Abstract
Endogenous nociceptin/orphanin FQ (N/OFQ) inhibits the activity of dopamine neurons in the substantia nigra and affects motor behavior. In this study we investigated whether a N/OFQ receptor (NOP) antagonist, J-113397, can modify movement in naive mice and nonhuman primates and attenuate motor deficits in MPTP-treated parkinsonian animals. J-113397 facilitated motor activity in naïve mice at low doses (0.1–1 mg/kg) and inhibited it at higher ones (10 mg/kg). Likewise, in MPTP-treated mice, J-113397 reversed motor deficit at 0.01 mg/kg but worsened hypokinesia at higher doses (1 mg/kg). In naïve nonhuman primates, J-113397, ineffective up to 1 mg/kg, produced inconsistent motor improvementsat 3 mg/kg. Conversely, in parkinsonian primates J-113397 (0.01 mg/kg) reversed parkinsonism, being most effective against hypokinesia. We conclude that endogenous N/OFQ modulates motor activity in mice and nonhuman primates and contributes to parkinsonian symptoms in MPTP-treated animals. NOP receptor antagonists may represent a novel approach to Parkinson’s disease.
Keywords: J-113397, L-DOPA, MPTP, Nociceptin/orphanin FQ, NOP-/- mice, NOP receptor, Parkinson’s disease
Introduction
Nociceptin/orphanin FQ (N/OFQ) is an opioid-like neuro-peptide (Meunier et al., 1995; Reinscheid et al., 1995) which activates the NOP receptor (Cox et al., 2000). The N/OFQ-NOP receptor system is expressed in motor areas of the rodent (Neal et al., 2001) and primate brain (Bridge et al., 2003) and, particularly, in the substantia nigra (SN), which contains dopamine (DA) neurons that degenerate in Parkinson’s disease (PD). N/OFQ inhibits the activity of DA neurons located in the SN compacta (SNc; Marti et al., 2004a) and impairs spontaneous (Reinscheid et al., 1995; Devine et al., 1996) and exercise-induced locomotion (Marti et al., 2004a) in rodents. Conversely, selective NOP receptor antagonists, such as [Nphe1, Arg14, Lys15]N/OFQ-NH2 (UFP-101; Calò et al., 2002) and 1-[(3R,4R)-1-cyclooctylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1,3-dihydro-2H benzimidazol-2-one (J-113397 or compound B; Kawamoto et al., 1999) increase striatal DA release and rotarod performance (Marti et al., 2004a), indicating that endogenous N/OFQ exerts an inhibitory control on physiologically-stimulated locomotion. We have proposed that endogenous N/OFQ plays a pathogenic role in PD (Marti et al., 2005). Indeed, N/OFQ transmission was found to be up-regulated in the DA-depleted SN of 6-hydroxydopamine (6-OHDA) hemi-lesioned rats (Marti et al., 2005). Moreover, NOP receptor antagonists alleviated akinesia/bradykinesia induced by haloperidol administration (Marti et al., 2004b) and 6-OHDA lesion (Marti et al., 2005), and enhanced the antiparkinsonian effect of L-DOPA when co-administered to rats (Marti et al., 2007). Finally, NOP receptor and ppN/OFQ knockout mice were found to be more resistant than wild-type to haloperidol-induced akinesia and 1-methyl-4-phenyl 1,2,3,6 tetrahydropyridine (MPTP) induced toxicity, respectively (Marti et al., 2005). Here we explored the hypothesis that the NOP antagonist J-113397 alleviates motor deficit in MPTP-treated mice and nonhuman primates. The advantage of the MPTP models is that systemic administration produces a bilateral DA denervation and, in nonhuman primates, MPTP induces symptoms that closely resemble those observed in parkinsonian patients. We used NOP receptor knockout (NOP-/-) mice to confirm the specificity of the in vivo action of J-113397.
Materials and methods
Subjects
Mice
Male Swiss, C57BL/6J (20–25 g; 12 week old, Harlan Italy, S. Pietro al Natisone, Italy), CD1/C57BL6J/129 NOP+/+ and NOP−/− mice (20–25 g; 12–15 week old; Nishi et al.,1997) were used. Mice were kept under standard conditions (12 h dark:12 h light cycle, free access to food and water) and the experimental protocols were approved by the Italian Ministry of Health (licence n° 71-2004-B).
Mice were trained for a week in order to obtain a stable performance in the three behavioral tests used to assess motor performance. Mice were then treated acutely with MPTP (4 × 20 mg/kg, 90 min apart). Pharmacological tests were performed 7 days after MPTP treatment, i.e. when DA cell loss stabilizes (Jackson-Lewis and Przedborski, 2007). Mice were later sacrificed for tyrosine hydroxylase (TH) immunohistochemistry (7th day post-MPTP).
Nonhuman primates
Four young adult male macaques (Macaca fascicularis) were included in the study. Animals were housed individually at the New England Primate Research Center. All the studies were done following NIH guidelines for animal welfare and were approved by the IACUC at Harvard Medical Area and the New England Regional Primate Research Center. After completion of experiments in the naïve state, animals were rendered parkinsonian by systemic administration of MPTP (0.3 mg/kg/week i.v. for 7.5±2.5 weeks) as described (Jenkins et al., 2004; Sanchez-Pernaute et al., 2007). Pharmacological tests were performed at least 3 months after the last MPTP dose.
Behavioral assessment
Mice
Motor activity was evaluated blindly using a battery of previously validated behavioral tests specific for different motor abilities under static (bar test; Sanberg et al., 1988) and dynamic (drag test and fixed-speed rotarod test; Rozas et al., 1997; Marti et al., 2004a, 2005) conditions. On the day of experiment, the tests were performed before (control session) and 10 and 60 min after drug or vehicle injection. Each animal was taken as its own control.
