Abstract
Purpose
The inhibitor of apoptosis proteins (IAPs) are overexpressed in hormone-refractory prostate cancer, rendering the cancer cells resistant to radiation. This study aims to investigate the radiosensitizing effect of small molecule IAP-inhibitor both in vitro and in vivo in androgen-independent prostate cancer, and the possible mechanism of radiosensitization.
Experimental Design
Radiosensitization of SH-130 in human prostate cancer DU-145 cells was determined by clonogenic survival assay. Combination effect of SH-130 and ionizing radiation was evaluated by apoptosis assays. Pull-down and immunoprecipitation assay were employed to investigate the interaction between SH-130 and IAPs. DU-145 xenografts in nude mice were treated with SH-130, radiation or in combination, and tumor suppression effect was determined by caliper measurement or bioluminescence imaging. NF-κB activation was detected by luciferase reporter assay and quantitative real-time PCR.
Results
SH-130 potently enhanced radiation-induced caspase activation and apoptosis in DU-145 cells. Both XIAP and cIAP-1 can be pulled down by SH-130, but not inactive SH-123. Moreover, SH-130 interrupted interaction between XIAP/cIAP-1 and Smac. In a nude mouse xenograft model, SH-130 potently sensitized the DU-145 tumors to X-ray radiation without increasing systemic toxicity. The combination therapy suppressed tumor growth more significantly than either treatment alone, with over 80% of complete tumor regression. Furthermore, SH-130 partially blocked TNFα- and radiation-induced NF-κB activation in DU-145 cells.
Conclusions
Our results demonstrate that small-molecule inhibitors of IAPs can overcome apoptosis-resistance and radiosensitize human prostate cancer with high levels of IAPs. Molecular modulation of IAPs may improve the outcome of prostate cancer radiotherapy.
Introduction
Androgen-independent (AI) disease is the main obstacle to improved survival and quality of life in patients with advanced prostate cancer. There is an urgent need for novel therapeutic strategies to overcome radioresistance in the treatment of advanced prostate cancer by specifically targeting the fundamental molecular basis of androgen-independent prostate cancer.
Most of the current anticancer therapies work, at least in part, through inducing apoptosis in cancer cells, including ionizing irradiation (1). Lack of appropriate apoptosis due to defects in the normal apoptosis machinery plays a crucial role in the resistance of cancer cells to a wide variety of current anticancer therapies. Radioresistance markedly impairs the efficacy of cancer radiotherapy and involves anti-apoptotic signal transduction pathways that prevent radiation-induced cell death (2). The aggressive cancer cell phenotype is the result of a variety of genetic and epigenetic alterations leading to deregulation of intracellular signaling pathways, including an impaired ability of the cancer cell to undergo apoptosis (3). Primary or acquired resistance of hormone-refractory prostate cancer to current treatment protocols has been associated with apoptosis-resistance in cancer cells and is linked to therapy failures (4, 5).
Current and future efforts toward designing new therapies to improve survival rates and quality of life for cancer patients will include strategies that specifically target cancer cell resistance to apoptosis. The inhibitors of apoptosis proteins (IAPs) is an important class of intrinsic cellular apoptosis inhibitors (6, 7). IAPs potently suppress apoptosis against a large variety of apoptotic stimuli, including chemotherapeutics, radiation, and immunotherapy in cancer cells (8). The IAPs function as potent endogenous apoptosis inhibitors by directly binding to and effectively inhibiting three members of the caspase family of enzymes: two effector caspases (-3 and -7) and one initiator caspase-9 (9). The X-linked IAP protein (XIAP) is perhaps the best characterized IAP member due to its potent activity (10). XIAP effectively inhibits both intrinsic and extrinsic apoptosis pathways by binding and inhibiting both initiator and effector caspases, whose activity is crucial for the execution of apoptosis (7, 11). Because effector caspase activity is both necessary and sufficient for irrevocable programmed cell death, XIAP functions as a gatekeeper to this final stage of the process. XIAP is widely expressed in cancer cell lines and tumor tissues and a high level of XIAP makes cancer cells apoptosis-resistant to a wide variety of therapeutic agents (12). cIAP-1/-2 also inhibits both caspase-3 and caspase-7, although not as powerfully as XIAP (13).
Most components of the major cell death regulatory pathways have been implicated in radiation-induced cell death (14). It has been well established that IAPs, which are highly expressed in many types of cancer, including prostate cancer, appear to play a pivotal role in resistance to apoptosis induced by cancer therapy. Accumulating evidences demonstrate that XIAP and cIAP-1, two IAP members that are mostly studied for anti-apoptosis and cell survival signaling, play a crucial role in chemo- or radioresistance (7). Specifically, radiation triggers apoptosis mediated by mitochondria, resulting in the release of mitochondrial proteins into cytoplasm, including Smac (15). The released Smac binds to XIAP and other IAP proteins and abolishes their anti-apoptotic function. Because IAPs block apoptosis at the downstream effector phase, a point where multiple apoptosis signaling pathways converge, strategies targeting IAPs may prove to be highly effective in overcoming apoptosis-resistance of human prostate cancer overexpressing IAPs. This link between radiation resistance and IAPs is supported by recent studies in which the suppression of XIAP levels by RNA interference or antisense indeed sensitized XIAP-overexpressing cancer cells to death receptor-induced apoptosis as well as radiation (16, 17).
Smac-based peptide inhibitors effectively overcome apoptosis-resistance in different types of cancer cells with high levels of IAP and sensitize cancer cells to current therapeutic agents in vitro and in vivo, providing an important proof-of-concept for molecular therapy targeting IAPs (15, 18). Recently, we have designed Smac peptido-mimetics and non-peptidic mimetics (Smac-mimetics) based upon Smac peptide and the high-resolution experimental 3D structures of Smac in complex with the XIAP BIR3 domain, called SH-compounds (19, 20). These Smac mimetics are cell-permeable and have a much higher binding affinity to XIAP than the natural Smac peptide. Our preliminary results with newer and more potent Smac-mimetic IAP-inhibitors show a 1-20 nM binding affinity to XIAP BIR3 as well as potent cellular activity. These studies provide us with a solid foundation to determine if the IAPs are valid molecular targets for radiosensitization of human prostate cancer with IAP overexpression.
In this study, we evaluated the radiosensitizing effects of one of the Smac-mimetic IAP-inhibitors, SH-130, in human prostate cancer cells with high levels of IAPs. We hypothesized that a Smac-mimetic targeting the IAP family of proteins would be highly effective in overcoming prostate cancer resistance to radiation therapy. We also investigated the potential molecular targets and mechanisms involved in the SH-130-mediated radiosensitization.
Materials and Methods
Reagents
Smac-mimetic compound SH-130, its negative control compound SH-123, and their biotin-labeled derivatives were dissolved in dimethyl sulfoxide (DMSO), stored as small aliquots at −20°C, and then diluted as needed in a cell culture medium. Recombinant human tumor necrosis factor alpha (TNFα) was obtained from Roche Applied Science (Indianapolis, IN). MG132 was provided by BIOMOL (Plymouth Meeting, PA). High glucose DMEM, fetal bovine serum (FBS), penicillin, streptomycin, and LipofectAMINE 2000 were purchased from Invitrogen (Grand Island, NY). Pan-caspase inhibitor zVAD, Caspase 3 substrate DEVD-AFC, and anti-Smac polyclonal antibody were purchased from Biovision (Mountain View, CA). Crystal violet and β-actin antibody were purchased from Sigma-Aldrich (St. Louis, MO). Antibodies against poly(ADP-ribose) polymerase (PARP), pro-Caspase 3, p65/RelA, IκBα, and β-tubulin were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-XIAP and cIAP-1/-2 antibodies were purchased from BD Biosciences (San Jose, CA). Survivin antibody was supplied by Novus Biologicals (Littleton, CO).
