Abstract
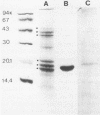
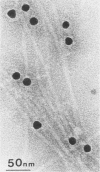
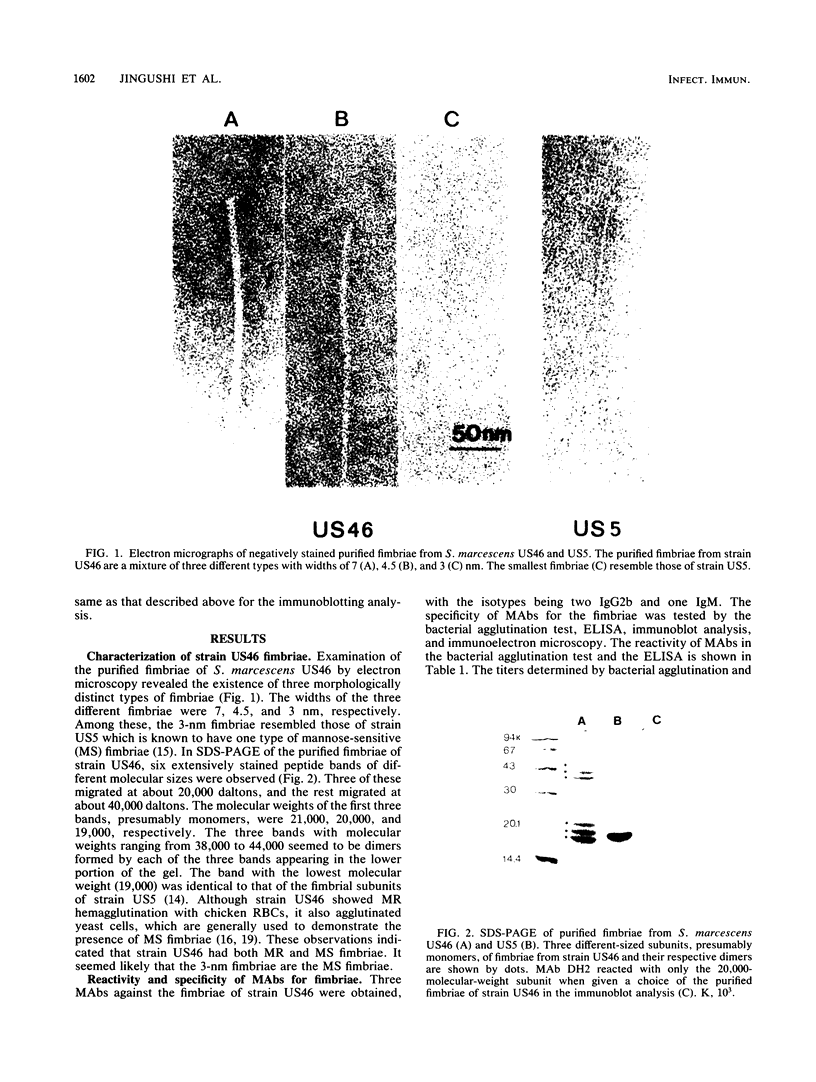
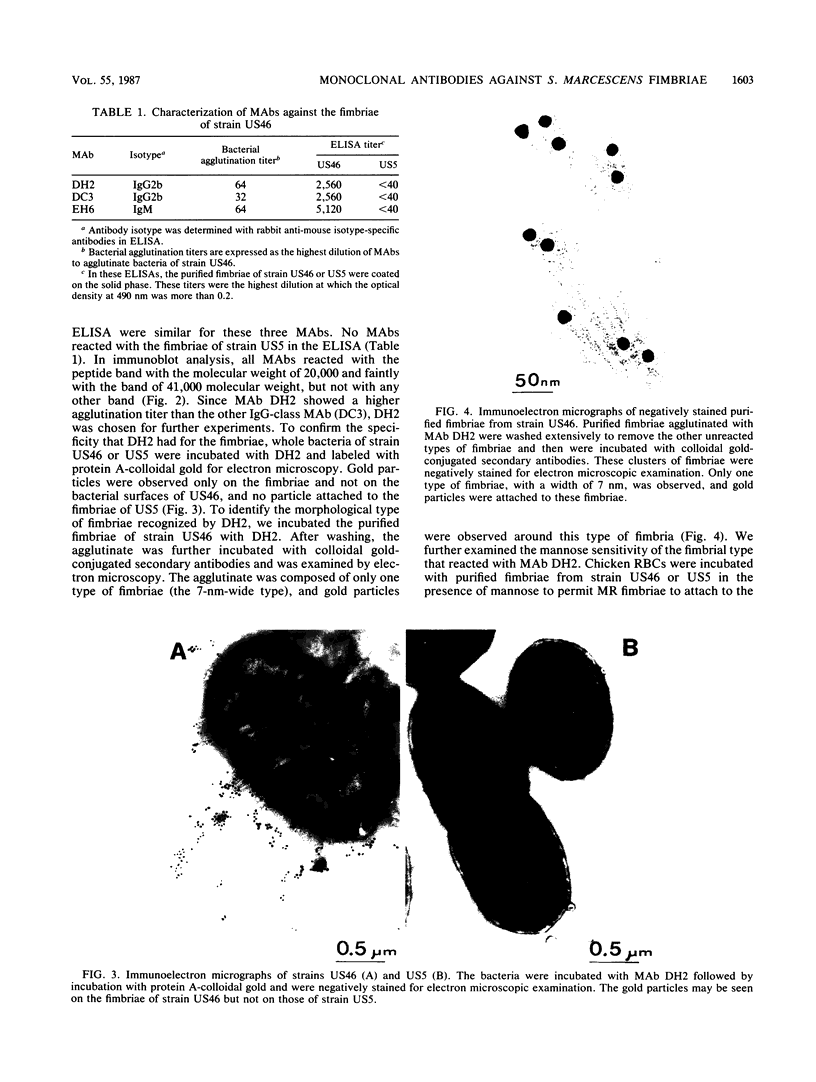
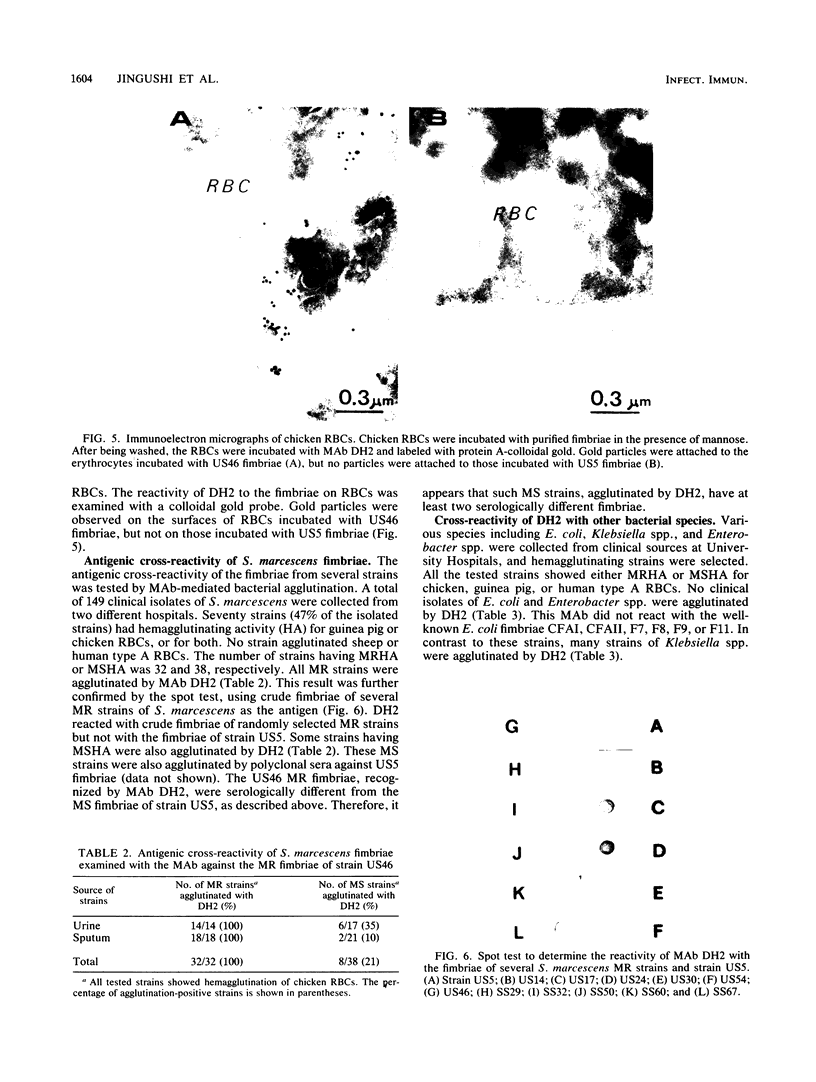
Monoclonal antibodies (MAbs) were raised against the purified fimbriae of Serratia marcescens US46, a strain expressing three morphologically distinct fimbriae. The widths of these fimbriae were 7, 4.5, and 3 nm, respectively. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the purified fimbriae showed three bands with molecular weights of 21,000, 20,000, and 19,000, respectively. This strain had mannose-resistant (MR) hemagglutinating activity and was agglutinated by yeast cells. Therefore, strain US46 appeared to have both MR and mannose-sensitive fimbriae. In the immunoblot analysis, all MAbs reacted with the 20,000-molecular-weight subunit when given a choice of three differently sized subunits. Immunoelectron microscopy showed these MAbs attached to the MR fimbriae with the largest width (7 nm). The antigenic cross-reactivity of fimbriae was examined by an MAb-mediated agglutination test. All MR strains of S. marcescens and some mannose-sensitive strains were agglutinated by the MAbs. The serological homogeneity of MR fimbriae was confirmed by a spot test, using the crude purified fimbriae from several MR strains of S. marcescens. In other gram-negative rods, clinical isolates of Klebsiella spp. with hemagglutinating activity were agglutinated, but clinical isolates of Escherichia coli and Enterobacter spp. were not.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adegbola R. A., Old D. C. New fimbrial hemagglutinin in Serratia species. Infect Immun. 1982 Oct;38(1):306–315. doi: 10.1128/iai.38.1.306-315.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. P., Clegg S., Wilson M. I. The fimbrial and non-fimbrial haemagglutinins of Escherichia coli. J Med Microbiol. 1979 May;12(2):213–227. doi: 10.1099/00222615-12-2-213. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Clegg S., Pauley J. A. Purification and characterization of the CFA/I antigen of enterotoxigenic Escherichia coli. Infect Immun. 