Abstract
Chitin, the second most abundant polysaccharide in nature, is commonly found in lower organisms such as fungi, crustaceans and insects, but not in mammals. Although the non-specific anti-viral and anti-tumor activities of chitin/chitin derivatives were described two decades ago, the immunological effects of chitin have been only recently been addressed. Recent studies demonstrated that chitin has complex and size-dependent effects on innate and adaptive immune responses including the ability to recruit and activate innate immune cells and induce cytokine and chemokine production via a variety of cell surface receptors including macrophage mannose receptor, toll-like receptor 2 (TLR-2), and Dectin-1. They also demonstrated adjuvant effects of chitin in allergen-induced Type 1 or Type 2 inflammation and provided insights into the important roles of chitinases and chitinase-like proteins (C/CLP) in pulmonary inflammation. The status of the field and areas of controversy are highlighted.
Keywords: chitin, chitinases, chitinase-like protein, innate and adaptive immunity
Chitin in Nature
Chitin, β-(1-4)-poly-N-acetyl D-glucosamine, is widely distributed in nature and is the second most abundant polysaccharide in nature after cellulose. It is found in the cell walls of bacteria and fungi, the exoskeleton of crustaceans (crabs, shrimp, etc.) and insects, the microfilarial sheath of parasitic nematodes, and the lining of the digestive tracts of many insect [1–8]. In these locations, chitin is used by chitin-containing organisms to protect it from the harsh conditions in its environment and host anti-parasite/pathogen immune responses. The mammalian counterpart of chitin has not been described. As some chitin derivatives are known to be non-toxic, non-allergenic, not biodegradable, and biocompatible, a number of prostheses such as artificial skin, contact lenses and surgical stitches have been produced from chitin derivatives and are widely used in medical practice [9]. Interestingly chitin is also a common component of allergy triggering antigens including those in shrimp, crab, cockroaches, and house dust mite[10–12]. Thus, it is very common for humans to be exposed to chitin/chitin derivatives in daily life.
Early Studies of Chitin Immunologic Activity
The first immune stimulatory activity of chitin and chitin derivatives was discovered and extensively explored in the middle to late 1980s. They initially formulated the idea that chitin or chitin derivatives [13–15] (Figure 1) might stimulate non-specific host resistance to bacterial and viral infections, and contain anti-tumor activity like N-acetylmuramyl-L-alanyl-D-isoglutamine (MDP), a known carbohydrate adjuvant [16–18]. They demonstrated that chitin and chitosan (deacetylated chitin) activate peritoneal macrophages and NK cells to express a number of pro-inflammatory cytokines such as interleukin-1 (IL-1), colony stimulating factor (CSF), and gamma interferon (IFN-γ) [13,19]. One of the intriguing observations made in these early studies was that the anti-viral and anti-tumor activities of chitin were impressively dependent on the composition of the chitin mixtures. They found that a mixture of 30% chitin and 70% chitosan had a greater ability to induce macrophage activation than pure chitin, pure chitosan or different chitin: chitosan combinations. Other investigators also noted that chitin can have dose-dependent protective or provocative effects against infections such as candidiasis [19]. Thus, these early studies clearly indicated that chitin and/or chitin derivatives have important immunologic effects in vitro and in vivo.
Figure 1.
Basic chemical structure of chitin and chitosan. In nature, chitin exists in two major forms which are characterized by the antiparallel (α-chitin) or parallel (β-chitin) arrangements of N-acetylglucosamine chains. Chitosan is derived from chitin by de-N-acetylation.
Chitin Activation of Innate Immune Responses
In keeping with its use in prosthetics, large chitin polymers have been shown to be biologically inert (Da Silva CA and Elias JA, unpublished observation). In contrast, chitin fragments are now known to regulate innate immune responses. This was initially highlighted by Shibata et al. who evaluated the immunological effects of chitin in vivo and in vitro using phagocytosable small (1 to 10 μm) chitin particles. These studies demonstrated that intravenous administration of fractionated chitin particles into the lung activated alveolar macrophages to express cytokines such as IL-12, tumor necrosis factor (TNF)-α, and IL-18, leading to INF-γ production mainly by NK cells [20]. Subsequent studies by the same group of investigators demonstrated that cytokine production was mediated by a mannose-receptor-dependent phagocytic process [21]. The mannose receptors also mediated the internalization of the chitin particles that were eventually degraded by macrophage lysozyme and N-acetyl-β-glucosaminidase [22]. Those studies were the first to demonstrate direct interactions between chitin and cell surface receptors.