Bar test
Each mouse was placed on a table and the contralateral and ipsilateral forepaws were placed alternatively on blocks of increasing heights (1.5, 3 and 6 cm). Total time (in seconds) spent by each paw on the blocks was recorded (cut-off time of 20 s).
Drag test
Each mouse was lifted by the tail (allowing forepaws on the table) and dragged backwards at a constant speed (∼20 cm/s) for a fixed distance (100 cm). The number of steps made by each paw was counted by two separate observers.
Rotarod test
A protocol that allowed to detect both facilitatory and inhibitory drug effects was used (Marti et al., 2004a). Mice were tested at increasing speeds (usually from 5 to 45 rpm for naïve and from 5 to 35 rpm for MPTP-treated mice; 180 s each) and motor activity was calculated by comparing the performance over a speed range (usually 30–45 rpm for naïve and 20–35 rpm for MPTP-treated mice) causing a progressive decrement of performance to ∼40% of the maximal response (i.e. the experimental cut-off time).
Nonhuman primates
Animals were trained to perform a computerized timed reaching task that measures the speed of arm movements (MAP test; Jenkins et al., 2004). Training was carried out for an average of 6 days for the platform task and 8–12 days for the straight rod task, until the performance (time to retrieve the treats) was stable. For pharmacological evaluation, animals were tested 30 min after the administration of vehicle (saline) for 2 days and then with either saline or the active drugs. In addition, global motor activity data was obtained using activity monitors (Actiwatch) for a week at the naïve and parkinsonian stages (Table 1). These tests provide objective measures of bradykinesia and hypokinesia, respectively. For motor evaluation, animals were transferred to a Plexiglas observation cage where they were videotaped. Motor behavior was rated according to a scale based on the motor subscale of the UPDRS (Unified Parkinson’s Disease Rating Scale), as described (Jenkins et al., 2004; Sanchez-Pernaute et al., 2007). The following signs were scored from 0 to 3: bradykinesia in the left and right arms (L/R), tremor L/R, rigidity L/R, hypokinesia, posture/balance (for a total score from 0 to 24). Scores were obtained at 30–45 min after each drug or vehicle administration.
Table 1.
Individual characteristics of MPTP-lesioned primates
| Animal # | Weight (kg) |
PRS score after MPTP |
PRS score 3rd month post-MPTP |
Activity d/n (% from baseline) |
|---|---|---|---|---|
| Mf23 | 4.4 | 17 | 18.25 | -76/-60 |
| Mf25 | 5.4 | 19 | 21.5 | -77/-62 |
| Mf30 | 6.1 | 21 | 20 | -68/-32 |
| Mf34 | 5.3 | 18 | 18.5 | -5/-60 |
Histological evaluation in mice
Mice were anaesthetised with ketamine 85 mg/kg and xylazine 15 mg/kg (i.p.), transcardially perfused with 20 mM phosphatebuffered saline (PBS) and fixed with 4% paraformaldehyde in PBS at pH 7.4. The brains were removed, post-fixed overnight and cryoprotected in 20% sucrose (solution in PBS). Serial coronal sections of 30 μm thickness were made in the striatum (−0.8 to +1.3 from bregma) and every second section processed for TH immunohistochemistry, as described (Marti et al., 2007). Briefly, sections were preincubated in blocking serum (5% normal horse serum and 0.3% Triton X 100 in PBS), incubated overnight in anti-TH mouse monoclonal antibody solution (1:2000, Chemicon, Temcula, CA), then in biotinylated horse anti-mouse IgG secondary antibody (1:200; Vector Laboratories, Burlingame, CA) for 1 h and finally treated with avidin—biotin-peroxidase complex (Vector Laboratories). Immunoreactivity was visualized by incubating the sections in a solution containing 0.05% 3,3-diaminobenzidine (DAB) in 0.013% H2O2. Images from every section were acquired with a Polaroid DMC camera mounted in a Zeiss Axioskop (Carl Zeiss, Oberkochen, Germany) and the optical density of TH-immunoreactive fibres in the striatum was analysed at five AP levels for each animal (−0.10,+ 0.20,+ 0.50,+ 0.80,+ 1.10) using ImageJ software (Wayne Rasband; NIH, USA). Region of interest for estimation of optical density was performed in the dorsolateral area of the caudate-putamen. Optical density (corrected for non-specific background measured in the corpus callosum) was first calculated for each animal as the mean of the 5 striatal levels. Group means (as presented in figure) were then obtained by averaging each individual value.
Data presentation and statistical analysis
Mice
Motor performance was calculated as time on bar or on rod (in seconds) and number of steps (drag test). Data are expressed as a percent±SEM of the control session. Statistical analysis was performed (CoStat 6.3, CoHort Software, Monterey, CA, USA) on percent data by repeated measure (RM) analysis of variance (ANOVA) followed by contrast analysis and the sequentially rejective Bonferroni’stest.
Nonhuman primates
Statistical analysis was performed (StatView Software, SAS, CA), using t tests and ANOVA (followed by the PLSD test) to determine the effect of the drugs on motor performance as indicated in the text. Data are shown as mean values±SEM.
Materials
L-DOPA methylester, benserazide and MPTP were purchased from Sigma Chemical Company (St Louis, MO, USA). J-113397 was synthesized as a racemic mixture in our laboratories (Marti et al., 2004a). All drugs were freshly dissolved in isoosmotic saline solution prior to use.