Cell culture
Human prostate cancer cell lines PC-3 and DU-145, and human normal fibroblast cell line WI-38 were purchased from American Type Culture Collection (Manassas, VA). Luciferase stably transfected DU-145 cells (DU-145Lux) were established as described previously (21). All types of cell lines were routinely cultured in high glucose DMEM supplemented with 10% FBS in a 5% CO2 humidified incubator at 37°C. All media were also supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin. Human normal prostate epithelial cell line PrEC was purchased from Lonza (Baltimore, MD) and was maintained following the manufacturer’s instruction.
Clonogenic cell survival assay
DU-145 cells were seeded in 6-well plates at different cell densities (100∼10,000 cells/well) and treated with SH-130 or SH-123 and X-ray radiation individually or in combination. Solvent DMSO was used as a vehicle control. Each sample was tested in triplicate, and the cell medium was replaced 7 days later. After another 5-7 days’ incubation the plates were gently washed with PBS and stained with a 0.1% crystal violet solution. Colonies with over 50 cells were manually counted. The cell survival enhancement ratio was calculated as we previously described (22).
Western blot analysis
To determine the levels of protein expression in prostate cancer cell lines, cells were harvested and lysed in a RIPA lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.1% SDS, 1% NP-40, 0.25% Sodium deoxycholate and 1 mM EDTA) with freshly added protease inhibitor cocktail (Roche) for 15 min on ice, then centrifuged at 13,000 rpm for 10 min. Whole cell extract was measured for total protein concentration using Bradford reagent (Bio-Rad, Hercules, CA), and proteins were resolved by SDS-PAGE (Bio-Rad). For non-reducing and non-denaturing SDS-PAGE, samples were mixed with loading buffer (no reducing agent) and directly loaded on a gel without boiling. After electrophoresis, the proteins were electrotransferred to nitrocellulose membranes (Bio-Rad), probed with the relevant primary antibody followed by HRP (horseradish peroxidase) conjugated secondary antibody (Pierce), and detected with the SuperSignal West Pico chemiluminescence substrate (Pierce). Intensity of the desired bands was analyzed using TotalLab software (Nonlinear Dynamics, Durham, NC).
Apoptosis assay
To detect apoptosis, we used an Annexin V-FITC and propidium iodide (PI) double staining kit (Trevigen, Gaithersburg, MD). In brief, DU-145 cells were seeded into 6-well plates and treated with SH-130 or negative control SH-123 with or without pretreatment with the pan-Caspase inhibitor zVAD (Biovision). X-ray irradiation was performed immediately after drug addition. Twenty-four hours after radiation the cells were collected, gently washed twice with cold PBS, stained with Annexin V and propidium iodide (PI) as per manufacturer’s instruction, and analyzed with a flow cytometer (FACSCalibur; BD Biosciences) in the Flow Cytometry Core at the University of Michigan Cancer Center. Other aliquoted samples were processed for Caspase function and Western blot analysis.
Caspase-3 function assay
Caspase activation of treated DU-145 cells was determined following the instructions of a Caspase-3 detection kit (BioVision). Cells were lysed in a lysis buffer, and whole cell lysates (20 μg) were incubated with 25 μM fluorogenic substrate DEVD-AFC in a reaction buffer (containing 5 mM DTT) at 37°C for 2 h. Proteolytic release of AFC was monitored at λex = 405 nm and λem = 500 nm using a microplate reader (BMG LABTECH, Durham, NC). Fold increase of the fluorescence signal was calculated for each treated sample by dividing its normalized signal activity by that of the untreated control.
Pull-down and immunoprecipitation assay
DU-145 cells were washed with ice-cold PBS, harvested and resuspended in a lysis buffer [50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, and protease inhibitor tablet (Roche)). For pull-down assay, after preclearing the streptavidin agarose gel (Invitrogen) at 4 °C for 2 h, whole cell lysate (1 mg total proteins) was incubated with biotin-labeled SH-130 (20 μM) with or without the addition of an unlabeled SH-130 competitor (200 μM). DMSO was used as a solvent control. After rotation at 4 °C for 3 h, the lysate was incubated overnight with the gel (50 μl) at 4 °C. For immunoprecipitation (IP) assay, the lysate (1 mg total proteins) was incubated with 1 μM and 10 μM SH-130, or 10 μM SH-123, at 4 °C for 1 h. Ten microliter of antibody was then added, and the sample incubated for 1 h before an overnight incubation with 50 μl Protein A/G-Agarose gel (Invitrogen). The immunoprecipitates were washed 5 times with desired wash buffer (Roche), eluted with SDS-PAGE sample buffer and analyzed by Western blot.
Animal studies
The general procedure for in vivo experiments was described previously (22). Briefly, female athymic NCr-nu/nu nude mice (5 to 6 weeks old) were inoculated subcutaneously (s.c) on both sides of the lower back above the tail with 3×106 /0.2 ml of DU-145 cells. When tumors reached 50∼100 mm3, the mice were randomized into 4 groups (8 mice per group) and treated with either SH-130 intravenously (i.v) injected with 50 mg/kg for q.d. × 5 × 2 weeks, or X-ray irradiation with 2 Gy daily for q.d. × 5 × 2 weeks, or a combination of SH-130 and radiation. The vehicle control and radiation-only groups received the same amount of DMSO solvent. Tumor size and body weight were measured twice a week. Tumor volume was calculated using the formula V= a× b2/2, where a and b represented both the long and vertical short diameter of the tumor. For in vivo imaging experiments, mice were transplanted s.c with 5×106 cells/0.2 ml of DU-145Lux cells. Treatment started (Day 0) when the average tumor volume reached 50 mm3 using the following regimen: SH-130 50 mg/kg i.v. q.o.d. × 3 weeks, or X-ray irradiation 2 Gy, q.d. × 5 × 3 weeks, or a combination of SH-130 and radiation. On Days 0, 8, 17, 24, 41, and 120, mice were imaged 12 min after intraperitoneal (i.p) injection of luciferin using the Xenogen Bio-Luminescence Imaging System (Cranbury, NJ) in the University of Michigan Small Animal Imaging Core. Light intensity was quantified using LivingImage™ software on a red (high intensity/cell number) to blue (low intensity/cell number) visual scale. All animal experiments were done according to protocols approved by the University of Michigan Committee for Use and Care of Animals.
NF-κB activity assay
Two independent assays were performed to determine the effect of SH-130 on TNFα-induced NF-κB activation, as shown below.
NF-κB reporter assay
DU-145 cells were seeded into a 24-well plate 24 h before transfection. For each well, cells were transiently co-transfected with 0.4 μg of an NF-κB reporter construct (pNF-κB) (Panomics, Redwood City, CA) or a control reporter plasmid (pControl) (Panomics), together with 0.2 μg of a β-galactosidase reporter vector (Promega, Madison, WI), which was used to normalize NF-κB reporter gene activity, using Lipofectamine 2000 (Invitrogen). Twenty-four hours after transfection, cells were pre-treated with SH-130 with or without the caspase inhibitor zVAD or SH-123 for 1 h, followed by TNFα stimulation for 4 h. The proteasome inhibitor MG132 was used as a positive control. Cell lysates were prepared using Reporter Lysis Buffer (Promega). For luciferase and β-galactosidase assays, the samples were measured on a microplate luminometer (BMG), using the Bright-Glo luciferase kit (Promega) and β-galactosidase enzyme assay kit (Promega) respectively, according to the manufacturer’s instructions. Fold increase was calculated for each treated sample by dividing normalized luciferase activity by that of the untreated control.
