Abstract
Mitochondria display considerable structural diversity, particularly in terms of the folding of the energy-transducing inner membrane. The hypothesis is forwarded that the topology of the mitochondrial inner membrane is not random but rather is a regulated cell parameter. Its effects on internal diffusion of metabolites and soluble proteins can influence such mitochondrial processes as ATP production and protein release during apoptosis. Progress towards understanding the factors that control mitochondrial inner membrane curvature and dynamics (fusion and fission) is summarized, with a focus on remodeling events associated with apoptosis and oxidative stress.
Keywords: Mitochondria, crista, electron microscopy, electron tomography, membrane dynamics, membrane topology, apoptosis, cardiolipin, Mgm1, OPA1, t-Bid, adenine nucleotide translocator, ATP synthase, dominant optic atrophy, oxidative stress, reactive oxygen species
Mitochondria are the organelles that are the primary site of energy transduction and ATP generation in eukaryotes. These organelles are composed of two distinctly different membranes, a limiting outer membrane and an inner membrane that encloses a protein-rich matrix. The molecular machinery of chemiosmosis is associated with the inner membrane. Although mitochondria serve fundamentally the same bioenergetic function in all cells, they display striking interspecies and inter-tissue variations in shape, connectivity, and inner-membrane morphology.1,2 The morphologic dexterity of isolated mitochondria in vitro is equally impressive. In the late 1960s and early 1970s, respiratory-state-linked changes in inner-membrane morphology were reported – first by Hackenbrock3 – leading to speculation that they might reflect changes in the “energization” state of the membrane integral to energy transduction.4 However, the consensus view was that observed transitions in inner-membrane morphology represented random refolding of a flexible membrane in response to osmotically driven changes in matrix volume.
In the last decade, advances in light and electron microscopy have led to a renewed interest in the structural diversity and dynamics of mitochondria, as well as in their interactions with other cellular components. These new imaging techniques are allowing systematic monitoring of changes in mitochondrial shape and ultrastructure during particular biological processes, and in so doing are revealing a coupling between structure and function not previously appreciated. From the perspective of inner-membrane morphologic changes, the old view of mitochondria as a passive “osmometer”,5 while still valid, is incomplete. Studies involving electron tomography have revealed dramatic remodelings of the inner membrane that correlate, in the case of apoptosis, with cytochrome c release6 and organelle fragmentation.7 The emerging paradigm is that mitochondrial structure and dynamics are under active control of the cell at all times to effect optimal mitochondrial performance and response to stimuli.8
This report provides an overview of the current understanding of mitochondrial inner membrane topology and some recent findings related to its integration into two processes, one that is already a topic of considerable interest and debate (apoptosis-linked changes), and another that is just now coming to light (changes associated with oxidative stress).
INNER MEMBRANE TOPOLOGY AND INTERNAL DIFFUSION
The inner membranes of mitochondria contain invaginations called cristae (Latin for crests). The cristae are not random folds in the membrane (as typically depicted) but rather micro-compartments that open through narrow tubular membrane segments into the peripheral region of the membrane (Figure 1).9–13 In tomograms of intact, frozen-hydrated rat liver mitochondria,11 the inner diameter of the tubular “crista junctions” is 10–15 nm (Figure 1). While this bore is wide enough to pass metabolites and many soluble proteins, the narrow inner-membrane junctions might nonetheless restrict internal diffusion rates. For example, computer simulations indicate that the steady-state level of ADP inside large cristae with long narrow junctions can drop below the Km for the adenine nucleotide translocator, leading to a local drop in ATP generation.11 Likewise, t-Bid-induced remodeling of the inner membrane of isolated mouse liver mitochondria (Figure 1) correlates with mobilization of a large fraction of the internal pool of cytochrome c, indicated by increased rates of reduction by the NADH-cytochrome b5 redox system on the outer membrane of the organelle.6 The inner-membrane remodeling involves, in part, five-fold widening of the crista junctions, suggesting that diffusion of cytochrome c between intracristal and peripheral (intermembrane) compartments is normally rate-limiting. These results indicate that the topology of the mitochondrial inner membrane can have a profound effect on mitochondrial activities, by influencing the kinetics of diffusion of metabolites and soluble proteins between the internal compartments defined by this membrane.10,14
Figure 1.