Results
Studies in mice
Naïve Swiss and C57BL/6J mice
To investigate the role of endogenous N/OFQ in the regulation of motor activity, J-113397 was administered systemically in Swiss mice (Fig. 1A). RM ANOVA showed a significant effect of treatment (F5,40= 20.90, p<0.0001), time (F1,5 = 4.80, p = 0.033) and a significant time×treatment interaction (F5,48=3.51, p = 0.0087). Post-hoc analysis showed that J-113397 facilitated rotarod performance in the 0.1–1 mg/kg dose range (maximal increase ∼34%) while inhibited it at 10 mg/kg (∼29%). Both effects were evident up to 60 min after administration. The complete battery of behavioral tests was then used to characterize J-113397 action in C57BL/6J mice (Figs. 1B–D). RM ANOVA on the immobility time as in the bar test (Fig. 1B), revealed a main effect of treatment (F4,28=12.49, p<0.0001) but not time (F1,4=0.04, p = 0.85) and a non significant time×treatment interaction (F4,39 = 0.09, p = 0.98). J-113397 increased the immobility time at 10 mg/kg, lower doses being ineffective. RM ANOVA on the number of steps as in the drag test (Fig. 1C) revealed a main effect of treatment (F4,28= 23.81, p<0.0001), time (F1,4= 27.65, p<0.0001) and a significant time×treatment interaction (F4,39 = 14.00, p<0.0001). Likewise, RM ANOVA on the rotarod performance (Fig. 1D) showed a main effect of treatment (F4,28 = 9.81, p<0.0001), time (F1,4 = 9.02, p = 0.0046) and a significant time×treatment interaction (F4,39 = 15.23, p<0.0001). Post-hoc analysis revealed that J-113397 enhanced the number of steps (Fig. 1C) and the exercise-stimulated locomotion (Fig. 1D) at 0.3 and 1 mg/kg while reducing them at 10 mg/kg. No effects were observed 60 min after drug administration.
Fig. 1.
J-113397 (0.03–10 mg/kg, i.p.) dually modulated rotarod performance in naïve Swiss and C57BL/6J mice. Mice were trained daily on the rotarod until their motor performance was reproducible (see Materials and methods). On the day of the experiment, three behavioral sessions were carried out, before (control session) and after (10 and 60 min) drug administration. The time spent on the rod was calculated (in seconds). Data are expressed as percent of the performance in the control session and represent the mean ±SEM of 8–10 determinations for each group. *p<0.05; different from saline (RM ANOVA followed by contrast analysis and the sequentially rejective Bonferroni’s test).
Naïve NOP−/− mice
Since high doses of J-113397 (10 mg/kg) have been reported to exert effects independent of NOP receptors (Koizumi et al., 2004), we investigated the specificity of the J-113397 action (Fig. 2). RM ANOVA in NOP+/+ mice (Fig. 2A) revealed a significant effect of treatment (F4,28 = 72.24, p<0.0001), time (F1,4 = 15.66, p = 0.0003) and a significant time×treatment interaction (F4,39 = 7.42, p = 0.0002). J-113397 facilitated rotarod performance at 0.1 and 1 mg/kg and inhibited it at 10 mg/kg. No major difference was observed between Swiss and NOP+/+ mice in terms of sensitivity to J-113397 or duration of the response. Conversely, J-113397 was not effective in NOP−/− mice at any of the doses tested (Fig. 2B), suggesting that both the facilitation and the inhibition observed in NOP+/+ mice were due to NOP receptor blockade.
Fig. 2.

Motor effects of J-113397 were dependent on NOP receptor. J-113397 (0.03–10 mg/kg, i.p.) dually modulated rotarod performance in wild-type mice (NOP+/+; panel A) but was ineffective in NOP receptor knockout mice (NOP−/−; panel B). Mice were trained daily on the rotarod until their motor performance was reproducible (see Materials and methods). On the day of the experiment, three behavioral sessions were carried out, before (control session) and after (10 and 60 min) drug administration. The time (in seconds) spent on the rod was calculated. Data are expressed as percent of the performance in the control session and are mean±SEM of 8–10 determinations for each group. *p<0.05; different from saline (RM ANOVA followed by contrast analysis and the sequentially rejective Bonferroni’s test).
MPTP-treated C57BL/6J mice
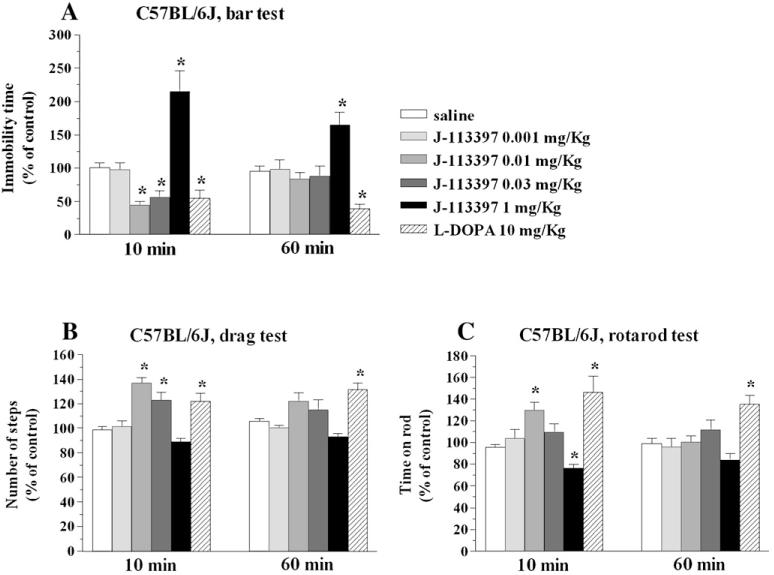
Seven days after MPTP intoxication, mice displayed increased immobility time (Fig. 3A), reduced number of steps (Fig. 3B) and impaired rotarod performance (Fig. 3C) compared to pre-treatment values. This behavior was associated with a ∼60% loss of striatal TH-positive nerve terminals (Figs. 3D—F). Motor deficit induced by MPTP was reversed by L-DOPA and J-113397 (Fig. 4). Thus, RM ANOVA on immobility time (Fig. 4A) revealed a significant effect of treatment (F5,40 = 19.92, p<0.0001) but not time (F1,5 = 0.01, p = 0.92), and a significant time×treatment interaction (F5,48 = 4.15, p = 0.0032). L-DOPA (10 mg/kg) and J-113397 (0.01–0.03 mg/kg) reduced the immobility time. However, J-113397 increased it at 1 mg/kg. RM ANOVA on the number of steps (Fig. 4B) revealed a significant effect of treatment (F5,40 = 13.32, p<0.0001) but not time (F1,5 = 0.12, p = 0.73), and a significant time×treatment interaction (F5,48 =3.73, p = 0.0062). L-DOPA and J-113397 (0.01–0.03 mg/kg) increased the number of steps. Higher doses of J-113397 (1 mg/kg) were found ineffective. Finally, RM ANOVA on the rotarod performance (Fig. 4C) showed a significant effect of treatment (F5,40 = 13.07, p<0.0001) but not time (F1,5 = 2.54, p = 0.11), and a time×treatment interaction at the limit of significance (F5,48 = 2.40, p = 0.05). L-DOPA and J-113397 (0.01 mg/kg) increased the rotarod performance. However, J-113397 (1 mg/kg) inhibited it. Differently from L-DOPA, J-113397 was no longer effective after 60 min in all tests.