Quantitive real-time PCR (qRT-PCR)
Quantitive PCR was performed to determine the TNF gene’s expression level as an NF-κB target gene (23). Total RNA was extracted from DU-145 cells using TRIZOL reagent (Invitrogen) according to the manufacturer’s instructions. Reverse transcription reaction with 1 μg of total RNA in 100 μl was done following the instructions of the TaqMan Reverse Transcription Kit (Applied BioSystems). For quantitative PCR reactions, FAM probe TNF primer (Applied BioSystems) was used for detection of target gene expression, and SYBR Green-labeled actin (Applied BioSystems) was used as an internal control, with the primers designed as: forward, 5′ATGCAGAAGGAGATCACTGC3′; reverse, 5′TCATAGTCCGCCTAGAAGCA3′. All reactions with TaqMan PCR Master Mix (Applied BioSystems) were performed on the Mastercycler realplex system (Eppendorf, Westbury, NY). Fold increase of gene expression was calculated for each treated sample by dividing normalized TNF expression activity with that from the untreated control.
Statistical analysis
Two-tailed Student’s t test and two-way ANOVA were employed to analyze the in vitro and in vivo data respectively, using Prism 5.0 software (GraphPad Prism, San Diego, CA). A threshold of P < 0.05 was defined as statistically significant.
Results
Synergistic induction of radiation-induced cancer cell growth inhibition by the small molecule IAP-inhibitor
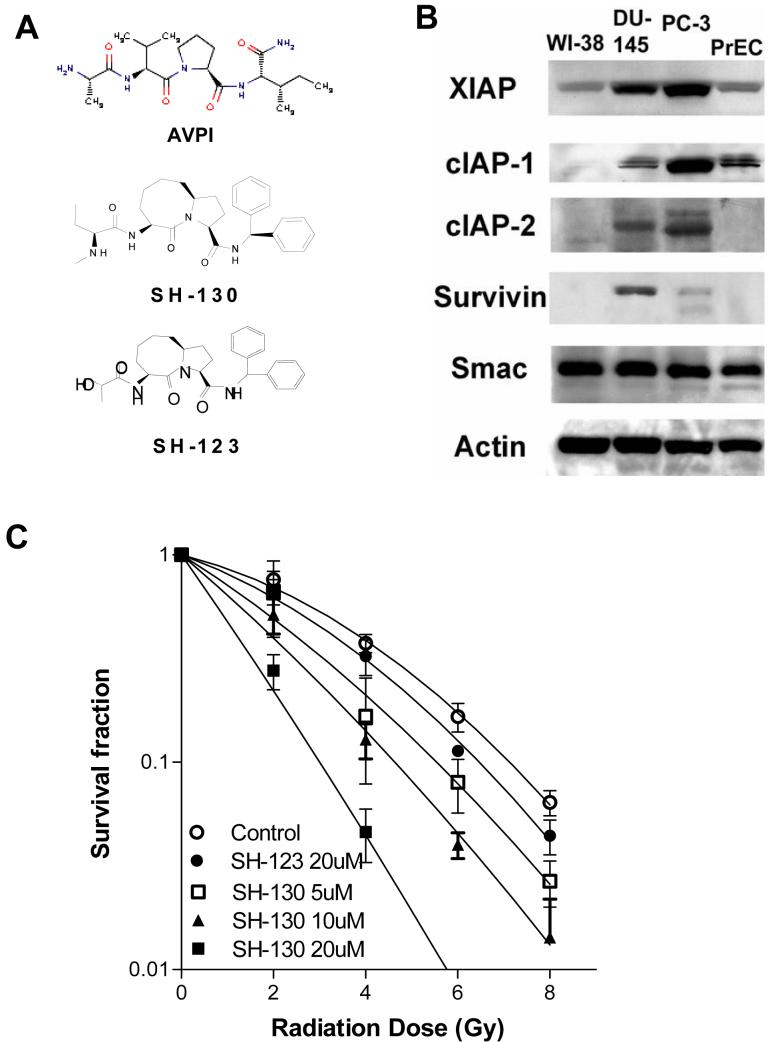
As previously reported (19, 20), a series of compounds were designed to mimic AVPI, the critical N-terminal tetrapeptide on the active Smac protein (Fig. 1A). SH-130, a lead compound of these Smac-mimetics, was found to be 20-30 times more potent in binding to XIAP than the cell-permeable Smac peptide pSMAC-8c, a positive control for serial SH-compounds used previously (Fig. 1A). Another analogue, SH-123, which was almost 1,000 times less active than SH-130, was used as an inactive control (Fig. 1A). Human prostate cancer DU-145 and PC-3 cell lines have medium to high levels of most of IAPs, while the normal human fibroblast cell line WI-38 and normal human prostate epithelial cells PrEC show low levels of IAPs. (Fig. 1B). To determine whether the Smac-mimetic compound could potentiate radiation-induced inhibition of cancer cell growth, we examined the radiosensitization of SH-130. SH-130 promoted radiation-induced clonogenic cell death dose-dependently, while SH-123 exhibited much less effect even at a high dose (Fig.1C). Radiobiological parameters were calculated and summarized in Table 1. The radiosensitizing activity of SH-130 was not observed in normal cells PrEC and WI-38 that have low IAPs (data not shown). We have also carried out MTT-based cytotoxicity assay (22) and SH-130 alone showed IC50 > 100uM in prostate cancer DU-145 and PC-3 cells as well as WI-38 cells, indicating that SH-130 is not a cytotoxic compound, consistent with other small molecule Smac-mimetics we reported earlier (19, 20).
Fig. 1.
,A, structure of the Smac-mimetic compound SH-130 and an inactive control compound, SH-123. B, Western blot analysis of the expression of IAPs in human prostate cancer cells DU-145 and PC3, with normal prostate epithelial cells PrEC and human fibroblast WI-38. C, SH-130-mediated radiosensitization of DU-145 cells by clonogenic survival assay. Cells seeded in 6-well plates were treated with 5, 10, or 20 μM of SH-130 or 20 μM of SH-123, respectively, followed by 2, 4, 6, or 8 Gy X-ray radiation within one hour. Each sample was tested in triplicate. After 14 days’ incubation, colonies were stained by crystal violet and counted manually. Data were normalized and expressed as the mean ± SD (n=3), and represented one of three independent experiments.
Table 1.
Radiobiological parameters from surviving curves
| MID | ER | SF (2Gy) | Gy (1%) | |
|---|---|---|---|---|
| Control | 3.66 | 0.70 | 10.83 | |
| SH-123 (20 μM) |
2.96 | 1.24 | 0.57 | 10.49 |
| SH-130 (5 μM) |
2.53 | 1.45 | 0.49 | 9.53 |
| SH-130 (10 μM) |
2.05 | 1.79 | 0.40 | 8.42 |
| SH-130 (20 μM) |
1.28 | 2.86 | 0.21 | 5.63 |
NOTE: Radiobiological parameters were calculated from survival curves in Fig. 1C based on linear-quadratic model. MID, mean inactivation dose; ER, enhancement ratio, calculated from MID in control group divided by that in treated group; SF (2 Gy), survival fraction at 2 Gy; Gy (1%), radiation dose leading to 1% cell survival.