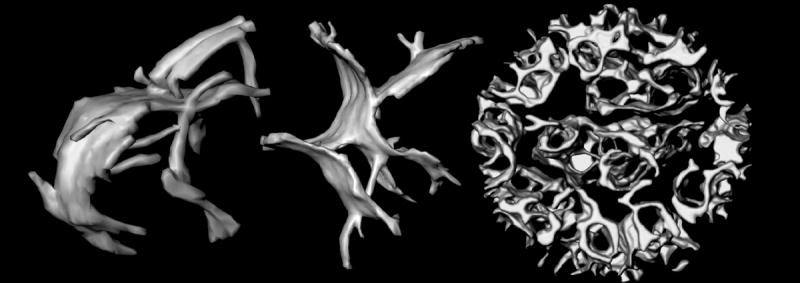
Topology of mitochondrial inner membranes. Left and middle: cristae of isolated, intact, frozen-hydrated rat-liver mitochondria.11 The large crista compartments appear to be formed by fusion of tubular membranes. These cristae are approximately 600 nm in length. Right: Inner membrane of a mouse liver mitochondrion pre-treated with the pro-apoptotic protein t-Bid.6 Rather than forming discrete compartments, the intracristal space is essentially one continuous compartment. The diameter of this mitochondrion is 860 nm
MITOCHONDRIAL INNER MEMBRANE DYNAMICS
Isolated mitochondria are often described as occurring in one of two morphologic states, condensed, characterized by a contracted, very dense matrix compartment and wide cristae, and orthodox, having an expanded, less-dense matrix and more compact crista compartments.3 Changes between these membrane morphologies, detectable in real time by light scattering, can be induced by simply adjusting the osmotic pressure of the external medium, causing water to flow into or out of the matrix. A reversible condensed-to-orthodox transition also occurs during respiration when ADP, added in excess, has been fully phosphorylated.3 The corresponding changes in inner membrane profiles observed in electron micrographs were interpreted initially as passive unfoldings and refoldings of the inner membrane. However, 3D images of rat liver mitochondria provided by electron tomography indicate that something more interesting is going on.8,15 Condensed rat liver mitochondria have large pleiomorphic cristae that can have multiple junctions to each other and to the peripheral region of the inner membrane, i.e, the region apposed to the outer membrane. Orthodox rat liver mitochondria have cristae that are either tubular or flattened lamellae, both types usually having only one junction to the periphery of the inner membrane. To account for the observed topological transition, the inner membranes must undergo fusion and fission, with tubular forms merging into the larger cisternae during matrix condensation. That large lamellar compartments are formed via crista fusion is strongly suggested by their appearance in tomograms of frozen-hydrated mitochondria (Figure 1). Thus, the structural adjustments that mitochondria undergo in response to osmotic and metabolic changes involve not only reciprocal contraction and dilation of the matrix and intracristal spaces defined by the inner membrane, but also remodeling of the inner membrane itself. A review of mitochondrial morphologies associated with a variety of osmotic, metabolic and disease states suggests that inner membrane topology represents a balance between fusion and fission, with defects (such as crista vesiculation) corresponding to an imbalance in this process.8
PROTEINS THAT AFFECT INNER MEMBRANE MORPHOLOGY
It is likely that some of the proteins responsible for maintenance of normal crista morphology and dynamics are the same as those that mediate inter-mitochondrial fusion and organelle division, since these processes involve fusion and fission of the inner as well as the outer membranes. For example, the dynamin-like GTPase called Mgm1p in yeast and OPA1 in mammalian cells is required for the fusion of mitochondria that gives rise to their typical elongated form in most cell types.16,17 Mutations in this protein cause a progressive, autosomal dominant retinopathy, Dominant Optic Atrophy,18,19 underscoring the physiological importance of mitochondrial dynamics. Mutation or down-regulation of Mgm1/OPA1 in yeast,16,17,20,21 C. elegans,22 and HeLa cells23 results in mitochondria with structural phenotypes consistent with defective membrane fusion, i.e., spherical mitochondria with morphologically simple inner membranes. In some cases, such as a C. elegans OPA1 mutant (Figure 2), the inner membranes form unattached vesicles indicative of unbalanced membrane fission. These vesicular inner membrane compartments can have extended regions of close apposition, suggesting that any failure of the inner membranes to fuse is not simply due to lack of proximity. In fact, there is growing evidence that Mgm1/OPA1 plays multiple roles in mitochondrial fusion and maintenance of normal inner membrane morphology, associated in part with its considerable structural polymorphism (splicing variants, proteolytic processing, complex formation).21,24–26
Figure 2.