Fig. 3.
MPTP administration produced motor impairment and loss of striatal DA terminals in C57BL/6J mice. Motor activity was evaluated by three behavioral tests: the bar (panel A), drag (panel B) and rotarod (panel C) test. Mice were trained daily for a week until their motor activity was reproducible (see Materials and methods). Before MPTP (20 mg/kg) or saline administration (4 injections, 90 min apart), motor activity was evaluated, and the time spent (seconds) on the blocks (bar test), the number of steps made by the forelimbs (drag test) and the time spent on the rod (rotarod test) were calculated. Another session was repeated 7 days after MPTP administration. Tyrosine hydroxylase (TH) immunohistochemistry was then performed in striatal slices (panels D—F). Photomicrograph of TH-positive fibres in the striata of saline (panel D) and MPTP-injected (panel E) mice. Optical density of TH-positive fibres in the striatum (panel F). Values are presented as means±SEM, obtained from 4 saline- and 6 MPTP-treated mice. Data are expressed in absolute values and are mean±SEM of 9 determinations for each group. *p<0.05; different from saline-injected mice (panels A—C, ANOVA followed by the Newman—Keuls test; panel F, Student’s t-test).
Fig. 4.
J-113397 attenuated motor deficits in MPTP-treated C57BL/6J mice. Motor activity was evaluated by three behavioral tests: the bar (panel A), drag (panel B) and rotarod (panel C) test. Mice were trained daily until their motor activity was reproducible (see Materials and methods), then they were treated with MPTP. Seven days after MPTP mice were challenged with saline, J-113397 (0.01–1 mg/kg, i.p.) or L-DOPA (10 mg/kg plus benserazide 2.5 mg/kg, i.p.). Three behavioral sessions were carried out, before (control session) and after (10 and 60 min) drug or saline administration. The time spent (seconds) on the blocks (bar test), the number of steps made by the forelimbs (drag test) and the time spent on the rod (rotarod test) were calculated. Data are expressed as percent of the performance in the control session and are mean±SEM of 8–10 determinations for each group. *p<0.05; different from saline (RM ANOVA followed by contrast analysis and the sequentially rejective Bonferroni’s test).
Studies in nonhuman primates
Naïve macaques
The NOP receptor antagonist, J-113397 did not affect motor performance in naïve macaques at 0.1 and 1 mg/kg (Fig. 5). At a higher dose (3 mg/kg) J-113397 induced a faster performance in the straight rod test in two of the animals (Fig. 5A), although the other two did not perform the test. Therefore we tested these animals on a simpler task (i.e. the platform task) but at this dose animals were inattentive and performance was slightly slower than normal (Fig. 5B).
Fig. 5.

J-113397 (0.1–3 mg/kg, i.m.) modulated motor activity in naïve nonhuman primates. Motor performance was evaluated in the Movement Analysis Panel (MAP) test. Average time in seconds to retrieve a treat in the straight rod (panel A) test was significantly improved in 2 animals at the higher dose of J-113397 tested, but at this dose the other 2 animals showed side effects (distractibility, scratching and “wet dog” shakes) and failed to perform. These 2 animals were subsequently tested in the platform (easier) task (panel B) of the MAP test in which they performed significantly worse at the high dose. *p<0.05; different from saline (ANOVA followed by PLSD test).
MPTP-treated macaques
We first examined the response to four doses of J-113397 (0.01, 0.03, 0.1 and 1 mg/kg) in two stable parkinsonian animals (data not shown). From 0.03 to 1 mg/kg we did not observe any beneficial effect on either parkinsonian score or MAP platform performance; at 1 mg/kg both animals displayed long episodes of akinesia (freezing) similar to the effect of high doses of J-113397 observed in 6-OHDA rats (30 mg/kg, Morari and Marti, unpublished observation) and did not perform the reaching test. No major side effects were observed at 0.03 mg/kg but one animal did not test and the other did not show any improvement in the reaching task. With the lower dose (0.01 mg/kg) both animals showed an improvement in the MAP platform reaching task and therefore we selected this dose for the rest of the experiments.