Enhancement of radiation-induced apoptosis by inhibition of IAPs
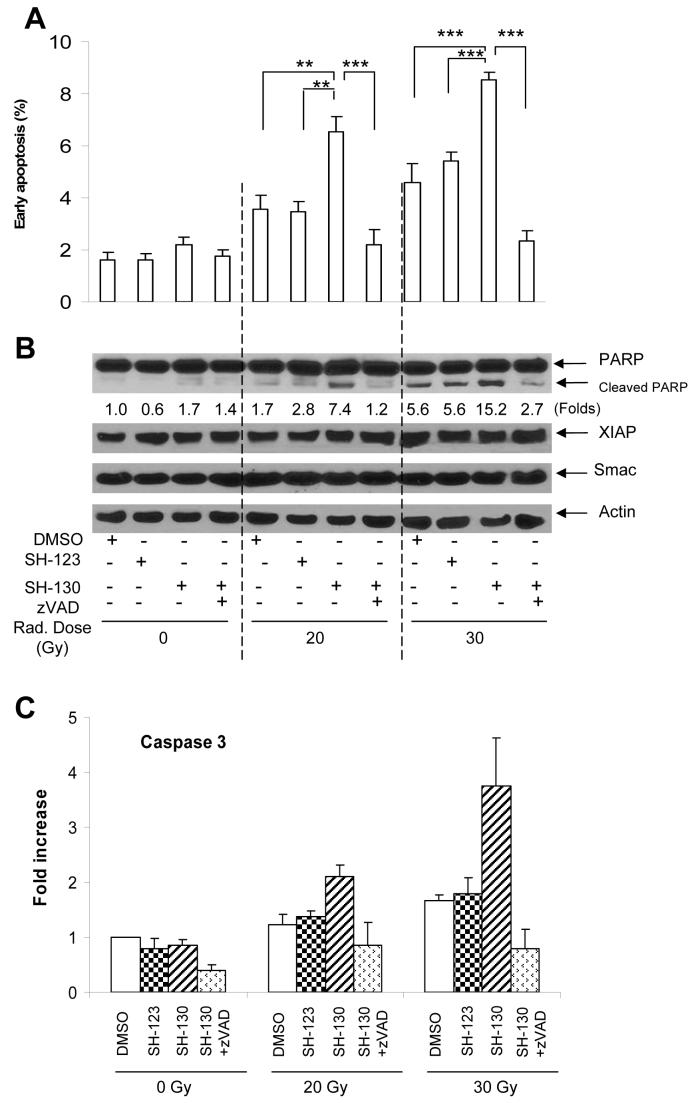
Preliminary data indicated that in cell-free systems SH-130, but not SH-123, abolished the inhibitory action of IAPs on both caspase-9 and caspase-3 in a dose- and time-dependent manner, showing a potent effect on competing IAPs and thus activating caspases. To provide further evidence for the role of SH-130 in mediating radiation-induced apoptosis, DU-145 cells were treated with SH-130 or SH-123 with or without X-ray radiation. As shown in Fig. 2A, induction of early apoptotic events (Annexin V positive) became evident when cells were treated with either 20 Gy or 30 Gy together with 10 μM of SH-130. A 4∼5-fold increase was seen compared with the untreated control, and a 2-fold increase over radiation alone (P < 0.01). Pretreatment with the pan-caspase inhibitor zVAD abolished this increase in radiation-induced early apoptosis by SH-130 (P < 0.001, Fig. 2A), indicating that SH-130-mediated radiosensitization is caspase—dependent. In contrast, neither SH-130 nor SH-123 alone induced significant apoptosis, suggesting a non-toxic profile of these compounds. We also evaluated total (early plus late) apoptotic events and obtained similar results (Fig. S1). Furthermore, in the cells treated with radiation plus SH-130, a clear PARP cleavage was observed (Fig. 2B), as compared to the cells treated with radiation alone. Since PARP is one of the substrates of caspase-3, our functional assay also showed similar profile on caspase-3 activation (Fig. 2C). Here again, zVAD efficiently blocked the SH-130 effect on caspase-3 activation and PARP cleavage. For all the samples tested (Fig. 2B), the expression level of XIAP and Smac did not change significantly, indicating that SH-130 acts on the apoptosis pathway, not by inhibiting the IAPs’ expression.
Fig. 2.
SH-130 enhances radiation-induced apoptosis. DU-145 cells were seeded into 6-well plates at the concentration of 2×105/ml, pre-treated with zVAD (2.5 μM) for 1 h, incubated with 10 μM of SH-130 or SH-123, and irradiated at doses of 0, 20 or 30 Gy, respectively. Twenty-four hours after incubation, cells were harvested and processed for further detection. Data represented one of three independent experiments. A, early apoptotic cell populations after treatment. DU-145 cells were stained with Annexin V-FITC and PI (Trevigen) and determined by flow cytometry. Data represented the mean ± SD (n=3), and were analyzed by Student’s t test. ** P<0.01, *** P<0.001. B, Western blot analysis of apoptosis-related proteins. Samples were probed with antibodies against PARP, caspase 3, XIAP, and Smac. Actin is shown as a loading control. Band intensity of cleaved PARP was normalized to Actin. C, caspase 3 activation after treatment. Whole cell lysates (20 μg) were reacted with fluorogenic substrate DEVD-AFC. After incubation at 37 °C for 2 h, released AFC was monitored by a microplate reader (BMG LABTECH). Data were shown as mean ± SD (n=3). Fold increase of fluorescence signal was expressed by normalizing activity to untreated control.
Blockade of XIAP/ cIAP-1 and Smac interaction by the IAP-inhibitor
To further examine whether the small molecule IAP-inhibitor, SH-130, binds to the IAP family of proteins in cells as designed, we carried out IAP pull-down assays using biotin-labeled compounds. SH-130 was labeled with biotin (SH-130BL) via chemical synthesis. A fluorescence polarization-based binding assay confirmed that SH-130BL had the same binding affinity to XIAP as unlabeled SH-130 (unpublished data). SH-130BL successfully pulled down both XIAP and cIAP-1 in DU-145 cells (Fig. 3A). Competition with unlabeled SH-130 effectively blocked the pull-down of both XIAP and cIAP-1 by SH-130BL. Furthermore, we conducted immunoprecipitation (IP) assays to see if SH-130 interfered with XIAP (or cIAP-1) and Smac interaction. Interestingly, under non-reducing and nondenaturing conditions, XIAP and Smac formed a complex at molecular weight ranging from approximately 80 kD to 160 kD (Fig. 3B), consistent with the tetramer model (24). XIAP-Smac complex levels were decreased by the addition of SH-130, but not SH-123, with increasing levels of free XIAP and Smac (Fig. 3B). Furthermore, SH-130 dose-dependently interrupted interaction between Smac and XIAP or cIAP-1 (Fig. 3C). Interestingly, when mature Smac was pulled down together with XIAP, two bands always seemed to be visualized by Western blot (Fig. 3C). The upper band (∼28 kD) level showed the same alteration as the lower band (∼21 kD), which is thought to be the cleaved or activated Smac. Compared with the artificial transfection with tag-labeled system (25), our condition is more likely to mimic the native intracellular protein behavior. These data confirm that the IAP-inhibitors can bind to XIAP/cIAP-1, thus interrupting the interaction between endogenous XIAP/cIAP-1 and Smac in prostate cancer cells.
Fig. 3.
SH-130 interrupts interaction between XIAP/cIAP-1 and Smac. DU-145 cells were collected and disrupted in a lysis buffer. Pull-down or IP was done as described in Materials and Methods. Immunoprecipitates were analyzed by Western blot. Endogenous XIAP, cIAP-1, and Smac were visualized by relevant antibodies. A, biotin-labeled SH-130 (SH-130BL) was incubated with cell lysates with or without non-labeled SH-130, followed by incubation with precleared Streptavidin-Agarose beads. B, cell lysates were incubated with 1 μM and 10 μM of SH-130 or 10 μM of SH-123 for 1 h. Lysates were mixed with a sample buffer (Bio-Rad, without reducing agent) and directly analyzed by Western blot without boiling. Actin was used as a loading control. C, cell lysates from B were incubated with an anti-Smac or an anti-XIAP antibody, and mixed with Protein A (or Protein G)-Agarose beads. Eluents were analyzed by Western blot and probed with specified antibodies. IgG heavy chain (IgH) was probed as a control. PD, pull-down; IP, immunoprecipitation; IB, immunoblotting. * ns, non-specific. Data shown were one of three independent experiments.