Recent studies by Reese et al. also addressed the in vivo immune effects of chitin [23]. The investigators administrated chitin/chitosan coated beads directly into the lungs of mice expressing a green fluorescent protein (GFP)-enhanced transcript of IL-4 (4get mice). They noted that after several hours of chitin exposure, IL-4 GFP positive cells, in particular eosinophils (GFP+, siglec F+) and basophils (GFP+, IgE+, cKit−), were recruited to the lungs of these mice. They also demonstrated that chitin-induced eosinophil recruitment was not tissue-specific because intra-peritoneal chitin also induced eosinophilic peritonitis. On the other hand, neutrophils and mast cells were significantly recruited to the peritoneum in response to this chitin preparation, while there were no significant changes of these inflammatory cells in the lung. In these experiments, eosinophil trafficking was dependent on leukotriene B4, because recruitment was significantly decreased in the leukotriene B4 receptor (BLT1) null mice. These studies also demonstrated that chitin induced alternative macrophage activation, and that macrophage depletion with clodronate liposome treatment prevented the recruitment of eosinophils. These studies strongly suggest that chitin can contribute to the development of allergic type inflammation by activating a number of innate immune cells including macrophages, eosinophils and basophils and by enhancing the generation of alternatively activated macrophages. They also demonstrated that chitin-induced alternatively activated macrophages (AAM) play a central role in the recruitment of eosinophils and other innate cells into the lung thereby augmenting Th2 cytokine production and type 2 inflammatory responses. These studies, however, did not differentiate the direct and indirect effects of chitin. For example, it is not clear if chitin or the Th2 cytokines that it induced were responsible for the generation of the AAM. In addition, the technical, and biologic factors that explain the different responses that were noted by Reese et al. and Shibata and colleagues have not been defined.
Chitin Regulation of Adaptive Type 2 Immune Responses
In keeping with the noted ability of chitin to stimulate the production of type 1 cytokines, and the known ability of type I cytokines to inhibit type 2 inflammation [24,25], several lines of evidence suggest that chitin can regulate type 2 immune responses. Initially, Shibata et al. demonstrated that orally administered chitin inhibited allergen-induced IgE production and lung inflammation in a ragweed-immunized allergic animal model [26]. In these experiments the production of type 2 cytokines by allergen stimulated spleen cells, was also decreased by the addition of chitin to in vitro culture and, the inhibitory effects were shown to be mediated by IFN-γ produced by NK cells and ragweed-specific Th1 cells. In a separate study, the same group of investigators demonstrated that chitin is a strong Th1 adjuvant that up-regulates heat-killed Mycobacterium bovis Calmette-Guerin bacillus-induced Th1 immunity, and down regulates mycobacterial protein-induced Th2 immunity [27]. Chitin micro-particles (CMP) have also been shown to be Th1 adjuvants in the induction of viral specific immunity [28]. Direct instillation of CMP into the lung also significantly down-regulated allergic responses to Dermatophagoids pteronyssinus (Der p) and Aspergillus fumigatus including IgE levels, IL-4 production, peripheral eosinophilia, airway hyper-responsiveness, and lung inflammation while increasing the levels of IL-12, IFN-γ and TNF [29]. Ozdemir et al. also demonstrated that microgram quantities of CMP prevented and ameliorated the histopathologic changes in the airways of “asthmatic” mice [30]. In accord with these findings, intranasal application of water soluble chitosan also significanlty attenuated Dermatophagoids farinae (Der f)-induced lung inflammation and mucus production [29]. When viewed in combination, these studies strongly support the contention that chitin can have inhibitory effects on the development of adaptive type 2 allergic responses. Further support for this concept comes form recent studies that demonstrate that thymic stromal lymphopoietin (TSLP) and arginase I play critical roles in Th2 polarization and tissue remodeling responses respectively [31,32] and that they are both inhibited at sites of allergen-induced inflammation by water soluble chitosan [33]. It is important to point out however that, there is also data that suggests that chitin can also augment Th2 responses. This includes the data noted above by Reese et al. that highlights Type 2-like innate immune responses elicited by chitin beads. It also includes studies from our laboratory that demonstrate that appropriately sized chitin fragments stimulate macrophage IL-17 and IL-23 production via a TLR-2-dependent and TLR-4-independent mechanism [34] and that chitin can be a potent adjuvant in the ovalbumin mouse model of Th2 inflammation (Da Silva CA and Elias JA, unpublished observation). In combination, these studies suggest that chitin may not have a unidirectional effect on Th2 immunity in vivo. However, the experimental and biologic factors that explain the different responses that have been seen have not been adequately defined.