Both J-113397 (0.01 mg/kg) and L-DOPA (30 mg/kg) induced a significant benefit in parkinsonian scores in the 4 animals (F2,9 = 13.5, p<0.01; Fig. 6A). The overall improvement after J-113397 administration (∼19±3%, p = 0.015) was more moderate than that achieved with L-DOPA (∼46±3%, p = 0.001). Although as a group, the difference between L-DOPA and J-113397 improvement in global PRS score did not reach significance (p = 0.07), only in one animal (Mf25) was the response to both drugs not significantly different (Fig. 6A). There was no significant effect of the baseline PRS score (i.e. severity of the parkinsonian signs) on the response to either drug. We further analysed the therapeutic effect on parkinsonian symptoms (Fig. 6B). The largest improvement induced by J-113397 (∼30%) was observed on hypokinesia and the L-DOPA effect on this particular symptom was not significantly different from J-113397. All other symptoms (rigidity, tremor and bradykinesia) improved significantly more with L-DOPA (Fig. 6B).
Fig. 6.

Effect of J-113397 on motor symptoms in MPTP-treated primates. Panel A. Effect of J-113397 (0.01 mg/kg) and L-DOPA (30 mg/kg) on the global PRS score. The average improvement over the baseline score (n=2–3 pharmacological tests for each compound) is shown for each animal. The improvement after J-113397 administration (∼19±3%) was more moderate than that achieved with L-DOPA (∼46±3%, Fisher PLSD p=0.001). Comparative analysis of the pharmacological effects of L-DOPA and J-113397 on parkinsonian symptoms (panel B) showed that all symptoms improved more with L-DOPA except for hypokinesia.
Finally, we evaluated the performance of the parkinsonian animals in the MAP test (Fig. 7). MPTP induced a significant increase in the time needed to complete the platform task in 3 out of 4 animals (Mf23, Mf25 and Mf30). These animals showed a significant improvement in the time to retrieve treats from the platform with L-DOPA and one of them had also a significant improvement in performance after J-113397 (Fig. 7).
Fig. 7.
Effect of J-113397 on the MAP test in MPTP-treated primates. MPTP induced a significant increase in the time needed to complete the platform task (animals were unable to perform the straight rod task) in 3 out of 4 animals (Mf23, Mf25 and Mf30). In these animals L-DOPA improvement on task performance was significant. Only one of them (Mf23) showed a significant improvement in response to J-113397. *p<0.05; different from post-MPTP performance (ANOVA followed by PLSD test). #p<0.05; different from J-113397 (ANOVA followed by PLSD test).
Discussion
The most important finding of the present study is that the NOP receptor antagonist J-113397 reversed motor disabilities in MPTP-treated mice and nonhuman primates. These data reinforce the view that endogenous N/OFQ plays a role in motor symptoms in parkinsonism across species. Moreover, the efficacy of J-113397 in some MPTP-treated primates raises the possibility of a therapeutic effect of NOP receptor antagonists in PD patients, although the unusual dual action of J-113397 calls for evaluation of other (more selective?) NOP antagonists.
Role of endogenous N/OFQ in modulation of locomotion under physiological conditions
Motor impairment has been one of the main biological effects observed after intracerebroventricular injection of N/OFQ in rodents. It was observed at high N/OFQ doses (nmol) and replicated by systemic administration of nonpeptide NOP receptor agonists. (Jenck et al., 2000; Varty et al., 2005). Reports that lower N/OFQ doses (pmol) facilitate motor performance in rats (Florin et al., 1996; Kuzmin et al., 2004) and mice (Sakoori and Murphy, 2004) have also been published. Although this effect is much milder than the inhibitory one, and observed in a narrower dose range, it confers to the N/OFQ dose-response curve a biphasic profile. The finding that J-113397 dually modulated exercise-induced locomotion in Swiss, C57BL/6J and NOP+/+ but not NOP−/− mice suggests that endogenous N/OFQ can both facilitate and inhibit motor activity through NOP receptor activation. These effects, however, do not appear to be physiologically equivalent, motor inhibition being predominant (Marti et al., 2004a). Systemic injection of NOP receptor antagonists or deletion of the NOP receptor gene failed to affect spontaneous locomotion (Gavioli et al., 2003; Kuzmin et al., 2004; Marti et al., 2004a; Rizzi et al., 2007). In line with this view, we did not detect motor facilitation by J-113397 in the bar test (a static test), while there was a prominent effect in tests engaging the animals in repetitive and prolonged movements (i.e. the drag and rotarod test). These data reinforce the hypothesis that endogenous N/OFQ produces a functional inhibition during induced motor activity and not at rest. Indeed, extracellular N/OFQ levels rise during rotarod performance (Marti et al., 2005).
In line with a previous study (Ko et al., 2006), J-113397 did not exert motor effects in naïve macaques up to 1 mg/kg. However, higher doses (3 mg/kg) improved arm speed (straight rod test) in two animals while the remaining two could not perform the test, looking distracted and slightly “hallucinated”. Although the inconsistency of response prevents from drawing firm conclusions on the role of endogenous N/OFQ, the data obtained in two animals are in line with the view that the peptide plays an inhibitory role on motor activity. Nevertheless, high doses of J-113397 may also activate sigma receptors (Chiou et al., 2007), causing loss of attention and hallucinations (Okuyama et al., 1994).