1979 Aug;25(2):738–748. doi: 10.1128/iai.25.2.738-748.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Silver R. P., Evans D. J., Jr, Chase D. G., Gorbach S. L. Plasmid-controlled colonization factor associated with virulence in Esherichia coli enterotoxigenic for humans. Infect Immun. 1975 Sep;12(3):656–667. doi: 10.1128/iai.12.3.656-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., DuPont H. L. Hemagglutination patterns of enterotoxigenic and enteropathogenic Escherichia coli determined with human, bovine, chicken, and guinea pig erythrocytes in the presence and absence of mannose. Infect Immun. 1979 Feb;23(2):336–346. doi: 10.1128/iai.23.2.336-346.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Young L. S., Pitt J. Hemagglutination typing of Escherichia coli: definition of seven hemagglutination types. J Clin Microbiol. 1980 Aug;12(2):235–242. doi: 10.1128/jcm.12.2.235-242.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinée P. A., Jansen W. H. Behavior of Escherichia coli K antigens K88ab, K88ac, and K88ad in immunoelectrophoresis, double diffusion, and hemagglutination. Infect Immun. 1979 Mar;23(3):700–705. doi: 10.1128/iai.23.3.700-705.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagblom P., Segal E., Billyard E., So M. Intragenic recombination leads to pilus antigenic variation in Neisseria gonorrhoeae. Nature. 1985 May 9;315(6015):156–158. doi: 10.1038/315156a0. [DOI] [PubMed] [Google Scholar]
- Harn D. A., Mitsuyama M., David J. R. Schistosoma mansoni. Anti-egg monoclonal antibodies protect against cercarial challenge in vivo. J Exp Med. 1984 May 1;159(5):1371–1387. doi: 10.1084/jem.159.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Arita M., Miwatani T. Characterization of new hydrophobic pili of human enterotoxigenic Escherichia coli: a possible new colonization factor. Infect Immun. 1984 Mar;43(3):959–965. doi: 10.1128/iai.43.3.959-965.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P., Orskov I., Orskov F. F7 and type 1-like fimbriae from three Escherichia coli strains isolated from urinary tract infections: protein chemical and immunological aspects. Infect Immun. 