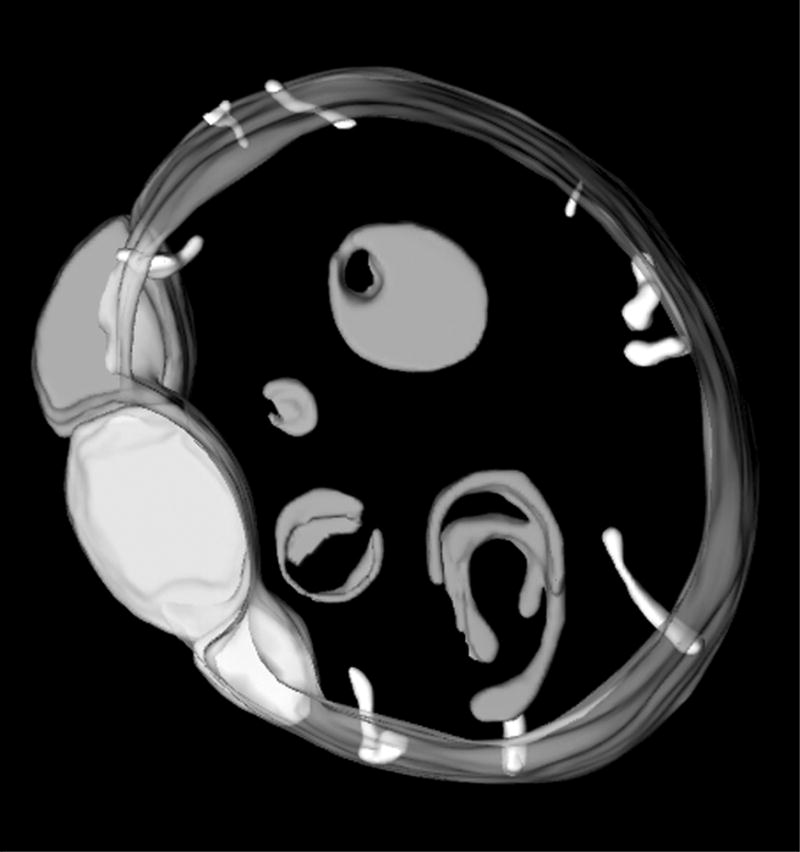
Internal mitochondrial membranes of an OPA1 deficient mutant of C. elegans.22 These mitochondria are characterized by numerous vesicular compartments, characteristic of a membrane fusion defect. The group of close-packed inner membrane vesicles at left is similar to features seen in apoptotic mitochondria7. The diameter of this mitochondrion is 1.6 μm.
A second protein postulated to directly influence inner membrane topology is F1F0 ATP synthase. Mutations in subunits e or g of the F0 domain interrupt lateral dimerization and subsequent oligomerization of these inner membrane complexes, and are associated with unusual concentrically wrapped cristae lacking tubular junctions.27 The same structural phenotype occurs with down-regulation of the protein mitofilin, suggesting that it might somehow regulate interactions of the ATP synthase.28 A mechanism for inner-membrane tubularization has been suggested by single-particle electron microscopic analyses of detergent-isolated complexes.29,30 In synthase dimers, close packing of the bulky extra-membrane F1 domains causes the smaller, intra-membrane F0 domains to adopt an angle of 40–70°, which could induce local bending of the inner membrane. Interestingly, Mgm1/OPA1 has been reported to have a chaperone-like function for subunit e of the ATP synthase.20 The loss of this protective function in Mgm1/OPA1 mutants inhibits ATP synthase dimer formation,20 which could explain the deficiency of normal tubular crista junctions in these mitochondria, noted above.
MEMBRANE REMODELING ASSOCIATED WITH APOPTOSIS
Mitochondria play a pivotal role in the cascade of events associated with the intrinsic apoptotic pathway.31 The key event is the release from mitochondria of soluble proteins like cytochrome c that participate in the downstream proteolytic processes following induction of apoptosis. The release mechanism is not yet completely described but it is known to be regulated by members of the Bcl-2 protein family, including the pro-apoptotic proteins Bid, Bax and Bak. Truncation of Bid by a caspase (apoptosis-associated protease) causes Bax oligomerization and relocation from the cytoplasm to the mitochondrial surface, where it and Bak (resident on the outer membrane) create large pores that have been characterized electrophysiologically.32 The first evidence for mitochondrial inner-membrane remodeling associated with these events came from the experiments of Scorrano and colleagues (mentioned above) that correlated the 3D ultrastructure of mouse-liver mitochondria with changes in internal diffusion of cytochrome c following incubation with t-Bid.6 These experiments indicated that a large internal pool of cytochrome c was mobilized in conjunction with a dramatic transition in the topology of the inner membrane (Figure 1). The crista junctions were wider and slot-like, and the curvature of the membrane was reversed in many regions, forming tubes that enclosed matrix. (The invaginations of the inner membrane normally curve away from the matrix and define the intracristal space.)