Mammalian Chitinases and Chitinase-Like Proteins (C/CLP)
Any discussion of the biologic effects of chitin and chitin fragments must also address the C/CLP that cleave and or bind to chitin polysaccharides. In keeping with the fact that mammals and other higher organisms lack chitin and chitin synthase, it was long assumed that mammals also lacked chitinases. Recent studies in humans and rodents, however, have refuted this belief. They have identified a family of C/CLP genes in both species referred to as the 18-glycosyl hydrolase family. Acidic mammalian chitinase (AMCase), chitotriosidase, oviductin, HcGP-39/YKL-40 and YKL-39 have been described in humans while YM-1, YM-2, AMCase, oviductin, and BRP-39 have been described in mice [2,3,35–37]. Recent studies from our laboratory have also described mouse chitotriosidase [38]. YM-1 and YM-2 may be mouse-specific because comparable genes have yet to be described in man. AMCase is produced by lung epithelial cells, macrophages and eosinophils at sites of Th2 inflammation [39]. Interestingly, IL-13 is necessary and sufficient for the induction of this chitinase [39]. In all cases these moieties have a moderate degrees of sequence homology with lower life form chitinases. However, in contrast to the chitinases in lower life forms, only a minority of the members of this family (chitotriosidase and AMCase) have true chitin-degrading activity [2]. Because of mutations in their highly conserved putative enzyme sites, BRP-39/HcGP-39/YKL-40 and the other CLPs bind but do not cleave chitin [36,37].
One of the most pressing issues in chitinase biology relates to our almost complete lack of understanding of the functions of these strongly conserved (and therefore presumably biologically important) moieties in mammals and man [37]. Mammalian C/CLP are induced at sites of inflammation (such as parasitic infections) [36] and remodeling [40]. This raises the possibility that these molecules play active roles in human anti-parasite and anti-infective defense and repair responses. To gain insights into the biology of these moieties their regulation in a variety of diseases has been studied and modeling systems have been used to define the effector profiles of selected molecules. These studies have focused most intently on AMCase and the murine-human homologues, BRP-39/YKL-40. In accord with our murine studies, AMCase was shown to be expressed in an exaggerated fashion in epithelial cells and inflammatory cells in tissues from patients with moderate-severe asthma [39]. Genetic studies in human populations have also linked polymorphisms in AMCase to asthma susceptibility in children, suggesting that inherent defects in this chitinase could underlie airway inflammation and allergic responses [41]. Recently, a compelling story has also been developing with the CLP, YKL-40. These studies demonstrated that the levels of serum and tissue YKL-40 were increased in asthma where expression correlates with disease severity and the degree of airway fibrosis [42]. These studies also defined polymorphisms in the chitinase 3 like 1 gene that encodes YKL-40 that were associated with increased levels of serum YKL-40 and the presence of asthma [16,42]. When viewed in combination these studies suggest that chitin, chitinases and CLP play important roles in Th2 responses. The mechanism(s) that underlies this association, however are still not adequately understood. This can be readily appreciated with AMCase which has been shown to play an important role in the pathogenesis of adaptive Th2 inflammation and stimulate epithelial chemokine production in chitin-free experimental systems and inhibit chitin-induced innate Th2 responses in chitin-rich systems [23,39].
Controversies and Unanswered Questions: The “Blind Man and the Elephant”
The studies that have been reported to date are generally in agreement that large chitin polymers are inert while chitin fragments can have powerful effects on innate and adaptive immunity. As described above, however, the direction of these regulatory events can be different in different situations. To understand this literature one needs to understand why these different results have been seen. We believe we are only beginning to understand the biology of chitin and the 18 glycosyl hydrolase family members and, as a result, are experiencing “the blind man and the elephant “ syndrome. Specifically, we have only been able to define parts of what is a big and diverse field in immunology and biology and the pieces that have been defined do not mirror one another. We also believe that what looks like controversy now will likely make sense once we know more about the technical aspects and biology of the systems that are being studied. Support for this contention can be seen in 2 areas where recent studies have provided insights and allowed for new testable hypotheses.