Role of endogenous N/OFQ in modulation of MPTP-induced parkinsonism
The MPTP-lesioned nonhuman primate (macaque) model reproduces many PD motor symptoms faithfully and is used to assess the therapeutic potential of novel antiparkinsonian drugs (Dauer and Przedborski, 2003). Conversely, the neurochemical and behavioral outcomes of MPTP administration in mice are highly variable, mainly depending on strain, age, gender, route and protocol of administration (Sedelis et al., 2001; Meredith and Kang, 2006). Thus, a direct comparison with data obtained in our model is quite difficult. Nevertheless, we found that mice displayed increased immobility time (likely reflecting increased akinesia), reduced number of steps (possibly reflecting increased akinesia, bradykinesia and rigidity) and overall impaired motor performance (possibly reflecting loss of coordination and overall gait ability) at 7 days after MPTP intoxication. These data are consistent with that reported by other authors using the bar (Kato et al., 2004; Watanabe et al., 2008) and the treadmill (Petzinger et al., 2007) test at 7 days after MPTP administration (4×20 mg/kg). In the same study, no differences in rotarod performance after MPTP intoxication were observed. The discrepancy may be explained on the basis of technical issues such as rod diameter (3 cm vs 8 cm in our model) and exercise protocol, which appear more challenging for mice in our study (from 5 to 35 rpm, for a total of 21 min) compared to the previous one (30 rpm for 200 s; Petzinger et al., 2007). Actually, the difficulty and duration of motor task appear crucial to detect changes in rotarod performance. Indeed, Sedelis et al. (2000) did not find difference in rotarod performance 4 days after MPTP intoxication (4×15 mg/kg) using a less strenuous protocol (fixed speed of 12 rpm for 120 s; rod 2.5 cm). It is noteworthy that motor impairment in our study was DA-dependent since it was associated with significant (∼60%) loss of striatal DA terminals and was reversed by L-DOPA. In these tests, J-113397 reproduced the antiparkinsonian action of L-DOPA. Thus, in addition to reversing the neuroleptic and 6-OHDA-induced parkinsonism (Marti et al., 2004b, 2005, 2007), J-113397 was effective both in acute (mice) and chronic (macaques) paradigms of MPTP administration, overall suggesting that endogenous N/OFQ plays a role in experimental parkinsonism independent of the species and models used. Interestingly, not only DA loss did not prevent the antiparkinsonian action of J-113397 but it enhanced sensitivity to J-113397, resulting in a leftward shift of the dose-response curve. In 6-OHDA hemi-lesioned rats, this phenomenon was associated with up-regulation of N/OFQ expression and release (Marti et al., 2005). In parkinsonian macaques, J-113397 was less effective than L-DOPA, although it improved hypokinesia comparably to L-DOPA, indicating a general depressive effect of endogenous N/OFQ on movement. In keeping with this notion, we reported that exogenous N/OFQ depressed motor cortex excitability and motor output in vivo via activation of nigral NOP receptors (Viaro et al., 2006). From a clinical perspective, however, the narrow therapeutic range is quite disappointing, as the antiparkinsonian effects of 0.01 mg/kg J-113397 vanished at higher doses, turning into motor inhibition at 1 mg/kg. The fact that this phenomenon was observed in mice (in the same dose range) suggests that it is mediated by NOP receptors. Nevertheless, we cannot elucidate whether this is a peculiarity of the compound (e.g. kinetics of interaction with the NOP receptor, brain penetrability) or a “class effect” until other NOP receptor antagonists, preferably chemically unrelated, have been tested.
Neurobiological substrates of J-113397 action
Systemic administration of J-113397 in naïve rats increased rotarod performance and elevated striatal DA release, suggesting that endogenous DA tonically inhibits nigrostriatal DA transmission (Marti et al., 2004a). This is in line with the findings that exogenous N/OFQ inhibited activity of nigral DA cells, an effect associated with hypolocomotion (Marti et al., 2004a). Thus, it can be proposed that the antiparkinsonian action of J-113397 is mediated by blockade of inhibitory NOP receptors expressed on residual nigral DA cells resulting in increased DA transmission. However, NOP receptor antagonists are effective also under conditions of DA depletion and DA receptor blockade (Marti et al., 2004b; Marti et al., 2005) suggesting that endogenous N/OFQ causes motor depressant responses also via non-DA mechanisms. Indeed, NOP receptors are also present on serotonergic, GABAergic and glutamatergic terminals in SN (M. Morari and M. Marti, personal communication). In particular, we found that the antiakinetic effect of J-113397 was associated with increased GABA and reduced GLU release in the SNr, leading to an impairment of nigrothalamic GABAergic transmission and, possibly, thalamic disinhibition (Marti et al., 2007). Within this frame, motor inhibitory actions of J-113397 could be attributed to an excessive or more prolonged degree of NOP receptor blockade or to blockade of different subsets of NOP receptors (Kuzmin et al., 2004) (facilitating or inhibiting motor activity) located either along the same or different motor pathways. The fact that motor facilitation induced by exogenous N/OFQ was blocked by haloperidol or DA depletion (Florin et al., 1996; Kuzmin et al., 2004) suggests the involvement of the same mesencephalic DA areas that mediate locomotion.
Concluding remarks
The NOP receptor antagonist J-113397 produced a dose-dependent effect (facilitation at low doses and inhibition at high) of motor activity in naïve and MPTP-induced parkinsonian mice and nonhuman primates. This is consistent with the notion that endogenous N/OFQ is predominantly involved in motor inhibition, particularly during exercise-induced activity. Although the dual action of J-113397 in parkinsonian primates needs to be further characterized, our data support the view that NOP receptor is a new target in the therapy of PD.
Acknowledgments
This work has been supported by a grant from the Michael J. Fox Foundation for Parkinson’s Research (Fast Track 2004) to M. Morari, and by grants from NINDS Parkinson’s Disease Research Center of Excellence (P50 NS39793), the Michael Stern Foundation, and N.E.P.R.C. Center (Grant P51RR00168) to O. Isacson. We gratefully acknowledge the contribution of Jennifer Pagel and Jack McDowell in the acquisition of primate data.