Several lines of evidence suggest that the observed t-Bid-induced effects on mitochondria involve the prominent inner-membrane phospholipid cardiolipin. In particular, cardiolipin has been shown to target to t-Bid in the mitochondrial inner membrane,33 and interrupting this binding of t-Bid to cardiolipin inhibits cytochrome c release from mitochondria.34 From a structural perspective, t-Bid causes reversal of the curvature of cardiolipin-containing membranes,35 consistent with its effects on mouse-liver inner membranes. In a recent electron tomographic study of mitochondria from patients with Barth syndrome, a cardiolipin-deficiency disorder, inner membranes display reversed curvature (inverted, matrix filled tubes) similar to that seen in t-Bid treated mitochondria.36 This lends further support to the hypothesis that the morphologic change in the inner membrane caused by t-Bid is related to alterations in cardiolipin organization or content. Finally, it is well known that several respiratory chain complexes as well as the adenine nucleotide translocator (ANT) have a specific dependency on cardiolipin. The action of t-Bid at cardiolipin is supported by the finding that t-Bid inhibits ANT activity of yeast mitochondria in a cardiolipin-dependent manner.37 Interestingly, an inhibitor of the adenine nucleotide translocator was shown in the 1970s to cause a remodeling of the inner membrane of beef heart mitochondria that is essentially identical to that induced by t-Bid in mouse liver mitochondria.38
In an elegant correlative light-electron microscopic study, Frey and co-workers have described structural changes in the mitochondria of Hela cells associated with apoptosis.7 They report accumulation of vesicular inner membranes in apoptotic mitochondria which, they hypothesize, is a precursor to organellar fragmentation, a requirement for apoptosis to proceed.39 The occurrence of the inner membrane vesicles is attributed to widening of crista junctions into progressively longer slits that eventually circumscribe the organelle. Interestingly, the formation of elongated slit-like membrane junctions had been shown to be induced by t-Bid in the earlier liver mitochondria study6, suggesting that this structural change in apoptotic HeLa mitochondria might result from alterations to cardiolipin organization triggered by t-Bid. Likewise, the accumulation of independent inner membrane vesicles inside apoptotic mitochondria is similar to that seen in mitochondria of Mgm1/OPA1-defective mutants in a variety of cell-types (e.g., Figure 2). The apparent imbalance of mitochondrial inner membrane fission (absence of fusion) in apoptotic HeLa cells could be attributable to loss of OPA1 from HeLa mitochondria during apoptosis.40
A question raised in the HeLa study is whether remodeling of the mitochondrial inner membrane is, in fact, a requirement for rapid and complete release of cytochrome c during apoptosis. Mitochondrial cytochrome c release occurred in control HeLa cells at a stage when a significant fraction of the mitochondria displayed vesicular cristae, suggesting crista junction elongation had occurred, consistent with the result of the earlier study with t-Bid treated liver mitochondria.6 However, HeLa cells pretreated with a caspase inhibitor fully released cytochrome c in the absence of inner membrane vesiculation and, according to tomograms of three mitochondria, without crista junction elongation. Issue can be taken with the latter conclusion based on small sample size and concerns about possible experimental artifacts associated with the modified cytochrome c expressed in these cells as a reporter molecule. Likewise, it is unclear why caspase activation would be needed for inner membrane remodeling, since that is clearly not the case with liver mitochondria. Suffice it to say that further investigations of cristae remodeling during apoptosis are underway in several laboratories that should lead to eventual resolution of this lively dispute. For example, recent results by Scorrano and colleagues, using mouse embryonic fibroblasts, point to Mgm1/OPA1 playing a key role in the modulation of crista openings and mitochondrial cytochrome c release during apoptosis.25,26
INNER MEMBRANE REMODELING IN RESPONSE TO OXIDATIVE STRESS
Mitochondria in the amoeba Chaos carolinensis undergo a remarkable structural transition during fasting. Their normal tubular cristae transform within 1–2 days of fasting into a membrane continuum with wide (over 100-nm diameter), interconnected compartments related by cubic symmetry.41 This inner membrane remodeling was found to correlate with increased production of reactive oxygen species (ROS) during fasting, probably associated with a change in metabolism to oxidation of internal protein and lipid stores.42 It was hypothesized at the time that this membrane reorganization might be a protective response of the organism that expedites efflux (or catalytic deactivation) of ROS from mitochondria and/or reduces damage to the inner membranes by decreasing membrane curvature. These organisms survive for weeks in the fasting state, and the mitochondrial membranes revert to their normal tubular morphology shortly after food organisms are made available, suggesting that the drastic changes in membrane topology are physiological and reversible.