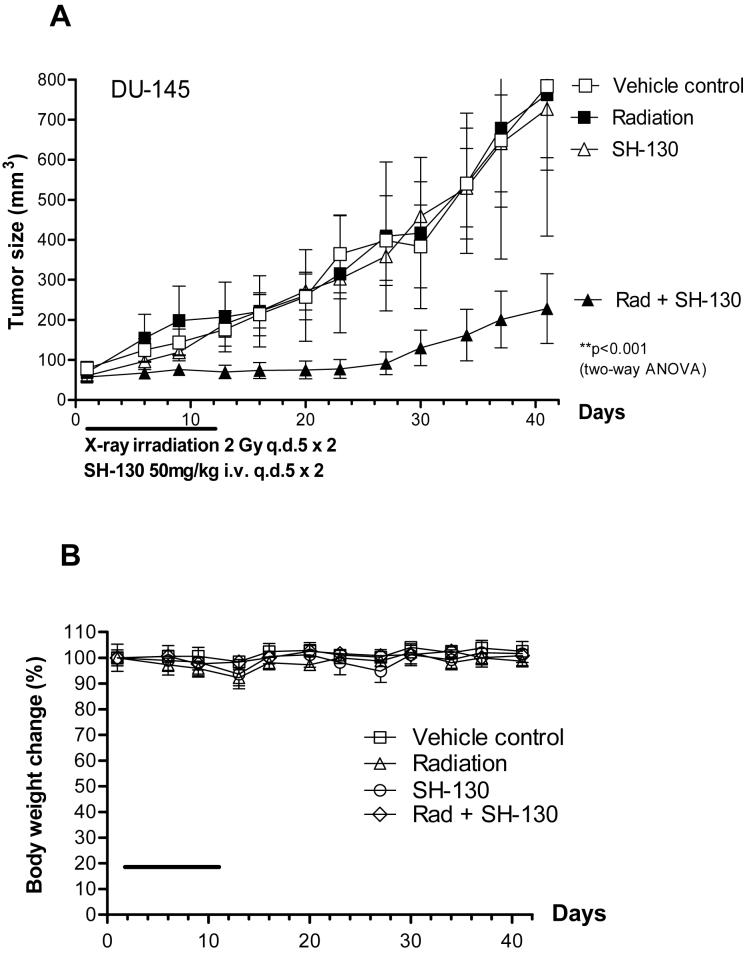
In vivo tumor suppression effect of the IAP-inhibitor with radiation
To evaluate the radiosensitization potential of SH-130 in vivo, a DU-145 tumor model was established as we previously described (4, 22). The DU-145 tumors were resistant to radiation, and SH-130 alone didn’t show tumor suppression effect (Fig. 4A). However, SH-130 synergistically sensitized the DU-145 tumors to radiation without showing systemic toxicity (Fig. 4B). The combination therapy inhibited tumor growth significantly more effectively than either treatment alone (P < 0.001, n=14), and no obvious animal toxicity was observed. Notably, 5 out of 14 tumors in the combination group showed complete regression that did not grow back 5 months after the therapy, whereas the radiation-alone group had only 2 out of 10 tumors with complete regression. No complete regression in the SH-130-alone or vehicle control groups was observed.
Fig. 4.
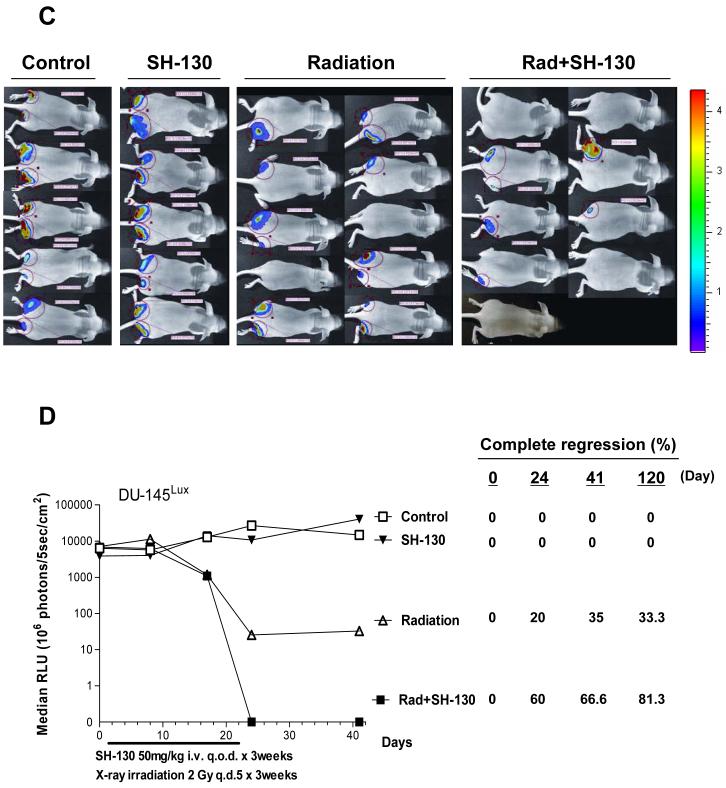
SH-130 potentiates DU-145 tumor regression induced by X-ray radiation, with caliper measurement (A and B) or bioluminescence imaging (BLI) (C and D). Nude mice bearing DU145 cells were treated with either SH-130, or X-ray irradiation, or a combination. Tumor size (A) and body weight (B) were measured twice a week, and curves were plotted up to Day 41. Data were shown as mean ± SE (n=16) for tumor volume (A), and mean ± SE (n=8) for body weight (B). C and D, SH-130 combined with radiation eradicated DU-145 tumors by BLI. Nude mice bearing DU-145Lux cells were treated as described in Materials and Methods. Imaging was carried out to visualize bioluminescent tumors on Days 0, 8, 17, 24, 41 and 120, respectively. C, BLI of DU-145Lux on Day 41. The light intensity of each photo was quantified to an optimized visual scale (from red to blue). D, summary of quantitative data of bioluminescence intensity. Absolute photon counts of tumors were measured and expressed as a median of relative light unit (RLU). Percentage of complete regression was calculated by dividing the number of eradicated tumors with total tumors. Rad, X-ray radiation.
In the second experiment we wished to extend the treatment period to a more clinically relevant three-week course of therapy. For this experiment, we used the DU-145Lux tumor model and employed Bio-Luminescence Imaging (BLI) to evaluate tumor response. SH-130 plus radiation led to complete tumor regression (undetectable signal in bioluminescence imaging in Fig. 4C). By quatification, the combination therapy resulted in a 2-log reduction in bioluminescence signal as compared with the radiation-alone group, and a 4-5-log reduction as compared with the vehicle control group (Fig. 4D). Bioluminescence imaging was also conducted on Days 0, 24, and 120 (Fig. S2), and percentage of complete tumor regression is summarized in Fig. 4D. The percentage of complete tumor regression in the radiation-alone group was highest (35%) on Day 41 and dropped to 33.3% on Day 120, indicating tumor recurrence over time. For the combination therapy group, the percentage of complete tumor regression continued to increase up to Day 120 (81.3%), indicating a potent and long-term efficacy of the combination treatment. Thus, two in vivo studies demonstrated a similar and promising in vivo radiosensitization efficacy of the IAP-inhibitor.