References
- Bridge KE, Wainwright A, Reilly K, Oliver KR. Autoradio-graphic localization of (125)i[Tyr(14)] nociceptin/orphanin FQ binding sites in macaque primate CNS. Neuroscience. 2003;118:513–523. doi: 10.1016/s0306-4522(02)00927-2. [DOI] [PubMed] [Google Scholar]
- Calò G, Rizzi A, Rizzi D, Bigoni R, Guerrini R, Marzola G, Marti M, McDonald J, Morari M, Lambert DG, Salvatori S, Regoli D. [Nphe1,Arg14,Lys15]Nociceptin-NH2, a novel potent and selective antagonist of the nociceptin/orphanin FQ receptor. Br. J. Pharmacol. 2002;136:303–311. doi: 10.1038/sj.bjp.0704706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou LC, Liao YY, Fan PC, Kuo PH, Wang CH, Riemer C, Prinssen EP. Nociceptin/orphanin FQ peptide receptors: pharmacology and clinical implications. Curr. Drug Targets. 2007;8:117–135. doi: 10.2174/138945007779315605. [DOI] [PubMed] [Google Scholar]
- Cox BM, Chavkin C, Christie MJ, Civelli O, Evans C, Hamon MD, Hoellt V, Kieffer B, Kitchen I, Mcknight AT, Meunier JC, Portoghese PS. Opioid receptors. In: Girdlestone D, editor. The IUPHAR Compendium of Receptor Characterization and Classification. IUPHAR Media Ltd; London: 2000. [Google Scholar]
- Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Devine DP, Taylor L, Reinscheid RK, Monsma FJJ, Civelli O, Akil H. Rats rapidly develop tolerance to the locomotor-inhibiting effects of the novel neuropeptide orphanin FQ. Neurochem. Res. 1996;21:1387–1396. doi: 10.1007/BF02532380. [DOI] [PubMed] [Google Scholar]
- Florin S, Suaudeau C, Meunier JC, Costentin J. Nociceptin stimulates locomotion and exploratory behaviour in mice. Eur. J. Pharmacol. 1996;317:9–13. doi: 10.1016/s0014-2999(96)00707-8. [DOI] [PubMed] [Google Scholar]
- Gavioli EC, Marzola G, Guerrini R, Bertorelli R, Zucchini S, De Lima TC, Rae GA, Salvadori S, Regoli D, Calò G. Blockade of nociceptin/orphanin FQ-NOP receptor signalling produces antidepressant-like effect: pharmacological and genetic evidences from the mouse forced swimming test. Eur. J. Neurosci. 2003;17:1987–1990. doi: 10.1046/j.1460-9568.2003.02603.x. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat. Protoc. 2007;2:141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- Jenck F, Wichmann J, Dautzenberg FM, Moreau JL, Ouagazzal AM, Martin JR, Lundstrom K, Cesura AM, Poli SM, Roever S, Kolczewski S, Adam G, Kilpatrick G. A synthetic agonist at the orphanin FQ/nociceptin receptor ORL1: anxiolytic profile in the rat. Proc. Natl. Acad. Sci. U. S. A. 2000;97:4938–4943. doi: 10.1073/pnas.090514397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins BG, Sanchez-Pernaute R, Brownell AL, Chen YC, Isacson O. Mapping dopamine function in primates using pharmacologic magnetic resonance imaging. J. Neurosci. 2004;24:9553–9560. doi: 10.1523/JNEUROSCI.1558-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Kurosaki R, Oki C, Araki T. Arundic acid, an astrocyte-modulating agent, protects dopaminergic neurons against MPTP neurotoxicity in mice. Brain Res. 2004;1030:66–73. doi: 10.1016/j.brainres.2004.09.046. [DOI] [PubMed] [Google Scholar]
- Kawamoto H, Ozaki S, Itoh Y, Miyaji M, Arai S, Nakashima H, Kato T, Ohta H, Iwasawa Y. Discovery of the first potent and selective small molecule opioid receptor-like (ORL1) antagonist: 1-[3R, 4R)-1-cyclooctylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1,3-dihydro-2H benzimidazol-2-one (J-113397) J. Med. Chem. 1999;42:5061–5063. doi: 10.1021/jm990517p. [DOI] [PubMed] [Google Scholar]
- Ko MC, Wie H, Woods JH, Kennedy RT. Effects of intrathecally administered nociceptin/orphanin FQ in monkeys: behavioral and mass spectrometric studies. J. Pharmacol. Exp. Ther. 2006;318:1257–1264. doi: 10.1124/jpet.106.106120. [DOI] [PubMed] [Google Scholar]
- Koizumi M, Sakoori K, Midorikawa N, Murphy NP. The NOP (ORL1) receptor antagonist Compound B stimulates mesolimbic dopamine release and is rewarding in mice by a non-NOP-receptor-mediated mechanism. Br. J. Pharmacol. 2004;143:53–62. doi: 10.1038/sj.bjp.0705906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin A, Sandin J, Terenius L, Ogren SO. Evidence in locomotion test for the functional heterogeneity of ORL-1 receptors. Br. J. Pharmacol. 2004;141:132–140. doi: 10.1038/sj.bjp.0705583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti M, Mela F, Veronesi C, Guerrini R, Salvadori S, Federici M, Mercuri NB, Rizzi A, Franchi G, Beani L, Bianchi C, Morari M. Blockade of nociceptin/orphanin FQ receptor signaling in rat substantia nigra pars reticulata stimulates nigrostriatal dopaminergic transmission and motor behavior. J. Neurosci. 2004a;24:6659–6666. doi: 10.1523/JNEUROSCI.0987-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti M, Mela F, Guerrini R, Calò G, Bianchi C, Morari M. Blockade of nociceptin/orphanin FQ transmission in rat substantia nigra reverses haloperidol-induced akinesia and normalizes nigral glutamate release. J. Neurochem. 2004b;91:1501–1504. doi: 10.1111/j.1471-4159.2004.02843.x. [DOI] [PubMed] [Google Scholar]