While this phenomenon of inner membrane remodeling in response to oxidative stress was thought at the time to be limited to amoeba, recent findings suggest that it might be more generally relevant. In particular, Walker and Benzer have described a structural change in the mitochondria of Drosophila flight muscle during hyperoxic conditions that involves formation of a “swirl” of expanded cristae.43 Preliminary analysis of these mitochondria by electron tomography indicates that membranes comprising the swirls are closely packed, dilated tubes (diameters 30–40 nm), interconnected to lamellar regions of the inner membrane (Figure 3).44 The local membrane curvature and packing geometry (hexagonal) of the fly “swirl” mitochondrial inner membranes are different from those of fasting amoeba, but the effect of the two topological transitions is the same: creation of an interconnected network of expanded crista compartments. In addition, very similar “swirl”-like tubular inner membranes occur in mitochondria of steroidogenic tissue, 45,46 in which the P450 system generates considerable ROS.47,48 Finally, numerous dilated cristae are found in neuronal mitochondria of a transgenic mouse model for amyotrophic lateral sclerosis with mutant mitochondrial superoxide dismutase (mSOD).49
Figure 3.
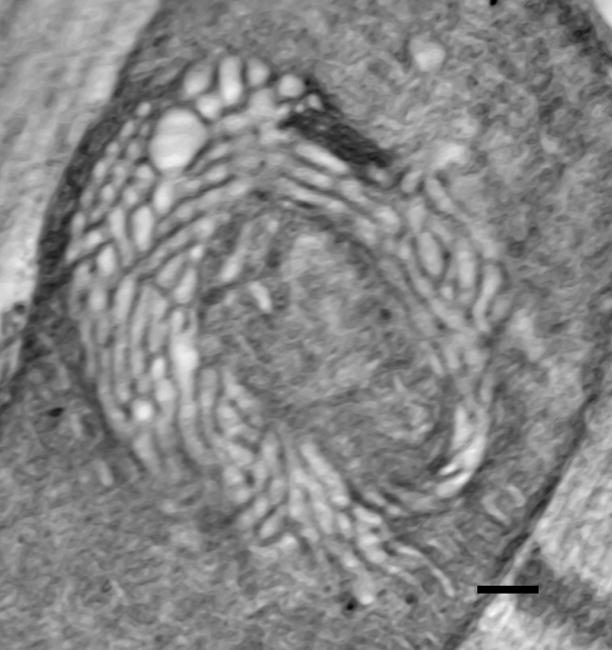
Slice from a tomogram of a “swirl” mitochondrion from Drosophila flight muscle.43,44 Tomographic analysis indicates that the swirl region is comprised of dilated, interconnected tubular cristae. (Specimen provided by Drs. S. Benzer and D. Walker.) The diameter of the mitochondrion is 900 nm.
While this survey is far from exhaustive, it suggests that cristae dilation and increased interconnectivity is associated with oxidative stress, either caused by elevated ROS production (e.g., fasting amoeba and steroidogenic tissue) or reduced ROS protective mechanisms (e.g., mSOD-deficient mice or a hyperswirl fly mutant that develops “swirl” cristae under normal oxygen tension43). There is no direct evidence that the widening of the diffusion pathways inside mitochondria is a protective response, perhaps allowing faster efflux of ROS, but it appears that the converse is true. One effect of mutation of OPA1 in the nematode is enhanced sensitivity of the organism to elevated ROS.22 The aberrant, vesiculated inner membranes of the mutant may have limited ability to clear the ROS generated during normal respiration, leading to increased sensitivity of these mitochondria to the damaging effects of ROS.
CONCLUSIONS
There is growing evidence that changes in the topology of the mitochondrial inner membrane are not merely random unfoldings and refoldings of a flexible membrane. Rather, these morphologic changes are remodelings, associated with altered functional states of the mitochondrion, that involve fusion and fission of this membrane and alter internal diffusion pathways. The emerging hypothesis is that mitochondrial inner-membrane topology is a parameter that is regulated by the cell to optimize mitochondrial performance and response to stimuli. While some progress has been made in identifying the proteins and lipids involved in mitochondrial inner membrane remodelings, considerably more work is needed to identify the sensors and describe the feedback mechanisms by which inner membrane topology is regulated.
Acknowledgments
Special thanks are due to colleagues at Wadsworth’s Resource for Visualization of Biological Complexity (RVBC) for development of techniques of electron tomography, supported by the National Center for Research Resources, NIH, grant RR01219 (PI: Joachim Frank). In particular, Michael Marko, Karolyn Buttle, Chyongere Hsieh, and Christian Renken have been instrumental in the successful application of electron tomography to the mitochondrial studies cited herein. The assistance of Michael Watters (Howard Hughes Medical Institute) with producing Figure 1 is gratefully acknowledged.
