis. J Clin Invest. 2006 May;116(5):1274–83. doi: 10.1172/JCI26372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuluaga AF, Salazar BE, Rodriguez CA, Zapata AX, Agudelo M, Vesga O. Neutropenia induced in outbred mice by a simplified low-dose cyclophosphamide regimen: characterization and applicability to diverse experimental models of infectious diseases. BMC Infect Dis. 2006;6:55. doi: 10.1186/1471-2334-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hosseinimehr SJ, Karami M. Chemoprotective effects of captopril against cyclophosphamide-induced genotoxicity in mouse bone marrow cells. Arch Toxicol. 2005 August;79(8):482–6. doi: 10.1007/s00204-005-0655-7. [DOI] [PubMed] [Google Scholar]
- 29.Salem ML, Kadima AN, El-Naggar SA, Rubinstein MP, Chen Y, Gillanders WE, Cole DJ. Defining the ability of cyclophosphamide preconditioning to enhance the antigen-specific CD8+ T-cell response to peptide vaccination: creation of a beneficial host microenvironment involving type I IFNs and myeloid cells. J Immunother (1997) 2007 January;30(1):40–53. doi: 10.1097/01.cji.0000211311.28739.e3. [DOI] [PubMed] [Google Scholar]
- 30.Brode S, Raine T, Zaccone P, Cooke A. Cyclophosphamide-induced type-1 diabetes in the NOD mouse is associated with a reduction of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006 November 15;177(10):6603–12. doi: 10.4049/jimmunol.177.10.6603. [DOI] [PubMed] [Google Scholar]
- 31.Murphy TJ, Paterson HM, Kriynovich S, Zang Y, Kurt-Jones EA, Mannick JA, Lederer JA. Linking the “two-hit” response following injury to enhanced TLR4 reactivity. J Leukoc Biol. 2005 January;77(1):16–23. doi: 10.1189/jlb.0704382. [DOI] [PubMed] [Google Scholar]
- 32.Stark JM, Stark MA, Colasurdo GN, LeVine AM. Decreased bacterial clearance from the lungs of mice following primary respiratory syncytial virus infection. J Med Virol. 2006 June;78(6):829–38. doi: 10.1002/jmv.20631. [DOI] [PubMed] [Google Scholar]
- 33.Hiramatsu M, Hotchkiss RS, Karl IE, Buchman TG. Cecal ligation and puncture (CLP) induces apoptosis in thymus, spleen, lung, and gut by an endotoxin and TNF-independent pathway. Shock. 1997 April;7(4):247–53. doi: 10.1097/00024382-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Neumann B, Emmanuilidis K, Stadler M, Holzmann B. Distinct functions of interferon-gamma for chemokine expression in models of acute lung inflammation. Immunology. 1998 December;95(4):512–21. doi: 10.1046/j.1365-2567.1998.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amano H, Morimoto K, Senba M, Wang H, Ishida Y, Kumatori A, Yoshimine H, Oishi K, Mukaida N, Nagatake T. Essential contribution of monocyte chemoattractant protein-1/C-C chemokine ligand-2 to resolution and repair processes in acute bacterial pneumonia. J Immunol. 2004 January 1;172(1):398–409. doi: 10.4049/jimmunol.172.1.398. [DOI] [PubMed] [Google Scholar]
- 36.Hildebrandt GC, Duffner UA, Olkiewicz KM, Corrion LA, Willmarth NE, Williams DL, Clouthier SG, Hogaboam CM, Reddy PR, Moore BB, Kuziel WA, Liu C, Yanik G, Cooke KR. A critical role for CCR2/MCP-1 interactions in the development of idiopathic pneumonia syndrome after allogeneic bone marrow transplantation. Blood. 2004 March 15;103(6):2417–26. doi: 10.1182/blood-2003-08-2708. [DOI] [PubMed] [Google Scholar]
- 37.Dessing MC, de Vos AF, Florquin S, van der PT. Monocyte chemoattractant protein 1 does not contribute to protective immunity against pneumococcal pneumonia. Infect Immun. 2006 December;74(12):7021–3. doi: 10.1128/IAI.00977-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janardhan KS, Sandhu SK, Singh B. Neutrophil depletion inhibits early and late monocyte/macrophage increase in lung inflammation. Front Biosci. 2006;11:1569–76. doi: 10.2741/1904. [DOI] [PubMed] [Google Scholar]