Size-dependent and pathway specific effects of chitin fragments
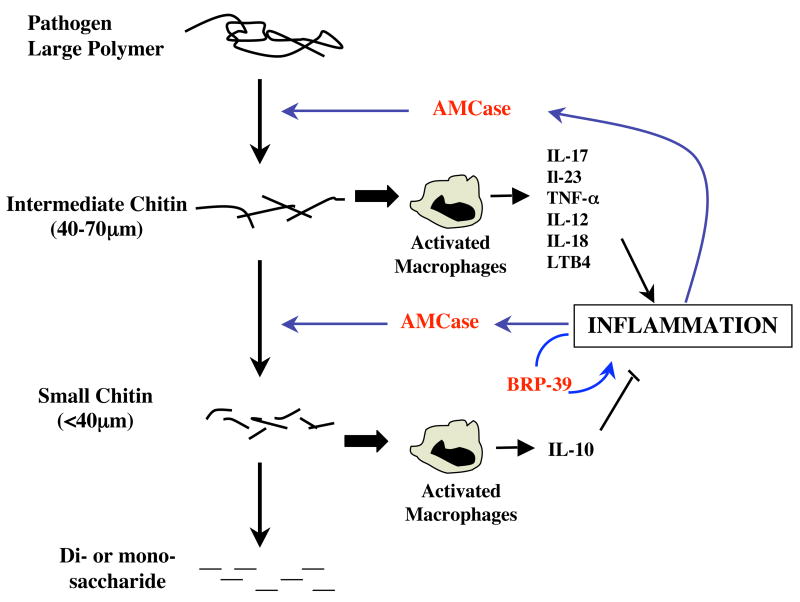
The studies noted above suggest that the composition of the chitin preparation (% chitin versus chitosan etc) and its route of administration may be important determinants of its effects in vivo and in vitro. They also suggest that the size of the chitin fragment is a critical determinant of the effector responses that it elicits. This can be readily seen in comparisons of large chitin polymers that are biologically inert and intermediately sized (40–70 μm) fragments which are PAMPS (pathogen associated molecular patterns) which trigger inflammation and IL-17, TNF and IL-23 production via the pattern recognition receptor TLR-2 and the NF-κB signaling pathway [34]. Interestingly, smaller (<40 μM), but not larger fragments, stimulate macrophage IL-10 production via a pathway that involves the Dectin-1 and Syk kinase (Da Silva CA and Elias JA, unpublished observation). These observations provide insights into the mechanisms that may underlie the different effects of chitin by demonstrating that chitin has size-dependent effects on immune cell function and that different sized chitin binds to different receptors and activates different intracellular signaling pathways. An appropriate anti-pathogen response needs to have an innate immune response that is rapid enough and powerful enough to control the invading pathogen. On the other hand it also needs to shut off when the pathogen is killed or controlled to ensure that ongoing inflammation does not cause excess tissue injury and scarring. The observation that large chitin polymers are inert, smaller fragments are proinflammatory and even smaller fragments stimulate the production of anti-inflammatory cytokine IL-10 allows for an interesting hypothesis regarding the importance of the size dependent effects of chitin in this response. It is tempting to speculate that, when chitin containing pathogens enter a host, the innate anti-pathogen response contains oxidants and chitinases that induce chitin fragmentation. The resulting intermediate sized fragments, in turn, serve as an alarm signal to induce and amplify local inflammation by activating pattern recognition receptors and pathways like NF-κB. This would continue until the invader has been successfully dealt with and smaller chitin fragments are generated. These small fragments would induce molecules like IL-10 which feedback to control the local inflammatory response (Figure 2). Interestingly, this hypothesis is very similar to the established biology of another polysaccharide, hyaluronin which also serves as an alarm signal after degradation [43]. Thus, the ability of appropriately sized polysaccharides to induce inflammation may be a general principle of glycobiology.
Figure 2.