References
- 1.Munn EA. The Structure of Mitochondria. Academic Press; London: 1974. [Google Scholar]
- 2.Fawcett DW. An Atlas of Fine Structure. W.B. Saunders Co.; Philadelphia: 1966. [Google Scholar]
- 3.Hackenbrock CR. Ultrastructural bases for metabolically linked mechanical activity in mitochondria. I Reversible ultrasturctural changes with change in metabolic steady state in isolated liver mitochondria. J Cell Biol. 1966;30:269–297. doi: 10.1083/jcb.30.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green DE, Ji S. Tranductional and structural principles of the mitochondrial transducing unit. Proc Natl Acad Sci (USA) 1973;70:904–908. doi: 10.1073/pnas.70.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tedeschi H, Harris DL. The osmotic behavior and permeability to non-electrolytes of mitochondria. Arch Biochem Biophys. 1955;58:52–67. doi: 10.1016/0003-9861(55)90092-8. [DOI] [PubMed] [Google Scholar]
- 6.Scorrano L, Ashiya M, Buttle K, Oakes SA, Mannella CA, Korsmeyer SJ. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Devel Cell. 2002;2:55–67. doi: 10.1016/s1534-5807(01)00116-2. [DOI] [PubMed] [Google Scholar]
- 7.Sun MG, Williams J, Munoz-Pinedo C, Perkins GA, Brown JM, Ellisman MH, Green DR, Frey TG. Correlated three-dimensional light and electron microscopy reveals transformation of mitochondria during apoptosis. Nature Cell Biol. 2007;9:1057–1065. doi: 10.1038/ncb1630. [DOI] [PubMed] [Google Scholar]
- 8.Mannella CA. Structure and dynamics of the mitochondrial inner membrane cristae. Biochim Biophys Acta. 2006;1763:542–548. doi: 10.1016/j.bbamcr.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Mannella CA, Marko M, Penczek P, Barnard D, Frank J. The internal compartmentation of rat-liver mitochondria: tomographic study using the high-voltage transmission electron microscope. Microscopy Res Tech. 1994;27:278–283. doi: 10.1002/jemt.1070270403. [DOI] [PubMed] [Google Scholar]
- 10.Mannella CA, Buttle K, Marko M. Reconsidering mitochondrial structure: new views of an old organelle. Trends Biochem Sci. 1997;22:37–38. doi: 10.1016/s0968-0004(96)30050-9. [DOI] [PubMed] [Google Scholar]
- 11.Mannella CA, Pfeiffer DR, Bradshaw PC, Moraru I, Slepchenko B, Loew LM, Hsieh C, Buttle K, Marko M. Topology of the mitochondrial inner membrane: dynamics and bioenergetic implications. IUBMB Life. 2001;52:93–100. doi: 10.1080/15216540152845885. [DOI] [PubMed] [Google Scholar]
- 12.Perkins G, Renken C, Martone ME, Young SJ, Ellisman M, Frey T. Electron tomography of neuronal mitochondria: three-dimensional structure and organization of cristae and membrane contacts. J Struct Biol. 1997;119:260–272. doi: 10.1006/jsbi.1997.3885. [DOI] [PubMed] [Google Scholar]
- 13.Frey TG, Mannella CA. The internal structure of mitochondria. Trends Biochem Sci. 2000;25:319–324. doi: 10.1016/s0968-0004(00)01609-1. [DOI] [PubMed] [Google Scholar]
- 14.Mannella CA. Our changing views of mitochondria. J Bioenerget Biomembr. 2000;32:1–4. doi: 10.1023/a:1005562109678. [DOI] [PubMed] [Google Scholar]
- 15.Mannella CA. The relevance of mitochondrial membrane topology to mitochondrial function. Biochim Biophys Acta. 2006;1762:140–147. doi: 10.1016/j.bbadis.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Sesaki H, Southard SM, Yaffe MP, Jensen RE. Mgm1p a dynamin-related GTPase, is essential for fusion of the mitochondrial outer membrane. Molec Biol Cell. 2003;14:2342–2356. doi: 10.1091/mbc.E02-12-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong ED, Wagner JA, Scott SV, Okreglak V, Holewinske TJ, Cassidy-Stone A, Nunnari J. The intramitochondrial dynamin-related GTPase, Mgm1p, is a component of a protein complex that mediates mitochondrial fusion. J Cell Biol. 2003;160:303–311. doi: 10.1083/jcb.200209015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexander C, Votruba M, Pesch UEA, Thiselton DL, Mayer S, Moore A, Rodriguez M, Kellner U, Leo-Kottler B, Auburger G, et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominat optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26:211–215. doi: 10.1038/79944. [DOI] [PubMed] [Google Scholar]