1982 May;36(2):462–468. doi: 10.1128/iai.36.2.462-468.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno K., Yamamoto T., Kuroiwa A., Amako K. Purification and characterization of Serratia marcescens US5 pili. Infect Immun. 1984 Nov;46(2):295–300. doi: 10.1128/iai.46.2.295-300.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Black R. E., Brinton C. C., Jr, Clements M. L., Fusco P., Hughes T. P., O'Donnell S., Robins-Browne R., Wood S., Young C. R. Reactogenicity, immunogenicity and efficacy studies of Escherichia coli type 1 somatic pili parenteral vaccine in man. Scand J Infect Dis Suppl. 1982;33:83–95. [PubMed] [Google Scholar]
- Mirelman D., Altmann G., Eshdat Y. Screening of bacterial isolates for mannose-specific lectin activity by agglutination of yeasts. J Clin Microbiol. 1980 Apr;11(4):328–331. doi: 10.1128/jcm.11.4.328-331.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hanley P., Lark D., Falkow S., Schoolnik G. Molecular basis of Escherichia coli colonization of the upper urinary tract in BALB/c mice. Gal-Gal pili immunization prevents Escherichia coli pyelonephritis in the BALB/c mouse model of human pyelonephritis. J Clin Invest. 1985 Feb;75(2):347–360. doi: 10.1172/JCI111707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORSKOV I., ORSKOV F., SOJKA W. J., WITTIG W. K ANTIGENS K88AB(L) AND K88AC(L) IN E. COLI. A NEW O ANTIGEN: 0147 AND A NEW K ANTIGEN: K89(B). Acta Pathol Microbiol Scand. 1964;62:439–447. doi: 10.1111/apm.1964.62.3.439. [DOI] [PubMed] [Google Scholar]
- Olafson R. W., McCarthy P. J., Bhatti A. R., Dooley J. S., Heckels J. E., Trust T. J. Structural and antigenic analysis of meningococcal piliation. Infect Immun. 1985 May;48(2):336–342. doi: 10.1128/iai.48.2.336-342.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old D. C. Inhibition of the interaction between fimbrial haemagglutinins and erythrocytes by D-mannose and other carbohydrates. J Gen Microbiol. 1972 Jun;71(1):149–157. doi: 10.1099/00221287-71-1-149. [DOI] [PubMed] [Google Scholar]