Suppression of NF-κB activation by inhibiting IAPs
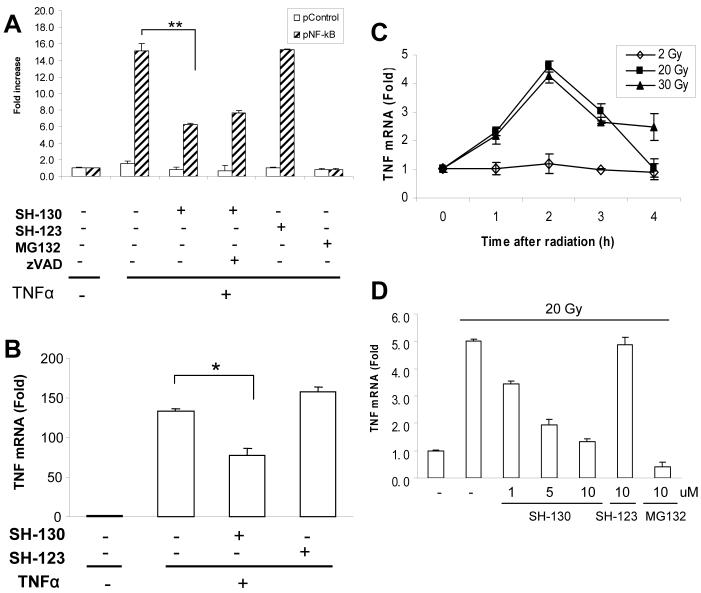
To test the hypothesis that SH-130 may also work on the NF-κB pathway, we analyzed the effects of SH-130 on NF-κB activation induced by TNFα and X-ray radiation in DU-145 cells. Using luciferase-based NF-κB reporter assay (26), SH-130 inhibited TNFα-induced NF-κB activation by more than 50% (P<0.01) and the inhibition could not be blocked by zVAD (Fig. 5A), indicating that the IAP-inhibitor-mediated NF-κB inhibition is caspases-independent. Moreover, SH-130 partially inhibited NF-κB activation, even at a high concentration, as compared with MG132 (Fig. 5A). SH-130 inhibited TNFα-induced IκBα degradation (Fig. S3), yet without a significant change in RelA nuclear translocation (data not shown), suggesting that SH-130-mediated inhibition of NF-κB activation is indirect. qRT-PCR assay further confirmed that TNF gene expression was downregulated 50% by SH-130 (P < 0.05), but not by negative control compound SH-123, after TNFα stimulation (Fig. 5B), indicating that SH-130 clearly although partially blocked NF-κB target gene expression.
Fig. 5.
SH-130 inhibits NF-κB activation induced by TNFα (A and B) and radiation (C and D). A, DU-145 cells were transiently co-transfected with pNF-κB or pControl (0.4 μg/well), together with β-galactosidase plasmid (0.2 μg/well). Cells were then pre-treated with SH-130 (20 μM) with or without zVAD (2.5 μM), or SH-123 (20 μM) for 1 h, followed by TNFα stimulation for 4 h. MG132 (10 μM) was used as a positive control. Luciferase and beta-galactosidase activities were measured as described in Materials and Methods. Fold increase was calculated as the means ± SD (n=2). Data shown are from one of three independent experiments. ** P < 0.01. B, cells were pre-treated with 20 μM of SH-130 or SH-123 for 1 h, before 10 min pulse stimulation of TNFα. Total RNA was extracted and quantitative PCR was performed as described in Materials and Methods. Fold increase was calculated by dividing the normalized TNF expression activity by that of the untreated control. Columns, mean; bars, SD (n = 3); * P < 0.05. C, cells were irradiated with the indicated doses of X-ray radiation. TNF mRNA expression level at desired time points was detected and normalized as described in B. D, cells were pre-treated with the indicated doses of compounds for 1 h and TNF mRNA expression level was determined 2 h after 20 Gy radiation. MG132, positive control.
Next we examined if SH-130 has similar suppression effect on X-ray radiation induced NF-κB target gene expression. Twenty Gray radiation was employed to trigger NF-κB activation as high radiation doses (20 Gy and 30 Gy) exhibited activation of TNF gene expression by 4∼5 folds more than low dose (2 Gy) (Fig. 5C). Similar to TNFα, radiation induced TNF gene expression could also be partially suppressed by SH-130 in a dose-dependent manner (Fig. 5D), as compared with its negative analog, SH-123. These data demonstrate that SH-130 can block both TNFα- and radiation-induced NF-κB activation in terms of inhibiting NF-κB target genes expression.
Discussion
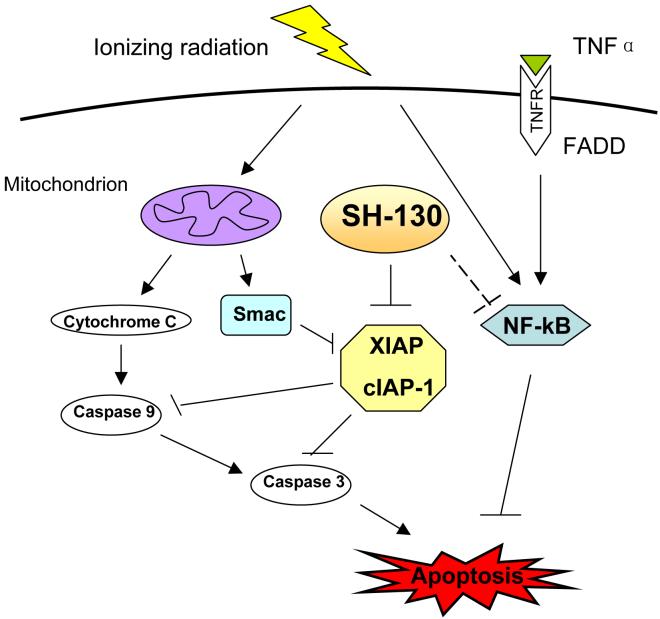
In this study, we have found that a small molecular IAP-inhibitor potently sensitizes prostate cancer DU-145 cells to X-ray irradiation and increases radiation-induced cell death and tumor growth delay, both in vitro and in vivo. Such effects may be due to activation of the apoptosis pathway. Moreover, the IAP-inhibitor SH-130 also blocked activation of the NF-κB partially and indirectly, suggesting a potential cross-talk between apoptosis pathway and NF-κB pathway (Fig. 6). The data confirmed our hypothesis that molecular modulation of IAP family proteins may improve the outcome of prostate cancer radiotherapy and represents a promising molecularly targeted therapy for hormone-refractory prostate cancer.
Fig. 6.
Working model of the Smac-mimetic IAP-inhibitors in radiosensitization. SH-130 enhances ionizing radiation-induced apoptosis via the intrinsic (mitochondrion) pathway, by inhibiting XIAP/cIAP-1 activity and blocking caspase activation. On the other hand, SH-130 blocks NF-κB activation induced by TNFα as well as ionizing radiation, indicating the suppression effect of radiation-mediated pro-survival signaling that attenuates apoptosis.
Small molecule inhibitors of IAPs are a promising choice for functionally blocking IAPs and overcoming chemo/radioresistance of cancer cells with high levels of IAPs. Our previous studies showed that the Smac-mimetic tetrapeptide pSmac-8c (used as the positive control) significantly sensitized both AI DU-145 and PC-3 cells to chemotherapeutic agents (19, 20), indicating that IAP is a valid target for overcoming chemoresistance in prostate cancer cells, and our current study supports that modulating IAPs is a promising approach on radiosensitization. Future work will focus on comparing androgen-dependent (AD) LNCaP and its AI derivative cell line CL-1, on their response to radiation and SH-130. Expected data on this isogenic cell model will further support that IAPs may play an essential role in the transition from AD to AI prostate cancer, and overcoming resistance of radiation-induced apoptosis can be achieved by down-regulating IAPs.
Our in vivo studies showed consistently that IAP-inhibition exhibits promising radiosensitivity in the DU-145 xenografted model, although IAP-inhibitor itself is non-toxic and shows no tumor suppression effect alone. Data from regular tumor volume measurement and BLI imaging are consistent in exhibiting a synergistic efficacy when SH-130 is used together with X-ray radiation. By BLI, mice in the combination group showed an increasing trend towards complete tumor regression that lasted up to 4 months after treatment, indicating the long-term tumor-suppressing efficacy of combination therapy over radiation alone. Using BLI for the efficacy study is critically important, especially when the tumor disappears as a complete tumor regression.
Conventional tumor measurement by hand will still detect a scar or nodule, but no live tumor cells are detectable, as the BLI will detect no light emission. On the other hand, some tumors may appear to have completely disappeared and will not be detectable by hand, but BLI can still detect light emission and reveal the presence of live tumor cells. Therefore, the more sensitive and quantitative BLI will yield robust and quantitative efficacy data, especially when evaluating the complete tumor regression in the current study.