- 19.Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, Belenguer P, Pelloquin L, Grosgeorge J, Turc-Carel C, Perret E, et al. Nuclear gene OPA1, encoding a mitochondrial dynanim-related protein, is mutated in dominant optic atrophy. Nat Genet. 2002;26:207–210. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- 20.Amutha B, Gordon DM, Gu Y, Pain D. A novel role of Mgm1p, a dynamin-related GTPase, in ATP synthase assembly and cristae formation/maintenance. Biochem J. 2004;381:19–23. doi: 10.1042/BJ20040566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meeusen S, DeVay R, Block J, Cassidy-Stone A, Wayson S, McCafferey JM, Nunnari J. Mitochondrial inner-membrane fusion and crista maintenance requires the dynamin-related GTPase Mgm1. Cell. 2006;127:383–395. doi: 10.1016/j.cell.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 22.Kanazawa T, Zappaterra MD, Hasegawa A, Wright AP, Newman-Smith ED, Buttle KF, McDonald K, Mannella CA, van der Bliek AM. The C. elegans Opa1 homologue EAT-3 is essential for resistance to free radicals. PLoS Biology. 2007 doi: 10.1371/journal.pgen.1000022. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griparic L, van der Welt NN, Orozco IJ, Peters PJ, van der Bliek AM. Loss of the intermembrane space protein Mgm1/OPA1 induces swelling and localized constrictions along the lengths of mitochondria. J Biol Chem. 2004;279:18792–18798. doi: 10.1074/jbc.M400920200. [DOI] [PubMed] [Google Scholar]
- 24.Ishihara N, Fujita Y, Oka T, Mihara K. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J. 2006;25:2966–2977. doi: 10.1038/sj.emboj.7601184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De Strooper B, Scorrano L. OPA1 Controls Apoptotic Cristae Remodeling Independently from Mitochondrial Fusion. Cell. 2006;126:177–189. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 26.Olichon A, Elachouri G, Baricault L, Delettre C, Belenguer P, Lenaers G. OPA1 alternate splicing uncouples an evolutionary conserved function in mitochondrial fusion from a vertebrate restricted function in apoptosis. Cell Death Differ. 2007;14:682–692. doi: 10.1038/sj.cdd.4402048. [DOI] [PubMed] [Google Scholar]
- 27.Paumard P, Vallier J, Coulary B, Schaeffer J, Soubannier V, Mueller DM, Brethes D, di Rago JP, Velours J. The ATP synthase is involved in generating mitochondrial cristae morphology. EMBO J. 2002;21:221–230. doi: 10.1093/emboj/21.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.John GB, Shang Y, Li L, Renken C, Mannella CA, Selker JML, Rangall L, Bennett MJ, Zha J. The mitochondrial inner membrane protein mitofilin controls cristae morphology. Molec Biol Cell. 2005;16:1543–1554. doi: 10.1091/mbc.E04-08-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minauro-Sanmiguel F, Wilkens S, Garcia JJ. Structure of dimeric mitochondrial ATP synthase: Novel F0 bridging features and the structural basis of mitochondrial cristae biogenesis. Proc Natl Acad (USA) 2005;102:12356–12358. doi: 10.1073/pnas.0503893102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dudkina N, Heinemeyer J, Keegstra W, Boekema EJ, Braun HP. Structure of dimeric ATP synthase from mitochondria: An angular association of monomers induces the strong curvature of the inner membrane. FEBS Lett. 2005;579:5769–5772. doi: 10.1016/j.febslet.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 31.Spierings D, McStay G, Saleh M, Bender C, Chipuk J, Maurer U, Green DR. Connected to death: The (unexpurgated) mitochondrial pathway of apoptosis. Science. 2005;310:66–67. doi: 10.1126/science.1117105. [DOI] [PubMed] [Google Scholar]
- 32.Pavlov EV, Priault M, Pietkiewicz D, Cheng EHY, Antonsson B, Manon S, Korsmeyer SJ, Mannella CA, Kinnally KW. A novel, high-conductance channel linked to apoptosis in mammalian cells and Bax expression in yeast. J Cell Biol. 2001;155:725–732. doi: 10.1083/jcb.200107057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lutter M, Fang M, Luo X, Nishijima M, Xie X, Wang X. Cardiolipin provides specificity for targeting of tBid to mitochondria. Nat Cell Biol. 2000;2:754–761. doi: 10.1038/35036395. [DOI] [PubMed] [Google Scholar]