Schematic illustrating the proposed roles of chitin size in anti-pathogen responses. . Chitin containing pathogens contain large chitin polymers that are felt to be biologically inert. The antiparasite response causes chitin fragmentation. The larger fragments (termed intermediate chitin) interact with a variety of macrophage receptor systems including, the macrophage mannose receptor and TLR-2 to produce a variety of proinflammatory cytokines and mediators including IL-17, IL-23, TNF and LTB4. The resulting inflammatory response can, in turn, stimulate the production of C/CLP. This includes AMCase which can further degrade the chitin polymers. This results in the accumulation of even smaller molecules (termed small chitin) which can induce the production of the anti-inflammatory cytokine IL-10 via Dectin -1. It also includes BRP-39/YKL-40 which can control inflammatory cell death. In an appropriately regulated response, the anti-inflammatory effects of molecules like IL-10 and the degradation of chitin to smaller saccharides (for example; di-saccharides and mono-saccharides) likely contribute to the resolution of the response once the pathogen has been controlled.
The effects of AMCase
As noted above, AMCase has been shown to play an important role in the pathogenesis of and inhibit chitin-induced type 2 inflammatory responses [23,39]. On superficial analysis these observations can appear to conflict with one another. A deeper evaluation, however, suggests that this interpretation is not correct because one study evaluated the role of AMCase in the pathogenesis of the adaptive immune responses induced by a chitin-free aeroallergen [39] while the other evaluated the role of AMCase in chitin-induced innate immune responses [23]. Studies from our laboratory and others have also demonstrated that 18 glycosyl hydrolases can regulate cellular apoptosis [44] (Lee CG and Elias JA, unpublished observation). This allows for an interesting hypothesis that ties chitin, chitinases, CLPs and both views of the biology of AMCase together at sites of invasion with a chitin-containing pathogen (Figure 2). Specifically, while the host anti-inflammatory response is generating intermediate sized chitin fragments that augment local tissue inflammation, the inflammatory response stimulates the production of 18 glycosyl hydrolases like AMCase and BRP-39/YKL-40. The true chitinase generates additional immunostimulatory chitin fragments. These molecules also regulate the intensity and chronicity of local inflammation by regulating local inflammatory cell apoptosis. Over time, AMCase would also help to limit local injury by fostering the more complete degradation of chitin to IL-10-inducing small chitin and eventually even smaller inert polysaccharide moieties.
Conclusion
Recent studies clearly demonstrate that chitin and chitin derivatives can stimulate innate immune cells – macrophages, basophils and eosinophils -and modulate adaptive Type I or Type 2 responses. They also demonstrated that chitin moieties mediate their cellular and tissue effects in a size-dependent and pathway-specific manner and highlight the ability of chitin to serve as a PAMP that activates macrophages via TLR-2. However, the exact in vivo immune regulatory effects of chitin remain controversial and the mechanisms that mediate these complex effects are still poorly defined. Additional experimentation will be required to define the biology and immunobiology of chitin, chitinases and CLP. Experimentation will also be required to determine if chitin- or C/CLP-based interventions can have beneficial effects on inflammatory responses like those in asthma and other lung disorders.
Acknowledgments
These work was supported by NIH Grants HL-081639 (J.A. Elias), HL-084225 (Lee C.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Araujo AC, Souto-Padron T, de Souza W. Cytochemical localization of carbohydrate residues in microfilariae of Wuchereria bancrofti and Brugia malayi. J Histochem Cytochem. 1993;41:571–578. doi: 10.1177/41.4.8450196. [DOI] [PubMed] [Google Scholar]