- Marti M, Mela F, Fantin M, Zucchini S, Brown JM, Witta J, Di Benedetto M, Buzas B, Reinscheid RK, Salvadori S, Guerrini R, Romualdi P, Candeletti S, Simonato M, Cox BM, Morari M. Blockade of nociceptin/orphanin FQ transmission attenuates symptoms and neurodegeneration associated with Parkinson’s disease. J. Neurosci. 2005;95:9591–9601. doi: 10.1523/JNEUROSCI.2546-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti M, Trapella C, Viaro R, Morari M. The nociceptin/orphanin FQ receptor antagonist J-113397 and L-Dopa additively attenuate experimental parkinsonism through overinhibition of the nigrothalamic pathway. J. Neurosci. 2007;27:1297–1307. doi: 10.1523/JNEUROSCI.4346-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Kang UJ. Behavioral models of Parkinson’s disease in rodents: a new look at an old problem. Mov. Disord. 2006;21:1595–1606. doi: 10.1002/mds.21010. [DOI] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, Mazarguil H, Vassart G, Parmentier M, Costentin J. Isolation and structure of the endogenus agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Neal CRJ, Akil H, Watson SJ. Expression of orphanin FQ and the opioid receptor-like (ORL1) receptor in the developing human and rat brain. J. Chem. Neuroanat. 2001;22:219–249. doi: 10.1016/s0891-0618(01)00135-1. [DOI] [PubMed] [Google Scholar]
- Nishi M, Houtani T, Noda Y, Mamiya T, Sato K, Doi T, Kuno J, Takeshima H, Nukada T, Nabeshima T, Yamashita T, Noda T, Sugimoto T. Unrestrained nociceptive response and disregulation of hearing ability in mice lacking the nociceptin/orphanin FQ receptor. EMBO J. 1997;16:1858–1864. doi: 10.1093/emboj/16.8.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama S, Imagawa Y, Sakagawa T, Nakazato A, Yamaguchi K, Katoh M, Yamada S, Araki H, Otomo S. NE-100, a novel sigma receptor ligand: effect on phencyclidine-induced behaviors in rats, dogs and monkeys. Life Sci. 1994;55:PL133–PL138. doi: 10.1016/0024-3205(94)00749-7. [DOI] [PubMed] [Google Scholar]
- Petzinger GM, Walsh JP, Akopian G, Hogg E, Abernathy A, Arevalo P, Turnquist P, Vucković M, Fisher BE, Togasaki DM, Jakowec MW. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J. Neurosci. 2007;27:5291–5300. doi: 10.1523/JNEUROSCI.1069-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr., Civelli O. Orphanin FQ: a neuropeptide that activates an opioid-like G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Rizzi A, Gavioli EC, Marzola G, Spagnolo B, Zucchini S, Ciccocioppo R, Trapella C, Regoli D, Calò G. Pharmacological characterization of the nociceptin/orphanin FQ receptor antagonist SB-612111 [(−)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl] methyl]-6,7,8,9-tetrahydro-5H benzocyclohepten-5-ol]: in vivo studies. J. Pharmacol. Exp. Ther. 2007;32:968–974. doi: 10.1124/jpet.106.116780. [DOI] [PubMed] [Google Scholar]
- Rozas G, Guerra MJ, Labandeira-Garcia JL. An automated rotarod method for quantitative drug-free evaluation of overall motor deficits in rat models of parkinsonism. Brain Res. Protoc. 1997;2:75–84. doi: 10.1016/s1385-299x(97)00034-2. [DOI] [PubMed] [Google Scholar]
- Sakoori K, Murphy NP. Central administration of nociceptin/orphanin FQ blocks the acquisition of conditioned place preference to morphine and cocaine, but not conditioned place aversion to naloxone in mice. Psychopharmacology. 2004;172:129–136. doi: 10.1007/s00213-003-1643-3. [DOI] [PubMed] [Google Scholar]
- Sanberg PR, Bunsey MD, Giordano M, Norman AB. The catalepsy test: its ups and downs. Behav. Neurosci. 1988;102:748–759. doi: 10.1037//0735-7044.102.5.748. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pernaute R, Jenkins BG, Choi JK, Iris Chen YG, Isacson O. In vivo evidence of D3 dopamine receptor sensitization in parkinsonian primates and rodents with l-DOPA-induced dyskinesias. Neurobiol. Dis. 2007;27:220–227. doi: 10.1016/j.nbd.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedelis M, Hofele K, Auburger GW, Morgan S, Huston JP, Schwarting RKW. MPTP susceptibility in the mouse: behavioural, neurochemical and histological analysis of gender and strain differences. Behav. Genet. 2000;30:171–182. doi: 10.1023/a:1001958023096. [DOI] [PubMed] [Google Scholar]
- Sedelis M, Schwarting RKW, Huston JP. Behavioral phenotyping of the MPTP mouse model of Parkinson’s disease. Behav. Brain Res. 2001;125:109–125. doi: 10.1016/s0166-4328(01)00309-6. [DOI] [PubMed] [Google Scholar]
- Varty GB, Hyde LA, Hodgson RA, Lu SX, McCool MF, Kazdoba TM, Del Vecchio RA, Guthrie DH, Pond AJ, Grzelak ME, Xu X, Korfmacher WA, Tulshian D, Parker EM, Higgins GA. Characterization of the nociceptin receptor (ORL-1) agonist, Ro64-6198, in tests of anxiety across multiple species. Psycopharmacology (Berl.) 2005;182:132–143. doi: 10.1007/s00213-005-0041-4. [DOI] [PubMed] [Google Scholar]
- Viaro R, Marti M, Morari M, Franchi G. Nociceptin/orphanin FQ receptors modulate locomotion and motor cortex excitability in adult rats. Acta Physiol. 2006;188:5. [Google Scholar]
- Watanabe Y, Kato H, Araki T. Protective action of neuronal nitric oxide synthase inhibitor in the MPTP mouse model of Parkinson’s disease. Metab. Brain Dis. 2008;23:51–69. doi: 10.1007/s11011-007-9080-3. [DOI] [PubMed] [Google Scholar]