- 34.Kim TH, Zhao Y, Ding WX, Shin JN, He X, Seo YW, Chen J, Rabinowich H, Amoscato AA, Yin XM. Bid-cardiolipin interaction at mitochondrial contact site contributes to mitochondrial cristae reorganization and cytochrome c release. Molec Biol Cell. 2004;15:3061–3072. doi: 10.1091/mbc.E03-12-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Epand RF, Martinou J-C, Fornallaz-Mulhauser M, Hughes DW, Epand RM. The apoptotic protein tBid promotes leakage by altering membrane curvature. J Biol Chem. 2002;277:32632–32639. doi: 10.1074/jbc.M202396200. [DOI] [PubMed] [Google Scholar]
- 36.Acehan D, Xu Y, Stokes DL, Schlame M. Comparison of lymphoblast mitochondria from normal subjects and patients with Barth syndrome using electron microscopic tomography. Lab Invest. 2007;87:40–48. doi: 10.1038/labinvest.3700480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalvez F, Pariselli F, Dupaigne P, Budihardjo I, Lutter M, Antonsson B, Diolez P, Manson S, Martinou JC, Goubern M, Wang X, Bernard S, Petit PX. tBid interaction with cardiolipin primarily orchestrates mitochondrial dysfunctions and subsequently activates Bax and Bak. Cell Death Different. 2005;12:614–626. doi: 10.1038/sj.cdd.4401571. [DOI] [PubMed] [Google Scholar]
- 38.Klingenberg M, Scherer B, Stengel-Rutkowski L, Buchholz M, Grebe K. Experimental demonstration of the reorienting (mobile) carrier mechanism exemplified by the mitochondrial adenine nucleotide translocator. In: Azzone GF, et al., editors. Mechanisms in Bioenergetics. Academic Press, Inc.; New York and London: 1973. pp. 257–284. [Google Scholar]
- 39.Youle RJ, Karbowski M. Mitochondrial fission in apoptosis. Nature Rev. 2005;6:657–663. doi: 10.1038/nrm1697. [DOI] [PubMed] [Google Scholar]
- 40.Arnoult D, Groder A, Lee YJ, Estaquier J, Blackstone C. Release of OPA1 during apoptosis participates in the rapid and complete release of cytochrome c and subsequent mitochondrial fragmentation. J Biol Chem. 2005;280:35742–35750. doi: 10.1074/jbc.M505970200. [DOI] [PubMed] [Google Scholar]
- 41.Deng Y, Marko M, Buttle K, Leith A, Mieczkowski M, Mannella CA. Cubic membrane structure in amoeba (Chaos carolinensis) mitochondria determined by electron microscopic tomography. J Struct Biol. 1999;127:231–239. doi: 10.1006/jsbi.1999.4147. [DOI] [PubMed] [Google Scholar]
- 42.Deng Y, Kohlwein S, Mannella CA. Fasting induces cyanide-resistant respiration and oxidative stress in the amoeba Chaos carolinensis: Implications for the cubic structural transition in mitochondrial membranes. Protoplasma. 2002;219:160–167. doi: 10.1007/s007090200017. [DOI] [PubMed] [Google Scholar]
- 43.Walker DW, Benzer S. Mitochondrial “swirls” induced by oxygen stress and in the Drosophila mutant hyperswirl. Proc Natl Acad Sci (USA) 2004;101:10290–10295. doi: 10.1073/pnas.0403767101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mannella CA, Buttle K, Walker DW, Benzer S. Microscopy and Microanalysis. suppl 2. Vol. 13. Microscopy Society of America; 2007. Electron tomographic studies of mitochondrial crista topology: “Swirl” mitochondria of Drosophila flight muscle; pp. 4–5. [Google Scholar]
- 45.Prince FP. Lamellar and tubular associations of the mitochondrial cristae: unique forms of the cristae present in steroid-producing cells. Mitochondrion. 2002;1:381–389. doi: 10.1016/s1567-7249(01)00038-1. [DOI] [PubMed] [Google Scholar]
- 46.Prince FP, Buttle KF. Mitochondrial structure in steroid-producing cells: three-dimensional reconstruction of human Leydig cell mitochondria by electron microscopic tomography. Anat Record, Part A. 2004;278:454–461. doi: 10.1002/ar.a.20019. [DOI] [PubMed] [Google Scholar]
- 47.Quinn PG, Payne AH. Steroid product-induced, oxygen-mediated damage of microsomal cytochrome P-450 enzymes in Leydig cell cultures. Relationship to desensitization. J Biol Chem. 1985;260:2092–2099. [PubMed] [Google Scholar]
- 48.Peltola V, Huhtaniemi I, Metsa-Ketela T, Ahotupa M. Induction of lipid peroxidation during steroidogenesis in the rat testis. Endocrinology. 1996;137:105–112. doi: 10.1210/endo.137.1.8536600. [DOI] [PubMed] [Google Scholar]
- 49.Sasaki S, Warita H, Abe K, Iwata M. Impairment of axonal transport in the axon hillock and the initial segment of anterior horn neurons in transgenic mice with a G93A mutatnt SOD1 gene. Acta Neuropathol. 2005;110:48–56. doi: 10.1007/s00401-005-1021-9. [DOI] [PubMed] [Google Scholar]