- 2.Boot RG, Blommaart EF, Swart E, Ghauharali-van der Vlugt K, Bijl N, Moe C, Place A, Aerts JM. Identification of a novel acidic mammalian chitinase distinct from chitotriosidase. J Biol Chem. 2001;276:6770–6778. doi: 10.1074/jbc.M009886200. [DOI] [PubMed] [Google Scholar]
- 3.Boot RG, Renkema GH, Verhoek M, Strijland A, Bliek J, de Meulemeester TM, Mannens MM, Aerts JM. The human chitotriosidase gene. Nature of inherited enzyme deficiency. J Biol Chem. 1998;273:25680–25685. doi: 10.1074/jbc.273.40.25680. [DOI] [PubMed] [Google Scholar]
- 4.Debono M, Gordee RS. Antibiotics that inhibit fungal cell wall development. Annu Rev Microbiol. 1994;48:471–497. doi: 10.1146/annurev.mi.48.100194.002351. [DOI] [PubMed] [Google Scholar]
- 5•.Elias JA, Homer RJ, Hamid Q, Lee CG. Chitinases and chitinase-like proteins in T(H)2 inflammation and asthma. J Allergy Clin Immunol. 2005;116:497–500. doi: 10.1016/j.jaci.2005.06.028. A brief review on the role of chitinases and chitinsae-like proteins in allergic animal models and potential implications of chitinase family proteins in the pathogenesis of human asthma. [DOI] [PubMed] [Google Scholar]
- 6.Fuhrman JA, Piessens WF. Chitin synthesis and sheath morphogenesis in Brugia malayi microfilariae. Mol Biochem Parasitol. 1985;17:93–104. doi: 10.1016/0166-6851(85)90130-6. [DOI] [PubMed] [Google Scholar]
- 7.Neville AC, Parry DA, Woodhead-Galloway J. The chitin crystallite in arthropod cuticle. J Cell Sci. 1976;21:73–82. doi: 10.1242/jcs.21.1.73. [DOI] [PubMed] [Google Scholar]
- 8.Shahabuddin M, Kaslow DC. Plasmodium: parasite chitinase and its role in malaria transmission. Exp Parasitol. 1994;79:85–88. doi: 10.1006/expr.1994.1066. [DOI] [PubMed] [Google Scholar]
- 9.Muzzarelli RA. Human enzymatic activities related to the therapeutic administration of chitin derivatives. Cell Mol Life Sci. 1997;53:131–140. doi: 10.1007/PL00000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arlian LG, Platts-Mills TA. The biology of dust mites and the remediation of mite allergens in allergic disease. J Allergy Clin Immunol. 2001;107:S406–413. doi: 10.1067/mai.2001.113670. [DOI] [PubMed] [Google Scholar]
- 11.Chew GL, Perzanowski MS, Canfield SM, Goldstein IF, Mellins RB, Hoepner LA, Ashby-Thompson M, Jacobson JS. Cockroach allergen levels and associations with cockroach-specific IgE. J Allergy Clin Immunol. 2008;121:240–245. doi: 10.1016/j.jaci.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Matsuo H, Morita E. Cross-reactivity among shrimp, crab and scallops in a patient with a seafood allergy. J Dermatol. 2006;33:174–177. doi: 10.1111/j.1346-8138.2006.00040.x. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura K, Nishimura S, Nishi N, Saiki I, Tokura S, Azuma I. Immunological activity of chitin and its derivatives. Vaccine. 1984;2:93–99. doi: 10.1016/s0264-410x(98)90039-1. [DOI] [PubMed] [Google Scholar]
- 14•.Nishimura S, Nishi N, Tokura S, Nishimura K, Azuma I. Bioactive chitin derivatives. Activation of mouse-peritoneal macrophages by O-(carboxymethyl)chitins. Carbohydr Res. 1986;146:251–258. doi: 10.1016/0008-6215(86)85044-3. First comprehensive studies on the general immunologic activity of chitin and chitin derivatives. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki K, Okawa Y, Hashimoto K, Suzuki S, Suzuki M. Protecting effect of chitin and chitosan on experimentally induced murine candidiasis. Microbiol Immunol. 1984;28:903–912. doi: 10.1111/j.1348-0421.1984.tb00746.x. [DOI] [PubMed] [Google Scholar]
- 16•.Ober C, Tan Z, Sun Y, Possick JD, Pan L, Nicolae R, Radford S, Parry RR, Heinzmann A, Deichmann KA, et al. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med. 2008;358:1682–1691. doi: 10.1056/NEJMoa0708801. Population genetic studies demonstrating genetic variations of a chitnase like protein YKL-40 are significantly associated with prevalence of asthma and severity of the disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellouz F, Adam A, Ciorbaru R, Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1974;59:1317–1325. doi: 10.1016/0006-291x(74)90458-6. [DOI] [PubMed] [Google Scholar]