Ionizing radiation activates the NF-κB signaling pathway, and blocking this pathway can sensitize cancer cells response to radiation (27, 28). Classically, cytokines such as TNFα trigger NF-κB activation, typically mediated by TNF receptor associated factor 2 (TRAF2). It has been demonstrated that TRAF2 physically interacts with cIAP-1, another IAP protein that is commonly studied along with XIAP (29). In addition, embelin, a natural IAP-inhibitor first reported by us (30), sequentially inhibits NF-κB activation at multiple levels (26). Moreover, XIAP could induce TAK1-dependent NF-κB activation (31). Thus it is reasonable to postulate that IAPs may function as “bridging” molecules mediating cross-talk between the apoptosis pathway and NF-κB pathway. Our data demonstrate that SH-130 indeed inhibits NF-κB activation, partially and indirectly, by TNFα and X-ray radiation. This partial effect of an IAP-inhibitor on NF-κB activation is consistent with an earlier report showing that partial NF-κB inhibition by another class of Smac-mimetics (32). Inhibition of IκBα degradation by SH-130 reflects an effect at the level of the IκBα kinase (IKK) complex, as demonstrated by embelin (26). Currently, we are delineating the detailed signaling pathways and molecules involved in the cross-talk, either via cIAP-1 or XIAP. This may have important implications for the rational design of novel therapies targeting IAPs and optimal recruitment of the patient population that will benefit the most from such therapy.
DU-145 cells are highly resistant to radiation, potentially due to high levels of IAPs as well as constitutive active NF-κB pathway (33). It is conceivable that the dose of radiation used might affect NF-κB activation as well as radiation-induced cell death, where the high dose and low dose may stimulate different cellular responses. In the clinic, the dose of radiation therapy on patients is 1.8-2 Gy daily fractionation with total doses of 20-70 Gy. Thus in the current study, we followed the clinical regimen with totaling 20 Gy and 30 Gy in two animal studies. Consistent with previous reports that a relative high dose (15-20 Gy) of X-ray radiation could induce NF-κB activation in human tumor cells in vitro (34, 35), we found that in our system, high dose radiation (20 and 30 Gy) effectively induced apoptosis as well as NF-κB activation, but not the low doses (2-4 Gy). Furthermore, RelA was clearly observed at doses > 15 Gy (data not shown). Taken together, the radiosensitization effect of Smac-mimetic SH-130 is due, at least in part, to the simultaneous inhibition of IAPs and the NF-κB survival signaling pathway, which is constitutively active in androgen-independent prostate cancer cells.
Very recently, three groups reported that several Smac-mimetic IAP-inhibitors can induce TNFα-dependent apoptosis in sensitive cell lines, via cIAP-1 down-regulation and NF-κB activation (36-38). However most of the androgen-independent prostate cancer cells are resistant to TNFα and have constitutive active NF-κB signaling (33). How these resistant cancer cells respond to IAP-inhibitors remains to be investigated. In our hands, those cells, including DU-145, PC-3 and CL-1, are highly resistant to our Smac-mimetic IAP-inhibitors such as SH-130, which has a IAP-binding affinity comparable to that of the compounds used in the three reports (36-38). To our knowledge, this is the first report that an IAP-inhibitor blocks radiation-induced NF-κB activation in these TNFα—resistant cells. Moreover, SH-130 treatment does not induce cIAP-1 down-regulation, TNFα upregulation and NF-κB activation in these resistant cells (Fig. S4). This mode of action is distinct from that in TNFα—sensitive cells reported recently (36-38). Our study has significant clinical implications and provides important impetus for utilizing IAP-inhibitors as an adjuvant therapy for the TNFα—resistant, NF-κB constitutively active cancers that account for the majority of patients who are refractory to conventional therapy.
In conclusion, our data provide strong support that modulating IAPs may be a promising novel approach to radiosensitization of human prostate cancer, especially among hormone-refractory, locally advanced, high risk patients. In a clinical context, although hormone-refractory prostate cancer still exerts response to high dose palliative radiation therapy to achieve the local control in patients (39), the patient population that could benefit the most would be those with a high Gleason score (> 8) and high PSA (> 20) without metastatic disease, and with a high failure rate even with high dose radiation. Showing a reasonable relevance to prostate cancer therapy in clinic, our reported small-molecule IAP-inhibitor has a promising implication on overcoming radiation resistance of human prostate cancer with high levels of IAPs.
Supplementary Material
Acknowledgements
Grant support: This study was supported in part by Department of Defense Prostate Cancer Research Program W81XWH-04-1-0215 and W81XWH-06-1-0010 (L. X.), NIH R01 CA121830-01 and R21 CA128220-01 (to L. X.), and by NIH through the University of Michigan’s Cancer Center Support Grant (5 P30 CA46592). J. D. is a University of Michigan Undergraduate Research Opportunity Program student.
We wish to thank Drs. Dian Wang, Jiaxin Zhang and Mu Li for technical support in the experiments; Dr. Shaomeng Wang for kindly providing IAP-inhibitors in our study; Dr. Lori Roberts in the UMCCC Unit of Laboratory Animal Medicine for measuring tumor sizes; Susan Harris for help with the manuscript; the University of Michigan Comprehensive Cancer Center (UMCCC) Flow Cytometry Core for flow cytometry analysis, and the University of Michigan Biomedical Imaging Core for bioluminescence imaging of animals.
Footnotes
Statement of Clinical Relevance Despite initial response to local therapy with surgery or radiation, many prostate cancer patients experience recurrence with advanced disease. Androgen ablation therapy produces only temporary responses because of the development of androgen independence. Failure to respond to radiation represents a critical problem in radiotherapy of hormone-refractory human prostate cancer (HRPC). HRPC is resistant to apoptosis and IAPs have been demonstrated to play a key role in apoptosis-resistance. This study aims to investigate the radiosensitizing potential of a novel small molecule IAP-inhibitor both in vitro and in vivo in androgen-independent prostate cancer, and the possible mechanism of radiosensitization. Our results show that the small molecule IAP-inhibitor radiosensitizes HRPC, partly due to the enhanced apoptosis and inhibition of NF-kB induced by radiation. The data suggest that functional down-modulation of IAPs may be a promising approach for overcoming radiation resistance of HRPC. More importantly, the success of this strategy will pave the way to develop the small molecule IAP-inhibitors as an entirely new class of anticancer therapy for radiosensitizing human prostate cancer. The combination of IAP-targeting molecular therapy and conventional radiotherapy may become a promising novel strategy to enhance the radiation efficacy and ultimately improve the survival of HRPC patients.