- Orskov I., Orskov F. Episome-carried surface antigen K88 of Escherichia coli. I. Transmission of the determinant of the K88 antigen and influence on the transfer of chromosomal markers. J Bacteriol. 1966 Jan;91(1):69–75. doi: 10.1128/jb.91.1.69-75.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottow J. C. Ecology, physiology, and genetics of fimbriae and pili. Annu Rev Microbiol. 1975;29:79–108. doi: 10.1146/annurev.mi.29.100175.000455. [DOI] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Orci L. Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J Histochem Cytochem. 1978 Dec;26(12):1074–1081. doi: 10.1177/26.12.366014. [DOI] [PubMed] [Google Scholar]
- Rothbard J. B., Fernandez R., Schoolnik G. K. Strain-specific and common epitopes of gonococcal pili. J Exp Med. 1984 Jul 1;160(1):208–221. doi: 10.1084/jem.160.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter J. M., Jones G. W. Protection against enteric disease caused by Escherichia coli--a model for vaccination with a virulence determinant? Nature. 1973 Apr 20;242(5399):531–532. doi: 10.1038/242531a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virji M., Everson J. S., Lambden P. R. Effect of anti-pilus antisera on virulence of variants of Neisseria gonorrhoeae for cultured epithelial cells. J Gen Microbiol. 1982 May;128(5):1095–1100. doi: 10.1099/00221287-128-5-1095. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Ariyoshi A., Amako K. Fimbria-mediated adherence of Serratia marcescens strain US5 to human urinary bladder surface. Microbiol Immunol. 1985;29(7):677–681. doi: 10.1111/j.1348-0421.1985.tb00871.x. [DOI] [PubMed] [Google Scholar]
- Zak K., Diaz J. L., Jackson D., Heckels J. E. Antigenic variation during infection with Neisseria gonorrhoeae: detection of antibodies to surface proteins in sera of patients with gonorrhea. J Infect Dis. 1984 Feb;149(2):166–174. doi: 10.1093/infdis/149.2.166. [DOI] [PubMed] [Google Scholar]
- de Ree J. M., Schwillens P., van den Bosch J. F. Monoclonal antibodies that recognize the P fimbriae F71, F72, F9, and F11 from uropathogenic Escherichia coli. Infect Immun. 1985 Dec;50(3):900–904. doi: 10.1128/iai.50.3.900-904.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]