- 18.Azuma I, Sugimura K, Taniyama T, Yamawaki M, Yamamura Y. Adjuvant activity of mycobacterial fractions: adjuvant activity of synthetic N-acetylmuramyl-dipeptide and the related compounds. Infect Immun. 1976;14:18–27. doi: 10.1128/iai.14.1.18-27.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iida J, Une T, Ishihara C, Nishimura K, Tokura S, Mizukoshi N, Azuma I. Stimulation of non-specific host resistance against Sendai virus and Escherichia coli infections by chitin derivatives in mice. Vaccine. 1987;5:270–274. doi: 10.1016/0264-410x(87)90150-2. [DOI] [PubMed] [Google Scholar]
- 20.Shibata Y, Foster LA, Metzger WJ, Myrvik QN. Alveolar macrophage priming by intravenous administration of chitin particles, polymers of N-acetyl-D-glucosamine, in mice. Infect Immun. 1997;65:1734–1741. doi: 10.1128/iai.65.5.1734-1741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21•.Shibata Y, Metzger WJ, Myrvik QN. Chitin particle-induced cell-mediated immunity is inhibited by soluble mannan: mannose receptor-mediated phagocytosis initiates IL-12 production. J Immunol. 1997;159:2462–2467. These studies demonstrate 1–10 μm chitin particles activate alveolar macrophages to express IL-12, TNF-α and IFN-γ via mannose receptor mediated pathway. It is the first study that demonstrate chitin uses specific receptor to stimulate macrophage response. [PubMed] [Google Scholar]
- 22.Bourbouze R, Raffi F, Dameron G, Hali-Miraftab H, Loko F, Vilde JL. N-acetyl-beta-D-glucosaminidase (NAG) isoenzymes release from human monocyte-derived macrophages in response to zymosan and human recombinant interferon-gamma. Clin Chim Acta. 1991;199:185–194. doi: 10.1016/0009-8981(91)90110-x. [DOI] [PubMed] [Google Scholar]
- 23••.Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, Locksley RM. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–96. doi: 10.1038/nature05746. Intranasal administration of chitin beads induces accumulation of innate effector cells such as eosinophils and basophils via Stat6- and Rag- independent BLT1- dependent mechanisms. They further demosntrate AMCase prevents chitin-induced eosinophil and basophil recruitment and alternative activation of macrophages is essential in chitin-mediated in vivo responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sur S, Lam J, Bouchard P, Sigounas A, Holbert D, Metzger WJ. Immunomodulatory effects of IL-12 on allergic lung inflammation depend on timing of doses. J Immunol. 1996;157:4173–4180. [PubMed] [Google Scholar]
- 25.Gavett SH, O’Hearn DJ, Li X, Huang SK, Finkelman FD, Wills-Karp M. Interleukin 12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. J Exp Med. 1995;182:1527–1536. doi: 10.1084/jem.182.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibata Y, Foster LA, Bradfield JF, Myrvik QN. Oral administration of chitin down-regulates serum IgE levels and lung eosinophilia in the allergic mouse. J Immunol. 2000;164:1314–1321. doi: 10.4049/jimmunol.164.3.1314. [DOI] [PubMed] [Google Scholar]
- 27•.Shibata Y, Honda I, Justice JP, Van Scott MR, Nakamura RM, Myrvik QN. Th1 adjuvant N-acetyl-D-glucosamine polymer up-regulates Th1 immunity but down-regulates Th2 immunity against a mycobacterial protein (MPB-59) in interleukin-10-knockout and wild-type mice. Infect Immun. 2001;69:6123–6130. doi: 10.1128/IAI.69.10.6123-6130.2001. Fragmented chitin administration in vivo enhances Th1 immunity while suppressing Th2 immunity, suggesting an adjuvant role of chitin in immune regulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamajima K, Kojima Y, Matsui K, Toda Y, Jounai N, Ozaki T, Xin KQ, Strong P, Okuda K. Chitin Micro-Particles (CMP): a useful adjuvant for inducing viral specific immunity when delivered intranasally with an HIV-DNA vaccine. Viral Immunol. 2003;16:541–547. doi: 10.1089/088282403771926355. [DOI] [PubMed] [Google Scholar]
- 29•.Strong P, Clark H, Reid K. Intranasal application of chitin microparticles down-regulates symptoms of allergic hypersensitivity to Dermatophagoides pteronyssinus and Aspergillus fumigatus in murine models of allergy. Clin Exp Allergy. 2002;32:1794–1800. doi: 10.1046/j.1365-2222.2002.01551.x. Intranasal application of chitin significantly regulate house dust mite or Aspergillus-associated type 2 allergic responses. [DOI] [PubMed] [Google Scholar]