References
- 1.Szostak MJ, Kyprianou N. Radiation-induced apoptosis: predictive and therapeutic significance in radiotherapy of prostate cancer (review) Oncol Rep. 2000;7:699–706. doi: 10.3892/or.7.4.699. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence TS, Davis MA, Hough A, Rehemtulla A. The Role of Apoptosis in 2′,2′-Difluoro-2′-deoxycytidine (Gemcitabine)-mediated Radiosensitization. Clin Cancer Res. 2001;7:314–9. [PubMed] [Google Scholar]
- 3.Denmeade SR, Lin XS, Isaacs JT. Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. Prostate. 1996;28:251–65. doi: 10.1002/(SICI)1097-0045(199604)28:4<251::AID-PROS6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 4.Xu L, Frederik P, Pirollo KF, et al. Self-assembly of a virus-mimicking nanostructure system for efficient tumor-targeted gene delivery. Hum Gene Ther. 2002;13:469–81. doi: 10.1089/10430340252792594. [DOI] [PubMed] [Google Scholar]
- 5.DiPaola RS, Patel J, Rafi MM. Targeting apoptosis in prostate cancer. Hematology - Oncology Clinics of North America. 2001;15:509–24. doi: 10.1016/s0889-8588(05)70229-x. [DOI] [PubMed] [Google Scholar]
- 6.Amantana A, London CA, Iversen PL, Devi GR. X-linked inhibitor of apoptosis protein inhibition induces apoptosis and enhances chemotherapy sensitivity in human prostate cancer cells. Mol Cancer Ther. 2004;3:699–707. [PubMed] [Google Scholar]
- 7.Devi GR. XIAP as target for therapeutic apoptosis in prostate cancer. Drug News Perspect. 2004;17:127–34. doi: 10.1358/dnp.2004.17.2.829046. [DOI] [PubMed] [Google Scholar]
- 8.Salvesen GS, Duckett CS.IAP proteins: blocking the road to death’s door. [Review] [154 refs]. Nature Reviews Molecular Cell Biology 2002. Jun;36401–10. [DOI] [PubMed] [Google Scholar]
- 9.Schimmer AD. Inhibitor of Apoptosis Proteins: Translating Basic Knowledge into Clinical Practice. Cancer Res. 2004;64:7183–90. doi: 10.1158/0008-5472.CAN-04-1918. [DOI] [PubMed] [Google Scholar]
- 10.Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–4. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 11.Tenev T, Zachariou A, Wilson R, Ditzel M, Meier P. IAPs are functionally non-equivalent and regulate effector caspases through distinct mechanisms. Nat Cell Biol. 2005;7:70–7. doi: 10.1038/ncb1204. [DOI] [PubMed] [Google Scholar]
- 12.Notarbartolo M, Cervello M, Poma P, Dusonchet L, Meli M, D’Alessandro N. Expression of the IAPs in multidrug resistant tumor cells. Oncol Rep. 2004;11:133–6. [PubMed] [Google Scholar]
- 13.Deveraux QL, Roy N, Stennicke HR, et al. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO Journal. 1998;17:2215–23. doi: 10.1093/emboj/17.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou L, Yuan R, Serggio L. Molecular mechanisms of irradiation-induced apoptosis. Front Biosci. 2003;8:d9–19. doi: 10.2741/927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 16.Ohnishi K, Scuric Z, Schiestl RH, Okamoto N, Takahashi A, Ohnishi T. siRNA targeting NBS1 or XIAP increases radiation sensitivity of human cancer cells independent of TP53 status. Radiat Res. 2006;166:454–62. doi: 10.1667/RR3606.1. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi Y, Shiraki K, Fuke H, et al. Targeting of X-linked inhibitor of apoptosis protein or survivin by short interfering RNAs sensitize hepatoma cells to TNF-related apoptosis-inducing ligand- and chemotherapeutic agent-induced cell death. Oncol Rep. 2005;14:1311–6. [PubMed] [Google Scholar]
- 18.Srinivasula SM, Datta P, Fan XJ, Fernandes-Alnemri T, Huang Z, Alnemri ES. Molecular determinants of the caspase-promoting activity of Smac/DIABLO and its role in the death receptor pathway. Journal of Biological Chemistry. 2000;275:36152–7. doi: 10.1074/jbc.C000533200. [DOI] [PubMed] [Google Scholar]
- 19.Sun H, Nikolovska-Coleska Z, Yang CY, et al. Structure-based design, synthesis, and evaluation of conformationally constrained mimetics of the second mitochondria-derived activator of caspase that target the X-linked inhibitor of apoptosis protein/caspase-9 interaction site. J Med Chem. 2004;47:4147–50. doi: 10.1021/jm0499108. [DOI] [PubMed] [Google Scholar]
- 20.Sun H, Nikolovska-Coleska Z, Yang CY, et al. Structure-based design of potent, conformationally constrained Smac mimetics. J Am Chem Soc. 2004;126:16686–7. doi: 10.1021/ja047438+. [DOI] [PubMed] [Google Scholar]
- 21.Kalikin LM, Schneider A, Thakur MA, et al. In vivo visualization of metastatic prostate cancer and quantitation of disease progression in immunocompromised mice. Cancer Biol Ther. 2003;2:656–60. [PubMed] [Google Scholar]
- 22.Xu L, Yang D, Wang S, et al. (-)-Gossypol enhances response to radiation therapy and results in tumor regression of human prostate cancer. Mol Cancer Ther. 2005;4:197–205. [PubMed] [Google Scholar]
- 23.Maine GN, Burstein E. COMMD proteins and the control of the NF kappa B pathway. Cell Cycle. 2007;6:672–6. doi: 10.4161/cc.6.6.3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srinivasula SM, Hegde R, Saleh A, et al. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis.[comment][erratum appears in Nature 2001. Jun 28;41168411081.Nature. 2001;410:112–6. doi: 10.1038/35065125.
- 25.Liu Z, Sun C, Olejniczak ET, et al. Structural basis for binding of Smac/DIABLO to the XIAP BIR3 domain. Nature. 2000;408:1004–8. doi: 10.1038/35050006. [DOI] [PubMed] [Google Scholar]
- 26.Ahn KS, Sethi G, Aggarwal BB. Embelin, an inhibitor of X chromosome-linked inhibitor-of-apoptosis protein, blocks nuclear factor-kappaB (NF-kappaB) signaling pathway leading to suppression of NF-kappaB-regulated antiapoptotic and metastatic gene products. Mol Pharmacol. 2007;71:209–19. doi: 10.1124/mol.106.028787. [DOI] [PubMed] [Google Scholar]
- 27.Magne NTR, Bottero V, Didelot C, Houtte PV, Gerard JP, Peyron JF. NF-kappaB modulation and ionizing radiation: mechanisms and future directions for cancer treatment. Cancer Lett. 2006;231:158–68. doi: 10.1016/j.canlet.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Voboril R, Weberova-Voborilova J. Constitutive NF-kappaB activity in colorectal cancer cells: impact on radiation-induced NF-kappaB activity, radiosensitivity, and apoptosis. Neoplasma. 2006;53:518–23. [PubMed] [Google Scholar]
- 29.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–3. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 30.Nikolovska-Coleska Z, Xu L, Hu Z, et al. Discovery of embelin as a cell-permeable, small-molecular weight inhibitor of XIAP through structure-based computational screening of a traditional herbal medicine three-dimensional structure database. J Med Chem. 2004;47:2430–40. doi: 10.1021/jm030420+. [DOI] [PubMed] [Google Scholar]
- 31.Lu M, Lin SC, Huang Y, et al. XIAP induces NF-kappaB activation via the BIR1/TAB1 interaction and BIR1 dimerization. Mol Cell. 2007;26:689–702. doi: 10.1016/j.molcel.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 2004;305:1471–4. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 33.Suh J, Rabson AB. NF-kappaB activation in human prostate cancer: important mediator or epiphenomenon? J Cell Biochem. 2004;91:100–17. doi: 10.1002/jcb.10729. [DOI] [PubMed] [Google Scholar]
- 34.Jung M, Dritschilo A. NF-kappa B signaling pathway as a target for human tumor radiosensitization. Semin Radiat Oncol. 2001;11:346–51. doi: 10.1053/srao.2001.26034. [DOI] [PubMed] [Google Scholar]
- 35.Brach MA, Hass R, Sherman ML, Gunji H, Weichselbaum R, Kufe D. Ionizing radiation induces expression and binding activity of the nuclear factor kappa B. J Clin Invest. 1991;88:691–5. doi: 10.1172/JCI115354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varfolomeev E, Blankenship JW, Wayson SM, et al. IAP Antagonists Induce Autoubiquitination of c-IAPs, NF-kappaB Activation, and TNFalpha-Dependent Apoptosis. Cell. 2007;131:669–81. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 37.Vince JE, Wong WW, Khan N, et al. IAP Antagonists Target cIAP1 to Induce TNFalpha-Dependent Apoptosis. Cell. 2007;131:682–93. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 38.Petersen SL, Wang L, Yalcin-Chin A, et al. Autocrine TNFalpha signaling renders human cancer cells susceptible to smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–56. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hindson B, Turner S, Do V. Palliative radiation therapy for localized prostate symptoms in hormone refractory prostate cancer. Australas Radiol. 2007;51:584–8. doi: 10.1111/j.1440-1673.2007.01897.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.