- 30.Ozdemir C, Yazi D, Aydogan M, Akkoc T, Bahceciler NN, Strong P, Barlan IB. Treatment with chitin microparticles is protective against lung histopathology in a murine asthma model. Clin Exp Allergy. 2006;36:960–968. doi: 10.1111/j.1365-2222.2006.02515.x. [DOI] [PubMed] [Google Scholar]
- 31.MacDonald AS, Maizels RM. Alarming dendritic cells for Th2 induction. J Exp Med. 2008;205:13–17. doi: 10.1084/jem.20072665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, Malefyt Rde W, Omori M, Zhou B, Ziegler SF. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 33.Chen CL, Wang YM, Liu CF, Wang JY. The effect of water-soluble chitosan on macrophage activation and the attenuation of mite allergen-induced airway inflammation. Biomaterials. 2008;29:2173–2182. doi: 10.1016/j.biomaterials.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 34••.Da Silva CA, Hartl D, Liu W, Lee CG, Elias JA. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. J Immunol. 2008;181:4279–4286. doi: 10.4049/jimmunol.181.6.4279. Chitin trigger inflammation and IL-17, TNF-α and IL-23 production via the recognition receptor TLR-2 and the NF-κB signaling pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ward JM, Yoon M, Anver MR, Haines DC, Kudo G, Gonzalez FJ, Kimura S. Hyalinosis and Ym1/Ym2 gene expression in the stomach and respiratory tract of 129S4/SvJae and wild-type and CYP1A2-null B6, 129 mice. Am J Pathol. 2001;158:323–332. doi: 10.1016/S0002-9440(10)63972-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang NC, Hung SI, Hwa KY, Kato I, Chen JE, Liu CH, Chang AC. A macrophage protein, Ym1, transiently expressed during inflammation is a novel mammalian lectin. J Biol Chem. 2001;276:17497–17506. doi: 10.1074/jbc.M010417200. [DOI] [PubMed] [Google Scholar]
- 37.Bleau G, Massicotte F, Merlen Y, Boisvert C. Mammalian chitinase-like proteins. Exs. 1999;87:211–221. doi: 10.1007/978-3-0348-8757-1_15. [DOI] [PubMed] [Google Scholar]
- 38.Zheng T, Rabach M, Chen NY, Rabach L, Hu X, Elias JA, Zhu Z. Molecular cloning and functional characterization of mouse chitotriosidase. Gene. 2005;357:37–46. doi: 10.1016/j.gene.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 39••.Zhu Z, Zheng T, Homer RJ, Kim YK, Chen NY, Cohn L, Hamid Q, Elias JA. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2004;304:1678–1682. doi: 10.1126/science.1095336. Acidic mammalian chitinase regulate Th2 and IL-13-iduced inflammation. AMCase is required for the increased expression of chemokines involved in the recruitment of monocytes, macrophages, eosinophils and neutrophils. [DOI] [PubMed] [Google Scholar]
- 40.Ostergaard C, Johansen JS, Benfield T, Price PA, Lundgren JD. YKL-40 is elevated in cerebrospinal fluid from patients with purulent meningitis. Clin Diagn Lab Immunol. 2002;9:598–604. doi: 10.1128/CDLI.9.3.598-604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bierbaum S, Nickel R, Koch A, Lau S, Deichmann KA, Wahn U, Superti-Furga A, Heinzmann A. Polymorphisms and haplotypes of acid mammalian chitinase are associated with bronchial asthma. Am J Respir Crit Care Med. 2005;172:1505–1509. doi: 10.1164/rccm.200506-890OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42•.Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, Dziura JD, Reed J, Coyle AJ, Kiener P, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007;357:2016–2027. doi: 10.1056/NEJMoa073600. Population study demonstrating a chitnase-like protein YKL-40 expression in serum and lung tissues is significantly elevated in the asthmatic patients and their YKL-40 expression levels are correlated with asthma severity. [DOI] [PubMed] [Google Scholar]
- 43.Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, Henson PM, Noble PW. Resolution of lung inflammation by CD44. Science. 2002;296:155–158. doi: 10.1126/science.1069659. [DOI] [PubMed] [Google Scholar]
- 44.Ling H, Recklies AD. The chitinase 3-like protein human cartilage glycoprotein 39 inhibits cellular responses to the inflammatory cytokines interleukin-1 and tumour necrosis factor-alpha. Biochem J. 2004;380:651–659. doi: 10.1042/BJ20040099. [DOI] [PMC free article] [PubMed] [Google Scholar]